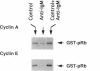Abstract
Cross-linking surface immunoglobulin (Ig)M on the WEHI-231 B-cell lymphoma results in decreased cell size, G1/S growth arrest, and finally DNA cleavage into oligonucleosomal fragments that are the classical features of apoptotic cells. Treatment of WEHI-231 cells with anti-IgM in early G1 phase prevents phosphorylation of the retinoblastoma gene product (pRb) and inhibits entry into S phase. Using unsynchronized cells, we previously demonstrated that cyclin A-associated and Cdk2-dependent GST-pRb kinase activity were inhibited in WEHI-231 cells treated with anti-IgM. We now show that progression of elutriated early G1 phase WEHI-231 cells from early into late G1 phase is accompanied by an increase in the abundance of cyclin A protein and cyclin A-associated kinase activity. Treatment of early G1 cells with anti-IgM prevented this increase in cyclin A-associated kinase activity at late G1, despite minimal changes in the overall level of cyclin A and Cdk2 proteins. Late G1 cells, which already possess high cyclin A-associated kinase activity, were insensitive to anti-IgM treatment and were able to complete the cell cycle. We also found that anti-IgM-treated cells contained increased amounts of the Cdk inhibitor protein p27Kip1. Essentially all of the cyclin A in treated cells was associated with p27, a result which we propose explains the lack of cyclin A/Cdk2 kinase activity. Accumulation of p27 in cyclin A kinase complexes, however, did not decrease the amount of Cdk2 bound to cyclin A. Thus, cross-linking IgM on growth-inhibitable B-cell lymphomas affects cyclin A kinase activity by increasing the levels of p27 in this complex, thus preventing productive pRb phosphorylation and leading to cell cycle arrest and subsequent apoptosis. These results are discussed in terms of the cell cycle restriction points that regulate lymphocyte function, as well as the lineage-specific differences in cell cycle control.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ohuchi T., Sumida S., Matsumoto K., Toyoshima K. Phosphorylation of the retinoblastoma protein by cdk2. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7900–7904. doi: 10.1073/pnas.89.17.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprelikova O., Xiong Y., Liu E. T. Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase. J Biol Chem. 1995 Aug 4;270(31):18195–18197. doi: 10.1074/jbc.270.31.18195. [DOI] [PubMed] [Google Scholar]
- Benhamou L. E., Cazenave P. A., Sarthou P. Anti-immunoglobulins induce death by apoptosis in WEHI-231 B lymphoma cells. Eur J Immunol. 1990 Jun;20(6):1405–1407. doi: 10.1002/eji.1830200630. [DOI] [PubMed] [Google Scholar]
- Buchkovich K., Duffy L. A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989 Sep 22;58(6):1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Bui K. C., Wu F., Buckley S., Wu L., Williams R., Carbonaro-Hall D., Hall F. L., Warburton D. Cyclin A expression in normal and transformed alveolar epithelial cells. Am J Respir Cell Mol Biol. 1993 Aug;9(2):115–125. doi: 10.1165/ajrcmb/9.2.115. [DOI] [PubMed] [Google Scholar]
- Bybee A., Thomas N. S. The synthesis of p58cyclin A and the phosphorylation of p34cdc2 are inhibited in human lymphoid cells arrested in G1 by alpha-interferon. Biochim Biophys Acta. 1992 Oct 6;1137(1):73–76. doi: 10.1016/0167-4889(92)90102-h. [DOI] [PubMed] [Google Scholar]
- Carbonaro-Hall D., Williams R., Wu L., Warburton D., Zeichner-David M., MacDougall M., Tolo V., Hall F. G1 expression and multistage dynamics of cyclin A in human osteosarcoma cells. Oncogene. 1993 Jun;8(6):1649–1659. [PubMed] [Google Scholar]
- Chen P. L., Scully P., Shew J. Y., Wang J. Y., Lee W. H. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989 Sep 22;58(6):1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- Connell-Crowley L., Solomon M. J., Wei N., Harper J. W. Phosphorylation independent activation of human cyclin-dependent kinase 2 by cyclin A in vitro. Mol Biol Cell. 1993 Jan;4(1):79–92. doi: 10.1091/mbc.4.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbon J. M., Devault A., Taviaux S., Fesquet D., Martinez A. M., Galas S., Cavadore J. C., Dorée M., Blanchard J. M. Cloning, expression and subcellular localization of the human homolog of p40MO15 catalytic subunit of cdk-activating kinase. Oncogene. 1994 Nov;9(11):3127–3138. [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Lynch D., Furukawa Y., Griffin J., Piwnica-Worms H., Huang C. M., Livingston D. M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989 Sep 22;58(6):1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- Dulić V., Kaufmann W. K., Wilson S. J., Tlsty T. D., Lees E., Harper J. W., Elledge S. J., Reed S. I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994 Mar 25;76(6):1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Durfee T., Becherer K., Chen P. L., Yeh S. H., Yang Y., Kilburn A. E., Lee W. H., Elledge S. J. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993 Apr;7(4):555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Whitehouse L. L., Livingston D. M. TGF beta inhibition of Cdk4 synthesis is linked to cell cycle arrest. Cell. 1993 Sep 24;74(6):1009–1020. doi: 10.1016/0092-8674(93)90723-4. [DOI] [PubMed] [Google Scholar]
- Ewen M. E. The cell cycle and the retinoblastoma protein family. Cancer Metastasis Rev. 1994 Mar;13(1):45–66. doi: 10.1007/BF00690418. [DOI] [PubMed] [Google Scholar]
- Firpo E. J., Koff A., Solomon M. J., Roberts J. M. Inactivation of a Cdk2 inhibitor during interleukin 2-induced proliferation of human T lymphocytes. Mol Cell Biol. 1994 Jul;14(7):4889–4901. doi: 10.1128/mcb.14.7.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G., Kent S. C., Joseph L., Green D. R., Scott D. W. Lymphoma models for B cell activation and tolerance. X. Anti-mu-mediated growth arrest and apoptosis of murine B cell lymphomas is prevented by the stabilization of myc. J Exp Med. 1994 Jan 1;179(1):221–228. doi: 10.1084/jem.179.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard F., Strausfeld U., Fernandez A., Lamb N. J. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991 Dec 20;67(6):1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- Hannon G. J., Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994 Sep 15;371(6494):257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Elledge S. J., Keyomarsi K., Dynlacht B., Tsai L. H., Zhang P., Dobrowolski S., Bai C., Connell-Crowley L., Swindell E. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995 Apr;6(4):387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbold J., Klaus G. G. Anti-immunoglobulin antibodies induce apoptosis in immature B cell lymphomas. Eur J Immunol. 1990 Aug;20(8):1685–1690. doi: 10.1002/eji.1830200810. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Brill J. A., Fink G. R., Weinberg R. A. Collaboration of G1 cyclins in the functional inactivation of the retinoblastoma protein. Genes Dev. 1994 Aug 1;8(15):1759–1771. doi: 10.1101/gad.8.15.1759. [DOI] [PubMed] [Google Scholar]
- Hinds P. W., Mittnacht S., Dulic V., Arnold A., Reed S. I., Weinberg R. A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992 Sep 18;70(6):993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- Hu Q. J., Lees J. A., Buchkovich K. J., Harlow E. The retinoblastoma protein physically associates with the human cdc2 kinase. Mol Cell Biol. 1992 Mar;12(3):971–980. doi: 10.1128/mcb.12.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994 Nov 18;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Ishida T., Kobayashi N., Tojo T., Ishida S., Yamamoto T., Inoue J. CD40 signaling-mediated induction of Bcl-XL, Cdk4, and Cdk6. Implication of their cooperation in selective B cell growth. J Immunol. 1995 Dec 15;155(12):5527–5535. [PubMed] [Google Scholar]
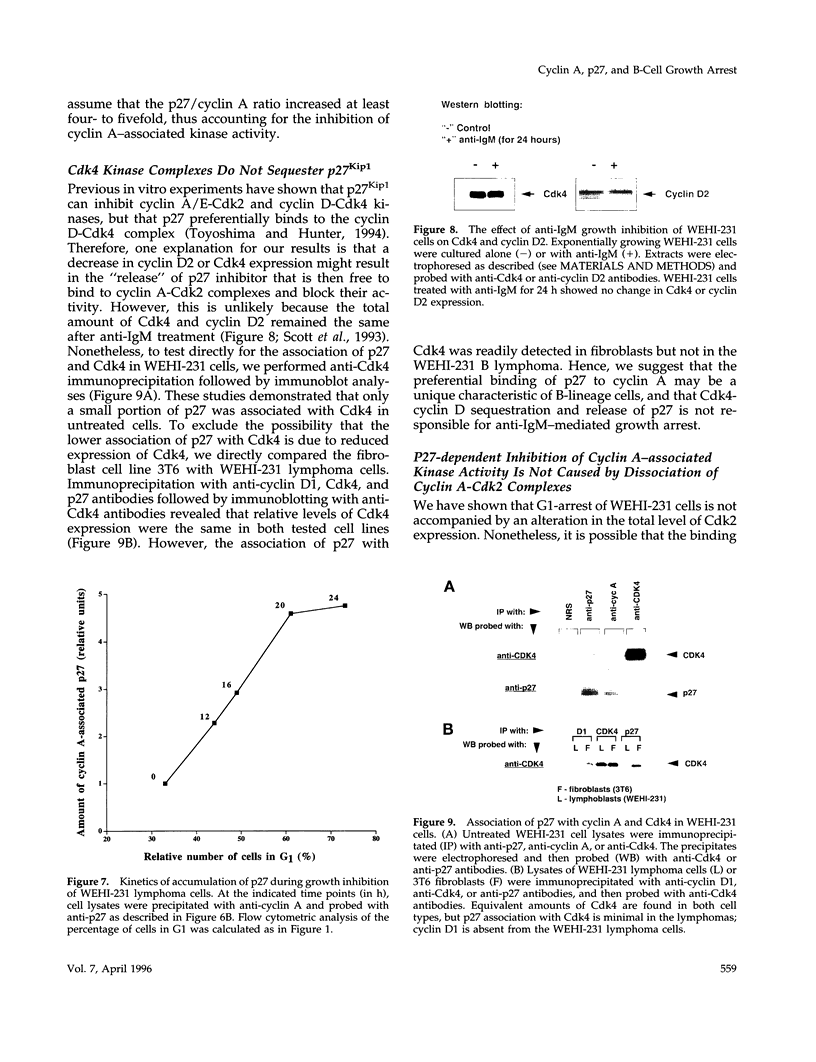
- Joseph L. F., Ezhevsky S., Scott D. W. Lymphoma models for B-cell activation and tolerance: anti-immunoglobulin M treatment induces growth arrest by preventing the formation of an active kinase complex which phosphorylates retinoblastoma gene product in G1. Cell Growth Differ. 1995 Jan;6(1):51–57. [PubMed] [Google Scholar]
- Kato J. Y., Matsuoka M., Polyak K., Massagué J., Sherr C. J. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994 Nov 4;79(3):487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Koff A., Giordano A., Desai D., Yamashita K., Harper J. W., Elledge S., Nishimoto T., Morgan D. O., Franza B. R., Roberts J. M. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992 Sep 18;257(5077):1689–1694. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- Koff A., Ohtsuki M., Polyak K., Roberts J. M., Massagué J. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-beta. Science. 1993 Apr 23;260(5107):536–539. doi: 10.1126/science.8475385. [DOI] [PubMed] [Google Scholar]
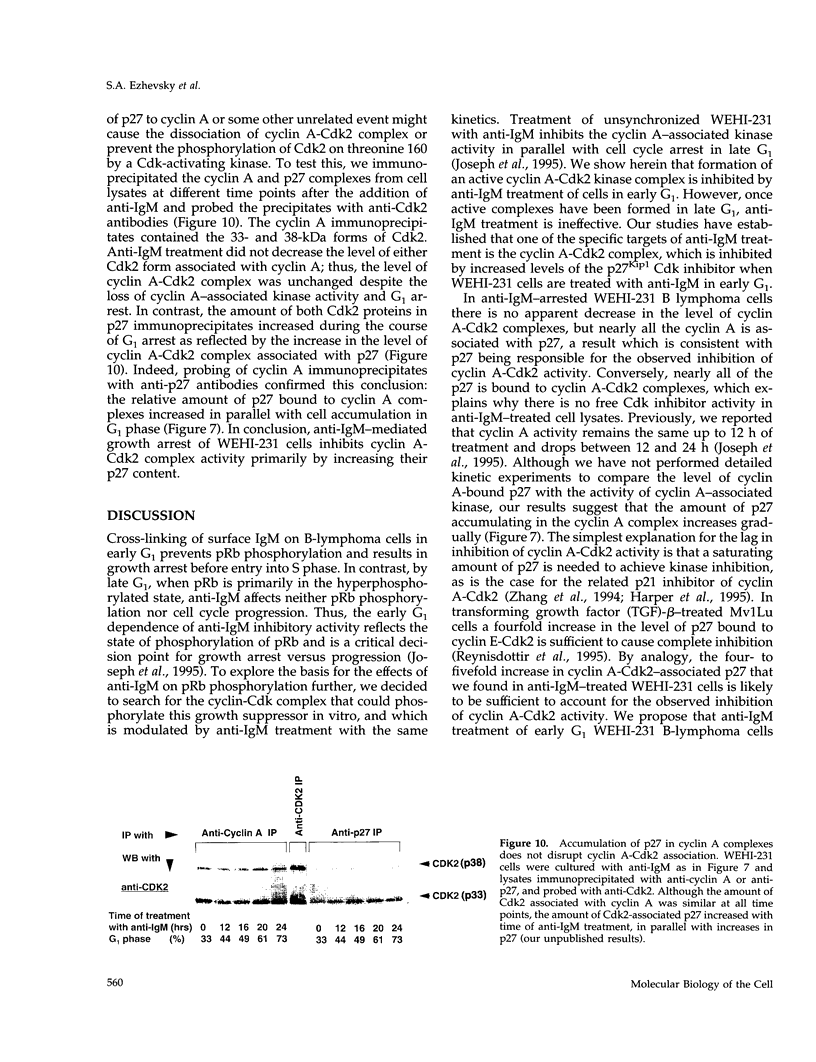
- La Thangue N. B. DRTF1/E2F: an expanding family of heterodimeric transcription factors implicated in cell-cycle control. Trends Biochem Sci. 1994 Mar;19(3):108–114. doi: 10.1016/0968-0004(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Lees E., Faha B., Dulic V., Reed S. I., Harlow E. Cyclin E/cdk2 and cyclin A/cdk2 kinases associate with p107 and E2F in a temporally distinct manner. Genes Dev. 1992 Oct;6(10):1874–1885. doi: 10.1101/gad.6.10.1874. [DOI] [PubMed] [Google Scholar]
- Lees J. A., Buchkovich K. J., Marshak D. R., Anderson C. W., Harlow E. The retinoblastoma protein is phosphorylated on multiple sites by human cdc2. EMBO J. 1991 Dec;10(13):4279–4290. doi: 10.1002/j.1460-2075.1991.tb05006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow J. W., Glendening C. L., Livingston D. M., DeCarprio J. A. Specific enzymatic dephosphorylation of the retinoblastoma protein. Mol Cell Biol. 1993 Jan;13(1):367–372. doi: 10.1128/mcb.13.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow J. W., Shon J., Pipas J. M., Livingston D. M., DeCaprio J. A. The retinoblastoma susceptibility gene product undergoes cell cycle-dependent dephosphorylation and binding to and release from SV40 large T. Cell. 1990 Feb 9;60(3):387–396. doi: 10.1016/0092-8674(90)90590-b. [DOI] [PubMed] [Google Scholar]
- Maheswaran S., McCormack J. E., Sonenshein G. E. Changes in phosphorylation of myc oncogene and RB antioncogene protein products during growth arrest of the murine lymphoma WEHI 231 cell line. Oncogene. 1991 Nov;6(11):1965–1971. [PubMed] [Google Scholar]
- Matsushime H., Ewen M. E., Strom D. K., Kato J. Y., Hanks S. K., Roussel M. F., Sherr C. J. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992 Oct 16;71(2):323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- Meyerson M., Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994 Mar;14(3):2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K., Cao X. R., Yen A., Chandler S., Driscoll B., Murphree A. L., T'Ang A., Fung Y. K. Cell cycle-dependent regulation of phosphorylation of the human retinoblastoma gene product. Science. 1989 Dec 8;246(4935):1300–1303. doi: 10.1126/science.2588006. [DOI] [PubMed] [Google Scholar]
- Mittnacht S., Weinberg R. A. G1/S phosphorylation of the retinoblastoma protein is associated with an altered affinity for the nuclear compartment. Cell. 1991 May 3;65(3):381–393. doi: 10.1016/0092-8674(91)90456-9. [DOI] [PubMed] [Google Scholar]
- Nourse J., Firpo E., Flanagan W. M., Coats S., Polyak K., Lee M. H., Massague J., Crabtree G. R., Roberts J. M. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994 Dec 8;372(6506):570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- Pagano M., Pepperkok R., Lukas J., Baldin V., Ansorge W., Bartek J., Draetta G. Regulation of the cell cycle by the cdk2 protein kinase in cultured human fibroblasts. J Cell Biol. 1993 Apr;121(1):101–111. doi: 10.1083/jcb.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M., Pepperkok R., Verde F., Ansorge W., Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992 Mar;11(3):961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M., Tam S. W., Theodoras A. M., Beer-Romero P., Del Sal G., Chau V., Yew P. R., Draetta G. F., Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995 Aug 4;269(5224):682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- Page D. M., DeFranco A. L. Antigen receptor-induced cell cycle arrest in WEHI-231 B lymphoma cells depends on the duration of signaling before the G1 phase restriction point. Mol Cell Biol. 1990 Jun;10(6):3003–3012. doi: 10.1128/mcb.10.6.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. Cyclins A and B1 in the human cell cycle. Ciba Found Symp. 1992;170:187–204. [PubMed] [Google Scholar]
- Polyak K., Kato J. Y., Solomon M. J., Sherr C. J., Massague J., Roberts J. M., Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994 Jan;8(1):9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Polyak K., Lee M. H., Erdjument-Bromage H., Koff A., Roberts J. M., Tempst P., Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994 Jul 15;78(1):59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Poon R. Y., Toyoshima H., Hunter T. Redistribution of the CDK inhibitor p27 between different cyclin.CDK complexes in the mouse fibroblast cell cycle and in cells arrested with lovastatin or ultraviolet irradiation. Mol Biol Cell. 1995 Sep;6(9):1197–1213. doi: 10.1091/mbc.6.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon R. Y., Yamashita K., Howell M., Ershler M. A., Belyavsky A., Hunt T. Cell cycle regulation of the p34cdc2/p33cdk2-activating kinase p40MO15. J Cell Sci. 1994 Oct;107(Pt 10):2789–2799. doi: 10.1242/jcs.107.10.2789. [DOI] [PubMed] [Google Scholar]
- Resnitzky D., Hengst L., Reed S. I. Cyclin A-associated kinase activity is rate limiting for entrance into S phase and is negatively regulated in G1 by p27Kip1. Mol Cell Biol. 1995 Aug;15(8):4347–4352. doi: 10.1128/mcb.15.8.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynisdóttir I., Polyak K., Iavarone A., Massagué J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 1995 Aug 1;9(15):1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J., Gu Y., Morgan D. O. Human cyclin-dependent kinase 2 is activated during the S and G2 phases of the cell cycle and associates with cyclin A. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2824–2828. doi: 10.1073/pnas.89.7.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. W. Analysis of B cell tolerance in vitro. Adv Immunol. 1993;54:393–425. doi: 10.1016/s0065-2776(08)60539-8. [DOI] [PubMed] [Google Scholar]
- Scott D. W., Livnat D., Pennell C. A., Keng P. Lymphoma models for B cell activation and tolerance. III. Cell cycle dependence for negative signalling of WEHI-231 B lymphoma cells by anti-mu. J Exp Med. 1986 Jul 1;164(1):156–164. doi: 10.1084/jem.164.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. W., Livnat D., Whitin J., Dillon S. B., Snyderman R., Pennell C. A. Lymphoma models for B cell activation and tolerance. V. Anti-Ig mediated growth inhibition is reversed by phorbol myristate acetate but does not involve changes in cytosolic free calcium. J Mol Cell Immunol. 1987;3(2):109–120. [PubMed] [Google Scholar]
- Sherr C. J. G1 phase progression: cycling on cue. Cell. 1994 Nov 18;79(4):551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Tanguay D. A., Chiles T. C. Cell cycle-specific induction of Cdk2 expression in B lymphocytes following antigen receptor cross-linking. Mol Immunol. 1994 Jun;31(9):643–649. doi: 10.1016/0161-5890(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Templeton D. J. Nuclear binding of purified retinoblastoma gene product is determined by cell cycle-regulated phosphorylation. Mol Cell Biol. 1992 Feb;12(2):435–443. doi: 10.1128/mcb.12.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima H., Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994 Jul 15;78(1):67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Tsai L. H., Lees E., Faha B., Harlow E., Riabowol K. The cdk2 kinase is required for the G1-to-S transition in mammalian cells. Oncogene. 1993 Jun;8(6):1593–1602. [PubMed] [Google Scholar]
- Warner G. L., Ludlow J. W., Nelson D. A., Gaur A., Scott D. W. Anti-immunoglobulin treatment of murine B-cell lymphomas induces active transforming growth factor beta but pRB hypophosphorylation is transforming growth factor beta independent. Cell Growth Differ. 1992 Mar;3(3):175–181. [PubMed] [Google Scholar]
- Zindy F., Lamas E., Chenivesse X., Sobczak J., Wang J., Fesquet D., Henglein B., Bréchot C. Cyclin A is required in S phase in normal epithelial cells. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1144–1154. doi: 10.1016/0006-291x(92)91851-g. [DOI] [PubMed] [Google Scholar]
- van den Heuvel S., Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993 Dec 24;262(5142):2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]