Abstract
Fungi of the genus Trichoderma are used as biocontrol agents against several plant pathogenic fungi like Rhizoctonia spp., Pythium spp., Botrytis cinerea and Fusarium spp. which cause both soil-borne and leaf- or flower-borne diseases of agricultural plants. Plant disease control by Trichoderma is based on complex interactions between Trichoderma, the plant pathogen and the plant. Until now, two main components of biocontrol have been identified: direct activity of Trichoderma against the plant pathogen by mycoparasitism and induced systemic resistance in plants. As the mycoparasitic interaction is host-specific and not merely a contact response, it is likely that signals from the host fungus are recognised by Trichoderma and provoke transcription of mycoparasitism-related genes.
In the last few years examination of signalling pathways underlying Trichoderma biocontrol started and it was shown that heterotrimeric G-proteins and mitogen-activated protein (MAP) kinases affected biocontrol-relevant processes such as the production of hydrolytic enzymes and antifungal metabolites and the formation of infection structures. MAPK signalling was also found to be involved in induction of plant systemic resistance in Trichoderma virens and in the hyperosmotic stress response in Trichoderma harzianum. Analyses of the function of components of the cAMP pathway during Trichoderma biocontrol revealed that mycoparasitism-associated coiling and chitinase production as well as secondary metabolism are affected by the internal cAMP level; in addition, a cross talk between regulation of light responses and the cAMP signalling pathway was found in Trichoderma atroviride.
Keywords: Trichoderma, biocontrol, mycoparasitism, signal transduction
Introduction
Fungal species of the genus Trichoderma occur worldwide and can be isolated from soil, decaying wood and other forms of plant organic material. Mycoparasitic Trichoderma species are used commercially as biological control agents against plant-pathogenic fungi such as Rhizoctonia solani, Botrytis cinerea, Sclerotium rolfsii, Sclerotinia sclerotiorum, Pythium spp., and Fusarium spp. in, among others, the United States, India, Israel, New Zealand, and Sweden as a promising alternative to chemical pesticides (Barak and Chet, 1986; Chet, 1987; Harman and Björkman, 1987; Howell, 2003).
What currently is defined as biocontrol is a combination of different mechanisms working synergistically to achieve disease control (Howell, 2003). Trichoderma mycoparasitism combines processes such as nutrient competition (Chet, 1987), the secretion of antifungal metabolites (e.g. Dennis and Webster, 1971; Claydon et al. 1987; Schirmböck et al. 1994; Lorito et al. 1996) and formation of morphological changes such as coiling around the host and development of appressorium-like structures (Elad et al. 1983; Lu et al. 2004). As mycoparasitism by Trichoderma results in penetration of the cell wall of the host fungus and utilization of its cellular contents, hydrolytic enzymes such as chitinases, glucanases, and proteases, which are at least partially induced before direct contact with the host, play major roles in biocontrol (Hjeljord and Tronsmo, 1998). Chitinase gene expression is induced in liquid culture by e.g. fungal cell walls, colloidal chitin, or the chitin monomer N-acetylglucosamine (Kubicek et al. 2001). In Trichoderma atroviride the N-acetylglucosaminidase-encoding gene nag1 has a major impact on the induction by chitin of other chitinases (Brunner et al. 2003). In mycoparasitic interactions between Trichoderma and R. solani, a diffusible factor released from the host is responsible for induction of ech42 (endochitinase 42-encoding) gene transcription before physical contact (Cortes et al. 1998; Zeilinger et al. 1999). Upon direct contact, lectins in the host’s cell wall can induce coiling of the mycoparasite around the host hyphae (Barak and Chet, 1986; Inbar and Chet, 1994). The current model suggests that both enzyme production and infection structure formation are induced responses triggered by molecules released from the host fungus (e.g. degradation products from its cell wall) or located on its surface (e.g. lectins) (Inbar and Chet, 1992; Zeilinger et al. 1999).
The expression of genes associated with bio-control appears to be regulated by intracellular signal transduction pathways, which are activated by the binding of host-derived ligands to as yet unidentified receptors. The elucidation of these pathways has recently begun and has confirmed the involvement of highly conserved signalling components.
The Role of G Protein Signalling in Trichoderma Biocontrol
Heterotrimeric G protein signalling is basically comprised of three parts: a G protein-coupled receptor (GPCR), a heterotrimeric G protein (α, β, γ subunits), and an effector (Neer, 1995). More than 20 years ago, the first GPCR-encoding gene was cloned and now more than 1000 have been described and characterized, mainly of vertebrate origin (Kolakowski, 1994). The overall amino acid sequence similarities between GPCRs are low, but these receptor proteins all have in common seven transmembrane domains and have the N-terminus outside and the C-terminus inside the cytoplasm. Ligand binding to the receptor results in a conformational change leading to release of the G protein and exchange of GDP for GTP on the Gα subunit. GTP-bound α dissociates from its βγ partner, allowing both signalling units to regulate the activities of downstream effectors (Kaziro et al. 1991; Birnbaumer et al. 1992; Neer, 1995; Gutkind, 1998).
Due to only minimal amino acid similarities between GPCRs, intensive research depended on the release of genome sequences. Numerous fungal genomes are available nowadays and comparative genomics pointed out that receptors can be classified into nine groups (Lafon et al. 2006): classes I and II comprise pheromone receptors with similarity to the yeast Ste2p and Ste3p receptors; classes III and V contain putative carbon source receptors and cAMP-sensors; class IV comprises Schizosaccharomyces pombe Stm1p-like nitrogen sensors; class VI, which is characteristic of filamentous fungi, comprises receptors with an RGS domain downstream of their transmembrane regions; classes VII and VIII have been identified only recently and share similarities with some vertebrate receptors; class IX comprises fungal opsins, similar to the bacterial retinal-binding rhodopsin, with Neurospora crassa NOP-1 and ORP-1 as prominent members (Borkovich et al. 2004). Preliminary investigations of the Trichoderma reesei (http://genome.jgi-psf.org/Trire2/Trire2.home.html) and T. atroviride (unpublished; release in progress) genomes revealed at least 16 putative proteins with 7-transmembrane domains, well distributed over all nine receptor classes (Brunner and Zeilinger, unpublished).
Highly conserved heterotrimeric G-proteins act as signal transducers that couple cell surface receptors to cytoplasmic effector proteins. In fungi, G-proteins are essential during sexual and pathogenic development and during secondary metabolism. They are part of the pheromone signalling cascade and also affect a number of developmental and morphogenetic processes which determine the virulence of fungi and plant fungal pathogens (e.g. Bölker, 1998).
G-protein α subunits can be classified into three major subgroups (Bölker, 1998). Members of subgroup I are homologues of the mammalian Gαi subunits that inhibit adenylate cyclase (Turner and Borkovich,1993). Only a few members of subgroup II of fungal Gα proteins, which are characterized by the lack of the consensus site for pertussis toxin-dependent ribosylation, have so far been associated with a biological function or a distinct phenotype. Members of the fungal subgroup III, which are homologues of the mammalian Gαs family, have been implicated to positively influence the internal cAMP level (Bölker, 1998).
Rocha-Ramirez et al. (2002) analyzed the T. atroviride subgroup I Gα subunit Tga1 by tga1 over-expression and tga1 gene silencing and showed that it is involved in both coiling and conidiation. tga1 gene deletion confirmed these findings but revealed that some of the observed effects were more distinct in the deletion mutant (Reithner et al. 2005). Further characterisation of the Δtga1 mutant showed that this G-protein α subunit affects processes like vegetative growth, production of antifungal metabolites, and chitinase formation (Reithner et al. 2005), which are at least partially involved in Trichoderma biocontrol. In liquid culture the Δtga1 mutant produced strongly decreased chitinase activities and showed a reduced transcription of the nag1 (N-acetyl-glucosaminidase-encoding) and ech42 (endochitinase 42-encoding) genes (Reithner et al. 2005). In antagonistic assays, the Δtga1 mutant was unable to overgrow and lyse host fungi such as R. solani, B. cinerea, and S. sclerotiorum, although infection structure formation was unaffected; nevertheless, it displayed an enhanced growth inhibition of the host fungi by over-producing and secreting low molecular weight metabolites. To get insights into the nature of these over-produced substances which caused the increase in host growth inhibition, the production of some main secondary metabolites produced by T. atroviride was analyzed. Interestingly, the production of 6-pentyl-α-pyrone and of metabolites with sesquiterpene structure was reduced in the Δtga1 mutant (Reithner et al. 2005), while it produced elevated amounts of peptaibols belonging to the trichorzianine family (Stoppacher, Zeilinger, Schuhmacher, unpublished), suggesting opposite roles of Tga1 in regulating the biosynthesis of different antifungal substances in T. atroviride.
In contrast to the role of Tga1 in influencing growth and conidiation in T. atroviride, its homologue TgaA did not affect these properties in Trichoderma virens. ΔtgaA mutants grew normally and sporulated like the wild type, but had a reduced ability to colonise S. rolfsii sclerotia, whereas they were fully pathogenic against R. solani (Mukherjee et al. 2004). No such host specificity could be observed in the T. atroviride Δtga1 mutant.
Mutants of T. virens lacking the TgaB protein (belonging to subgroup II Gα subunits) did not show major phenotypic defects: they grew and sporulated like the wild type and biocontrol against R. solani and sclerotia of S. sclerotiorum was unaffected (Mukherjee et al. 2004).
Characterization of T. atroviride Tga3 revealed involvement of this subgroup III Gα subunit in regulating vegetative growth, conidiation, and conidial germination (Zeilinger et al. 2005). Δtga3 mutants had reduced intracellular cAMP levels and were avirulent in direct confrontation assays against R. solani or B. cinerea. In addition, mycoparasitism-related infection structures were not formed, strongly suggesting a loss of host recognition. Δtga3 mutants produced reduced extracellular chitinase activity even though the chitinase-encoding genes ech42 and nag1 were transcribed at a significantly higher rate than they were in the wild type. The observed accumulation of chitinolytic enzymes inside the cell and their retention in the cell wall may be due to an influence of Tga3 on chitinase secretion. Addition of exogenous cAMP did not suppress the altered phenotype or restored mycoparasitic overgrowth, although it did restore the ability to produce infection structures (Zeilinger et al. 2005). In planta biocontrol assays confirmed that Δtga3 mutants were unable to protect bean plants against infection with R. solani (Lorito and Zeilinger, unpublished).
Mitogen-Activated Protein Kinases in Trichoderma Biocontrol
A variety of signals are transduced by mitogen-activated protein kinase (MAPK) cascades through sequential activation of serine/threonine protein kinases by phosphorylation resulting in the control of gene expression required by a plurality of biological processes in eukaryotes (Banuett, 1998; Schaeffer and Weber, 1999). MAPK cascades are evolutionarily conserved in all eukaryotes: they are typically organised in a three kinase architecture consisting of a MAPK, a MAPK activator (MEK, MKK or MAPK kinase) and a MEK activator (MEK kinase = MEKK or MAPK kinase kinase). In yeasts there are five MAPK genes transmitting signals for mating, filamentous growth, cell integrity, response to osmotic stress, and ascospore formation (Gustin et al. 1998). In fungi, genes encoding MAPK homologues are essential for developmental processes such as sporulation, mating, hyphal growth, and pathogenicity (Xu, 2000); nevertheless, examples of complete MAP kinase modules examined in filamentous fungi are rare.
The best studied MAPKs in Trichoderma belong to the family of yeast and fungal extracellular-related kinases (YERK1), a class also comprising MAPKs such as Pmk1 from Magnaporthe grisea, Fmk1 from Fusarium oxysporum, Bmp1 from B. cinerea, or Ubc3/Kpp2 from Ustilago maydis.
The T. virens MAPK homolog belonging to the YERK1 class was described by two different groups: although tmkA and tvk1 encode the same protein in different strains of T. virens, contradictory results concerning the role of this MAP kinase in the production of mycoparasitism-related enzymes have been reported (Mendoza-Mendoza et al. 2003; Mukherjee et al. 2003). Enzyme activities of chitinases and proteases were described to be elevated in Δtvk1 mutants, but ΔtmkA strains showed a delay and reduction in clearing a chitin-containing medium. Moreover, Mukherjee et al. (2003) postulated that ΔtmkA mutants lost their biocontrol potential in a host-specific manner as they exhibited mycoparasitic coiling and lysis of R. solani hyphae similar to the wild type T. virens IMI304061, whereas they had reduced antagonistic properties in confrontation assays against S. rolfsii and they failed to parasitize the sclerotia of this pathogen. On the other hand, Δtvk1 mutants of T. virens Gv29-8 were reported to show a clear increase in the expression level of mycoparasitism-related genes during direct confrontation with R. solani and they were described to be more effective in disease control than the wild type or the chemical fungicide Apron (Mendoza-Mendoza et al. 2003). Further studies on the role of Tvk1 revealed that this MAPK regulates conidiation, hydrophobicity and the expression of genes coding for cell wall proteins during development of T. virens (Mendoza-Mendoza et al. 2007).
Recently, the corresponding MAPK from T. atroviride (Tmk1) was also characterized and showed 98% identity with T. virens TmkA/Tvk1 (Reithner et al. 2007). Δtmk1 mutants exhibited decreased radial growth and showed light-independent conidiation. Results from direct plate confrontation assays against R. solani and B. cinerea as hosts suggested that T. atroviride Tmk1 – similar to T. virens TmkA – affected the host specificity as Δtmk1 mutants still could parasitize R. solani (although less effectively than the parental strain) whereas they were unable to attack B. cinerea. Microscopic analysis of hyphae of the T. atroviride Δtmk1 mutants during interaction with R. solani revealed that they were still able to attach and coil around the host. Moreover, deletion of tmk1 caused an extensive, host-independent coiling around the own hyphae and attachment to plain Nylon fibers. In Δtmk1 mutants, nag1 and ech42 transcript levels as well as extracellular chitinase activities were significantly elevated under chitinase-inducing conditions and the mutants also showed a de-regulation of the production of antifungal metabolites (e.g. increased production of 6-pentyl-α-pyrone and peptaibols). Consistent with the finding that mycoparasitism-related processes, such as infection structure formation (coiling) and chitinase and antifungal metabolite production, were enhanced upon tmk1 gene deletion, Δtmk1 mutants showed an improved ability to protect bean plants against R. solani infection (Reithner et al. 2007).
It has recently been recognized that the basis for plant protection by Trichoderma is not only the direct mycoparasitic interaction with the pathogen but also a systemic defence response induced in the plant by colonization of its roots by Trichoderma (Harman et al. 2004). The role of MAPK signalling in inducing plant systemic resistance during Trichoderma-plant interaction was recently examined using T. virens ΔtmkA mutants. When Trichoderma spores were germinated in proximity to cucumber roots, ΔtmkA mutants were able to colonize the plant roots as effectively as the wild type strain but they failed to induce full systemic resistance against the bacterial leaf pathogen Pseudomonas syringae pv. lacrymans suggesting that T. virens needs MAPK signalling in order to induce full systemic resistance in the plant (Viterbo et al. 2005).
T. harzianum ThHog1, which is highly similar to Hog1p controlling the osmotic stress response in S. cerevisiae (Brewster et al. 1993), was recently characterized (Delgado-Jarana et al. 2006). ThHog1 was shown to be phosphorylated under different stress conditions such as hyper-osmotic or oxidative stress and the protein was demonstrated to be localized in nuclei under these stress conditions. The main role of ThHog1 was proposed to be in the hyper-osmotic stress response since a hog1-silenced mutant was highly sensitive to osmotic stress and showed intermediate levels of resistance against oxidative stress (Delgado-Jarana et al. 2006). A T. harzianum mutant strain carrying a hog1 F315S allele was highly resistant to the calcineurin inhibitor cyclosporine A, suggesting links between the two pathways. In plate confrontation assays the two mutant strains had strongly reduced antagonistic activity against the plant pathogens Phoma betae and Colletotrichum acutatum but no changes in the mycoparasitic ability against B. cinerea, R. solani and S. sclerotiorum. It was proposed that ThHog1 could be involved in neutralizing stress agents, such as reactive oxygen species, produced by the parasitized fungi during the mycoparasitic action (Delgado-Jarana et al. 2006).
cAMP Signalling During Trichoderma Biocontrol
In fungi, cAMP signalling is involved in a variety of processes including the control of differentiation, sexual development, virulence, monitoring of the nutrititional status, and stress. The cAMP pathway also influences transcription and cell cycle progression (Kronstad et al. 1998). cAMP levels are regulated by a membrane-associated adenylate cyclase for synthesis and a cAMP-specific phosphodiesterase for degradation. Activity of adenylate cyclase, which synthesizes the intracellular messenger cAMP, has been shown to be regulated by α subunits of heterotrimeric G-proteins in most fungi. Most of the effects of cAMP in eukaryotes occur via the stimulation of cAMP-dependent protein kinases (PKA) which consist of two regulatory and two catalytic subunits (Dickman and Yarden, 1999).
In plant pathogenic fungi, processes like growth, morphogenesis, and virulence are known to involve functional PKA (e.g. Xu and Hamer, 1996; Dürrenberger et al. 1998).
cAMP was reported to act as a positive effector of endoglucanase induction by enhancing the efficacy of the induction process in T. reesei, a species which was recently shown to be able to antagonize and overgrow Pythium ultimum and to provide protection of zucchini plants against P. ultimum blight in planta (Sestak and Farkas, 1993; Seidl et al. 2006). In T. harzianum, application of exogenous cAMP increased coiling around nylon fibres in the biomimetic system (Omero et al. 1999) and substances which increase the intracellular levels of cAMP (e.g. dinitrophenol, caffeine, aluminium tetra fluoride) repressed the synthesis of N-acetyl-β-D- glucosaminidase (Silva et al. 2004).
The role of cAMP signalling during conidiation was investigated in T. viride and T. atroviride (Nemcovic and Farkas, 1998; Casas-Flores et al. 2006). The production of conidia is the main mechanism for Trichoderma to survive and disperse in the environment and conidiation is induced by environmental factors such as blue light and nutrient stresses in these mycoparasites. In T. viride, the photoinduction of conidiation is accompanied by a rapid temporal rise in the intracellular level of cAMP (Gresik et al. 1988), and exogenous cAMP stimulated the formation of conidia in both illuminated colonies and in colonies that were kept in the dark (Nemcovic and Farkas, 1998). Recently, a gene (pkr-1) encoding the regulatory subunit of protein kinase A (PKA) from T. atroviride was cloned and characterized and the authors demonstrated that PKA plays an important role in the regulation of light responses in this fungus (Casas-Flores et al. 2006). Mukherjee et al. (2007) recently reported on the cloning of an adenylate cyclase-encoding gene (tac1) of T. virens, whose deletion lowered the intracellular cAMP levels to below the detection limit. Δtac1 mutants showed heavily reduced growth rates on agar, did not sporulate in darkness, were unable to overgrow host fungi like S. rolfsii, R. solani, and Pythium sp., and exhibited reduced secondary metabolite production.
Conclusions
Mycoparasitism is a fungus-fungus interaction comprising host-pathogen cross-talk. Until now, only little information has been available on the role of conserved eukaryotic signalling pathways during this interaction.
Isolation and characterization of selected components involved in different signal transduction pathways of mycoparasitic and biocontrol-active Trichoderma strains recently started and revealed high sequence conservation to homologous proteins from other fungi.
Signalling pathways involving G-protein α subunits as well as mitogen-activated protein kinases have been repeatedly shown to be important for virulence in both plant pathogens as well as mycoparasites. Therefore, G protein-coupled receptors are promising targets as they are putatively involved in host recognition and may be responsible for triggering responses resulting in e.g. host attack.
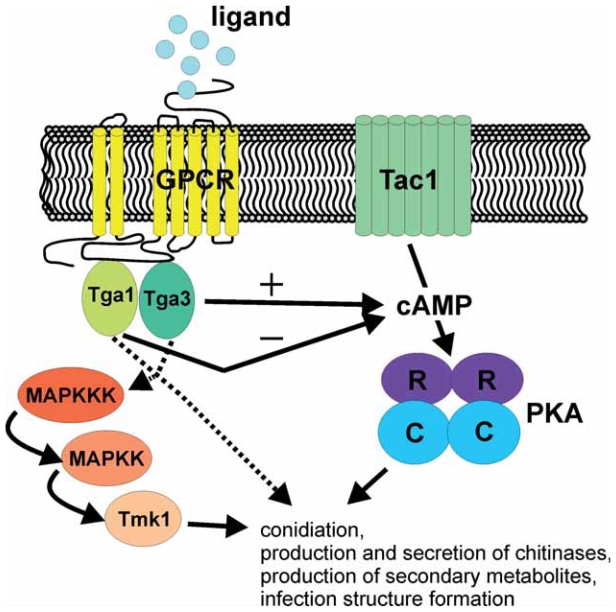
Research on G-protein signalling in Trichoderma spp. revealed that they have members of each fungal Gα subgroup. In T. atroviride, both the subgroup I and III Gα subunits Tga1 and Tga3 directly affect mycoparasitism-related processes by having overlapping roles in the regulation of mycoparasitism-related genes (Fig. 1). For both Tga1 and Tga3, an interaction with the cAMP pathway was found, as the Δtga1 mutant exhibited an elevated internal cAMP level, whereas Δtga3 mutants had reduced internal cAMP levels compared to the wild type control. Although not all processes contributing to mycoparasitism could be restored by exogenous cAMP in the T. atroviride G protein mutants, it was shown that Tga1 affected antifungal metabolite production by signalling via the cAMP pathway, whereas Tga3 is involved in infection structure formation in a cAMP-dependent manner. Furthermore, PKA was shown to play an important role in the regulation of light responses in T. atroviride, and adenylate cyclase was reported to be involved in growth, germination and mycoparasitism in T. virens.
Figure 1.
Schematic representation of selected components of mycoparasitism- related signalling pathways of T. atroviride. Tga1, Tga3: subgroup I and III G-protein α subunits; GPCR: G protein-coupled receptor; Tac1: adenylate cyclase; PKA: protein kinase A regulatory (R) and catalytic (C) subunits; Tmk1: MAP kinase
In addition to Gα proteins and the cAMP pathway, the T. atroviride MAPK Tmk1 (Fig. 1) and the T. virens MAPK TmkA/Tvk1 also directly affect transcription of mycoparasitism-related genes, suggesting that Tga1/TgaA and Tga3 could also be connected with the Tmk1/TmkA-containing MAP kinase cascade in regulating Trichoderma mycoparasitism-associated gene transcription.
Acknowledgements
The described work performed by S. Zeilinger and co-workers on T. atroviride signal transduction was supported by grants from the Fonds zur Förderung Wissenschaftlicher Forschung (FWF P15483 and P18109).
References
- Banuett F. Signalling in the yeasts: an informational cascade with links to the filamentous fungi. Microbiol Mol Biol Rev. 1998;62(2):249–74. doi: 10.1128/mmbr.62.2.249-274.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak R, Chet I. Determination, by fluorescein diacetate staining, of fungal viability during mycoparasitism. Soil Biol Biochem. 1986;18:315–9. [Google Scholar]
- Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for beta gamma dimers as well as alpha subunits. Cell. 1992;71(7):1069–72. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- Bolker M. Sex and crime: heterotrimeric G proteins in fungal mating and pathogenesis. Fungal Genet Biol. 1998;25(3):143–56. doi: 10.1006/fgbi.1998.1102. [DOI] [PubMed] [Google Scholar]
- Borkovich KA, Alex LA, Yarden O, et al. Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol Mol Biol Rev. 2004;68(1):1–108. doi: 10.1128/MMBR.68.1.1-108.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster JL, de Valoir T, Dwyer ND, et al. An osmosensing signal transduction pathway in yeast. Science. 1993;259(5102):1760–3. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Brunner K, Peterbauer C, Mach R, et al. The Nag1 N-acetylglucosaminidase of Trichoderma atroviride is essential for chitinase induction by chitin and of major relevance to biocontrol. Curr Genet. 2003;43(4):289–95. doi: 10.1007/s00294-003-0399-y. [DOI] [PubMed] [Google Scholar]
- Casas-Flores S, Rios-Momberg M, Rosales-Saavedra T, et al. Cross talk between a fungal blue-light perception system and the cyclic AMP signaling pathway. Eukaryot Cell. 2006;5(3):499–506. doi: 10.1128/EC.5.3.499-506.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chet I. Innovative Approaches to Plant Disease Control. New York: Wiley and Sons; 1987. Trichoderma-Application, mode of action, and potential as a biocontrol agent of soilborne pathogenic fungi; pp. 137–60. [Google Scholar]
- Claydon N, Allan M, Hanson JR, et al. Antifungal Alkyl Pyrones of Trichoderma harzianum. Transactions British Mycological Society. 1987;88(4):503–13. [Google Scholar]
- Cortes C, Gutierrez A, Olmedo V, et al. The expression of genes involved in parasitism by Trichoderma harzianum is triggered by a diffusible factor. Mol Genet Genomics. 1998;260:218–25. doi: 10.1007/s004380050889. [DOI] [PubMed] [Google Scholar]
- Delgado-Jarana J, Sousa S, Gonzalez F, et al. ThHog1 controls the hyperosmotic stress response in Trichoderma harzianum. Microbiology. 2006;152(6):1687–700. doi: 10.1099/mic.0.28729-0. [DOI] [PubMed] [Google Scholar]
- Dennis C, Webster J. Antagonistic properties of species-groups of Trichoderma. Transactions British Mycological Society. 1971;57(1):25–39. [Google Scholar]
- Dickman MB, Yarden O. Serine/threonine protein kinases and phosphatases in filamentious fungi. Fungal Genet Biol. 1999;26(2):99–117. doi: 10.1006/fgbi.1999.1118. [DOI] [PubMed] [Google Scholar]
- Durrenberger F, Wong K, Kronstad JW. Identification of a cAMP-dependent protein kinase catalytic subunit required for virulence and morphogenesis in Ustilago maydis. Proc Natl Acad Sci USA. 1998;95(10):5684–9. doi: 10.1073/pnas.95.10.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elad Y, Chet I Boyle. et al. Parasitism of Trichoderma spp. on Rhizoctonia solani and Sclerotium rolfsii – SEM studies and fluorescence microscopy. Phytopathology. 1983;73:85–8. [Google Scholar]
- Gresik M, Kolarova N, Farkas V. Membrane potential, ATP and cyclic AMP changes induced by light in Trichoderma viride. Exp Mycol. 1988;12:295–301. [Google Scholar]
- Gustin M C, Albertyn J, Alexander M, et al. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1998;62(4):1264–300. doi: 10.1128/mmbr.62.4.1264-1300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkind JS. Cell growth control by G protein-coupled receptors: from signal transduction to signal integration. Oncogene. 1998;17(11 Reviews):1331–42. doi: 10.1038/sj.onc.1202186. [DOI] [PubMed] [Google Scholar]
- Harman GE, Björkman T. Potential and existing uses of Trichoderma and Gliocladium for, plant disease control and plant growth enhancement. In: Harman GE, Kubicek CP, editors. Trichoderma and Gliocladium. Vol. 2. London: Taylor and Francis; 1987. pp. 229–65. [Google Scholar]
- Harman GE, Howell CR, Viterbo A, et al. Trichoderma species - opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- Hjeljord L, Tronsmo A. Trichoderma and Gliocladium in biological control: an overview. In: Harman GE, Kubicek CP, editors. Trichoderma and Gliocladium. London: Taylor and Francis; 1998. pp. 131–52. [Google Scholar]
- Howell CR. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Disease. 2003;87(1):4–10. doi: 10.1094/PDIS.2003.87.1.4. [DOI] [PubMed] [Google Scholar]
- Inbar J, Chet I. Biomimics of fungal cell-cell recognition by use of lectin-coated nylon fibers. J Bacteriol. 1992;174(3):1055–9. doi: 10.1128/jb.174.3.1055-1059.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar J, Chet I. A newly isolated lectin from the plant pathogenic fungus Sclerotium rolfsii: purification, characterization and role in mycoparasitism. Microbiology. 1994;140(3):651–7. doi: 10.1099/00221287-140-3-651. [DOI] [PubMed] [Google Scholar]
- Kaziro Y, Itoh H, Kozasa T, et al. Structure and function of signal-transducing GTP-binding proteins. Annu Rev Biochem. 1991;60:349–400. doi: 10.1146/annurev.bi.60.070191.002025. [DOI] [PubMed] [Google Scholar]
- Kolakowski LF., Jr GCRDb: a G-protein-coupled receptor database. Receptors Channels. 1994;2(1):1–7. [PubMed] [Google Scholar]
- Kronstad J, De Maria AD, Funnell D, et al. Signalling via cAMP in fungi: interconnections with mitogen-activated protein kinase pathways. Arch Microbiol. 1998;170(6):395–404. doi: 10.1007/s002030050659. [DOI] [PubMed] [Google Scholar]
- Kubicek CP, Mach RL, Peterbauer CK, et al. Trichoderma: from fenes to biocontrol. J Plant Pathol. 2001;83(2):11–23. [Google Scholar]
- Lafon A, Han KH, Seo JA, et al. G-protein and cAMP-mediated signaling in Aspergilli: a genomic perspective. Fungal Genet Biol. 2006;43(7):490–502. doi: 10.1016/j.fgb.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Lorito M, Woo SL, D’Ambrosio M, et al. Synergistic interaction between cell wall degrading enzymes and membrane affecting compounds. Mol Plant Microbe Interact. 1996;9:206–13. [Google Scholar]
- Lu Z, Tombolini R, Woo S, et al. In vivo study of Trichoderma-pathogen-plant interactions, using constitutive and inducible green fluorescent protein reporter systems. Appl Environ Microbiol. 2004;70(5):3073–81. doi: 10.1128/AEM.70.5.3073-3081.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Mendoza A, Pozo MJ, Grzegorski D, et al. Enhanced biocontrol activity of Trichoderma through inactivation of a mitogen-activated protein kinase. Proc Natl Acad Sci USA. 2003;100(26):15965–70. doi: 10.1073/pnas.2136716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Mendoza A, Rosales-Saavedra T, Cortes C. The MAP kinase TVK1 regulates conidiation, hydrophobicity and the expression of genes encoding cell wall proteins in the fungus Trichoderma virens. Microbiology. 2007;153:2137–47. doi: 10.1099/mic.0.2006/005462-0. [DOI] [PubMed] [Google Scholar]
- Mukherjee PK, Latha J, Hadar R, et al. TmkA, a mitogen-activated protein kinase of Trichoderma virens, is involved in biocontrol properties and repression of conidiation in the dark. Eukaryot Cell. 2003;2(3):446–55. doi: 10.1128/EC.2.3.446-455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Latha J, Hadar R, et al. Role of two G-protein alpha subunits, TgaA and TgaB, in the antagonism of plant pathogens by Trichoderma virens. Appl Environ Microbiol. 2004;70(1):542–9. doi: 10.1128/AEM.70.1.542-549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee M, Mukherjee PK, Kale SP. cAMP signalling is involved in growth, germination, mycoparasitism and secondary metabolism in Trichoderma virens. Microbiology. 2007;153:1734–42. doi: 10.1099/mic.0.2007/005702-0. [DOI] [PubMed] [Google Scholar]
- Neer EJ. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995;80(2):249–57. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- Nemcovic M, Farkas V. Stimulation of conidiation by derivates of cAMP in Trichoderma viride. Folia Microbiologica. 1998;43(4):399–402. [Google Scholar]
- Omero C, Inbar J, Rocha-Ramirez V, et al. G protein activators and cAMP promote mycoparasitic behaviour in Trichoderma harzianum. Mycol Res. 1999;103(12):1637–42. [Google Scholar]
- Reithner B, Brunner K, Schuhmacher R, et al. The G protein alpha subunit Tga1 of Trichoderma atroviride is involved in chitinase formation and differential production of antifungal metabolites. Fungal Genet Biol. 2005;42(9):749–60. doi: 10.1016/j.fgb.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Reithner B, Schuhmacher R, Stoppacher N, et al. Signaling via the Trichoderma atroviride mitogen-activated protein kinase Tmk1 differentially affects mycoparasitism and plant protection. Fungal Genet Biol. 2007 doi: 10.1016/j.fgb.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Ramirez V, Omero C, Chet I, et al. Trichoderma atroviride G-protein alpha-subunit gene tga1 is involved in mycoparasitic coiling and conidiation. Eukaryot Cell. 2002;1(4):594–605. doi: 10.1128/EC.1.4.594-605.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19(4):2435–44. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmböck M, Lorito M, Wang Y-L, et al. Parallel formation and synergism of hydrolytic enzymes and peptaibol antibiotics, molecular mechanisms involved in the antagonistic action of Trichoderma harzianum against phytopathogenic fungi. Appl Environ Microbiol. 1994;60:4364–70. doi: 10.1128/aem.60.12.4364-4370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl V, Schmoll M, Scherm B, et al. Antagonism of Pythium blight of zucchini by Hypocrea jecorina does not require cellulase gene expression but is improved by carbon catabolite derepression. FEMS Microbiol Lett. 2006;257(1):145–51. doi: 10.1111/j.1574-6968.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- Sestak S, Farkas V. Metabolic regulation of endoglucanase synthesis in Trichoderma reesei: participation of cyclic AMP and glucose-6-phosphate. Can J Microbiol. 1993;39(3):342–7. doi: 10.1139/m93-048. [DOI] [PubMed] [Google Scholar]
- Silva RN, da Silva SP, Brandao RL, et al. Regulation of N-acetyl-β-D-glucosaminidase produced by Trichoderma harzianum: evidence that cAMP controls its expression. Res Microbiol. 2004;155:667–71. doi: 10.1016/j.resmic.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Turner GE, Borkovich KA. Identification of a G protein alpha subunit from Neurospora crassa that is a member of the Gi family. J Biol Chem. 1993;268(20):14805–11. [PubMed] [Google Scholar]
- Viterbo A, Harel M, Horwitz BA, et al. Trichoderma mitogen-activated protein kinase signalling is involved in induction of plant systemic resistance. Appl Environ Microbiol. 2005;71(10):6241–6. doi: 10.1128/AEM.71.10.6241-6246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J-R. MAP kinases in fungal pathogens. Fungal Genet Biol. 2000;31:137–52. doi: 10.1006/fgbi.2000.1237. [DOI] [PubMed] [Google Scholar]
- Xu J-R, Hamer JE. MAP kinase and cAMP signalling regulate infection structure formation and pathogenic growth in the rice blast fungusMagnaporthe grisea. Genes Dev. 1996;10:2696–706. doi: 10.1101/gad.10.21.2696. [DOI] [PubMed] [Google Scholar]
- Zeilinger S, Galhaup C, Payer K, et al. Chitinase gene expression during mycoparasitic interaction of Trichoderma harzianum with its host. Fungal Genet Biol. 1999;26:131–40. doi: 10.1006/fgbi.1998.1111. [DOI] [PubMed] [Google Scholar]
- Zeilinger S, Reithner B, Scala V, et al. Signal transduction by Tga3, a novel G protein alpha subunit of Trichoderma atroviride. Appl Environ Microbiol. 2005;71(3):1591–7. doi: 10.1128/AEM.71.3.1591-1597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]