Abstract
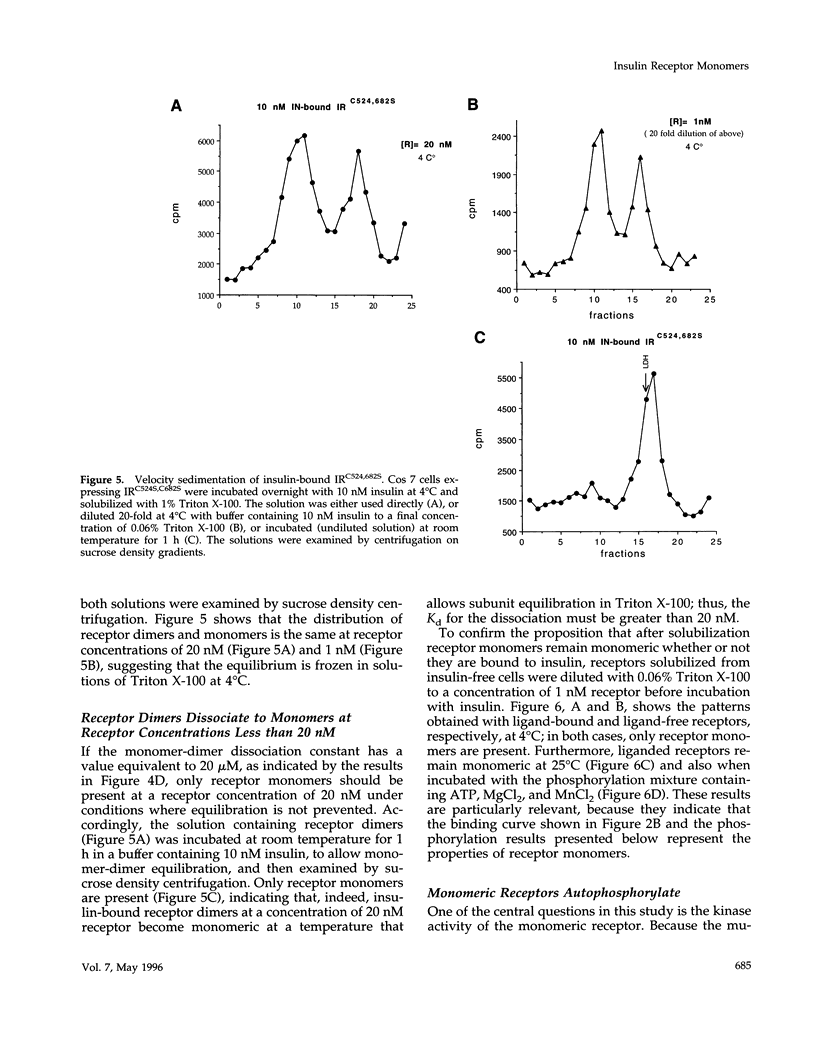
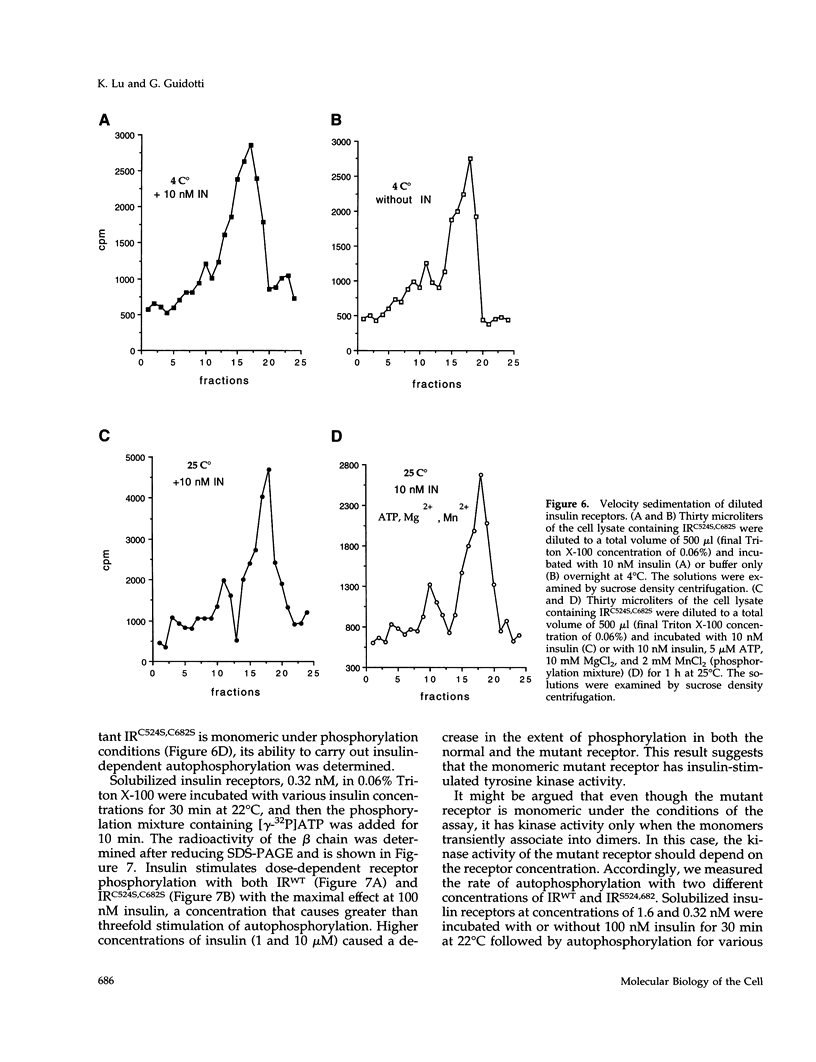
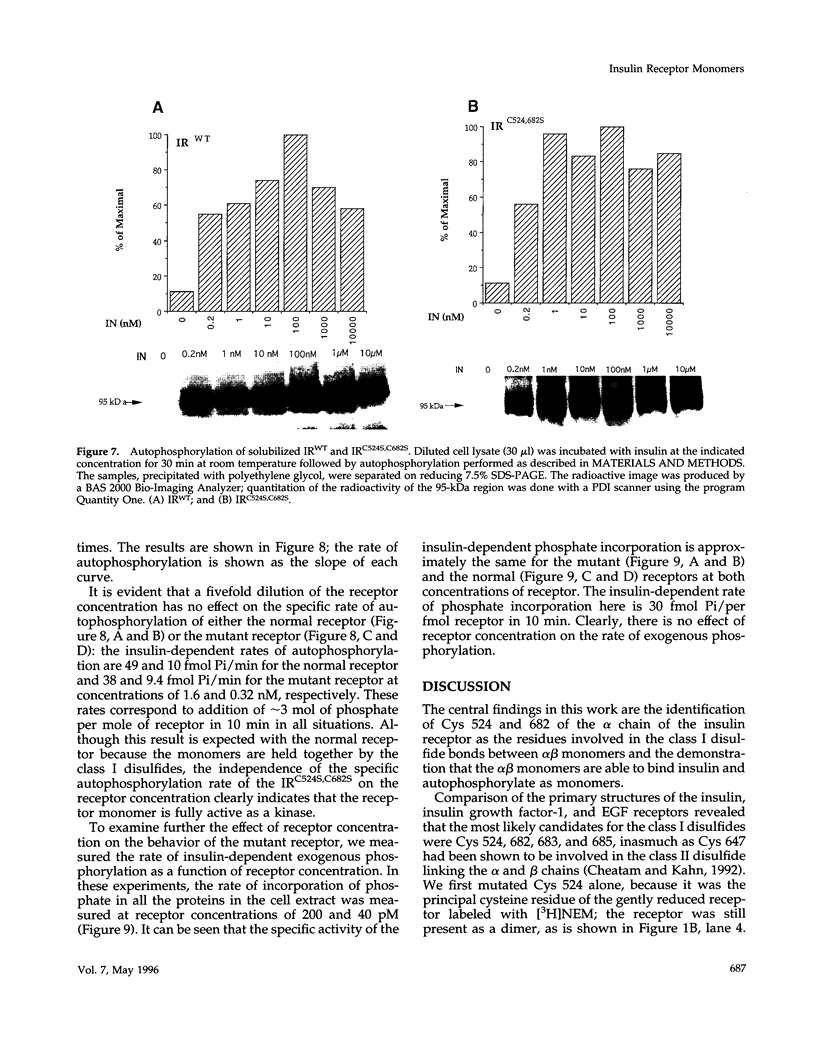
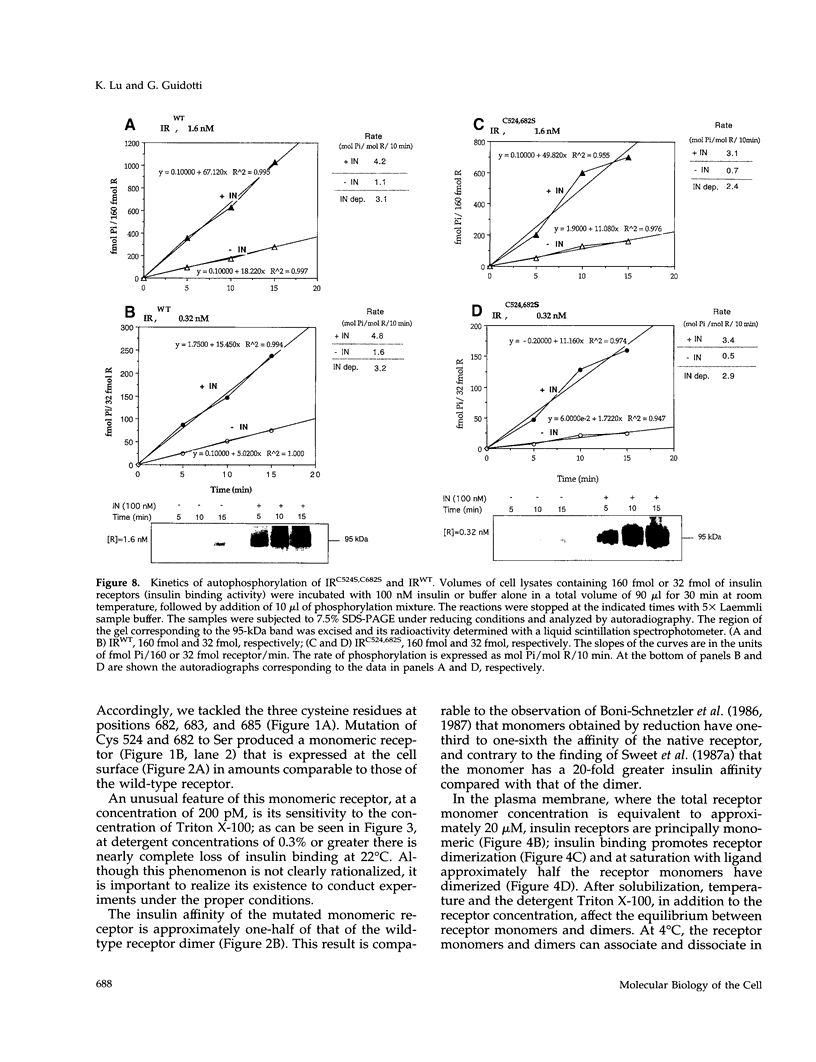
The cysteine residues involved in the class I disulfide bonds between the alpha subunits in the (alpha beta)2 dimer of the human insulin receptor have been identified by labeling with N-ethylmaleimide and by site-directed mutagenesis. Both cysteine 524 and cysteine 682 form interchain disulfide bonds; their conversion to serine residues results in the absence of receptor dimers and the presence of alpha beta monomers. The receptor monomers have a slightly lower affinity for insulin than the native receptor dimers. Insulin binding to the receptor monomers promotes their dimerization in the plasma membrane; at nanomolar concentrations of receptor, both unliganded and liganded receptors are monomers. Receptor monomers are stimulated by insulin to autophosphorylate and to phosphorylate exogenous subtrates with the same efficiency as the receptor dimers. The conclusion is that receptor dimerization is not required to activate the tyrosine kinase activity of the insulin receptor.
Full text
PDF


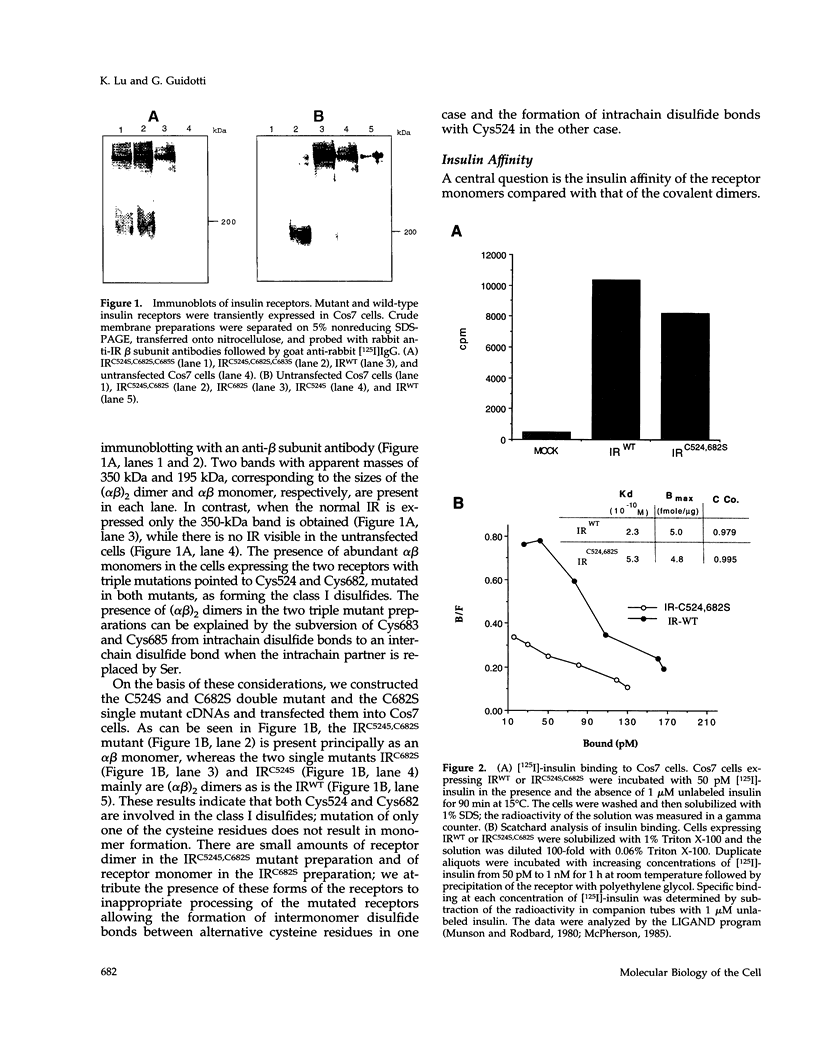


Images in this article
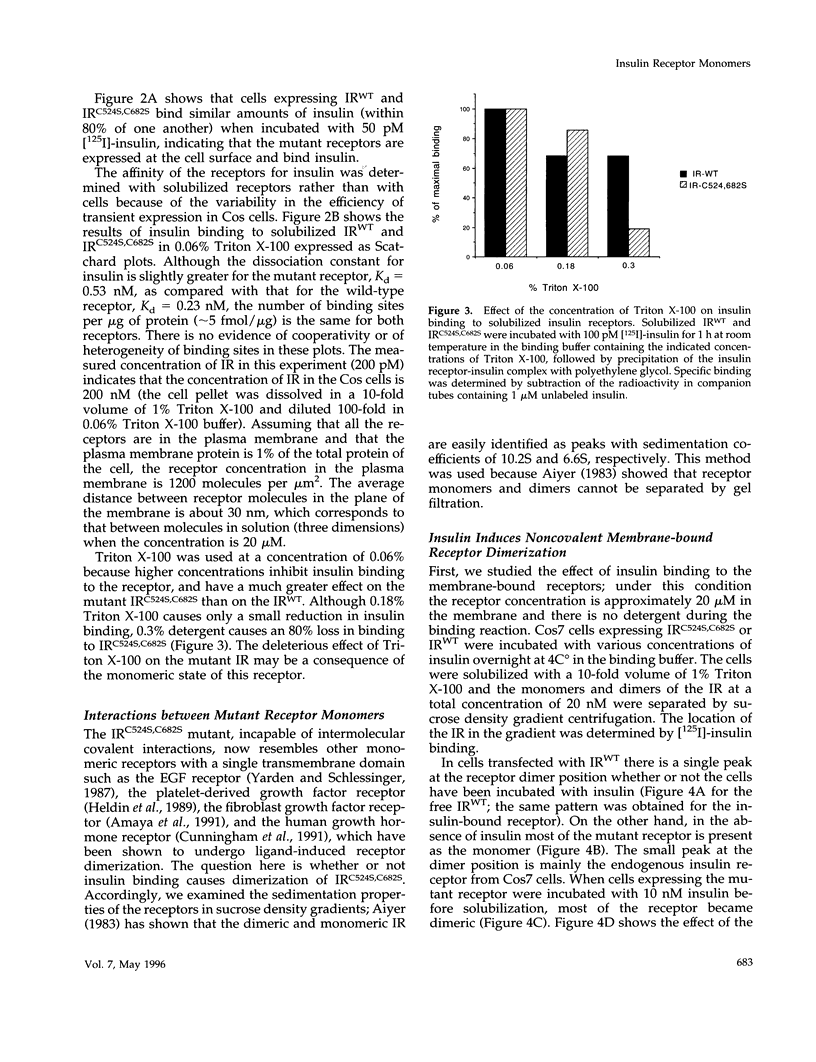
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyer R. A. Structural characterization of insulin receptors. I. Hydrodynamic properties of receptors from turkey erythrocytes. J Biol Chem. 1983 Dec 25;258(24):14992–14999. [PubMed] [Google Scholar]
- Amaya E., Musci T. J., Kirschner M. W. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991 Jul 26;66(2):257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Bilan P. J., Yip C. C. Unusual insulin binding to cells expressing an insulin receptor mutated at cysteine 524. Biochem Biophys Res Commun. 1994 Dec 30;205(3):1891–1898. doi: 10.1006/bbrc.1994.2891. [DOI] [PubMed] [Google Scholar]
- Böni-Schnetzler M., Kaligian A., DelVecchio R., Pilch P. F. Ligand-dependent intersubunit association within the insulin receptor complex activates its intrinsic kinase activity. J Biol Chem. 1988 May 15;263(14):6822–6828. [PubMed] [Google Scholar]
- Böni-Schnetzler M., Rubin J. B., Pilch P. F. Structural requirements for the transmembrane activation of the insulin receptor kinase. J Biol Chem. 1986 Nov 15;261(32):15281–15287. [PubMed] [Google Scholar]
- Böni-Schnetzler M., Scott W., Waugh S. M., DiBella E., Pilch P. F. The insulin receptor. Structural basis for high affinity ligand binding. J Biol Chem. 1987 Jun 15;262(17):8395–8401. [PubMed] [Google Scholar]
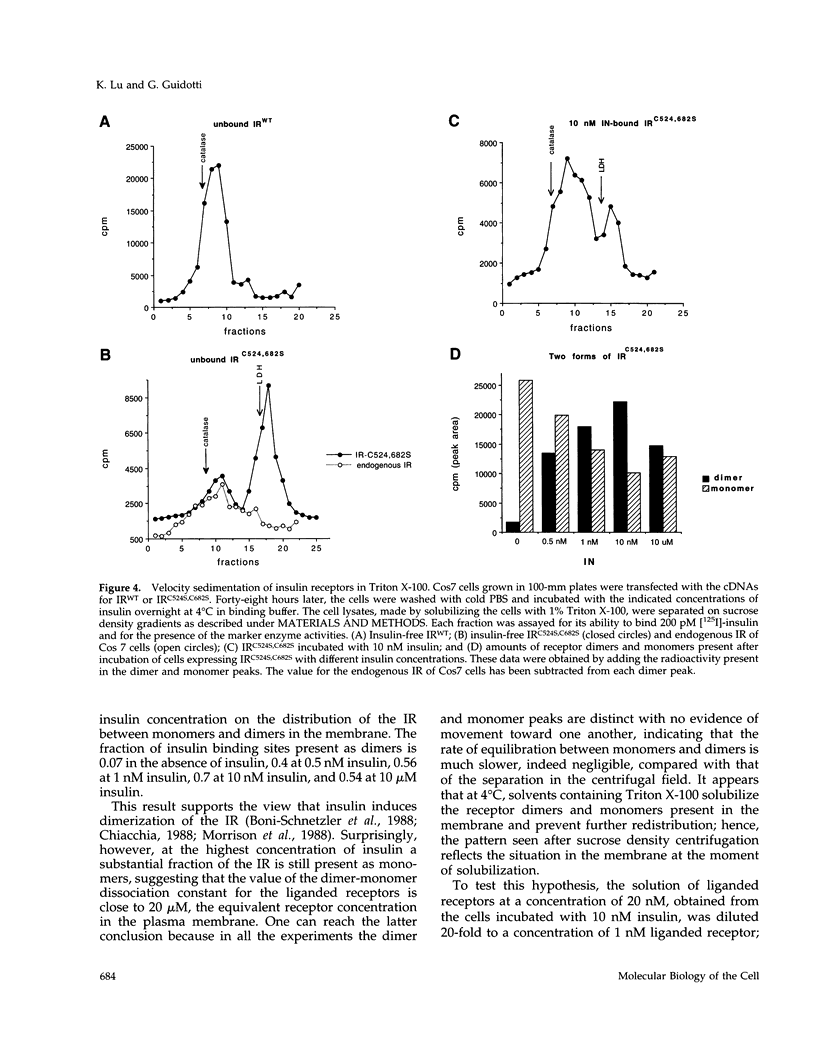
- Cheatham B., Kahn C. R. Cysteine 647 in the insulin receptor is required for normal covalent interaction between alpha- and beta-subunits and signal transduction. J Biol Chem. 1992 Apr 5;267(10):7108–7115. [PubMed] [Google Scholar]
- Chiacchia K. B. Quantitation of the class I disulfides of the insulin receptor. Biochem Biophys Res Commun. 1991 May 15;176(3):1178–1182. doi: 10.1016/0006-291x(91)90409-z. [DOI] [PubMed] [Google Scholar]
- Chiacchia K. B. Reoxidation of the class I disulfides of the rat adipocyte insulin receptor is dependent upon the presence of insulin: the class I disulfide of the insulin receptor is extracellular. Biochemistry. 1988 Jun 28;27(13):4894–4902. doi: 10.1021/bi00413a046. [DOI] [PubMed] [Google Scholar]
- Clarke S. The size and detergent binding of membrane proteins. J Biol Chem. 1975 Jul 25;250(14):5459–5469. [PubMed] [Google Scholar]
- Cunningham B. C., Ultsch M., De Vos A. M., Mulkerrin M. G., Clauser K. R., Wells J. A. Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule. Science. 1991 Nov 8;254(5033):821–825. doi: 10.1126/science.1948064. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Finn F. M., Ridge K. D., Hofmann K. Labile disulfide bonds in human placental insulin receptor. Proc Natl Acad Sci U S A. 1990 Jan;87(1):419–423. doi: 10.1073/pnas.87.1.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frattali A. L., Pessin J. E. Relationship between alpha subunit ligand occupancy and beta subunit autophosphorylation in insulin/insulin-like growth factor-1 hybrid receptors. J Biol Chem. 1993 Apr 5;268(10):7393–7400. [PubMed] [Google Scholar]
- Frattali A. L., Treadway J. L., Pessin J. E. Transmembrane signaling by the human insulin receptor kinase. Relationship between intramolecular beta subunit trans- and cis-autophosphorylation and substrate kinase activation. J Biol Chem. 1992 Sep 25;267(27):19521–19528. [PubMed] [Google Scholar]
- Heldin C. H., Ernlund A., Rorsman C., Rönnstrand L. Dimerization of B-type platelet-derived growth factor receptors occurs after ligand binding and is closely associated with receptor kinase activation. J Biol Chem. 1989 May 25;264(15):8905–8912. [PubMed] [Google Scholar]
- Hubbard S. R., Wei L., Ellis L., Hendrickson W. A. Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature. 1994 Dec 22;372(6508):746–754. doi: 10.1038/372746a0. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee J., O'Hare T., Pilch P. F., Shoelson S. E. Insulin receptor autophosphorylation occurs asymmetrically. J Biol Chem. 1993 Feb 25;268(6):4092–4098. [PubMed] [Google Scholar]
- Lemmon M. A., Schlessinger J. Regulation of signal transduction and signal diversity by receptor oligomerization. Trends Biochem Sci. 1994 Nov;19(11):459–463. doi: 10.1016/0968-0004(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Macaulay S. L., Polites M., Hewish D. R., Ward C. W. Cysteine-524 is not the only residue involved in the formation of disulphide-bonded dimers of the insulin receptor. Biochem J. 1994 Oct 15;303(Pt 2):575–581. doi: 10.1042/bj3030575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Czech M. P. Role of disulfides in the subunit structure of the insulin receptor. Reduction of class I disulfides does not impair transmembrane signalling. J Biol Chem. 1982 Jun 25;257(12):6729–6738. [PubMed] [Google Scholar]
- McPherson G. A. Analysis of radioligand binding experiments. A collection of computer programs for the IBM PC. J Pharmacol Methods. 1985 Nov;14(3):213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- Morrison B. D., Swanson M. L., Sweet L. J., Pessin J. E. Insulin-dependent covalent reassociation of isolated alpha beta heterodimeric insulin receptors into an alpha 2 beta 2 heterotetrameric disulfide-linked complex. J Biol Chem. 1988 Jun 5;263(16):7806–7813. [PubMed] [Google Scholar]
- Mortensen E. R., Drachman J. G., Guidotti G. The alpha beta monomer of the insulin receptor has hormone-responsive tyrosine kinase activity. Biochem J. 1991 Jan 1;273(Pt 1):49–56. doi: 10.1042/bj2730049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Signal transduction by allosteric receptor oligomerization. Trends Biochem Sci. 1988 Nov;13(11):443–447. doi: 10.1016/0968-0004(88)90219-8. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Ullrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992 Sep;9(3):383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- Schäffer L., Ljungqvist L. Identification of a disulfide bridge connecting the alpha-subunits of the extracellular domain of the insulin receptor. Biochem Biophys Res Commun. 1992 Dec 15;189(2):650–653. doi: 10.1016/0006-291x(92)92250-2. [DOI] [PubMed] [Google Scholar]
- Shoelson S. E., Boni-Schnetzler M., Pilch P. F., Kahn C. R. Autophosphorylation within insulin receptor beta-subunits can occur as an intramolecular process. Biochemistry. 1991 Aug 6;30(31):7740–7746. doi: 10.1021/bi00245a010. [DOI] [PubMed] [Google Scholar]
- Sweet L. J., Morrison B. D., Pessin J. E. Isolation of functional alpha beta heterodimers from the purified human placental alpha 2 beta 2 heterotetrameric insulin receptor complex. A structural basis for insulin binding heterogeneity. J Biol Chem. 1987 May 25;262(15):6939–6942. [PubMed] [Google Scholar]
- Sweet L. J., Morrison B. D., Wilden P. A., Pessin J. E. Insulin-dependent intermolecular subunit communication between isolated alpha beta heterodimeric insulin receptor complexes. J Biol Chem. 1987 Dec 5;262(34):16730–16738. [PubMed] [Google Scholar]
- Taouis M., Levy-Toledano R., Roach P., Taylor S. I., Gorden P. Structural basis by which a recessive mutation in the alpha-subunit of the insulin receptor affects insulin binding. J Biol Chem. 1994 May 27;269(21):14912–14918. [PubMed] [Google Scholar]
- Ueno H., Colbert H., Escobedo J. A., Williams L. T. Inhibition of PDGF beta receptor signal transduction by coexpression of a truncated receptor. Science. 1991 May 10;252(5007):844–848. doi: 10.1126/science.1851331. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Villalba M., Wente S. R., Russell D. S., Ahn J. C., Reichelderfer C. F., Rosen O. M. Another version of the human insulin receptor kinase domain: expression, purification, and characterization. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7848–7852. doi: 10.1073/pnas.86.20.7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh S. M., DiBella E. E., Pilch P. F. Isolation of a proteolytically derived domain of the insulin receptor containing the major site of cross-linking/binding. Biochemistry. 1989 Apr 18;28(8):3448–3455. doi: 10.1021/bi00434a045. [DOI] [PubMed] [Google Scholar]
- White M. F., Kahn C. R. The insulin signaling system. J Biol Chem. 1994 Jan 7;269(1):1–4. [PubMed] [Google Scholar]
- Wilden P. A., Morrison B. D., Pessin J. E. Relationship between insulin receptor subunit association and protein kinase activation: insulin-dependent covalent and Mn/MgATP-dependent noncovalent association of alpha beta heterodimeric insulin receptors into an alpha 2 beta 2 heterotetrameric state. Biochemistry. 1989 Jan 24;28(2):785–792. doi: 10.1021/bi00428a056. [DOI] [PubMed] [Google Scholar]
- Xu Q. Y., Paxton R. J., Fujita-Yamaguchi Y. Substructural analysis of the insulin receptor by microsequence analyses of limited tryptic fragments isolated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in the absence or presence of dithiothreitol. J Biol Chem. 1990 Oct 25;265(30):18673–18681. [PubMed] [Google Scholar]
- Yarden Y., Schlessinger J. Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor. Biochemistry. 1987 Mar 10;26(5):1443–1451. doi: 10.1021/bi00379a035. [DOI] [PubMed] [Google Scholar]