Abstract
Id3 belongs to the Inhibitor of differentiation family of HLH transcription factors, important in proliferation, differentiation, and apoptosis. We showed that Id3, but not Id2 or Id1, mediates the UVB-sensitization of immortalized keratinocytes by inducing caspase 9-dependent apoptosis. In the current study, quantitative PCR analysis revealed a time-dependent increase in Id3 mRNA induced by UVB, dependent on reactive oxygen species. UVB upregulated promoter activity of Id3, but not Id2, at early time points, as shown by reporter assays, and also stabilized Id3 mRNA, increasing its half-life from 10 to ~60 minutes. We next examined downstream events related to UVB-induced Id3 upregulation and investigated the effects of UVB or ectopic expression of Id3 on bax promoter activity. Regulatory elements in the bax promoter that mediate transcriptional activation by UVB and Id3, in the absence of p53, were identified. Bax promoter deletion analysis revealed that transcriptional activation by UVB involves a 738-bp region upstream from the transcription start site of bax. Mimicking the effects of UVB, ectopic expression of Id3 also upregulated bax mRNA and activated this 738-bp fragment. Mutational analysis of the transcription binding sites further showed that point mutations of the E-box region found in the 738-bp fragment, but not in a 174-bp fragment, completely abolished Id3- and UVB-inducible bax promoter activity, thus confirming the importance of Id3 and UVB-mediated Id3 upregulation in activating the bax promoter. These results suggest a mechanism whereby ROS upregulation of Id3 relieves repression of bax via E-box-binding factors.
Keywords: “Id proteins”, “apoptosis”, “immortalization”, “bax promoter”, “superoxide”
Introduction
Solar UVB is a DNA-damaging agent leading to the precancerous stage of actinic keratoses, and skin cancers, the most common human malignancies (1-3). UVA (>320 nm) contributes to DNA damage indirectly via the generation of reactive oxygen species (ROS) (4-7). UV-induced genetic alterations in the skin include both initiating mutations (e.g. p53), as well as promoting events that lead to the clonal expansion of mutated keratinocytes accompanied by apoptosis of normal cells (8). This provides aberrant cells a selective growth advantage over their normal counterparts. We showed that UVB inhibits differentiation in primary human keratinocytes, which could predispose them to tumorigenesis (9), and that immortalization may represent a transient stage in skin carcinogenesis during which the cells are sensitized to UVB-induced apoptosis (10). In subsequent studies we delineated the molecular events following UVB irradiation in immortalized keratinocytes and clarified that apical caspase-9 and upstream Bcl-2 family members, but not FADD, were necessary for UVB-induced apoptosis (11, 12).
Microarray analysis also revealed that 3 of the 4 members of Inhibitors of Differentiation/DNA binding (Id) family are differentially regulated in primary and immortalized keratinocytes upon UVB exposure (9). While Id1 was shown to be downregulated in both cell types, Id2 and Id3 were upregulated following UVB irradiation in primary and immortalized keratinocytes, respectively.
Id proteins belong to the helix-loop-helix (HLH) family of transcription factors. Other members of the HLH family termed basic helix-loop-helix (bHLH) transcription factors have an additional, DNA-binding, basic domain that recognizes E boxes (CANNTG) or N boxes (CACNAG). bHLH proteins include ubiquitously expressed E-proteins (HEB, E2-2, E2A) and tissue-specific ones (Myo D, myogenin; (13)), and form dimers via their HLH dimerization domain (14), binding to the promoter region of differentiation genes via their basic domain. Since Id proteins lack the DNA-binding basic domain, Id proteins regulate differentiation by binding to bHLH proteins, sequestering them away from their obligate tissue-specific binding partners (15). Given that bHLH factors may be transcriptional activators or inhibitors, Id proteins can consequently activate or inhibit gene expression. Id proteins have been shown to bind proteins other than bHLH factors, including Ets family proteins (Ets-2) and Rb, p107, and p130, involved in cell cycle regulation, differentiation, and tumor suppression (16). Ids have also been implicated in apoptosis (17), angiogenesis (18), and human carcinogenesis (19).
Id3 is induced by various factors, including: TGF-β (20), bFGF (21), cAMP (22), angiotensin II (23), and TCR (24), and plays different roles depending on the cell type. Id3-knockout mice exhibit defects in humoral immunity (25), whereas ectopic expression of Id3 induces apoptosis in rat fibroblasts (26), B-lymphocytes (27), immortalized human keratinocytes (12), and in response cisplatin in the sarcoma cell line MG-63 (28). Id3 has also been shown to increase cell proliferation and results in the expansion of the neural crest domain (29).
We previously showed that Id3 mRNA is induced by UVB and mediates the apoptotic effects of UVB in immortalized keratinocytes and oligomerization and mitochondrial localization of Bax (12). Bax has been implicated in carcinogenesis (30) and was shown to play an important role in conferring resistance to apoptosis in tumor cells (31).
We therefore investigated the mechanisms leading to Id3 mRNA induction following UVB irradiation. We demonstrate for the first time that Id3 mRNA is induced by UVB via ROS in immortalized keratinocytes. Further, Id3 is induced at the promoter level, as well as post-transcriptionally, by UVB in immortalized human keratinocytes. Downstream events related to Id3-induced apoptosis were also investigated. The bax promoter region contains several E-boxes and Ets (GGAA/T) sites, regions that can potentially be regulated by Id proteins. Promoter deletion and mutational analyses showed that a 738 bp fragment of the bax promoter is induced by ectopic expression of Id3 as well as UVB, and that deletion, or point mutation, of an E-box 202 bp upstream from the transcription start site of the bax gene abolishes all Id3- and UVB-induced bax promoter activity.
Materials and Methods
Cells
Keratinocytes were derived from human foreskins and grown in KSFM supplemented with EGF, bovine pituitary extract (Life Technologies Inc., Rockville, MD, USA), and 10 Hg/ml of Gentamicin (Cambrex Bio Science, Walkersville, MD, USA). Cells were transduced with an amphotropic LXSN retrovirus expressing HPV-16 E6 plus E7 genes, and selected in G418 (100 μg/ml) for 10 days. Primary and HPV16 E6/7 immortalized p30 human keratinocytes were grown to 75% confluency and passaged 1:4 at equal cell densities. Spontaneously immortalized HaCaT cells (32) were obtained from Dr. Richard Schlegel (Georgetown University).
UVB Irradiation
Immortalized keratinocytes were grown to 75% confluency and irradiated with 480 J/m2 UVB, using a UVB source with a peak wavelength of 312 nm (FS40 sunlamp; Philips, Somerset, NJ, USA) with a Kodacel filter (Kodak, Paris, France) to eliminate UV wavelengths <290 nm (UVC). Irradiation was monitored by IL1400A radiometer/photometer. Doses were selected to match reasonable range of solar UVB exposure to human skin (5-30 min typical sun exposure in the US; (12)). At indicated time points after UVB irradiation, cells were harvested, or treated with Actinomycin D and then harvested, for further analyses. For the promoter studies, cells were UVB-irradiated 24 hours after transfection and harvested 16 hours later.
ROS inducers and inhibitors
Cells were incubated with either vehicle control (ethanol) or 100 μM X/XO in the presence or absence of superoxide dismutase (SOD; 0.2 U/ml; Sigma). To inhibit ROS, cells were exposed to UVB (480 J/m2) in the presence of SOD, N-acetyl-cysteine (NAC; 10 mM; Acros Organics) or α-tocopherol (100 μg/ml; Acros Organics). To measure ROS, cells were pretreated with 100 nM CM-H2DCFDA dye (Molecular Probes) prior to UVB exposure.
Cloning of Id3 and Bax Promoter Constructs
Genomic DNA was extracted from immortalized keratinocytes and used to amplify the Id3 promoter constructs. The reverse primer, 5′-ATTAAGATCTTGCCCCAAAGAGAAAGAAAACC was used for both constructs. The 1445 bp construct was amplified using the forward primer 5′-ATTAGAGCTCCCAGGAAGAAATCTTTCTTGGG, while the 750 bp construct was amplified using the forward primer 5′-ATTAGAGCTCTTCCTGGTCTTTCAGATGGAGC. These constructs were cloned into pGL3-Basic vector at the Sac I and Bgl II sites. The pGL3/Id3-1445 construct was digested by Kpn I and Hind III to remove a 1069 bp fragment, blunt-ended by T4 DNA Polymerase, and religated to generate the pGL3/Id3-376 construct. Bax promoter constructs were amplified from the chromosome 19 clone CTD-2639E6 (Open Biosystems, Huntsville, AL, USA). The 738 bp construct reverse primer used was 5′-ACTGCTCGAGGTCACGTGACCGCACCTGC, while the forward primer used was 5′-ACTGGGTACCCATTCGAGTCATGACTGGG. This construct was cloned into the Kpn I/Xho I site of pGL3-Basic vector. The pGL3/Bax-174 construct was obtained by digesting the pGL3/Bax-738 construct with Kpn I and Sac I to remove a 564 bp fragment, blunt ended with T4 DNA Polymerase and then religated using T4 DNA Ligase. The pGL3/Bax-24 bp construct was obtained from pGL3/bax-738 using a Kpn I/Bso BI double digest.
Promoter Activity Assays and siRNA transfection
For Id3 promoter assays, immortalized keratinocytes were cotransfected with pRLTK Renilla luciferase and Id3 promoter-luciferase constructs (1:20 molar ratio) using Fugene Reagent (Roche Diagnostics, Indianapolis, IN), in a 40% (w/v) mixture of total DNA: Fugene. After 24 hours, cells were exposed to UVB, and extracts were either prepared for luciferase activity, or else RNA was prepared for quantitative reverse transcription-PCR (Q-PCR) of luciferase RNA. For bax promoter assays, immortalized keratinocytes were cotransfected with three plasmids: 1. pRLTK Renilla luciferase, 2. pGL3-derived bax promoter-luciferase construct (wild-type, deletion, or point mutants as described above), and 3. pLHCX/Id3 expressing Id3, or empty vector pLHCX (1:20:28 molar ratios). 24 hours after transfection, cells were either harvested, or exposed to 480 J/m2 of UVB and harvested 16 hours later. Luciferase activity was measured using the Dual Luciferase Reporter Assay System (Promega). siRNAs directed against Id3 or scrambled control were synthesized and transfected 48 hours prior to UVB exposure as previously described (12).
Mutagenesis
Two point mutations were introduced in the E-box at -202 in the bax promoter using the site-directed mutagenesis kit (Stratagene). The template mutagenic primers 5′-GGGCCTCTGAGCTTTTGCCCATGGTAATTCCTTCTGCGC (forward) and 5′-GCGCAGAAGGAATTACCATGGGCAAAAGCTCAGAGGCCC (reverse) were used to introduce the three point mutations A-201C, T-199A, and C-196G (outside the E-box region), creating an artificial NcoI site.
Reverse Transcription
RNA was extracted from harvested cells following Actinomycin D (for half-life experiments) and/or UVB treatment using the RNeasy kit (Qiagen, MD, USA). 2 μg RNA were reverse transcribed to cDNA using TaqMan Reverse Transcription reagents (Applied Biosystems, NJ, USA). After random hexamers annealed for 10 minutes at 25° C, cDNA synthesis was performed for 60 minutes at 37° C, followed by an inactivation step for 5 minutes at 95° C.
Q-PCR Amplification
Q-PCR amplifications were performed in a DNA Engine Opticon 2 System® for continuous fluorescence detection (MJ Research, Boston, USA). The reaction was conducted in a 96-well plate with each well containing a volume of 25 μl composed of SYBR Green PCR Master Mix (Applied Biosystems, CA, USA), 50 ng cDNA, 4.2 pmoles of each primer, and DEPC (0.1%) treated water. The Id3 forward primer 5′-CTAATCAGACAGCCGAGCTCACT and reverse primer 5′-TTTTGTCGTTGGAGATGACAAGTT were used to amplify a 51 bp region of Id3 mRNA. Luciferase transcript levels were measured by using the Luciferase forward primer 5′-AGGCAAGGATATGGGCTCACT and the luciferase reverse primer 5′-CATCCCCCTCGGGTGTAATC. Similarly, the levels of bax transcripts were measured by using the bax forward primer 5′- TGGAGCTGCAGAGGATGATTG and the bax reverse primer 5′-GAAGTTGCCGTCAGAAAACATG. Thermocycling was performed as 40 cycles of 15 seconds at 95° C, and 1 minute at 60° C. SYBR Green dye was used to monitor the accumulation of double-stranded 51 bp amplicon. Results of the Q-PCR assay were analyzed with Opticon 2 software supplied with the Opticon cycler®, obtaining fluorescence intensity vs. threshold cycle number curves. Cyclophilin amplification was done using the Human Cyclophilin Real Time PCR® Primer Set (Biosource, CA, USA).
Statistical analysis
Comparisons between treatment groups were made by performing T-tests on data derived from triplicates. P values of <0.05 were considered statistically significant, and are represented by one asterisk in all figures, while two and three asterisks represent p values of <.01 and < .001, respectively. The results are representative of at least three independent experiments with reproducible results.
Results
UVB induces Id3 mRNA expression in immortalized human keratinocytes
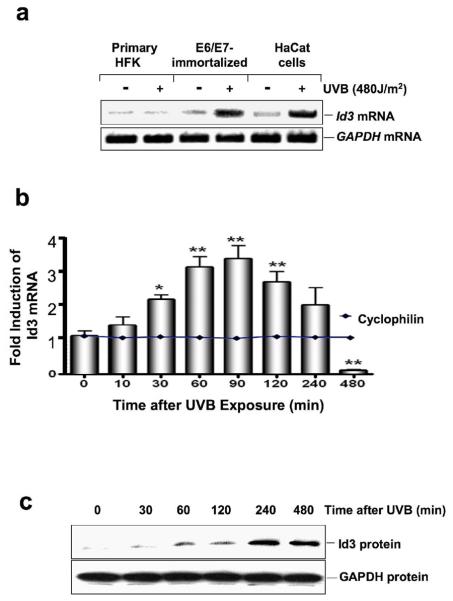
RT-PCR analysis of UVB-exposed and control cells was performed to confirm UVB induction of Id3 expression in HPV16 E6/E7-immortalized human keratinocytes, but not in primary keratinocytes (Fig. 1a). To further discount the possibility that E6/E7 per se might mediate the observed effect of UVB on Id3, we also used a different non-E6/E7 immortalized cell line (spontaneously immortalized HaCaT keratinocytes; (32)). The results demonstrate a significant increase in Id3 mRNA in both E6/E7-immortalized and HaCaT cells, but not in primary keratinocytes in response to UVB exposure (Fig. 1a). In contrast, there was no change in GAPDH mRNA, as loading control, in all cell types before or after UVB.
Figure 1.
UVB induces expression of Id3 in immortalized keratinocytes. Cells were irradiated with 480 J/m2 of UVB and then harvested at 4 h (a) or the indicated time points (b, c). Id3 mRNA steady state levels were quantified by RT-PCR and electrophoresis (a) or Q-PCR (b). (b) Fold expression of Id3/cyclophilin mRNA levels for each time point compared to unirradiated (Time=0) controls are shown and presented as mean ± S.D. of three replicates of a representative experiment; essentially the same results were obtained in three independent experiments. One, two, or three asterisks denote significance levels (compared to control) of p<.05, p<.01; or p<.001, respectively. (c) Id3 proteins levels were determined by immunoblot analysis following UVB irradiation.
We next investigated the changes in steady-state levels of endogenous Id3 mRNA over time in E6/E7-immortalized keratinocytes. At different times after UVB exposure, cell extracts were subjected to reverse transcription Q-PCR analysis (Fig. 1b). Id3 mRNA levels were normalized to cyclophilin mRNA, which did not change in response to UVB, further indicating specificity for Id3 induction. A significant increase in Id3 mRNA levels was seen 30 min after UVB exposure. After 60 minutes, Id3 mRNA levels peaked to about threefold the basal level, and remained elevated for at least 4 hours. After 8 hours, Id3 mRNA levels were significantly reduced below basal levels.
Immunoblot analysis further showed that the marked increase in Id3 transcript levels over time correlates with increased abundance of Id3 protein in these cells in response to UVB exposure (Fig.1c). As expected, the abundance of Id3 protein, but not GAPDH, increased markedly 60 min after UVB exposure, peaked after 240 min, and remained elevated after 4 h (Fig. 1c). Abundance of Id3 protein remained high 16 h after UVB (12).
UVB upregulates Id3 mRNA via ROS
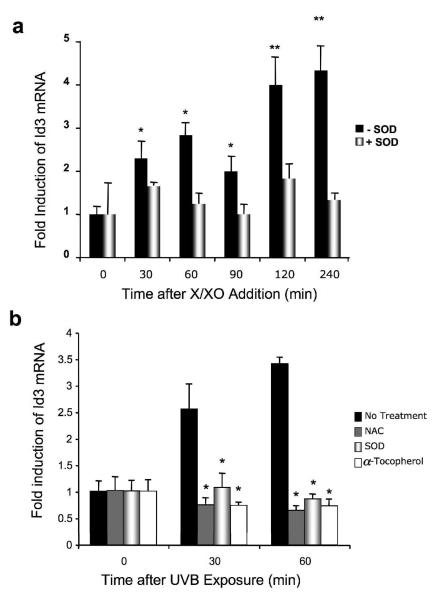
To test whether UVB upregulates Id3 via ROS, we assessed whether intracellular release of superoxide could induce Id3 expression in immortalized E6/E7 HFK. Cells were incubated with xanthin (100 μM) and xanthin oxidase 1.6 × 10−3 U/ml (100 μM X/XO) to induce production of superoxide. After the indicated times, Id3 mRNA was quantified by Q-PCR. X/XO induced a significant increase in Id3 expression within 30 min, which maximized after 2 h (four-fold induction; Fig. 2a). This upregulation was inhibited by SOD, which specifically scavenges superoxide.
Figure 2.
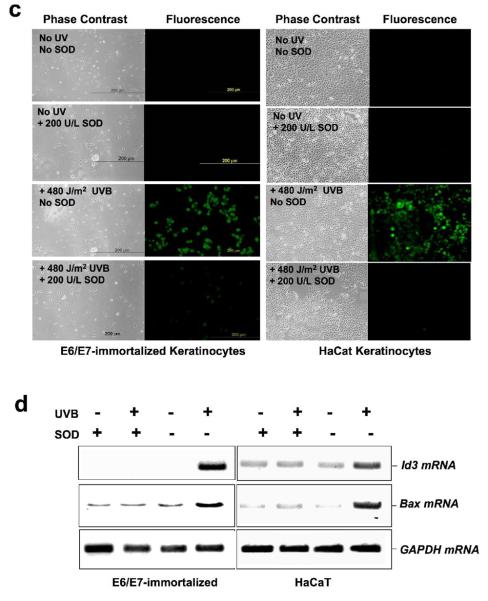
X/XO and UVB upregulate Id3 in immortalized keratinocytes via superoxide release. Immortalized E6/E7 keratinocytes (a-d) or HaCaT (c, d) were exposed to either vehicle (ethanol; control), 100 μM X/XO (a), or UVB (b-d) for the indicated times, in the presence or absence of the pharmacological inhibitor superoxide dismutase (SOD; 0.2 U/ml), or NAC, or α- tocopherol (b) followed by RNA isolation (a, b, d) and Id3 mRNA quantification by Q-PCR (a, b) or Id3 and bax mRNA by RT-PCR and electrophoresis (d). Results are mean ± S.D. of three replicates of a representative experiment; essentially the same results were obtained in three independent experiments. One or two asterisks denote significance levels corresponding to p<.05 or p<.01 when compared to +SOD (a) or to no treatment (b). (c) Pretreatment of cells with CM-H2DCFDA dye prior to UVB exposure reveals superoxide that is inhibited by SOD. Representative phase contrast and fluorescent images are shown. (d) ROS mediate UVB induction of Id3 and bax mRNAs. Immortalized keratinocytes were exposed to 480 J/m2 UVB with or without SOD, and RNA isolated after 4 hours and subjected to RTPCR using Id3, bax, or GAPDH primers, followed by electrophoresis.
We next examined the effects of ROS inhibitors on Id3. Cells were pretreated with ROS inhibitors superoxide dismutase (SOD; 0.2 U/mL), NAC (10 mM) or α-tocopherol (100 μg/ml) for 60 min and then exposed to UVB (480 J/m2), and Id3 mRNA quantified by Q-PCR. UVB exposure induced a two- to three-fold increase in Id3 expression within 30 min. The UVB response was significantly inhibited in the presence of SOD, suggesting that intracellular superoxide release may be involved in Id3 induction by UVB in these cells (Fig. 2b). In addition, α-tocopherol, as well as NAC, a reduced glutathione provider and a direct scavenger of ROS (33), also markedly inhibited UVB-induced upregulation of Id3 expression. To confirm that UVB induced ROS, which was inhibited by SOD, we loaded cells with CM-H2DCFDA dye that fluoresces in the presence of superoxide, prior to UVB exposure. Fig. 2c shows that both E6/7 and HaCaT cells reveal UVB-induced superoxide that is inhibited by SOD. Furthermore, the induction of both Id3 and bax mRNA by UVB is blocked by SOD in E6/7 and HaCaT cells (Fig. 2d).
Id3 promoter activity is UVB-inducible in E6/E7-immortalized keratinocytes
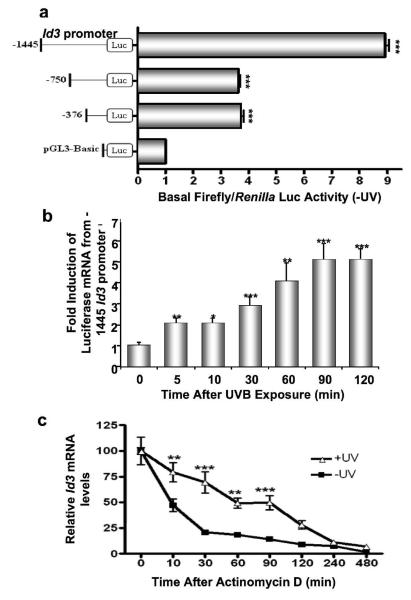
We next determined the effect of UVB exposure on the Id3 promoter. −1445 bp, −750 bp, and −376 bp Id3 upstream regions were cloned adjacent to the firefly luciferase cDNA, cotransfected with a Renilla reporter vector into immortalized keratinocytes, and exposed to UVB. After 16 hours, firefly luciferase assays were performed, and normalized against Renilla luciferase activity to control for transfection efficiency. The Id3-1445 construct showed greatest basal activity while upstream deletions reduced this level (Fig. 3a). To accurately determine UVB-induced Id3 promoter activity, we measured luciferase mRNA transcribed from the reporter constructs, via reverse transcription Q-PCR, following UVB-irradiation of cells transfected with the 1445 bp Id3 promoter. Induction was seen as early as 5 minutes after irradiation (Fig. 3b).
Figure 3.
UVB induces Id3 promoter expression and RNA stability. (a) Different Id3 promoter regions (1445 bp, 750 bp, and 376 bp) were cloned upstream to the firefly luciferase gene, and transiently cotransfected with the pRLTK Renilla vector into immortalized keratinocytes. 24 hours after transfection, cells were either harvested (a), or exposed to UVB and harvested at the indicated time points (b). (a) Luciferase assays were performed and normalized against Renilla luciferase activity to control for transfection efficiency. Fold increase in basal firefly/Renilla luciferase activity is shown for each mutant as a multiple of that obtained using the promoterless pGL3-Basic construct. (b) Immortalized keratinocytes were cotransfected with the Renilla luciferase reporter vector and the −1445 Id3 promoter-luciferase construct. After UVB exposure, RNA was extracted, and luciferase and cyclophilin mRNA levels were measured by QPCR. Fold expression of luciferase/cyclophilin mRNA compared to unirradiated (Time=0) controls are shown as mean ± S.D. of three replicates of a representative experiment. (c) Immortalized keratinocytes were exposed to 480 J/m2 UVB. Actinomycin-D (4 μM) was added 60 minutes after UVB irradiation. Cells were harvested at indicated time points after Actinomycin-D, RNA isolated, and endogenous levels of Id3 mRNA quantified by QPCR. Relative Id3/cyclophilin mRNA levels from UVB-irradiated and unirradiated (Time=0) controls are calculated as mean ± S.D. of three replicates of a representative experiment; essentially the same results were obtained in three independent experiments. One, two or three asterisks represent significance levels of p<.05, .01 or .001, respectively, for each promoter compared to pGL3-Basic (a), or when UVB-exposed is compared to unirradiated controls for each time point (b, c).
UVB stabilizes Id3 mRNA in HPV 16 E6/E7-immortalized keratinocytes
We next performed mRNA stability experiments. Immortalized keratinocytes were exposed to 480 J/m2 of UVB. Actinomycin D (4 μM) was added after 60 minutes, the peak of UVB-induced Id3 RNA synthesis (Fig. 1). Cells were harvested at different times, and levels of Id3 mRNA were quantified by reverse transcription Q-PCR (Fig. 3c). There is a notable increase in Id3 mRNA half-life in UVB-irradiated immortalized keratinocytes compared to their non UVB-irradiated counterparts (60 vs. 10 minutes). Therefore, UVB regulates Id3 mRNA by early transcriptional activation of the Id3 gene as well as stabilization of Id3 mRNA.
Id3- and UVB-induced bax promoter activities require an intact E-box at position −202
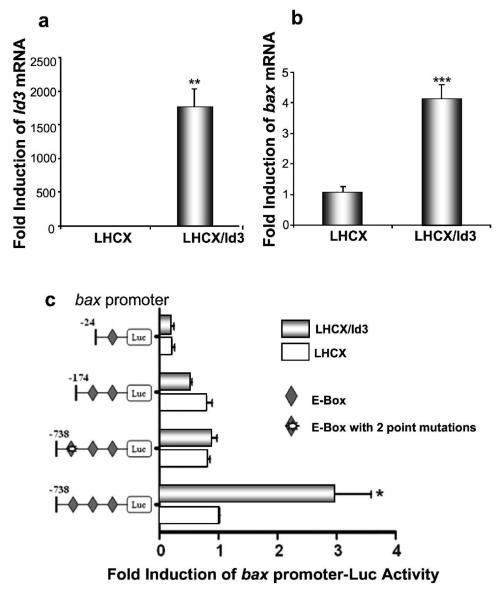
Transfection of the retroviral vector LHCX-Id3 followed by reverse transcription Q-PCR revealed a robust increase in both Id3 and bax mRNA levels (Fig. 4). The bax gene promoter contains several E-boxes, potential targets of Id3. We therefore cloned 24 bp, 178 bp, or 738 bp immediately upstream from the bax transcription start site, containing 1, 2, or 3 E-boxes, respectively, upstream of a Luciferase reporter. These constructs were cotransfected with a Renilla Luciferase reporter vector into E6/E7 immortalized keratinocytes, along with pLHCX/Id3, or empty vector LHCX. The bax-738-luc construct, with three E-boxes, can be induced by Id3, while constructs containing one (bax-24-luc), or two (bax-178-luc) E-boxes are no longer induced (Fig. 4c). The distal 3rd E-box, at position −202, is therefore important for induction of the bax promoter by Id3. To confirm this, we found that point mutations in this E-box abolished the Id3 response (Fig. 4c).
Figure 4.
Ectopic expression of Id3 in E6/7-immortalized keratinocytes induces endogenous bax mRNA and promoter via its E-boxes. Immortalized keratinocytes were transfected with the retroviral vectors LHCX-Id3 or LHCX. 24 hours after transfection, cells were harvested, and RNA was extracted and analyzed for levels of mRNA encoding Id3 (a) or bax (b) using Q-PCR. Relative Id3 or bax mRNA levels were calculated by normalizing for cyclophilin mRNA amplification. Fold change in Id3/cyclophilin or bax/cyclophilin mRNA is shown for LHCX/Id3 compared to LHCX vector alone, and calculated as the mean ± S.D. of three replicates of a representative experiment; essentially the same results were obtained in three independent experiments. (c) Immortalized keratinocytes were cotransfected with pRLTK Renilla luciferase, each of the pGL3-derived bax promoter-luciferase constructs shown (wild-type, deletion, or point mutants), along with pLHCX/Id3 expressing Id3, or empty vector pLHCX (1:20:28 molar ratios). Luciferase assays were performed after 24 hours to measure bax promoter activity, and normalized against Renilla luciferase activity to control for transfection efficiency. Fold change in firefly/Renilla luciferase activity is shown for LHCX/Id3 compared to LHCX vector alone, and calculated as the mean ± S.D. of three replicates of a representative experiment; essentially the same results were obtained in three independent experiments. One, two or three asterisks (a, b, c) represent significance levels of p<.05, .01, or .001, respectively, when Id3-transfected is compared to vector LHCX controls.
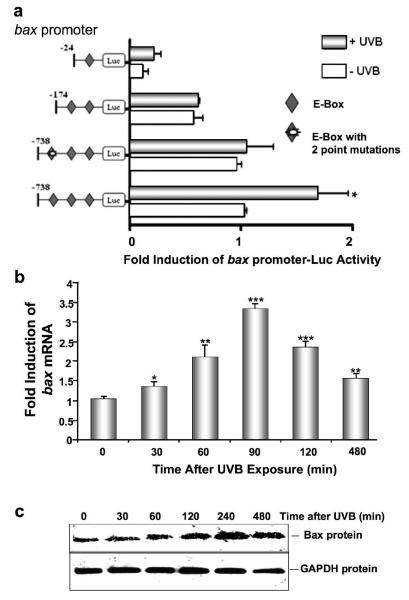
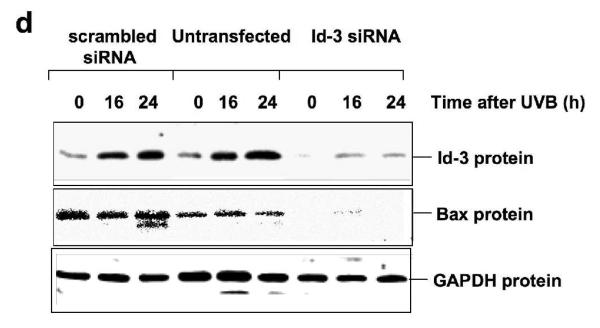
Since UVB upregulates Id3, which in turn induces the bax promoter, mRNA, and protein, we examined the ability of UVB to regulate the bax promoter. Cells were cotransfected with different bax promoter constructs, and exposed to UVB. Only the bax-738-luc construct was significantly induced by UVB, while shorter constructs, as well as the bax-738-luc construct with two point mutations in the distal 3rd E-box were completely unresponsive to UVB (Fig. 5a). Endogenous bax mRNA was also significantly upregulated by UVB, as determined by reverse transcription Q-PCR (Fig. 5b). The level of Bax protein was also induced in a time-dependent fashion by UVB (Fig. 5c). Furthermore, transfection of cells with Id3 siRNA prior to UVB exposure blocked both Id3 and Bax protein expression (Fig. 5d).
Figure 5.
UVB induces the bax promoter, mRNA, and protein, via Id3. (a) Immortalized keratinocytes were cotransfected with pRLTK Renilla luciferase, and each of the pGL3-derived bax promoter-luciferase constructs shown in a 1:20 molar ratio. After 24 hours, cells were UVB-irradiated and 16 hours later, extracts were used to measure bax promoter activity (firefly luciferase activity), and normalized against Renilla luciferase activity to control for transfection efficiency, using the Dual Luciferase Reporter Assay System. Fold change in firefly/Renilla luciferase activity is shown for UVB-irradiated cells compared to unirradiated controls, and calculated as the mean ± S.D. of three replicates of a representative experiment; essentially the same results were obtained in three independent experiments. (b) Following UVB irradiation, cells were harvested, and RNA was extracted and analyzed for levels of mRNAs encoding bax and cyclophilin using Q-PCR. Fold change in bax/cyclophilin mRNA is shown for UVB irradiated compared to unirradiated controls, and calculated as the mean ± S.D. of three replicates of a representative experiment; essentially the same results were obtained in three independent experiments. One, two or three asterisks represent significance levels of p<.05, .01, or .001, respectively, when UVB irradiated is compared to unirradiated controls (a, b). (c) Cells were UVB irradiated, protein extracted and immunoblot analysis performed utilizing antibodies monospecific for Bax or GAPDH. (d) E6/7 or HaCaT immortalized keratinocytes were transfected with Id3 siRNA or scrambled controls, and 48 hours later, UVB irradiated as described previously (12). Proteins were extracted and immunoblot analysis was performed utilizing antibodies monospecific for Id3, Bax or GAPDH
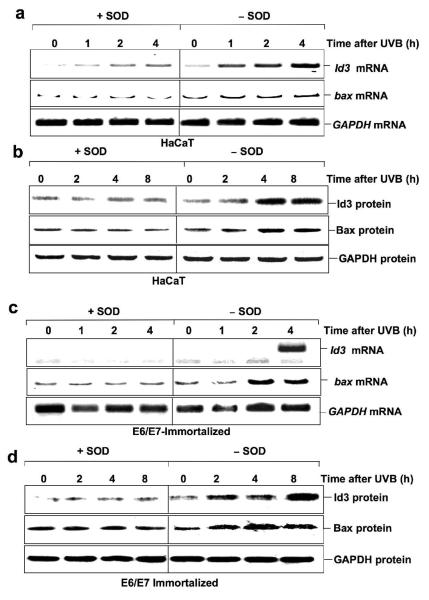
Finally, to determine if ROS mediates UVB-induced Id3 and Bax in non-E6/7 immortalized cells, both E6/7 and HaCaT immortalized cells were exposed to UVB with and without SOD pretreatment. Fig. 6 shows that Id3 and Bax mRNAs and proteins are induced by UVB, and this induction is blocked by SOD.
Figure 6.
Induction of Id3 and bax mRNA and protein is dependent on ROS. HaCaT (a, b) or E6/7 (c, d) immortalized keratinocytes were exposed to UVB in presence or absence of SOD. After the indicated times RNA or protein was extracted and subjected to RTPCR or immunoblot analysis using primers or antibodies specific for Id3, bax, or GAPDH.
Discussion
We show that UVB upregulates Id3 mRNA and protein in immortalized keratinocytes at both the promoter and post-transcriptional levels. Previous studies showed that a 180 bp promoter of the mouse Id3 gene confers high transcriptional activity in proliferating C2C12 cells (34). Another study localized promoter activity of mouse Id3 between −200 and + 54, and showed that membrane immunoglobulin induces Id3 in a B lymphoma cell line (35). Sp2 plays a role in regulating the mouse Id3 gene (36), but no Sp2 binding sites are located in the proximal promoter region of human Id3 gene. In this study the 1445 bp fragment had the highest basal transcriptional activity (Fig. 3a), and was induced 5 min after UVB exposure (Fig. 3b). Q-PCR analysis of Id3 mRNA showed that Id3 mRNA expression is maximal ~60 minutes after UVB irradiation. The half-life of Id3 mRNA increases from 10 to 60 minutes upon UVB irradiation (Fig. 3c), demonstrating regulation at both the promoter and posttranscriptional levels.
UVB irradiation affects a number of cellular functions, mediated by genes transcriptionally upregulated, including p21, p53 (37), interleukin-8 (38), platelet-derived endothelial cell growth factor (39), and GADD45 (40). The role of UVB in stabilizing mRNA messages has also been observed for IL-6 (41), and cyclooxygenase 2 (42), the latter of which is also regulated transcriptionally (43). Id3 is also post-transcriptionally regulated in response to vascular injury in rat and human atherosclerosis (44). Previously, it was shown that UVB induces apoptosis via ROS in human keratinocytes (45). In a different system, it was shown that superoxide induces Id3 mRNA in VSMC, following exposure to angiotensin II or to xanthin/xanthin oxidase (X/XO) (23, 46). Moreover, Id3 mRNA can be induced by ROS (23), which are generated upon UVB exposure, albeit not to the same extent as UVA, in different systems including human skin equivalent models (47).
In this paper we also investigated the mechanism of Id3 induction of bax. The promoter contains several E-boxes and Ets binding sites in the proximal promoter regions. The bax promoter was also found to be responsive to c-Myc via its E-boxes (48). In the current study, we found that only bax promoter constructs that span a region starting –738 bp upstream from the transcription start site containing three E-boxes are induced by Id3 and UVB. Upregulation of bax by UVB has been observed in several previous studies (49), and the bax gene was first identified as a primarily p53-responsive gene (50). Therefore, it is not surprising to see an upregulation of Bax upon UVB irradiation. However, in our system, immortalization was achieved via the stable overexpression of E6 and E7 that leads to the inactivation of p53 (10) as we have repeatedly verified by immunoblot analysis (9). Further, p53-null HaCaT cells responded similarly. To our knowledge, this is the first paper to describe an upregulation of Bax, in response to UVB irradiation, in a p53-independent manner.
Id3 may sequester an inhibitor of bax transcription. For example c-Myc, a bHLH protein, binds to the E-boxes in the bax promoter, leading to its activation (48), but the bHLH protein Mad1 binds to c-Myc resulting in transcriptional repression (51). Id2 was also able to relieve transcriptional repression of the promoter for murine p75 NGFR, by sequestering murine homologue of HEB (which also binds Id3) (52).
Skin cancer is the most common human cancer, and the role of UVB has been extensively studied. Immortalized cells are extremely sensitive to UVB-induced apoptosis (11), unlike primary and cancerous keratinocytes. However, after the accumulation of additional mutations they become fully tumorigenic, and resistant to UVB-induced apoptosis. At the physiological level, immortalized keratinocytes are similar to non-invasive precancerous cells derived from actinic keratoses, and their enhanced susceptibility to apoptosis upon UVB exposure could explain their relatively low incidence of progression to invasive squamous cell carcinoma ranging from 0.1% to 1% per year (53-55); compared to the high rates of spontaneously regressing actinic keratoses, estimated at 21% (56), 25.9% (53), and 29% per year (55). Identification of all the players involved in the UVB-induced apoptotic response would yield valuable information and may provide means for therapeutic interventions. Taken together, our studies determined that upregulation of Id3 is a potential mechanism for the selective UVB sensitivity of immortalized keratinocytes. As such, Id3 may therefore represent a therapeutic target for the selective elimination of initiated precancerous or cancerous cells.
Acknowledgements
Authors thank Richard Schlegel (Department of Oncology, Georgetown University) for the HPV16-E6/7 retroviral vectors. This work was supported in part by the NCI Grant 1RO1 CA100443-01A1 and the US Army Medical Research and Materiel Command contract DAMD17-00-C-0026 (to DSR).
References
- 1.Fears TR, Scotto J. Estimating increases in skin cancer morbidity due to increases in ultraviolet radiation exposure. Cancer Invest. 1983;1:119–126. doi: 10.3109/07357908309042414. [DOI] [PubMed] [Google Scholar]
- 2.Strickland PT, Vitasa BC, West SK, Rosenthal FS, Emmett EA, Taylor HR. Quantitative carcinogenesis in man: solar ultraviolet B dose dependence of skin cancer in Maryland watermen. J Natl Cancer Inst. 1989;81:1910–1913. doi: 10.1093/jnci/81.24.1910. [DOI] [PubMed] [Google Scholar]
- 3.Nomura T, Nakajima H, Hongyo T, et al. Induction of cancer, actinic keratosis, and specific p53 mutations by UVB light in human skin maintained in severe combined immunodeficient mice. Cancer Res. 1997;57:2081–2084. [PubMed] [Google Scholar]
- 4.Griffiths HR, Mistry P, Herbert KE, Lunec J. Molecular and cellular effects of ultraviolet light-induced genotoxicity. Crit Rev Clin Lab Sci. 1998;35:189–237. doi: 10.1080/10408369891234192. [DOI] [PubMed] [Google Scholar]
- 5.de Gruijl FR. Photocarcinogenesis: UVA vs UVB. Methods Enzymol. 2000;319:359–366. doi: 10.1016/s0076-6879(00)19035-4. [DOI] [PubMed] [Google Scholar]
- 6.Herrling T, Fuchs J, Rehberg J, Groth N. UV-induced free radicals in the skin detected by ESR spectroscopy and imaging using nitroxides. Free Radic Biol Med. 2003;35:59–67. doi: 10.1016/s0891-5849(03)00241-7. [DOI] [PubMed] [Google Scholar]
- 7.Herrling T, Jung K, Fuchs J. Measurements of UV-generated free radicals/reactive oxygen species (ROS) in skin. Spectrochim Acta A Mol Biomol Spectrosc. 2006;63:840–845. doi: 10.1016/j.saa.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Hanks AN, Boucher K, et al. UVB-induced apoptosis drives clonal expansion during skin tumor development. Carcinogenesis. 2005;26:249–257. doi: 10.1093/carcin/bgh300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simbulan-Rosenthal CM, Trabosh V, Velarde A, et al. Id2 protein is selectively upregulated by UVB in primary, but not in immortalized human keratinocytes and inhibits differentiation. Oncogene. 2005;4:5443–5458. doi: 10.1038/sj.onc.1208709. [DOI] [PubMed] [Google Scholar]
- 10.Simbulan-Rosenthal CM, Velena A, Veldman T, Schlegel R, Rosenthal DS. HPV-16 E6/7 immortalization sensitizes human keratinocytes to ultraviolet B by altering the pathway from caspase-8 to caspase-9-dependent apoptosis. J Biol Chem. 2002;277:24709–24716. doi: 10.1074/jbc.M200281200. [DOI] [PubMed] [Google Scholar]
- 11.Daher A, Simbulan-Rosenthal CM, Rosenthal DS. Apoptosis induced by ultraviolet B in HPV-immortalized human keratinocytes requires caspase-9 and is death receptor independent. Exp Dermatol. 2006;15:23–34. doi: 10.1111/j.0906-6705.2005.00384.x. [DOI] [PubMed] [Google Scholar]
- 12.Simbulan-Rosenthal CM, Daher A, Trabosh V, et al. Id3 induces a caspase-3- and -9-dependent apoptosis and mediates UVB sensitization of HPV16 E6/7 immortalized human keratinocytes. Oncogene. 2006;25:3649–3660. doi: 10.1038/sj.onc.1209407. Epub 2006 Jan 3630. [DOI] [PubMed] [Google Scholar]
- 13.Atchley WR, Fitch WM. A natural classification of the basic helix-loop-helix class of transcription factors. Proc Natl Acad Sci U S A. 1997;94:5172–5176. doi: 10.1073/pnas.94.10.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murre C, McCaw PS, Vaessin H, et al. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989;58:537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- 15.Norton JD. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci. 2000;113:3897–3905. doi: 10.1242/jcs.113.22.3897. [DOI] [PubMed] [Google Scholar]
- 16.Yates PR, Atherton GT, Deed RW, Norton JD, Sharrocks AD. Id helix-loop-helix proteins inhibit nucleoprotein complex formation by the TCF ETS-domain transcription factors. Embo J. 1999;18:968–976. doi: 10.1093/emboj/18.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotzer D, Krampert M, Sulyok S, et al. Id proteins: novel targets of activin action, which regulate epidermal homeostasis. Oncogene. 2006;25:2070–2081. doi: 10.1038/sj.onc.1209230. [DOI] [PubMed] [Google Scholar]
- 18.Benezra R, Rafii S, Lyden D. The Id proteins and angiogenesis. Oncogene. 2001;20:8334–8341. doi: 10.1038/sj.onc.1205160. [DOI] [PubMed] [Google Scholar]
- 19.Hasskarl J, Munger K. Id proteins--tumor markers or oncogenes? Cancer Biol Ther. 2002;1:91–96. doi: 10.4161/cbt.50. [DOI] [PubMed] [Google Scholar]
- 20.Kee BL, Rivera RR, Murre C. Id3 inhibits B lymphocyte progenitor growth and survival in response to TGF-beta. Nat Immunol. 2001;2:242–247. doi: 10.1038/85303. [DOI] [PubMed] [Google Scholar]
- 21.Chen B, Han BH, Sun XH, Lim RW. Inhibition of muscle-specific gene expression by Id3: requirement of the C-terminal region of the protein for stable expression and function. Nucleic Acids Res. 1997;25:423–430. doi: 10.1093/nar/25.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deleu S, Savonet V, Behrends J, Dumont JE, Maenhaut C. Study of gene expression in thyrotropin-stimulated thyroid cells by cDNA expression array: ID3 transcription modulating factor as an early response protein and tumor marker in thyroid carcinomas. Exp Cell Res. 2002;279:62–70. doi: 10.1006/excr.2002.5589. [DOI] [PubMed] [Google Scholar]
- 23.Mueller C, Baudler S, Welzel H, Bohm M, Nickenig G. Identification of a novel redox-sensitive gene, Id3, which mediates angiotensin II-induced cell growth. Circulation. 2002;105:2423–2428. doi: 10.1161/01.cir.0000016047.19488.91. [DOI] [PubMed] [Google Scholar]
- 24.Ko M, Ahn J, Lee C, et al. E2A/HEB and Id3 proteins control the sensitivity to glucocorticoid-induced apoptosis in thymocytes by regulating the SRG3 expression. J Biol Chem. 2004;279:21916–21923. doi: 10.1074/jbc.M402145200. [DOI] [PubMed] [Google Scholar]
- 25.Versnel MA. Id3 knockout mice as a new model for sjogren's syndrome: only a T cell defect or more? Immunity. 2004;21:457–458. doi: 10.1016/j.immuni.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Norton JD, Atherton GT. Coupling of cell growth control and apoptosis functions of Id proteins. Mol Cell Biol. 1998;18:2371–2381. doi: 10.1128/mcb.18.4.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kee BL. Id3 induces growth arrest and caspase-2-dependent apoptosis in B lymphocyte progenitors. J Immunol. 2005;175:4518–4527. doi: 10.4049/jimmunol.175.7.4518. [DOI] [PubMed] [Google Scholar]
- 28.Koyama T, Suzuki H, Imakiire A, Yanase N, Hata K, Mizuguchi J. Id3-mediated enhancement of cisplatin-induced apoptosis in a sarcoma cell line MG-63. Anticancer Res. 2004;24:1519–1524. [PubMed] [Google Scholar]
- 29.Kee Y, Bronner-Fraser M. To proliferate or to die: role of Id3 in cell cycle progression and survival of neural crest progenitors. Genes Dev. 2005;19:744–755. doi: 10.1101/gad.1257405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravi R, Bedi A. Requirement of BAX for TRAIL/Apo2L-induced apoptosis of colorectal cancers: synergism with sulindac-mediated inhibition of Bcl-x(L) Cancer Res. 2002;62:1583–1587. [PubMed] [Google Scholar]
- 31.Theodorakis P, Lomonosova E, Chinnadurai G. Critical requirement of BAX for manifestation of apoptosis induced by multiple stimuli in human epithelial cancer cells. Cancer Res. 2002;62:3373–3376. [PubMed] [Google Scholar]
- 32.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staal FJ, Roederer M, Herzenberg LA, Herzenberg LA. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990;87:9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeh K, Lim RW. Genomic organization and promoter analysis of the murine Id3 gene. Gene. 2000;254:163–171. doi: 10.1016/s0378-1119(00)00274-2. [DOI] [PubMed] [Google Scholar]
- 35.Li XJ, Hata K, Mizuguchi J. Engagement of membrane immunoglobulin enhances Id3 promoter activity in WEHI-231 B lymphoma cells. Acta Pharmacol Sin. 2005;26:486–491. doi: 10.1111/j.1745-7254.2005.00067.x. [DOI] [PubMed] [Google Scholar]
- 36.Wu J, Lim RW. Regulation of inhibitor of differentiation gene 3 (Id3) expression by Sp2-motif binding factor in myogenic C2C12 cells: downregulation of DNA binding activity following skeletal muscle differentiation. Biochim Biophys Acta. 2005;1731:13–22. doi: 10.1016/j.bbaexp.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Petrocelli T, Poon R, Drucker DJ, Slingerland JM, Rosen CF. UVB radiation induces p21Cip1/WAF1 and mediates G1 and S phase checkpoints. Oncogene. 1996;12:1387–1396. [PubMed] [Google Scholar]
- 38.Venner TJ, Sauder DN, Feliciani C, McKenzie RC. Interleukin-8 and melanoma growth-stimulating activity (GRO) are induced by ultraviolet B radiation in human keratinocyte cell lines. Exp Dermatol. 1995;4:138–145. doi: 10.1111/j.1600-0625.1995.tb00237.x. [DOI] [PubMed] [Google Scholar]
- 39.Howell BG, Wang B, Freed I, Mamelak AJ, Watanabe H, Sauder DN. Microarray analysis of UVB-regulated genes in keratinocytes: downregulation of angiogenesis inhibitor thrombospondin-1. J Dermatol Sci. 2004;34:185–194. doi: 10.1016/j.jdermsci.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Dazard JE, Gal H, Amariglio N, Rechavi G, Domany E, Givol D. Genome-wide comparison of human keratinocyte and squamous cell carcinoma responses to UVB irradiation: implications for skin and epithelial cancer. Oncogene. 2003;22:2993–3006. doi: 10.1038/sj.onc.1206537. [DOI] [PubMed] [Google Scholar]
- 41.de Vos S, Brach M, Budnik A, Grewe M, Herrmann F, Krutmann J. Post-transcriptional regulation of interleukin-6 gene expression in human keratinocytes by ultraviolet B radiation. J Invest Dermatol. 1994;103:92–96. doi: 10.1111/1523-1747.ep12391818. [DOI] [PubMed] [Google Scholar]
- 42.Tong X, Van Dross RT, Abu-Yousif A, Morrison AR, Pelling JC. Apigenin prevents UVB-induced cyclooxygenase 2 expression: coupled mRNA stabilization and translational inhibition. Mol Cell Biol. 2007;27:283–296. doi: 10.1128/MCB.01282-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang Q, Chen W, Gonzales MS, Finch J, Inoue H, Bowden GT. Role of cyclic AMP responsive element in the UVB induction of cyclooxygenase-2 transcription in human keratinocytes. Oncogene. 2001;20:5164–5172. doi: 10.1038/sj.onc.1204667. [DOI] [PubMed] [Google Scholar]
- 44.Matsumura ME, Li F, Berthoux L, et al. Vascular injury induces posttranscriptional regulation of the Id3 gene: cloning of a novel Id3 isoform expressed during vascular lesion formation in rat and human atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:752–758. doi: 10.1161/01.atv.21.5.752. [DOI] [PubMed] [Google Scholar]
- 45.Rezvani HR, Mazurier F, Cario-Andre M, et al. Protective effects of catalase overexpression on UVB-induced apoptosis in normal human keratinocytes. J Biol Chem. 2006;281:17999–18007. doi: 10.1074/jbc.M600536200. [DOI] [PubMed] [Google Scholar]
- 46.Nickenig G, Baudler S, Muller C, et al. Redox-sensitive vascular smooth muscle cell proliferation is mediated by GKLF and Id3 in vitro and in vivo. Faseb J. 2002;16:1077–1086. doi: 10.1096/fj.01-0570com. [DOI] [PubMed] [Google Scholar]
- 47.Hakozaki T, Date A, Yoshii T, Toyokuni S, Yasui H, Sakurai H. Visualization and characterization of UVB-induced reactive oxygen species in a human skin equivalent model. Arch Dermatol Res. 2007 doi: 10.1007/s00403-007-0804-3. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell KO, Ricci MS, Miyashita T, et al. Bax is a transcriptional target and mediator of c-myc-induced apoptosis. Cancer Res. 2000;60:6318–6325. [PubMed] [Google Scholar]
- 49.Kim YG, Kim HJ, Kim DS, et al. Up-Regulation and redistribution of Bax in ultraviolet B-irradiated melanocytes. Pigment Cell Res. 2000;13:352–357. doi: 10.1034/j.1600-0749.2000.130508.x. [DOI] [PubMed] [Google Scholar]
- 50.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 51.Gehring S, Rottmann S, Menkel AR, Mertsching J, Krippner-Heidenreich A, Luscher B. Inhibition of proliferation and apoptosis by the transcriptional repressor Mad1. Repression of Fas-induced caspase-8 activation. J Biol Chem. 2000;275:10413–10420. doi: 10.1074/jbc.275.14.10413. [DOI] [PubMed] [Google Scholar]
- 52.Chiaramello A, Neuman K, Palm K, Metsis M, Neuman T. Helix-loop-helix transcription factors mediate activation and repression of the p75LNGFR gene. Mol Cell Biol. 1995;15:6036–6044. doi: 10.1128/mcb.15.11.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marks R, Foley P, Goodman G, Hage BH, Selwood TS. Spontaneous remission of solar keratoses: the case for conservative management. Br J Dermatol. 1986;115:649–655. doi: 10.1111/j.1365-2133.1986.tb06644.x. [DOI] [PubMed] [Google Scholar]
- 54.Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet. 1988;1:795–797. doi: 10.1016/s0140-6736(88)91658-3. [DOI] [PubMed] [Google Scholar]
- 55.Frost C, Williams G, Green A. High incidence and regression rates of solar keratoses in a queensland community. J Invest Dermatol. 2000;115:273–277. doi: 10.1046/j.1523-1747.2000.00048.x. [DOI] [PubMed] [Google Scholar]
- 56.Harvey I, Frankel S, Marks R, Shalom D, Nolan-Farrell M. Non-melanoma skin cancer and solar keratoses. I. Methods and descriptive results of the South Wales Skin Cancer Study. Br J Cancer. 1996;74:1302–1307. doi: 10.1038/bjc.1996.534. [DOI] [PMC free article] [PubMed] [Google Scholar]