Abstract
A costimulatory signal is required for the full activation of T cells, in addition to the antigen-specific signal via the T cell receptor. The inducible costimulator, ICOS is one of the costimulatory molecules that play an essential role in this process, particularly in the expansion or the development of effector T cells. As blocking of the interaction between ICOS and its ligand, B7RP-1, suppresses the T cell response, it can be applied to the treatment of allograft rejection or autoimmune diseases. Here, we isolated four scFv clones that were specific to human B7RP-1 by biopanning a human antibody phage library. We found that three of these clones inhibited the interaction between ICOS-Fc and B7RP-1-Fc. These inhibitory clones not only recognized B7RP-1 molecules expressed on B cells, as assessed by FACS, but also exhibited inhibitory activity in a proliferation assay of T cells stimulated with anti-CD3 mAb and B7RP-1-Fc. Finally, the suppression effect of the scFv on the allogenic immune response was examined using a mixed lymphocyte reaction assay, which demonstrated a successful inhibition of the allogenic reaction, in spite of the high dose needed for complete inhibition (360 nM).
Keywords: costimulatory signal, B7RP-1, ICOS, T-cell proliferation, antibody fragment, scFv, allogenic reaction
Introduction
The interactions between T cells and APCs (antigen presenting cells) are essential for the T cell immune response, in which T cells require two distinct APC signals to fully activate or differentiate to effector cells. The primary signal is an antigen-specific signal delivered by the T cell receptor (TCR) that recognizes antigen peptides presented by the major histocompatibility complex (MHC) molecule on APCs. The secondary is the costimulatory signal exerted by the ligation between the costimulatory receptors on T cells and their pairwise ligands on APCs.1 CD28 is a costimulatory receptor that initializes T cell activation and its blocking leads to T cell anergy or apoptosis. The second member of the CD28 family, CTLA-4 (CD152), is a suppressive costimulatory receptor that regulates activated T cells, although these two contradictory receptors share common ligands: CD80 (B7-1) and CD86 (B7-2).2
The inducible costimulator (ICOS) is the third member of the CD28 superfamily and its expression is induced on activated T cells by stimulation mediated by the TCR signal.3 The ICOS signal, which is delivered by the ligation with B7RP-1 (ICOS ligand, B7-H2 or B7 h) on APCs, enhances the proliferation of both CD4 and CD8 T cells, the secretion of Th1- and/or Th2-type cytokines, and the expression of CD154 (CD40 ligand) on T cells.4 These functions, i.e., the antigen-stimulated T cell proliferation and IL-4 production, are abolished in an ICOS knockout mouse.5 The difference in the roles of CD28 and ICOS played in the immune response were examined using model experiments of mucosal bronchial inflammation induced in ovalbumin-immunized mice.6,7 The specific inhibition of the CD28 or ICOS signals using CTLA-4-Fc (i.e., fusion proteins of the extracellular domains of CTLA-4 and the Fc of IgG) or anti-ICOS antibodies leads to different effects on lymphocyte infiltration into tissues or in the pattern of cytokine production, depending on the administration time. These results clarified the different roles of CD28 and ICOS, that CD28 is essential for the priming of the T cell response and that ICOS promotes the subsequent effector responses.
The blocking of the ICOS signal controls the immune/inflammatory response very effectively in EAE and chronic allograft rejection,8,9 in which an anti-ICOS antibody or fusion proteins of the extracellular domains of ICOS or B7RP-1 and the Fc of IgG (ICOS-Fc or B7RP-1-Fc) were used as ICOS inhibitors. An anti-B7RP-1 monoclonal antibody (mAb) was also used as a therapy for collagen type II-induced arthritis or murine experimental autoimmune uveoretinitis and yielded a good treatment effect,10 which suggests a possible application of these approaches to the treatment of autoimmune diseases. However, these divalent molecules connected with antibody Fc fragments have the potential risk of functioning agonistically via receptor dimerization on the cell surface, which may result in unexpected and adverse side effects in vivo. In this respect, a monovalent antibody fragment may be an adequate inhibitor to regulate immune responses. Kolly et al.11 reported on an anti-CD86 monovalent small antibody fragment (single chain Fv, scFv) that successfully regulates the T cell proliferation induced by TCR stimulation with anti-CD3 antibody along with a CD28 costimulatory signal using CD86-expessing P815 cells.
Pan et al.12 described a combined immunotherary for allograft transplantation via the blocking of CD28 and ICOS using an adenoviral vector carrying CTLA-4-Fc and ICOS-Fc, which significantly prevented the cardiac arrest associated with allograft rejection by reducing inflammatory-cell infiltration into the graft. This synergistic effect of blocking both CD28 and ICOS signals has been observed for alloimmunity,13 for the model of glomerulonephritis autoimmune disease, and for vaccine responses.14,15 Here, we report the isolation of anti-human-B7RP-1 scFv from a human scFv antibody phage library and show its antagonistic effect on anti-CD3 antibody-stimulated T cell proliferation and on allograft rejection using a mixed lymphocyte reaction (MLR). Our results suggest that the scFv isolated may be an alternative candidate as ICOS inhibitor that could be used to regulate the excess immune response in allograft rejection or autoimmune disease.
Results
Isolation and binding specificity of human scFv antibodies specific to human B7RP-1.
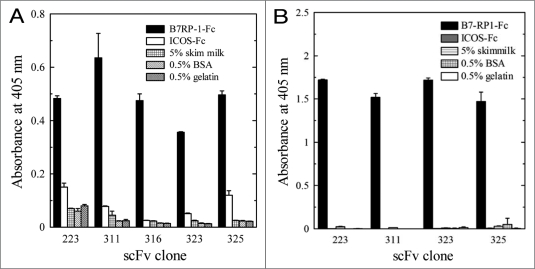
To isolate specific phages to B7RP-1 from the human scFv phage library, a biopanning was performed against the B7RP-1-Fc coated on the immuno tube. This procedure involving the step to deplete Fc-specific phages was repeated three times. The phages isolated after the second and third rounds of biopanning were cloned and subjected to the binding analysis using ELISA, which revealed that eight clones were positive among the 80 isolated clones. The DNA sequence of the scFv gene from each phage clone was determined and identified the presence of five unique clones. Figure 1A shows the binding activities of the five phage clones to B7RP-1-Fc using ELISA. We subsequently purified scFv fragments from E. coli HB2151 cells infected with each phage clone; however, clone 316 did not produce a detectable scFv in the bacterial culture. Thus, we proceeded with the binding analysis of the other four scFv fragments. As shown in Figure 1B, these four scFv exhibited binding activity to B7RP-1-Fc, but not to ICOS-Fc, which suggests that these scFv fragments were directed against the extracellular domain of B7RP-1 and not against the Fc region of the fusion molecules.
Figure 1.
Binding activity of the phage clones selected by biopanning (A) and of purified scFv (B) to human B7RP-1-Fc using ELISA. We examined the binding of the phage clones (1 × 1013 TU/ml) or of the purified scFv fragments (20 µg/ml) toward human B7RP-1-Fc, ICOS-Fc, and other proteins (skim milk, BSA and gelatin), which were coated onto the wells of the immuno plate. Binding phages were detected using the biotinylated anti-M13 mAb and AP-conjugated SA. The scFv fused C-terminally to an E-tag was detected using an anti-E-tag antibody and an AP-conjugated anti-Fc antibody.
Inhibition of the ICOS/B7RP-1 interaction by B7RP-1-specific scFv in ELISA and SPR.
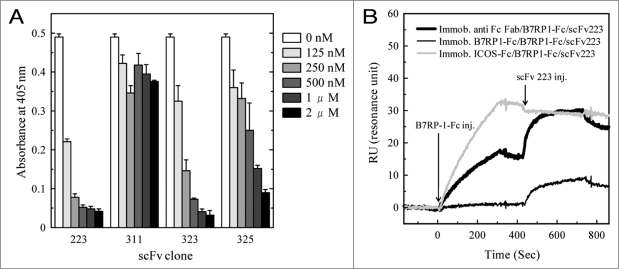
The inhibitory activity of the four clones specific for B7RP-1-Fc were examined against the binding between ICOS-Fc and B7RP-1-Fc. Figure 2 shows that clones 223, 323 and 325 dose-dependently interfered with the ICOS/B7RP-1 interaction, although clone 325 was less inhibitory than clones 223 and 323. On the other hand, clone 311 did not show inhibitory activity.
Figure 2.
Inhibitory activity of the B7RP-1-specific scFv fragment against the ICOS/B7RP-1 interaction, as assessed by ELISA (A) and by SPR analyses (B). (A) The binding of the biotinylated ICOS-Fc to the B7RP-1-Fc coated onto the plate was examined in the presence of varying concentrations of anti B7RP-1 scFv (0.125–2 µM). The biotinylated ICOS-Fc was detected using AP-conjugated SA. (B) The sensor chip was first immobilized with an anti-human Fc F(ab)2 antibody (thick line), B7RP-1-Fc (thin line), and ICOS-Fc (grey line), using the amine-coupling method (RU: 400–630). Subsequently, B7RP-1-Fc (2 µg/ml) was injected into the flow cells, and after washing for 120 s, scFv 223 (100 nM) was injected, as indicated by the arrows.
The SPR analysis of scFv 223 using the BIAcore 2000 apparatus also clearly showed its inhibitory activity against the ICOS/B7RP-1 interaction. Three types of the B7RP-1-Fc fixed on the sensorchip; (1) captured by the covalently immobilized anti-Fc antibody, (2) captured by the covalently immobilized ICOS-Fc or (3) directly immobilized on the sensorchip were prepared and subsequently scFv 223 was injected to start the association reaction. As shown in Figure 2B, a binding response of scFv 223 were observed to B7RP-1-Fc captured by the anti-Fc antibody (thick black line), but not to B7RP-1-Fc captured by ICOS-Fc (grey line). This observation indicates that the binding of scFv 223 to B7RP-1-Fc was disturbed by the formation of ICOS/B7RP-1 complex, supporting the inhibitory activity of scFv 223 against ICOS-Fc/B7RP-1-Fc interaction (Fig. 2A).
B-cell staining with human B7RP-1-specific scFv.
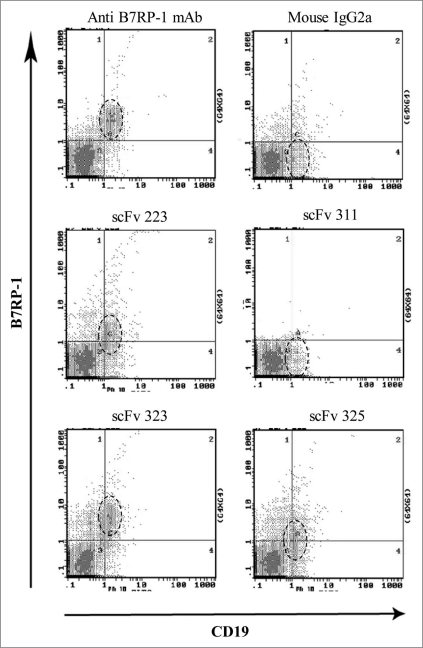
To test whether the isolated scFv actually recognized B7RP-1 molecules on the cell surface, human PBMCs were double stained with scFv and anti-CD19 mAb and analyzed by FACS (Fig. 3). Clones 223, 323 and 325 bound to a B-cell population that was stained with anti-CD19 mAb, indicating the binding capability to the extracellular domain of B7RP-1 expressed on B cells. Clone 323 had a relatively high binding activity (RMF, 14) when compared with clones 223 and 325 (RMF, 5.3 and 4.0, respectively) and with anti-B7RP-1 mouse mAb (RMF, 12) used as a positive control. In contrast, clone 311 did not bind to B cells, in spite of its clear binding to B7RP-1-Fc in ELISA (Fig. 1).
Figure 3.
Binding analysis of anti-B7RP-1 scFv to B cells from human PBMCs on FACS. Human PBMCs stimulated with PMA and PHA were gated according to their forward and side scatter (FSC/SSC) profiles and T cell/B cell subpopulations were analyzed. Cells were treated with FITC-conjugated anti-CD19 mAb (to stain B cells) and with B7RP-1-specific scFv, which was then detected using biotinylated anti-E-tag mAb and PE-labeled SA. Anti-human B7RP-1 mouse mAb and the isotypic mAb (mouse IgG2a) were used as positive and negative controls, respectively. The area surrounded by the broken line indicates the B-cell population stained with the anti-CD19 mAb.
Suppression of CD3-stimulated T cell proliferation by the B7RP-1-specific scFv fragment.
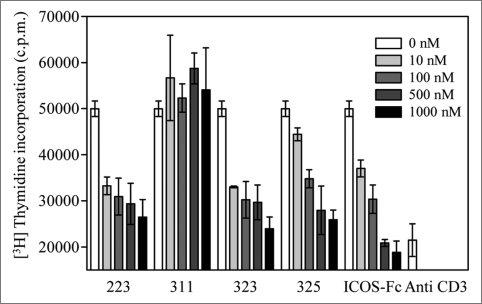
To estimate the function of scFv in the blocking of the ICOS-B7RP-1 signaling, T cell proliferation induced by the stimulation of the TCR and costimulatory signals with the anti-CD3 mAb and B7RP-1-Fc both coated on the well was examined in the presence of the different concentration (10–1,000 nM) of scFv (Fig. 4). The anti CD3 mAb-mediated stimulation resulted in a poor proliferative activity; however, the costimulation of ICOS with B7RP-1-Fc largely enhanced the response. Under these conditions, the scFv clones 223, 323 and 325 dose-dependently suppressed T cell proliferations in a manner similar to that observed for ICOS-Fc, which was used as a positive control inhibitor. In contrast, clone 311 did not show the inhibitory activity against T cell proliferation, which reflects the facts that it lacked the binding ability to B7RP-1 on cells (Fig. 3).
Figure 4.
Suppression of T cell proliferation by blockage of ICOS signaling with anti-B7RP-1 scFv clones 223, 311, 323 and 325. Human PBMCs (1 × 105 cells/well) were stimulated with an anti-CD3 mAb (OKT3) and B7RP-1-Fc, which were coated onto the 96-well culture plate in the presence of varying doses (10–1000 nM) of scFv fragments. Cell proliferation was estimated based on the radioactivity of the incorporated [3H] thymidine, after 48 h of cell culture. ICOS-Fc was used as a positive control for the blocking of the ICOS signal. Anti-CD3 (right) indicates the proliferative activity of T cells via exclusive stimulation with an anti-CD3 mAb. The radioactivity incorporated was measured in a scintillation counter and was expressed as cpm.
Assessment of the effect of scFv fragments on the allogenic reaction using MLR.
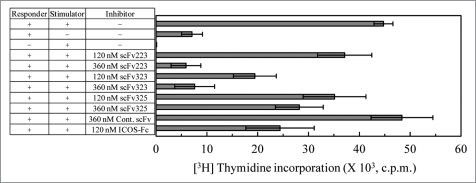
To confirm the effect of scFv fragments in the blocking of the ICOS/B7RP-1 signal during cell-cell interaction, we performed an MLR assay. The prestimulated corresponder cells were cultured with the irradiated stimulator cells (which were derived from another donor) for five days and the allogenic T cell proliferation was estimated. As shown in Figure 5, although the corresponder cells alone did not exhibit a significant proliferation, the addition of the irradiated stimulator cells largely augmented the proliferation of the corresponder cells in the presence of the control scFv (anti nuclear protein scFv). The addition of 120 and 360 nM B7RP-1-specific scFv led to the reduction of the allogenic T cell responses to 82 and 13% for clone 223, to 43 and 16% for clone 323, and to 78 and 62% for clone 325, respectively. This result indicates that at least scFv clones 223 and 323 effectively function to regulate the allogenic response between T cell responder and other donor-derived stimulator cells by blocking ICOS/B7RP-1 signals.
Figure 5.
Inhibition of proliferative responses in allogeneic MLR. Responder cells were prestimulated with an anti-CD3 mAb, which was coated onto the well, for 12 h. Stimulator cells prepared from another donor were treated with 15 Gy of irradiation and cultured with the prestimulated responder cells in the presence of anti B7RP-1 scFv or ICOS-Fc. After the cells were pulsed with [3H] thymidine for 18 h on day 5, the radioactivity incorporated into the cells was counted and expressed as cpm. As the control scFv, anti nuclear protein scFv was used.
Kinetic binding analysis of scFv using SPR analysis.
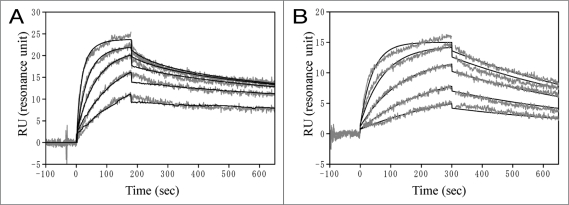
The kinetic binding analysis of scFv toward B7RP-1-Fc was examined on the BIAcore 2000 apparatus using a CM5 sensor chip. B7RP-1-Fc was directly immobilized on the sensor chip and scFv or ICOS-Fc binding was examined. The sensorgrams of the binding ICOS-Fc and scFv 223 to B7RP-1-Fc were shown in Figure 6 and the kinetic parameters evaluated were summarized in Table 1. The dissociation constant (Kd) of the binding between ICOS and B7RP-1 was 24 nM (Table 1). On the other hand, the Kd values for scFv were within 9–22 nM. The variations of the affinity among scFv seem to be caused by the variations in kd rather than ka values (Table 1). The high affinity scFv 323 and 223 showed slow dissociation rate (kd: 0.9 and 1.7 × 10−3 sec−1), while the low affinity scFv 325 had relatively fast dissociation rate (kd: 3.8 × 10−3 sec−1).
Figure 6.
SPR analysis of the binding between B7RP-1-Fc and ICOS-Fc (A) or scFv 223 (B) on BIAcore 2000. To the B7RP-1-Fc (RU: 332) immobilized on the CM5 sensorchip, ICOS-Fc solutions in (A) or scFv 223 solutions in (B) were injected at 25°C to monitor the association and the subsequent dissociation reaction. The sensorgrams of ICOS-Fc or scFv were analyzed using a bivalent or 1:1 Langmuir model on the BIAevaluation 3.2 software, respectively. The evaluated kinetic parameters are listed in Table 1. The grey and black lines indicate the experimental and simulated data.
Table 1.
Binding parameters of scFv fragments to B7RP-1-Fc by SPR
| scFv Clone | ka (M−1 sec−1) | kd (sec−1) | Kd (nM) |
| 223 | 1.3 × 105 | 1.7 × 10−3 | 14 |
| 323 | 1.1 × 105 | 0.9 × 10−3 | 9 |
| 325 | 1.7 × 105 | 3.8 × 10−3 | 22 |
| ICOS-Fc* | 3.7 × 104 | 8.9 × 10−3 | 24 |
The kinetic parameters of scFv and ICOS-Fc binding were calculated from the sensorgrams using 1:1 Langumuir binding model on BIAevaluation 3.2 software. As for the binding of ICOS-Fc (*), the ka1/ kd1 values evaluated using a bivalent model were listed.
Amino acid sequences of anti B7RP-1 scFv fragments.
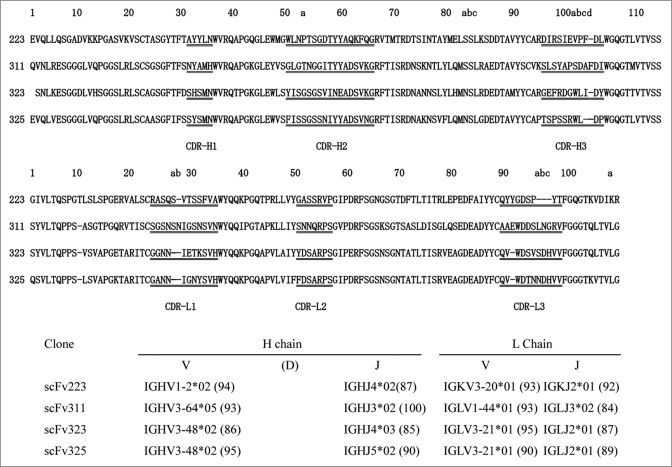
The amino acid sequences of the scFv fragments developed against human B7RP-1 were deduced from the DNA sequences carried by each phage clone, as listed in Figure 6. The V gene repertoires of each clone were analyzed using the DNA sequences on IMGT/V-QUEST database Web site.16 The heavy chain V genes used in clone 223 and in the other clones were VH1 and VH3, respectively. The light chain V genes for clones 223, 311, and the other two clones were Vkappa3, Vlamda1 and Vlamda3, respectively. The scFv 323 and 325 are most similar to each other with the amino acid sequence identities of 70, 85 and 76% in VH, VL and overall region, which might indicate the same binding properties of these two scFv and also reflect sharing the common origins of HV3-48*2, lamdaV3-21*01 and also lamdaJ2*01 genes between them. The comparison of the sequence in the hypevariable HCDR3 region suggests a vague similarity of the hydrophobic residues (WL, 103a-b in Kabat numbering) between scFv323 and 325, although the importance of this short sequence in the binding or specificity should be further investigated.
Discussion
We isolated four scFv phage clones that were specific for B7RP-1-Fc molecules from a naïve human scFv phage library. Among them, three clones inhibited the interaction between ICOS-Fc and B7RP-1-Fc in a dose-dependent manner (Fig. 2). The tertiary structure of the complex of ICOS and B7RP-1 has not been elucidated to date. However, mapping analysis of the binding site on the surface of ICOS17 and B7RP-1,18 using point mutations revealed that the binding mode of ICOS and B7RP-1 is approximately overlapped by that of CTLA-4 and CD80 or CD86 in the complex structures elucidated by X-ray crystallographic analysis.19,20 Wang et al.18 suggested that the conservative PPP region (residues 116–118) of the ICOS molecule interacts with CC′C″FG strands in the N-terminal IgV domain of B7RP-1 and demonstrated that only the N-terminal Ig-V domain of B7RP-1 retains the ability to bind to the ICOS molecule. These observations imply that the binding sites of the three inhibitory scFv fragments are possibly located in the N-terminal IgV-like domain of B7RP-1, although the precise epitope mapping will be nessesary for comfirming the binding site.
In contrast, scFv clone 311 showed no inhibitory activity against the interaction between ICOS-Fc and B7RP-1-Fc, in spite of its clear ability to bind to B7RP-1-Fc (Fig. 2). As this clone did not recognize the Fc region (Fig. 1), it is possible that its recognition site is directed to the C-terminal IgC-like domain or to the opposite side of the ICOS-binding site on the N-terminal domain of B7RP-1. Taking this into consideration, together with the fact that this clone also lost binding affinity for B7RP-1 molecules expressed on B cells (Fig. 3), it is possible that the recognition site of this clone may be not exposed to the outer side instead, it may be buried in the C-terminal domain, which lies proximal to the membrane surface.
Blocking studies of the ICOS/B7RP-1 signal have been performed using ICOS-Fc, B7RP-1-Fc, anti-ICOS mAb and anti-B7RP-1 mAb in the previous papers. While these full length antibodies or Fc-fusion molecules actually function as specific inhibitors of ICOS/B7RP-1 signaling, they might deplete the cells expressing the target molecules because of the Fc-mediated cellular cytotoxicity that is exerted by natural killer cells through Fc receptor or because of Fc-mediated complement activation in vivo. Furthermore, targeting ICOS with the anti-ICOS mAb or B7RP-1-Fc may result in an unexpected agonistic action on T cells by dimering receptor. In contrast, scFv fragment, which is monomeric and does not contain an Fc region, possesses the advantage of avoiding the depletion of B7RP-1-expressing cells and the adverse agonistic activity.
The affinity between B7RP-1 and ICOS in our experiment (Fig. 6A and Table 1) was estimated to be 24 nM, which was close to the reported Kd value of 33 nM.21 On the other hand, the Kd values for the binding of our scFv fragments to B7RP-1-Fc were 14, 9 and 22 nM for scFv 223, 323 and 325, which were lower than that of the monovalent ICOS/B7RP-1 interaction. These results suggest that these scFv are comparably good inhibitors for the ICOS/B7RP-1 interaction at least in affinity. In T cell proliferation assay or MLR assay, scFv 325 showed relatively weak inhibitory actibities, as compared with scFv 223 and 323. This may be partly caused by the lower affinity or rapid dissociation rate of scFv 325 in the binding with B7RP-1.
Our data demonstrated that scFv clones 223, 323 and 325 suppressed the CD3-stimulated (Fig. 4) or allogenic T cell response (Fig. 5) by blocking the costimulation exerted via the ICOS/B7RP-1 interaction. In transplantation models, blocking of the costimulatory signals of the CD28/B7 or CD40/CD154 (CD40 ligand) interaction reportedly induce tolerance to the grafted tissues;22 however, these blocking signals sometimes fail to induce complete tolerance because of chronic rejection.23,24 Nevertheless, both acute and chronic allograft rejection of heart or islet allografts in rats or mice were effectively evaded by administration of an anti-ICOS mAb in combination with CTLA4-Fc, anti CD154 mAb, or immunosuppressant agents (rapamycin and cyclosporine A).8,25,26 A recent report showed that the high expression of ICOS induced in CD8 memory T cells within grafted tissues becomes a trigger for allograft rejection via the activation of these cells.27 Therefore, ICOS is a specific target for the neutralization of the proinflammatory functions of endogenous CD8 memory T cells. Thus, blockage of the ICOS signal seems to be a promising way to bypass allograft rejection. The blocking of the ICOS/B7RP-1 interaction is an effective therapy for allograft rejection in tissue transplantation and also for autoimmune diseases and allergy.10,28,29
We found that scFv clones 223 and 323 possessed a clear inhibitory activity for the allogenic MLR (Fig. 5). This inhibition required relatively high concentrations of scFv (120–360 nM) when compared with the dose (10–100 nM) used for the inhibition of anti-CD3 mAb/B7RP-1-Fc-stimulated T cell proliferation. Logue et al.26 reported that soluble (extracellular) B7RP-1 was released by shedding from B cells via proteolytic digestion followed by ligation of B7RP-1 with ICOS on activated T cells and it worked for downregulating B7RP-1 on B cells to terminate the immune response. Also in our case, the high dose of scFv required for the inhibition of MLR may be related to the shedding of B7RP-1. It is possible that the added scFv fragment was expended by the binding with soluble B7RP-1 and could not sufficiently block B7RP-1 on the cell surface; however, this hypothesis should be confirmated by further studies.
Recently, Mets et al.30 reported the in vivo estimation of the immune response during the therapeutic blockade of B7RP-1 in mice. The administration of low levels of an anti-B7RP-1 mouse mAb (1B7v2), which was generated using hybridoma technology, efficiently repressed T cell-dependent immunoglobulin (IgG1 and IgG2a) production against the immunized antigen, i.e., Keyhole Limpet Hemocyanin (KLH), which demonstrates the in vivo efficacy of B7RP-1 blockage in the T cell dependent immune response. The scFv fragment described here may contribute to therapeutic strategies that use B7RP-1 blockage, although reconstruction/modification of the scFv fragment may be required for the optimization of its in vivo efficacy.
Materials and Methods
Biopanning for the isolation of antibody phage clones specific for B7RP-1.
The human naïve scFv phage library used here was constructed in pCANTAB5E phagemid vector, as described previously.31,32 Based on this library, B7RP-1/Fc-specific scFv phages were isolated using the following biopanning method. Ten micrograms of human B7RP-1-Fc (R & D systems, Minneapolis, MN) in 0.1 M NaHCO3 was used to coat the immuno tube (Nunc) at 4°C overnight. The tube was blocked with 0.5% gelatin in phasphae buffer saline (PBS) and washed three times with PBS containing 0.1% Tween 20 (PBST). The library solution, which contained 1012 transforming units (TU) of the phages, was added to the tube and incubated for 2 h. After removal of the solution, the tube was washed 20 times with PBST and the bound phages were eluted with 0.1 M glycine-HCl (pH 2.2) containing 0.1% bovine serum albumin (BSA). The eluate was immediately neutralized with 1 M Tris solution (pH 9.2) and was then incubated with human IgG-coated tube for 1 h to deplete the human Fc-specific phages. The unbound phages were infected into E. coli TG1 cells, expanded in culture at 30°C, and rescued by coinfection with M13KO7 helper phages. The phages recovered from the culture supernatant using polyethylene glycol (PEG) precipitation were dissolved in PBS, centrifuged, filtrated through a 0.45 µm nitrocellulose membrane, and used in the subsequent biopannings. Five percent skim milk and 0.5% BSA were used as blocking reagents in the second and third pannings. After the completion of the biopanning, phages were cloned, purified and used in the binding assay.
ELISA screening of scFv phage clones.
The phage solution (40 µl, 1 × 1013 TU/ml) was added to the well coated with human B7RP-1-Fc (80 ng/well), blocked with 0.5% gelatin in the immuno plate (Maxisorp, Nunc), and incubated for 2 h. The wells were washed with PBST and the bound phages were detected using a biotinylated anti-M13 mAb (GE healthcare) and alkaline phosphatase (AP)-conjugated streptavidin (SA). The colorimetric assay was performed using 1 mg/ml of p-nitrophenol phosphate (PNP) solution containing 10% iminodiethanol as a substrate solution, and the absorbance at 405 nm was measured on a multiplate reader (NJ-2001; Nunc).
Purification of B7RP1-specific scFv.
The phages were infected into E. coli HB2151 cells and the transformants were cultured at 30°C in 2TY-AG medium (2TY medium containing 100 µg/ml ampicillin and 2% glucose) for 1 h. The medium was then replaced with 2TY-AI (2TY medium containing 100 µg/ml ampicillin and 1 mM IPTG). After four hours of IPTG induction, the cells were removed by centrifugation and the scFv fragment was purified from the supernatant using anti-E-tag mAb affinity chromatography. The purified scFv was futher supplied to the size exclusion HPLC on Superdex75 (10/300 GL, GE Healthcare) equilibrated with 0.1 M phosphate buffer (pH 7.0) to collect the monomer fraction of scFv. The scFv solution was filtrated, used for the experiments and if nessesary stored at −20°C until further use. Endotoxin contamination was checked using the Toxicolor LS50M endotoxin assay kit (Seikagaku Kogyo, Tokyo) and lipopolysaccharide (LPS) of the E. coli 055:B5 strain (Sigma) as the standard material, to verify that the contaminant endotoxin level was between 0.5 and 1.5 ng LPS/mg of scFv protein.
Binding specificity of scFv and inhibition in ELISA.
A 96-well immuno plate (Maxisorp, Nunc) was coated with B7RP-1-Fc (40 ng/well) and blocked with 0.5% gelatin in PBS. After washing with PBST, the scFv fragment (20 µg/ml), which was premixed with the anti-E-tag mAb, was added to each well and incubated for 2 h. The binding was detected using an AP-conjugated antimouse IgG Fcgamma antibody (Jackson Laboratory). For the inhibitory experiment, the biotinylated ICOS-Fc (200 nM) was added to B7RP-1-Fc (40 ng/well) coated on the wells in the presence of various concentrations (125 nM to 2 µM) of anti-B7RP-1 scFv. AP-labeled SA was used for the detection of the bound biotinylated ICOS-Fc.
Surface plasmon resonance (SPR) analysis.
The binding analysis was performed on a BIAcore 2000 (GE Healthcare) using a CM5 sensor chip at 25°C. The flow cells on the sensor chip were coupled with B7RP-1-Fc, ICOS-Fc, CTLA-4-Fc and anti-human Fc (Fab)2 antibodies (each at 10 µg/ml) in 10 mM acetate buffer (pH 4.0), according to the amino-coupling protocol recommended by the supplier. Analytical injection of B7RP-1-Fc was carried out at a flow rate of 20 µl/ml. After washing for 120 s, the subsequent injection with scFv was done and the association/dissociation reactions were monitored. To determine the kinetic parameters of the reaction, B7RP-1-Fc was directly immobilized to the sensor chip and the different concentrations of ICOS-Fc (25, 50, 100, 200, 400 nM ) or scFv (12.5, 25, 50, 100, 200 nM) were injected at a flow rate of 30 µl/ml. The regeneration of the sensorchip was done with 0.1 M glycine-HCl (pH 3.0). The sensorgrams of association/dissociation reaction were analyzed to determine the kinetic binding parameters on the BIAevaluation 3.2 (BIAcore) software.
Flow cytometric analysis of B cells from human PBMCs.
Human peripheral blood mononuclear cells (PBMCs) were prepared from heparin-treated healthy blood using ficoll density gradient centrifugation. Cells were cultured for 40 h in RPMI 1640 medium containing 8% heat-inactivated FCS, 5 ng/ml phorbol myristate acetate (PMA), and 2 µg/ml of phytohemagglutinin (PHA). After blocking the human Fc receptor with human IgG, cells were treated with scFv (20 µg/ml) and biotinylated anti E-tag mouse mAb. Cells were stained with phycoerythrin (PE)-labeled SA (Becton Dickinson). To identify B cells, cells were also stained with FITC-labeled anti human CD19 antibody (Becton Dickinson). Flow cytometry was carried out on EPICS XL-MCL flow cytometer (Beckman Coulter). After lymphocytes were gated according to their forward and side scatter (FSC/SSC) profiles, T and B cell populations were analyzed for their expression of CD19 and B7RP-1. The binding strength of antibody to the cells was expressed as relative mean fluorescence (RMF) which is the ratio of the mean fluorescence with scFv versus without scFv.
T cell proliferation assay.
The T cell proliferation assay was performed using a partially modified published method.21 A 96-well culture plate was coated with 2 µg/ml of anti-CD3 mAb (OKT-3) and 2.4 µg/ml of anti human Fc (Fab')2 antibody (Jackson Immuno Research Laboratory) at 4°C for 12 h. Human B7RP-1/Fc (1 µg/ml) was then added and immobilized on the culture plate. Human PBMCs (1 × 105 cells/well) suspended in RPMI 1640 medium containing 8% heat-inactivated FBS and anti-B7RP-1 scFv or ICOS-Fc (10–1000 nM) were incubated for 30 min and were then added to the wells and cultured for 48 h at 37°C. After adding [3H>] thymidine (0.5 µCi/well), the cells were pulsed for 18 h. The radioactivity level in the proliferated cells was measured and expressed as mean counts per minute (cpm).
Allogenic mixed lymphocyte reaction (MLR) assay.
MLRs were performed using irradiated (15 Gy) PBMCs as stimulator cells and allogeneic PBMCs from another donor as responder cells, according to a modified previously published method.33 Responder cells were prestimulated with 1 µg/ml of anti-CD3 mAb coated onto the plate for 12 h at 37°C. Stimulator cells (1 × 105) suspended in RPMI 1640 medium containing 8% heat-inactivated FBS and 3.3 and 10 µg/ml scFv (120 and 360 nM) were mixed with 1 × 105 responder cells in a 96-well flat bottom plate and cultured at 37°C. After culture for 5 days, the cells were pulsed for 18 h with [3H] thymidine (0.5 µCi/well). The radioactivity of the proliferated cells was measured.
Figure 7.
Comparison of amino acid sequences of the four anti-human B7RP-1 scFv. The upper and middle panels display the amino acid sequences of VH and VL regions of the scFv. The sequences were aligned according to Kabat's numbering. The V and J genes used for the VH/VL fragments were automatically identified by searching the IMGT/V-QUEST database.16 The assigned genomic genes for clones 223, 311, 323 and 325 were summarized in the bottom panel. The numbers in the parentheses indicates % identities between the scFv genes and the genomic genes. The D genes of VH chain were not listed in the panel, because it was difficult to assign them correctly.
Abbreviations
- scFv
single chain Fv
- FITC
fluorescent isothiocyanate
- mAb
monoclonal antibody
- PE
phycoerythrin
- FACS
fluorescence-activated cell sorter
- SPR
surface plasmon resonance
- APC
antigen-presenting cells
- RMF
relative mean fluorescence intensity
- MHC
major histocompatibility complex
- PBS
phosphate buffer saline
- TU
transforming unit
- SA
streptavidin
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/9633
References
- 1.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science (New York, NY) 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 2.McAdam AJ, Schweitzer AN, Sharpe AH. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunological reviews. 1998;165:231–247. doi: 10.1111/j.1600-065x.1998.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 3.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 4.Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J. T cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 5.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 6.Wiley RE, Goncharova S, Shea T, Johnson JR, Coyle AJ, Jordana M. Evaluation of inducible costimulator/B7-related protein-1 as a therapeutic target in a murine model of allergic airway inflammation. American journal of respiratory cell and molecular biology. 2003;28:722–730. doi: 10.1165/rcmb.2002-0220OC. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalo JA, Tian J, Delaney T, Corcoran J, Rottman JB, Lora J, et al. ICOS is critical for T helper cell-mediated lung mucosal inflammatory responses. Nat Immunol. 2001;2:597–604. doi: 10.1038/89739. [DOI] [PubMed] [Google Scholar]
- 8.Rottman JB, Smith T, Tonra JR, Ganley K, Bloom T, Silva R, et al. The costimulatory molecule ICOS plays an important role in the immunopathogenesis of EAE. Nat Immunol. 2001;2:605–611. doi: 10.1038/89750. [DOI] [PubMed] [Google Scholar]
- 9.Ozkaynak E, Gao W, Shemmeri N, Wang C, Gutierrez-Ramos JC, Amaral J, et al. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat Immunol. 2001;2:591–596. doi: 10.1038/89731. [DOI] [PubMed] [Google Scholar]
- 10.Usui Y, Akiba H, Takeuchi M, Kezuka T, Takeuchi A, Hattori T, et al. The role of the ICOS/B7RP-1 T cell costimulatory pathway in murine experimental autoimmune uveoretinitis. European journal of immunology. 2006;36:3071–3081. doi: 10.1002/eji.200636138. [DOI] [PubMed] [Google Scholar]
- 11.Kolly R, Thiel MA, Herrmann T, Pluckthun A. Monovalent antibody scFv fragments selected to modulate T cell activation by inhibition of CD86-CD28 interaction. Protein Eng Des Sel. 2007;20:91–98. doi: 10.1093/protein/gzl058. [DOI] [PubMed] [Google Scholar]
- 12.Pan XC, Guo L, Deng YB, Naruse K, Kimura H, Sugawara Y, Makuuchi M. Further study of anti-ICOS immunotherapy for rat cardiac allograft rejection. Surgery today. 2008;38:815–825. doi: 10.1007/s00595-007-3734-y. [DOI] [PubMed] [Google Scholar]
- 13.Salama AD, Yuan X, Nayer A, Chandraker A, Inobe M, Uede T, Sayegh MH. Interaction between ICOS-B7RP1 and B7-CD28 costimulatory pathways in alloimmune responses in vivo. Am J Transplant. 2003;3:390–395. doi: 10.1034/j.1600-6143.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- 14.Okano K, Nitta K, Ogawa S, Horita S, Habiro K, Nihei H, Abe R. Effects of double blockade of CD28 and inducible-costimulator signaling on anti-glomerular basement membrane glomerulonephritis. The Journal of laboratory and clinical medicine. 2004;144:183–192. doi: 10.1016/j.lab.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 15.van Berkel ME, Schrijver EH, van Mourik A, Tesselaar K, van der Ley P, Steeghs L, Oosterwegel MA. A critical contribution of both CD28 and ICOS in the adjuvant activity of Neisseria meningitidis H44/76 LPS and lpxL1 LPS. Vaccine. 2007;25:4681–4688. doi: 10.1016/j.vaccine.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic acids research. 2008;36:W503–W508. doi: 10.1093/nar/gkn316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Zhu G, Tamada K, Chen L, Bajorath J. Ligand binding sites of inducible costimulator and high avidity mutants with improved function. The Journal of experimental medicine. 2002;195:1033–1041. doi: 10.1084/jem.20011607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chattopadhyay K, Bhatia S, Fiser A, Almo SC, Nathenson SG. Structural basis of inducible costimulator ligand costimulatory function: determination of the cell surface oligomeric state and functional mapping of the receptor binding site of the protein. J Immunol. 2006;177:3920–3929. doi: 10.4049/jimmunol.177.6.3920. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz JC, Zhang X, Fedorov AA, Nathenson SG, Almo SC. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature. 2001;410:604–608. doi: 10.1038/35069112. [DOI] [PubMed] [Google Scholar]
- 20.Stamper CC, Zhang Y, Tobin JF, Erbe DV, Ikemizu S, Davis SJ, et al. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410:608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- 21.Yoshinaga SK, Zhang M, Pistillo J, Horan T, Khare SD, Miner K, et al. Characterization of a new human B7-related protein: B7RP-1 is the ligand to the co-stimulatory protein ICOS. Int Immunol. 2000;12:1439–1447. doi: 10.1093/intimm/12.10.1439. [DOI] [PubMed] [Google Scholar]
- 22.Clarkson MR, Sayegh MH. T cell costimulatory pathways in allograft rejection and tolerance. Transplantation. 2005;80:555–563. doi: 10.1097/01.tp.0000168432.60022.99. [DOI] [PubMed] [Google Scholar]
- 23.Ensminger SM, Witzke O, Spriewald BM, Morrison K, Morris PJ, Rose ML, Wood KJ. CD8+ T cells contribute to the development of transplant arteriosclerosis despite CD154 blockade. Transplantation. 2000;69:2609–2612. doi: 10.1097/00007890-200006270-00022. [DOI] [PubMed] [Google Scholar]
- 24.Jones ND, Van Maurik A, Hara M, Spriewald BM, Witzke O, Morris PJ, Wood KJ. CD40-CD40 ligand-independent activation of CD8+ T cells can trigger allograft rejection. J Immunol. 2000;165:1111–1118. doi: 10.4049/jimmunol.165.2.1111. [DOI] [PubMed] [Google Scholar]
- 25.Nanji SA, Hancock WW, Anderson CC, Adams AB, Luo B, Schur CD, et al. Multiple combination therapies involving blockade of ICOS/B7RP-1 costimulation facilitate long-term islet allograft survival. Am J Transplant. 2004;4:526–536. doi: 10.1111/j.1600-6143.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- 26.Guillonneau C, Aubry V, Renaudin K, Seveno C, Usal C, Tezuka K, Anegon I. Inhibition of chronic rejection and development of tolerogenic T cells after ICOS-ICOSL and CD40-CD40L co-stimulation blockade. Transplantation. 2005;80:255–263. [PubMed] [Google Scholar]
- 27.Schenk AD, Gorbacheva V, Rabant M, Fairchild RL, Valujskikh A. Effector functions of donor-reactive CD8 memory T cells are dependent on ICOS induced during division in cardiac grafts. Am J Transplant. 2009;9:64–73. doi: 10.1111/j.1600-6143.2008.02460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu YL, Metz DP, Chung J, Siu G, Zhang M. B7RP-1 blockade ameliorates autoimmunity through regulation of follicular helper T cells. J Immunol. 2009;182:1421–1428. doi: 10.4049/jimmunol.182.3.1421. [DOI] [PubMed] [Google Scholar]
- 29.Chen YQ, Shi HZ. CD28/CTLA-4—CD80/CD86 and ICOS—B7RP-1 costimulatory pathway in bronchial asthma. Allergy. 2006;61:15–26. doi: 10.1111/j.1398-9995.2006.01008.x. [DOI] [PubMed] [Google Scholar]
- 30.Metz DP, Mohn D, Zhang M, Horan T, Kim H, Deshpande R, et al. Defining dose-response relationships in the therapeutic blockade of B7RP-1-dependent immune responses. European journal of pharmacology. 2009;610:110–118. doi: 10.1016/j.ejphar.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 31.Hashiguchi S, Nakashima T, Nitani A, Yoshihara T, Yoshinaga K, Ito Y, et al. Human Fc epsilon RIalpha-specific human single-chain Fv (scFv) antibody with antagonistic activity toward IgE/Fc epsilon RIalpha-binding. Journal of biochemistry. 2003;133:43–49. doi: 10.1093/jb/mvg001. [DOI] [PubMed] [Google Scholar]
- 32.Muraoka S, Ito Y, Kamimura M, Baba M, Arima N, Suda Y, et al. Effective induction of cell death on adult T cell leukaemia cells by HLA-DRbeta-specific small antibody fragment isolated from human antibody phage library. Journal of biochemistry. 2009;145:799–810. doi: 10.1093/jb/mvp039. [DOI] [PubMed] [Google Scholar]
- 33.Aicher A, Hayden-Ledbetter M, Brady WA, Pezzutto A, Richter G, Magaletti D, et al. Characterization of human inducible costimulator ligand expression and function. J Immunol. 2000;164:4689–4696. doi: 10.4049/jimmunol.164.9.4689. [DOI] [PubMed] [Google Scholar]