Abstract
Objective
To investigate systematically the natural history of visual outcome in branch retinal artery occlusion (BRAO).
Design
Cohort study.
Participants
199 consecutive untreated patients (212 eyes) with BRAO, first seen in our clinic from 1973 to 2000.
Methods
At first visit, all patients had a detailed ophthalmic and medical history, and comprehensive ophthalmic evaluation. Visual evaluation was done by recording visual acuity, using the Snellen visual acuity chart, and visual fields with a Goldmann perimeter. The same ophthalmic evaluation was performed at each follow-up visit.
Main Outcome Measures
Visual acuity and visual fields.
Results
BRAO was classified into permanent (133 eyes) and transient (18 eyes) BRAO and cilioretinal artery occlusion (CLRAO – 61 eyes). In eyes with permanent BRAO, of the 61 eyes seen within 7 days of onset, initial visual acuity was 20/40 or better in 74%, central scotoma in 20%, central inferior altitudinal defect in 13%, and inferior nasal and superior sector defects in 29% and 24% respectively. Of those with follow-up, in the eyes with visual acuity worse than 20/40, it improved in 79% (11 of 14), abnormal central visual field defect improved in 47%, and abnormal peripheral visual field defect improved in 52%. Of the 18 eyes with transient BRAO, initially 17 (94%) had visual acuity of 20/40 or better and one (6%) worse that 20/40, which improved to 20/30 on follow-up. Of the 11 eyes with nonarteritic CLRAO alone, visual acuity was worse than 20/40 in 3 eyes – that improved to 20/40 or better in all during follow-up. In CLRAO on follow-up of 9 eyes, the central field improved in 4. When CLRAO was associated with retinal vein occlusion (38 eyes) or giant cell arteritis (12 eyes), visual findings were influenced by the associated diseases.
Conclusion
These findings show that visual acuity of 20/40 or better is seen initially in 74% of permanent BRAO, 94% of transient BRAO and 73% of nonarteritic CLRAO alone; and finally on follow-up, in 89%, 100% and 100% respectively. The effectiveness of various treatment modalities for visual outcome has to be judged against this background.
Branch retinal artery occlusion (BRAO) is a common ocular vascular occlusive disorder. To evaluate the course and outcome of any disease compared to any therapy, the first essential is to know the natural history of the disease, so as to assess whether any treatment modality actually performs better than the natural history. In view of that, we conducted a PubMed search to find out information on natural history of visual outcome in a large cohort of BRAO eyes, and found to our surprise only three studies with large enough cohorts of patients, published in peer review journals. They were those of Hayreh and Podhajsky1 in 44 eyes, Yuzurihara and Iijima2 in 30 and Mason et al.3 in 52. In the present study, we have prospectively evaluated the visual outcome (both visual acuity and visual fields) in a cohort of 199 consecutive patients (212 eyes) with various types of BRAO seen in our Ocular Vascular Clinic from 1973 to 2000.
In the literature, all retinal artery occlusions are often lumped together as regards evaluation of visual outcome and management, and that has resulted in confusion and controversy. Our studies have shown not only that central retinal artery occlusion (CRAO)4,5 and BRAO have different pathogeneses, clinical characteristics and management but also that BRAO is not one clinical entity. For example, cilioretinal artery occlusion (CLRAO) has usually been included in BRAO; but CLRAO is a distinct clinical entity because the cilioretinal artery arises from the posterior ciliary artery, instead of the central retinal artery. Furthermore, etiologically CLRAO is of three distinct types: (i) nonarteritic CLRAO alone, (ii) arteritic CLRAO associated with giant cell arteritis (Fig.1-A)6–9, and (iii) CLRAO associated with central retinal vein occlusion (CRVO)/hemi-CRVO (Fig. 1-B)10. Therefore, to get valid information on the natural history of visual outcome in BRAO, one has to categorize it into: (a) permanent BRAO, (b) transient BRAO, and (c) three types of CLRAO.
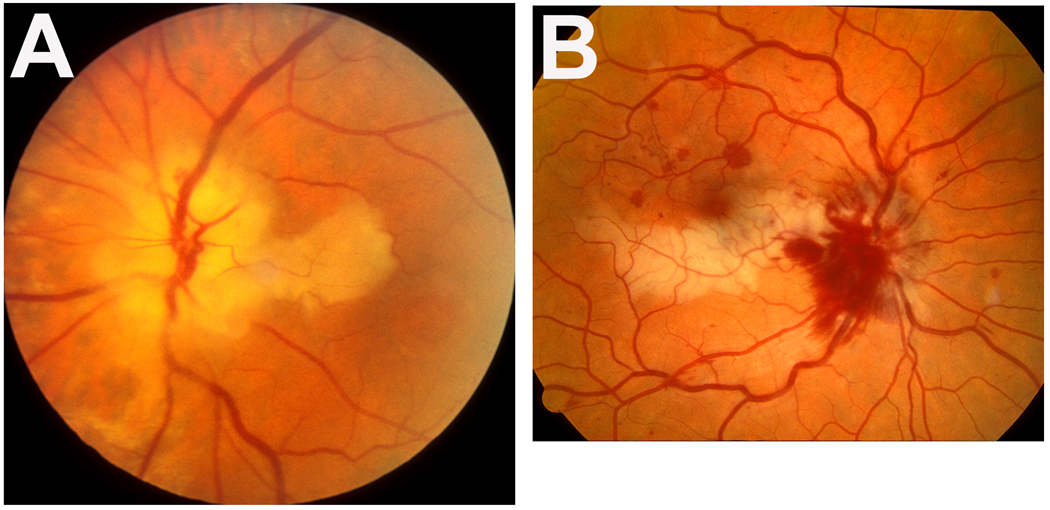
Figure 1.
Fundus photographs of two eyes with cilioretinal artery occlusion.
A: Left eye of a patient with giant cell arteritis, showing cilioretinal artery occlusion and arteritic anterior ischemic optic neuropathy. Note the presence of chalky white optic disc edema, which is diagnostic of arteritic anterior ischemic optic neuropathy. A combination of chalky white optic disc edema with cilioretinal artery occlusion is diagnostic of giant cell arteritis.
B: Right eye with cilioretinal artery occlusion associated with non-ischemic central retinal vein occlusion. Note the junction between the normal (upper) and infarcted (lower) retina lies in the foveal region.
Material and Methods
We investigated prospectively the natural history of visual outcome in patients with BRAO seen in the Ocular Vascular Clinic at the Tertiary Care University of Iowa Hospitals and Clinics since 1973. The present study was part of prospective studies on ocular vascular occlusive disorders funded by the National Institute of Health (RO1 grant) and approved by the Institutional Review Board. The study consists of a cohort of 199 consecutive patients (212 eyes), first seen in our clinic from 1973 to 2000, who fulfilled our inclusion criteria for this study. (A small number of these eyes, which fulfilled criteria for another study on BRAO, were included in a publication in 19821). The data were collected prospectively and systematically.
Inclusion criteria
A definite diagnosis of BRAO was based on the presence of its various classical clinical findings. These were recorded either in our clinic, or by the local referring ophthalmologist for patients who were not seen by us within the first few days after the onset of visual loss. These findings included the following. (1) There was a history of sudden onset of visual deterioration in the eye. (2) On initial ophthalmic evaluation, there was evidence of acute retinal ischemia in the distribution of the occluded branch retinal artery. (3) Fluorescein fundus angiography, performed soon after the onset, showed evidence of absence or marked stasis of circulation in the involved branch retinal arterial, except in eyes with transient BRAO. (4) No treatment was given for BRAO.
Exclusion criteria
A BRAO eye associated with any other retinal or optic nerve pathology was excluded. However, this did not apply to CLRAO since, as discussed below, in some eyes that is associated with arteritic anterior ischemic optic neuropathy (Fig. 1-A)6–9 or CRVO (Fig. 1-B)10.
Examinations performed
At the initial visit, all patients were seen by one of us (SSH) in the Ocular Vascular Clinic and had a detailed ocular and medical history, as well as a detailed ocular evaluation. The ocular examination included careful testing of the visual acuity, visual field plotting with a Goldmann perimeter, a detailed anterior segment examination, intraocular pressure recording with a Goldmann applanation tonometer, relative afferent pupillary defect, detailed fundus evaluation by indirect and direct ophthalmoscopy and if required by contact lens, and fluorescein fundus angiography. In addition, most patients had carotid Doppler and echocardiograhic studies performed to find any source of embolism. In CLRAO without associated CRVO or hemi-CRVO, giant cell arteritis was ruled out in all persons older than 55 years by urgently evaluating erythrocyte sedimentation and C-reactive protein (since 1985)7,9,11, because giant cell arteritis represents an ophthalmic emergency and requires immediate, intensive systemic corticosteroid therapy to prevent any further visual loss. None of the patients without giant cell arteritis had any treatment.
Visual Status Evaluation
Visual acuity (Snellen) testing was done under identical testing conditions, consistently almost invariably by the same person (SSH), encouraging the patient to look around and take his/her own time in responding, to ensure that the visual deterioration was genuine and not a testing artifact. The following steps of visual acuity were checked: 20/15, 20/20, 20/25, 20/30, 20/40, 20/50, 20/60, 20/70, 20/80, 20/100, 20/200, 20/400, counting fingers (CF), hand motion (HM), perception of light (PL), and no perception of light (NPL).
Visual fields were plotted in all patients with a Goldmann perimeter using I-2e, I-4e and V-4e targets regularly. These patients were mixed with other patients concurrently undergoing perimetry for a variety of diseases, and the perimetrists were unaware of the ophthalmic diagnosis. Patients with extensive central visual loss and difficulty in maintaining fixation on the fixation hole of the Goldmann perimeter were given a large black cross to view, placed in the perimeter bowl in the center of the visual field. Only eyes whose visual fields were judged reliable were included in the visual field evaluation. Visual fields plotted during the first visit to our clinic were examined and coded for the pattern of visual field defect(s). Visual defects were divided into: (a) peripheral visual field defect(s), and (b) various types of scotomas in the central 30°.
The follow-up ophthalmic evaluation (by SSH) was almost identical to those described in the initial visit examination, except that fluorescein fundus angiography was not usually repeated. Visual field grading was done as described elsewhere.12
Types of branch retinal artery occlusion
For data analysis in this study, BRAO was classified into the following categories:
BRAO (151 eyes): This was further classified into permanent (133 eyes) and transient (18 eyes).
-
Cilioretinal artery occlusion (CLRAO - 61 eyes): This type of BRAO is a distinct clinical entity different from the usual type of BRAO, because the cilioretinal artery arises from the posterior ciliary artery, instead of the central retinal artery. Etiologically it is of three distinct types:
Non-arteritic CLRAO alone (11 eyes).
Arteritic CLRAO associated with giant cell arteritis (Fig. 1-A; 12 eyes).6–9 This is due to thrombotic occlusion of the posterior ciliary artery by giant cell arteritis (Fig. 2).
CLRAO associated with CRVO (Fig. 1-B) /hemi-CRVO (38 eyes): This is a distinct clinical entity.10 It is due to transient hemodynamic blockage of the cilioretinal artery, caused by a sudden, sharp rise in intraluminal pressure in the retinal capillary bed (due to CRVO) above the level of that in the cilioretinal artery.10 Unlike regular non-arteritic CLRAO, there is no thrombotic or embolic occlusion of the artery in this type.
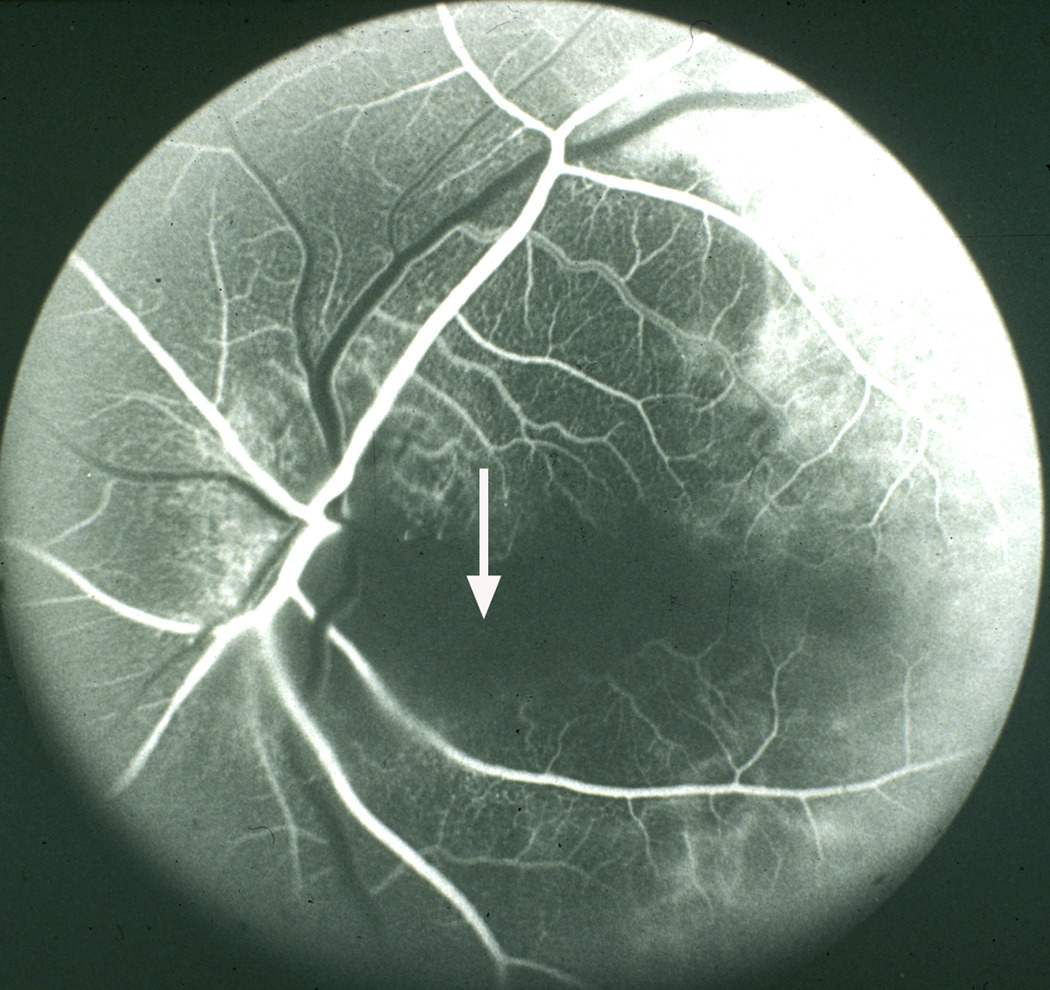
Figure 2.
Fluorescein fundus angiogram of left eye, of a patient with giant cell arteritis, with cilioretinal artery occlusion and arteritic anterior ischemic optic neuropathy. Note no filling of the choroid and optic disc supplied by the medial posterior ciliary artery and of the cilioretinal artery (arrow), with normal filling of central retinal artery and lateral posterior ciliary artery. A combination of posterior ciliary artery occlusion, cilioretinal artery occlusion and arteritic anterior ischemic optic neuropathy is diagnostic of giant cell arteritis.
Statistical data analysis
Descriptive statistics (mean, median, interquartile range) for the demographic characteristics and percentages to describe the distribution of BRAO types, initial visual acuity and visual field defects were computed. Estimates of the percent (95% confidence interval) improvement in visual acuity were also obtained. For defining improvement or deterioration, a change of at least 3 lines in the Snellen visual acuity chart was considered a significant change in either direction (i.e. improved or deteriorated), which is equivalent to a logMAR change of at least 0.30. Median time to infarct resolution and time to disc pallor were obtained from the empirical estimates of the distribution of these time variables that were computed using interval censored data.
RESULTS
The study comprised 199 patients (212 eyes) with various types of branch retinal artery occlusion (BRAO) (Table 1).
Table 1.
Types of retinal artery occlusion
| Type of Retinal Artery Occlusion | n patients (n eyes) |
|---|---|
| Branch Retinal Artery Occlusion | 140 (151) |
| (i) Permanent BRAO | 122 (133) |
| (ii) Transient BRAO | 18 (18) |
| Cilioretinal Artery Occlusion | 59 (61) |
| (i) Non-arteritic CLRAO alone | 10 (11) |
| (ii) Arteritic CLRAO with GCA | 11(12) |
| (iii) CLRAO with CRVO/hemi-CRVO | 38 (38) |
| Total | 199 (212) |
BRAO = Branch retinal artery occlusion
CLRAO = Cilioretinal Artery Occlusion
CRVO=Central Retinal Vein Occlusion
GCA=Giant Cell Arteritis
PERMANENT BRAO
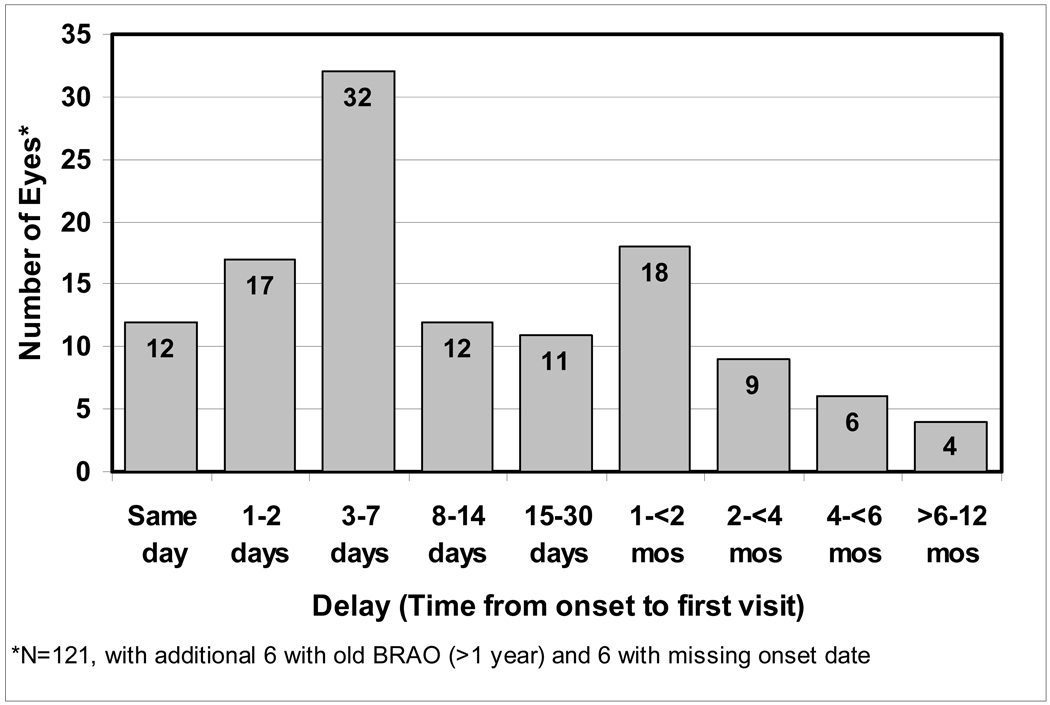
There were 122 patients (47 female, 75 male) with BRAO, 11 of whom had bilateral involvement. The mean age at first onset was 61±14(SD) years, with a range of 13 to 83 years. Of the 133 eyes with BRAO, 61 (46%) were first seen within 7 days of onset (Figure 3). 58% had follow-up of at least 6 months, with median follow-up of 2 years (interquartile range 3.8 months – 6.1 years). Table 2 (available at http://aaojournal.org) lists the various types of BRAO seen in our study. The two most common types were superior temporal BRAO (31%) and inferior temporal BRAO (28%).
Figure 3.
Distribution of the time interval between the onset of permanent branch retinal artery occlusion and the first clinic visit. BRAO = Branch retinal artery occlusion. mos = months.
Table 2.
Types of permanent branch retinal artery occlusion (BRAO)
| BRAO type | Count (%) n=133 eyes |
|---|---|
| Superior (involving superior half of retina) | 14 (11%) |
| Superior temporal | 41 (31%) |
| Superior temporal + Inferior nasal | 1 (1%) |
| Superior macular | 5 (4%) |
| Inferior (involving inferior half of retina) | 11 (8%) |
| Inferior temporal | 35 (26%) |
| Inferior temporal + Superior nasal | 1 (1%) |
| Inferior macular | 8 (6%) |
| Superior temporal + Inferior temporal | 7 (5%) |
| Superior temporal + Inferior temporal + Superior nasal | 1 (1%) |
| Superior nasal | 2 (2%) |
| Inferior nasal | 1 (1%) |
| Superior nasal + Inferior nasal | 1 (1%) |
| Temporal peripapillary arteriolar occlusion | 1 (1%) |
| Superior peripapillary arteriolar occlusion | 1 (1%) |
| Peripheral superior temporal | 2 (2%) |
| Peripheral inferior temporal | 1 (1%) |
Visual acuity at the first clinic visit was 20/40 or better in 74% of eyes seen within 7 days of onset, 64% in those with superior temporal BRAO and 85% in those with inferior temporal BRAO. Those whose initial visit was more than 1 week after onset showed similar visual acuity (Table 3). The types of central and peripheral visual field defects seen at initial visit are shown in Table 4 and Table 5 (available at http://aaojournal.org) respectively. Of those seen within 7 days of onset, 7% had no scotoma, 20% had central scotoma, and 13% had inferior central altitudinal defect. There was no peripheral defect in 15% of eyes seen within 1 week of onset, with peripheral defect in 29% in the inferior nasal sector and 24% in the superior nasal sector.
Table 3.
Initial visual acuity in permanent BRAO and the number of days from onset to initial visit
| BRAO Type | Initial visit within 7 days of onset | Initial visit >1 week–30days of onset | Initial visit >30 days from onset | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n eyes |
Initial VA | n eyes |
Initial VA | n eyes |
Initial VA | ||||||||||
| 20/40 or better |
20/50– 20/100 |
20/200– 20/400 |
CF | 20/40 or better |
20/50– 20/100 |
20/200 – 20/400 |
CF | 20/40 or better |
20/50– 20/100 |
20/200 – 20/400 |
CF | ||||
| Superior temporal | 28 | 18 (64%) |
7 (25%) |
0 | 3 (11%) |
9 | 6 (67%) |
3 (33%) |
0 | 0 | 21 | 16 (76%) |
2 (10%) |
1 (5%) |
2 (10%) |
| Inferior temporal | 28 | 23 (82%) |
2 (7%) |
0 | 3 (11%) |
12 | 10 (83%) |
0 | 1 (8%) |
1 (8%) |
14 | 12 (86%) |
1 (7%) |
0 | 1 (7%) |
|
Superior temporal + Inferior temporal |
3 | 2 (67%) |
0 | 1 (33%) |
0 | - | - | - | - | - | 5 | 5 (100%) |
0 | 0 | 0 |
| Nasal | - | - | - | - | - | 1 | 1 (100%) |
0 | 0 | 0 | 2 | 2 (100%) |
0 | 0 | 0 |
| Other types | 2 | 2 (100%) |
0 | 0 | 0 | 1 | 1 (100%) |
0 | 0 | 0 | 1 | 1 (100%) |
0 | 0 | 0 |
| Any BRAO* | 61 | 45 (74%) |
9 (15%) |
1 (2%) |
6 (8%) |
23 | 18 (78%) |
3 (13%) |
1 (4%) |
1 (4%) |
43 | 36 (84%) |
3 (7%) |
1 (2%) |
3 (7%) |
There were 6 eyes with missing onset date (3 superior temporal, 1 inferior temporal, 1 nasal, 1 other)
BRAO = Branch retinal artery occlusion
CF = Counting fingers
VA = Visual acuity
Table 4.
Types of central visual field defects in eyes with permanent branch retinal artery occlusion (BRAO)
| Visual Field Defect Type | Superior* | Superior temporal |
Superior macular |
Inferior** | Inferior temporal |
Inferior macular |
Superior Temporal+ Inferior Temporal |
Nasal/Other | Any BRAO |
|---|---|---|---|---|---|---|---|---|---|
|
Initial visit within 7 days of onset |
n=7 |
n=18 (1 missing) |
n=2 | n=3 |
n=20 (2 missing) |
n=3 | n=3 | n=2 |
n=58 (3 missing) |
| No scotoma | 1 (5%) | 2 (10%) | 1 (50%) | 4 (7%) | |||||
| Central scotoma | 3 (43%) | 4 (21%) | 1 (50%) | 1 (33%) | 7 (35%) | 2 (67%) | 2 (67%) | 20 (34%) | |
| Centrocecal scotoma | 1 (33%) | 1 (2%) | |||||||
| Paracentral scotoma | 1 (5%) | 1 (2%) | |||||||
| Central + Paracentral scotoma | 2 (10%) | 2 (4%) | |||||||
| Superior nasal defect | 3 (15%) | 1 (33%) | 4 (7%) | ||||||
| Inferior nasal defect | 4 (21%) | 1 (50%) | 5 (8%) | ||||||
| Inferior temporal defect | 1 (50%) | 1 (2%) | |||||||
| Superior altitudinal | 2 (67%) | 5 (25%) | 7 (12%) | ||||||
| Inferior altitudinal | 4 (57%) | 9 (47%) | 13 (22%) | ||||||
|
Initial visit >7 days from onset |
n=5 (1 missing) |
n=17 (4 missing) |
n=3 | n=8 |
n=12 (1 missing) |
n=5 |
n=4 (1 missing) |
n=5 |
n=59 (7 missing) |
| No scotoma | 3 (41%) | 1 (8%) | 1 (25%) | 2 (40%) | 7 (12%) | ||||
| Central scotoma | 1 (20%) | 10 (59%) | 3 (38%) | 4 (33%) | 4 (80%) | 1 (25%) | 1 (20%) | 24 (41%) | |
| Centrocecal scotoma | 1 (20%) | 2 (40%) | 3 (5%) | ||||||
| Paracentral scotoma | 1 (8%) | 1 (25%) | 2 (4%) | ||||||
| Central + Paracentral scotoma | 1 (12%) | 1 (2%) | |||||||
| Superior nasal defect | 6 (50%) | 1 (20%) | 7 (12%) | ||||||
| Inferior nasal defect | 1 (33%) | 1 (2%) | |||||||
| Superior altitudinal | 1 (6%) | 4 (50%) | 1 (25%) | 6 (10%) | |||||
| Inferior altitudinal | 3 (60%) | 3 (18%) | 2 (67%) | 8 (13%) | |||||
| ALL BRAO |
n=13, 1 unk onset (1 missing) |
n=37, 2 unk onset (5 missing) |
n=5 | n=11 |
n=33, 1 unk onset (3 missing) |
n=8 |
n=7 (1 missing) |
n=8 1 unk onset (1 missing, unk onset) |
n=122, 5 unk onset (11 missing) |
| No scotoma | 1 (8%) | 4 (11%) | 3 (9%) | 1 (14%) | 4 (50%) | 13 (27%) | |||
| Central scotoma | 4 (31%) | 14 (38%) | 1 (20%) | 4 (36%) | 11 (33%) | 6 (75%) | 3 (43%) | 1 (12%) | 44 (36%) |
| Centrocecal scotoma | 1 (8%) | 1 (12%) | 2 (25%) | 4 (3%) | |||||
| Paracentral scotoma | 1 (3%) | 2 (6%) | 1 (14%) | 4 (3%) | |||||
| Central + Paracentral scotoma | 1 (9%) | 2 (6%) | 3 (2%) | ||||||
| Superior nasal defect | 1 (3%) | 9 (27%) | 1 (12%) | 1 (14%) | 12 9%) | ||||
| Inferior nasal defect | 4 (11%) | 2 (40%) | 6 (5%) | ||||||
| Inferior temporal defect | 1 (12%) | 1 (1%) | |||||||
| Superior altitudinal | 1 (3%) | 6 (54%) | 6 (18%) | 1 (141%) | 14 (11%) | ||||
| Inferior altitudinal | 7 (54%) | 12 (32%) | 2 (40%) | 21 (17%) |
Superior temporal+superior nasal
Inferior temporal+inferior nasal. unk = unkown
Table 5.
Types of peripheral visual field defects in eyes with permanent branch retinal artery occlusion (BRAO)
| Visual Field Defect Type | Superior* | Superior temporal |
Superior macular |
Inferior** | Inferior temporal |
Inferior macular |
Superior Temporal+ Inferior Temporal |
Nasal/Other | Any BRAO |
|---|---|---|---|---|---|---|---|---|---|
|
Initial visit within 7 days of onset |
n=7 | n=19 | n=2 | n=3 |
n=20 (2 missing) |
n=3 | n=3 | n=2 |
n=59 (2 missing) |
| No peripheral defect | 2 (11%) | 1 (50%) | 3 (15%) | 2 (67%) | 1 (50%) | 9 (15%) | |||
| Superior nasal sector | 13 (65%) | 1 (33%) | 14 (24%) | ||||||
| Inferior nasal sector | 14 (74%) | 1 (50%) | 1 (5%) | 1 (50%) | 17 (29%) | ||||
| Superior temporal sector | 1 (33%) | 1 (2%) | |||||||
| Superior temporal and inferior nasal sector |
1 (5%) | 1 (2%) | |||||||
| Superior temporal sector and nasal |
1 (33%) | 1 (2%) | |||||||
| Nasal defect | 1 (5%) | 1 (33%) | 2 (3%) | ||||||
| Inferior field defect | 7 (100%) | 2 (10%) | 9 (15%) | ||||||
| Superior field defect | 3 (100%) | 2 (10%) | 5 (9%) | ||||||
|
Initial visit 8–30 days from onset |
n=5 (1 missing) |
n=18 (3 missing) |
n=3 | n=8 |
n=12 (1 missing) |
n=5 |
n=4 (1 missing) |
n=5 |
n=60 (6 missing) |
| No peripheral defect | 5 (14%) | 3 (25%) | 3 (60%) | 1 (25%) | 12 (20%) | ||||
| Superior nasal sector | 3 (38%) | 9 (75%) | 2 (40%) | 1 (25%) | 15 (25%) | ||||
| Inferior nasal sector | 2 (40%) | 13 (86%) | 2 (67%) | 17 (28%) | |||||
| Superior temporal sector | 1 (12%) | 1 (20%) | 2 (4%) | ||||||
| Inferior temporal sector | 1 (33%) | 1 (20%) | 2 (4%) | ||||||
| Nasal defect | 2 (50%) | 1 (20%) | 3 (5%) | ||||||
| Temporal defect | 1 (20%) | 1 (2%) | |||||||
| Inferior field defect | 3 (60%) | 1 (20%) | 4 (7%) | ||||||
| Superior field | 4 (50%) | 4 (7%) | |||||||
| ALL BRAO |
n=13, 1 unk onset (1 missing) |
n=39 (3 missing) |
n=5 | n=11 |
n=33, 1 unk onset (3 missing) |
n=8 |
n=7 (1 missing) |
n=8 1 unk onset (1 missing, unk onset) |
n=124, 4 unk onset (9 missing) |
| No peripheral defect | 7 (18%) | 1 (20%) | 6 (18%) | 5 (63%) | 1 (14%) | 1 (12%) | 21 (17%) | ||
| Superior nasal sector | 1 (3%) | 3 (27%) | 23 (70%) | 2 (25%) | 2 (29%) | 31 (25%) | |||
| Inferior nasal sector | 2 (15%) | 28 (72%) | 3 (60%) | 1 (3%) | 1 (12%) | 35 (28%) | |||
| Superior temporal and inferior nasal sector |
1 (3%) | 1 (1%) | |||||||
| Superior temporal sector | 1 (9%) | 1 (12%) | 1 (12%) | 3 (2%) | |||||
| Inferior temporal sector | 1 (8%) | 1 (20%) | 2 (25%) | 4 (3%) | |||||
| Superior temporal sector and nasal |
1 (14%) | 1 (1%) | |||||||
| Nasal defect | 1 (3%) | 3 (43%) | 1 (12%) | 5 (4%) | |||||
| Temporal defect | 1 (12%) | 1 (1%) | |||||||
| Inferior field defect | 10 (77%) | 2 (6%) | 1 (12%) | 13 (10%) | |||||
| Superior field defect | 7 (63%) | 2 (6%) | 9 (7%) |
Superior temporal+superior nasal
Inferior temporal+inferior nasal. unk = unkown
Follow- up information
Visual acuity improved in 79% (11 of 14 eyes; 95% CI: 49%, 95%) of the eyes with worse than 20/40 visual acuity that were first seen within 7 days of onset (Table 6). Improvement was seen in 3 of 4 (75%) eyes with inferior temporal BRAO, in 7 of 9 (78%) with superior temporal BRAO and 1 (of 1) with superior temporal + inferior temporal BRAO. Visual acuity worsened in only 3% (1 of 39 eyes; 95% CI: 0%, 13%) of the eyes with 20/40 or better vision that were seen within 7 days of onset. Final visual acuity of 20/40 or better was seen in 89% (47 of 53; 95% CI: 77%, 96%). Changes in central and peripheral visual field defects are listed in Table 7 (available at http://aaojournal.org). Abnormal central visual field defect improved in 47% of those seen within 7 days of onset, while only 6% got worse. Abnormal peripheral visual field defect improved in 52% and got worse in 3% of the eyes first seen within 7 days of onset. To determine if the visual acuity improvement was genuine or simply due to the patient learning to fixate eccentrically, improvement in both the visual acuity and central visual field were compared; of the 11 eyes with visual acuity improvement (see above), 5 had a corresponding improvement in central scotoma, i.e. in 54.5% visual acuity improvement was simply due to eccentric fixation.
Table 6.
Visual acuity change in permanent BRAO of the eyes with follow-up
| Time from Onset to Initial Visit |
Initial VA worse than 20/40 |
Initial VA 20/40 or better |
||
|---|---|---|---|---|
| n eyes | VA Improved | n eyes | VA worsened | |
| Within 7 days | 14 | 11 (79%) | 39 | 1 (3%) |
| >1 week – 30 days | 4 | 1 (25%) | 15 | 0 (0%) |
| >30 days | 3 | 2 (67%) | 25 | 0 (0%) |
BRAO = Branch retinal artery occlusion
VA = Visual acuity
Table 7.
Visual field change in eyes with permanent branch retinal artery occlusion (BRAO) that had a follow-up for visual field testing
| BRAO Type | Change in Central Visual Field | Change in Peripheral Visual Field | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (d)* | Better** | Stable | Normal# | Worse | n (d)* | Better** | Stable | Normal# | Worse | |
| Initial visit ≤7 days of onset | ||||||||||
| Superior | 7 (7) | 6 (86%) | 1 (14%) | 0 | 0 | 7 (7) | 7 (100%) | 0 | 0 | 0 |
| Superior temporal | 11 (10) | 3 (30%) | 7 (64%) | 0 | 1 (9%) | 10 (9) | 4 (44%) | 4 (40%) | 1 (10%) | 1 (10%) |
| Superior macular | 1 (1) | 1 | 0 | 0 | 0 | 2 (1) | 1 | 0 | 1 (50%) | 0 |
| Inferior | 3 (3) | 0 | 3 (100%) | 0 | 0 | 3 (3) | 0 | 3 (100%) | 0 | 0 |
| Inferior temporal | 16 (14) | 4 (29%) | 8 (50%) | 2 (12%) | 2 (12%) | 17 (14) | 7 (50%) | 7 (42%) | 3 (18%) | 0 |
| Inferior macular | 2 (2) | 1 (50%) | 1 (50%) | 0 | 2 (0) | -- | 0 | 2 (100%) | 0 | |
| Inferior temporal.+ Superior temporal. |
3 (3) | 1 (33%) | 2 (67%) | 0 | 0 | 3 (3) | 0 | 3 (100%) | 0 | 0 |
| Nasal/Other | 2 (1) | 1 | 0 | 1 (50%) | 0 | 1 (0) | 0 | 0 | 1 | 0 |
| Any BRAO | 45 (41) | 17 (41%) | 22 (49%) | 3 (7%) | 3 (7%) | 45 (37) | 19 (51%) | 17 (38%) | 8 (18%) | 1 (2%) |
| Initial visit >7 days of onset | ||||||||||
| Superior | 4 (4) | 0 | 3 (75%) | 0 | 1 (25%) | 4 (4) | 0 | 3 (75%) | 0 | 1 (25%) |
| Superior temporal | 14 (11) | 4 (36%) | 6 (43%) | 3 (21%) | 1 (7%) | 13 (9) | 1 (11%) | 8 (62%) | 4 (31%) | 0 |
| Superior macular | 3 (3) | 2 (67%) | 1 (33%) | 0 | 0 | 3 (3) | 1 (33%) | 2 (67%) | 0 | 0 |
| Inferior | 3 (3) | 2 (67%) | 1 (33%) | 0 | 0 | 3 (3) | 1 (33%) | 1 (33%) | 0 | 1 (33%) |
| Inferior temporal | 7 (6) | 1 (17%) | 4 (57%) | 1 (14%) | 1 (14%) | 9 (6) | 2 (33%) | 4 (44%) | 3 (33%) | 0 |
| Inferior macular | 4 (4) | 3 (75%) | 1 (25%) | 0 | 0 | 4 (1) | 0 | 0 | 3 (75%) | 1 (25%) |
| Inferior temporal + Superior temporal |
4 (3) | 1 (33%) | 2 (50%) | 1 (25%) | 0 | 3 (2) | 0 | 2 (67%) | 1 (33%) | 0 |
| Nasal | 4 (2) | 2 (100%) | 0 | 2 (50%) | 0 | 3 (3) | 2 (67%) | 0 | 0 | 1 (33%) |
| Any BRAO | 43 (36) | 15 (42%) | 18 (42%) | 7 (16%) | 3 (7%) | 42 (31) | 7 (23%) | 20 (48%) | 11 (26%) | 4 (10%) |
| ALL BRAO | ||||||||||
| Superior (1 unknown onset) | 12 (11) | 6 (55%) | 4 (33%) | 1 (8%) | 1 (8%) | 12 (12) | 8 (67%) | 3 (25%) | 0 | 1 (8%) |
| Superior temporal (1 unk onset) | 26 (22) | 7 (32%) | 14 (54%) | 3 (12%) | 2 (8%) | 24 (19) | 5 (26%) | 13 (54%) | 5 (21%) | 1 (4%) |
| Superior macular | 4 (4) | 3 (75%) | 1 (25%) | 0 | 0 | 5 (4) | 2 (50%) | 2 (40%) | 0 | 0 |
| Inferior | 6 (6) | 2 (33%) | 4 (67%) | 0 | 0 | 6 (6) | 1 (17%) | 4 (67%) | 0 | 1 (17%) |
| Inferior temporal (1 unk onset) | 24 (21) | 6 (29%) | 12 (50%) | 3 (12%) | 3 (12%) | 27 (21) | 10 (48%) | 11 (41%) | 6 (22%) | 0 |
| Inferior macular | 6 (6) | 4 (67%) | 2 (33%) | 0 | 0 | 6 (1) | 0 | 0 | 5 (83%) | 1 (17%) |
| Inferior temporal + Superior temporal |
7 (6) | 2 (33%) | 4 (57%) | 1 (14%) | 0 | 6 (4) | 0 | 5 (83%) | 1 (17%) | 0 |
| Nasal/Other (1 unk onset) | 7 (3) | 3 (100%) | 0 | 4 (57%) | 0 | 5 (4) | 2 (50%) | 1 (20%) | 1 (20%) | 1 (20%) |
| Any BRAO (4 unk onset) | 92 (79) | 33 (42%) | 41 (45%) | 12 (13%) | 6 (7%) | 91 (71) | 28 (39%) | 39 (43%) | 19 (21%) | 5 (5%) |
n = Number of Eyes with VF follow-up (d = Number with abnormal VF at first VF testing and have VF follow-up)
Denominator for percentage with better VF is (d), the number of eyes that have abnormal VF at initial visit (d)
VF stayed normal
unk = unkown
Of 93 eyes with follow-up data for resolution of the retinal infarct in the area of the BRAO, median time to resolution was between 4 to 4.4 weeks, with 20% resolving at 2.1 weeks and 80% resolved by 5.6 weeks. Of 76 with information on follow-up for optic disc pallor, median time to disc pallor was 6.7 to 6.9 weeks, with pallor present in 25% 4 to 4.6 weeks, 67% 12.7 to 13.6 weeks.
TRANSIENT BRAO
There were 18 patients (18 eyes) with transient BRAO (7 superior temporal, 6 inferior temporal, 1 superior temporal + inferior temporal, 1 peripheral superior temporal, 1 foveolar arteriolar, and 2 unknown). Only one presented with worse than 20/40 initial visual acuity, which, on follow-up, changed to 20/30, with the rest maintaining vision of 20/40 or better. Central and peripheral visual fields remained normal during the entire follow-up period. Three of the 18 eyes had retinal infarct at initial visit and one developed disc pallor during follow-up.
CILIORETINAL ARTERY OCCLUSION (CLRAO)
CLRAO was of three types: (1) nonarteritic CLRAO alone (11 eyes), (2) CLRAO associated with giant cell arteritis (12 eyes) and (3) CLRAO associated with CRVO/hemi-CRVO (38 eyes).
(1) Non-arteritic CLRAO alone
Ten patients (11 eyes) had CLRAO (5 superior, 2 inferior, and 4 macular). There were 3 eyes that presented with worse than 20/40 visual acuity, with all 3 improving during follow-up. All 11 eyes had central visual defect at initial visit (6 eyes with centrocecal scotoma, 3 with central scotoma, 1 with superior central altitudinal defect, and 1 with inferior central altitudinal defect). Peripheral visual field defect was seen in 3 eyes with cilioretinal arteries of large size: 1 inferior altitudinal, 1 superior altitudinal, and 1 superior nasal. Of the 9 eyes with follow-up, central field improved in 4 eyes, worsened in 1, and remained stable in 4. Of the 3 eyes with peripheral field defect, only 1 had follow-up and remained stable. The 8 eyes with normal peripheral field, all remained normal during the follow-up period. Ten of the 11 eyes had retinal infarct at initial visit and 9 developed disc pallor in the corresponding sector during follow-up.
(2) Arteritic CLRAO associated with giant cell arteritis
This was present in 11 patients (12 eyes) with temporal artery biopsy confirmed giant cell arteritis. There were 7 females and 4 males, with age range 57 to 79 (mean 69.4±6.8 SD) years. Three eyes had episode/s of amaurosis before permanent visual loss. Of the 12 eyes, 10 had associated arteritic anterior ischemic optic neuropathy (Fig. 1-A), one arteritic posterior ischemic optic neuropathy and in only one eye CLRAO was not associated with optic neuropathy. Initial deterioration of visual acuity was primarily due to associated arteritic anterior/posterior ischemic optic neuropathy in all but one where it was 20/70. In the remaining 11 eyes, initial visual acuity was 20/20 in 1, 20/25 in 2, 20/40 in 1, counting fingers in 2, hand motion in 1, light perception in 2 and no light perception in 2. All were treated with systemic corticosteroids without any visual change on follow-up.
(3) CLRAO associated with CRVO/hemi-CRVO
A detailed account of the results of this group of CLRAO eyes is given elsewhere.10 In summary, of the 38 eyes, 30 had nonischemic CRVO (Fig. 1-B), 5 ischemic CRVO and 3 nonischemic hemi-CRVO. Patients with nonischemic CRVO were significantly younger (mean 45.3±16.0 years) than those with ischemic CRVO (72.3 ± 9.2 years; P=0.001) and nonischemic hemi- CRVO (64.7 ± 7.5 years; P=0.018). At least one third of the patients gave a definite history of episode(s) of transient visual blurring before the onset of constant blurred vision. Initial deterioration of visual acuity in all the three groups was due to either the CLRAO involving the foveal region or macular edema caused by CRVO or hemi-CRVO; therefore, visual acuity data are not necessarily related to CLRAO in this type of CLRAO. By contrast, central visual field defects were usually due to CLRAO, and of the 38 eyes, there was centrocecal scotoma in 16, central scotoma in 7, paracentral scotoma in 5, inferior nasal defect in 5 eyes and other types in 5. During follow-up, visual acuity improved markedly in eyes with associated nonischemic CRVO (P<0.001; 95% CI: 0.22, 0.57) and nonischemic hemi-CRVO but deteriorated in those associated with ischemic CRVO (primarily due to ischemic CRVO). Like the visual acuity, visual field improvement was common in the nonischemic CRVO group, except in eyes where an area of retina had suffered irreversible ischemic damage. Of the 30 eyes with nonischemic CRVO, central visual fields improved in 21, remained stable in 4 and worsened in 2, with no data in the remaining 3 eyes; in nonischemic hemi-CRVO, the central field improved in 2 of the 3 eyes, and remained stable in one.
Initially the ophthalmoscopic and fluorescein angiographic findings were similar to those seen in CRVO and hemi-CRVO, except that all these eyes had retinal infarct in the distribution of the cilioretinal artery (Fig. 1-B) – its size and site varied widely. Fluorescein angiography typically showed only transient hemodynamic block in the cilioretinal artery10 and not the typical organic occlusion seen in classical CLRAO.
DISCUSSION
In the present study, briefly, of the 61 eyes with permanent BRAO seen within 7 days of onset, initial visual acuity was 20/40 or better in 74%, and there was central scotoma in 20%, central inferior altitudinal defect in 13%, and inferior nasal and superior sector defects in 29% and 24%, respectively. Of those with follow-up, visual acuity improved by at least 3 lines in 79% (11 of 14) of the eyes that presented with visual acuity worse than 20/40. Abnormal central visual field defect improved in 47% and abnormal peripheral visual field defect improved in 52%. Of the 18 eyes with transient BRAO, initially 17 (94%) had visual acuity of 20/40 or better and one (6%) worse than 20/40, which improved to 20/30 on follow-up. Of the 11 eyes with nonarteritic CLRAO alone, visual acuity was worse than 20/40 in 3 eyes – that improved to better than 20/40 in all of them during follow-up. In CLRAO initially central visual field defect was centrocecal scotoma in 6, central scotoma in 3 and central superior/inferior altitudinal field defect in one each. On follow-up of 9 of these eyes, the central field improved in 4 (44%). When CLRAO is associated with CRVO/hemi-CRVO or arteritic ischemic optic neuropathies, visual findings are influenced by the associated diseases.
Yuzurihara and Iijima2, in a retrospective study of 30 eyes with BRAO, reported final visual acuity of 20/40 or better in 80% and worse than 20/200 in 3%. They concluded, “visual acuity in patients with BRAO is far better both at presentation and at the final visit”. Mason et al.3, in a retrospective study of 52 eyes with BRAO, reported that 60% of all eyes had final visual acuity of 20/40 or better. They stated that of the eyes with initial visual acuity of 20/40 or better, 89% retained that, 25% of those with visual acuity of 20/50 or worse improved to 20/40 or better. They concluded: “Visual prognosis after BRAO seems to be correlated to presenting VA (visual acuity).” In our study, visual acuity of 20/40 or better was seen initially in 74% of permanent BRAO (Table 3), 94% of transient BRAO and 73% of nonarteritic CLRAO alone. On follow-up, in permanent BRAO eyes with initial visual acuity worse than 20/40, improvement by 3 or more Snellen lines (0.3 LogMAR) was seen in 79% (11 of 14). Using the criteria of improvement based on achieving visual acuity of 20/40 or better among those that presented with worse that 20/40 visual acuity, there were 9 of 14 (64%; 95% CI: 35%, 87%) that improved. Worsening of visual acuity was observed in 3% (1 of 39) (Table 6) of those with initial visual acuity of 20/40 or better. Thus, of those with follow-up, the final visual acuity of 20/40 or better was seen in 89% (47 of 53; 95% CI: 77%, 96%) of permanent BRAO. Though we also reported a high proportion of those that presented with visual acuity of 20/40 or better retained 20/40 or better all along, as in the Mason et at. 3 study, we differed in the outcome for those that had initial visual acuity of worse than 20/40, where we found a significantly greater proportion of these eyes improving to 20/40 or better compared to their study (64% vs. 25%; p=0.037). Thus, our study does not support the conclusion by Mason et at. 3 that “Visual prognosis after BRAO seems to be correlated to presenting VA (visual acuity).”.
We also found in our study that initial visual acuity was worse than 20/40 in 1 eye with transient BRAO and 3 eyes with CLRAO, and at follow-up all of them improved to 20/40 or better. Final visual acuity was 20/40 or better in 100% of both transient BRAO and nonarteritic CLRAO alone. This is similar to that reported by Brown et al.13 in 10 eyes with nonarteritic CLRAO alone of which 90% achieved 20/40 or better vision at final follow-up.
The findings of an excellent final visual acuity for patients with BRAO have important clinical implications, casting doubt on claims made from time to time about the beneficial effects on visual acuity of one treatment modality or another. For example, it has been claimed that YAG laser embolysis/embolectomy in BRAO resulted in visual acuity improvement.14,15 As discussed elsewhere16, there are flaws in the study by Opremcak and colleagues15 which invalidate their claims; moreover, the procedure resulted in complications in 47%, including vitreous/ subhyaloid hemorrhages. Similarly, Garcia-Arumi and colleagues17 claimed that in 4 of 6 eyes with temporal BRAO, “surgical embolus removal’ resulted in visual acuity improvement; as discussed elsewhere18, the claimed visual acuity improvement simply represented natural history, in addition to which the procedure had complications, including development of vitreous hemorrhage. The same applies to claims of visual acuity improvement with local fibrinolysis.19,20 Of the various treatments proposed so far, in other words, none has achieved a visual outcome any better than the natural history, and all are associated with serious complications.
Visual acuity improvement in BRAO and CLRAO depends upon the following factors:
When the junction between the normal and infarcted retina in BRAO (as is the case in the majority of temporal BRAO eyes – see Fig. 4) and CLRAO (see Fig. 1) passes through the fovea, the visual acuity may suddenly deteriorate initially, but marked spontaneous visual acuity improvement can occur within several days or weeks, from 20/200 or worse even to normal21. Supporters of proposed treatments, often see this spontaneous improvement (or improvement due to patient learning to fixate eccentrically) as the beneficial result of their therapy.15,17
The retina can recover/improve function only so long it is not irreversibly damaged by acute ischemia. Our experimental studies on CRAO22,23, in elderly, atherosclerotic and hypertensive rhesus monkey (similar to most patients with CRAO and BRAO) showed that the retina suffers no detectable damage with CRAO of 97 minutes but above that level, the longer the CRAO, the more extensive and irreversible the damage, so that CRAO lasting for about 240 minutes results in massive irreversible retinal damage. That must apply to BRAO and CLRAO too. Almost all of advocated modes of treatments claiming visual acuity improvement have been performed far longer than 4 hours after the onset, which indicates that their results simply reflected natural history.
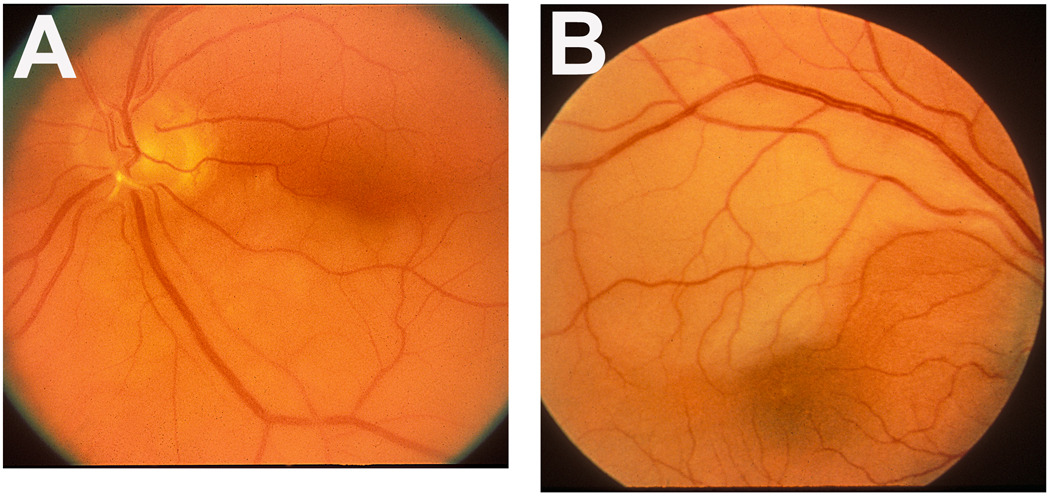
Figure 4.
Fundus photographs of two eyes with branch retinal artery occlusion.
A: Left eye with inferior branch retinal artery occlusion, with an embolus (white) impacted at its origin on the optic disc. Note the junction between the normal (upper half) and infarcted (lower half) retina lies in the foveal region.
B: Right eye with superior temporal branch retinal artery occlusion. Note the junction between the normal (lower) and infarcted (upper) retina lies in the foveal region.
Development of CLRAO in giant cell arteritis is an extremely important clinical entity. Giant cell arteritis is a prime ophthalmic emergency because with early diagnosis and prompt treatment with intensive corticosteroid therapy, any further visual loss is entirely preventable – it is preventable blindness., CLRAO in giant cell arteries has been erroneously diagnosed as “branch retinal artery occlusion”24, but the so-called “branch retinal arteries” are in fact arterioles, and giant cell arteritis is a disease of the medium-sized and large arteries and not of the arterioles8,9. We have seen patients with CLRAO occlusion diagnosed by ophthalmologists as ordinary BRAO and left untreated, resulting in catastrophic visual loss in both eyes, which could have been prevented, if the possibility of giant cell arteritis as one of its causes had been borne in mind. The posterior ciliary artery supplies the optic nerve head25 as well as the cilioretinal artery26. Giant cell arteritis has a selective tendency to involve the posterior ciliary artery (Fig. 4)6,8,9, resulting in its occlusion, which in turn results in simultaneous development of both arteritic anterior ischemic optic neuropathy and CLRAO (Fig.1-A)6–9. The arteritic anterior ischemic optic neuropathy causes massive visual loss in these eyes. They present with a classical, diagnostic clinical picture. A combination of chalky white optic disc edema, retinal infarct in the region of the occluded cilioretinal artery and presence of posterior ciliary artery occlusion on fluorescein angiography is diagnostic of giant cell arteritis (see Fig.1-A, Fig 2).6–9
Development of CLRAO in CRVO and hemi-CRVO is a distinct clinical entity (Fig. 1-B), and we have discussed its pathogenesis, clinical features and management in detail elsewhere.10
Acknowledgments
Supported by grant EY-1151 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This article contains online-only material. The following should appear online-only: Tables 2,4,5 and 7.”
The authors have no conflict of interest.
REFERENCES
- 1.Hayreh SS, Podhajsky P. Ocular neovascularization with retinal vascular occlusion II. Occurrence in central and branch retinal artery occlusion. Arch Ophthalmol. 1982;100:1585–1596. doi: 10.1001/archopht.1982.01030040563002. [DOI] [PubMed] [Google Scholar]
- 2.Yuzurihara D, Iijima H. Visual outcome in central retinal and branch retinal artery occlusion. Jpn J Ophthalmol. 2004;48:490–492. doi: 10.1007/s10384-004-0102-y. [DOI] [PubMed] [Google Scholar]
- 3.Mason JO, Shah AA, Vail RS, et al. Branch retinal artery occlusion: visual prognosis. Am J Ophthalmol. 2008;46:455–457. doi: 10.1016/j.ajo.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Hayreh SS, Zimmerman B. Central retinal artery occlusion: visual outcome. Am J Ophthalmol. 2005;140:376–391. doi: 10.1016/j.ajo.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 5.Hayreh SS, Zimmerman MB. Fundus changes in central retinal artery occlusion. Retina. 2007;27:276–289. doi: 10.1097/01.iae.0000238095.97104.9b. [DOI] [PubMed] [Google Scholar]
- 6.Hayreh SS. Anterior ischaemic optic neuropathy II. Fundus on ophthalmoscopy and fluorescein angiography. Br J Ophthalmol. 1974;58:964–980. doi: 10.1136/bjo.58.12.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayreh SS. Anterior ischaemic optic neuropathy: differentiation of arteritic from non-arteritic type and its management. Eye. 1990;4:25–41. doi: 10.1038/eye.1990.4. [DOI] [PubMed] [Google Scholar]
- 8.Hayreh SS, Podhajsky PA, Zimmerman B. Ocular manifestations of giant cell arteritis. Am J Ophthalmol. 1998;125:509–520. doi: 10.1016/s0002-9394(99)80192-5. [DOI] [PubMed] [Google Scholar]
- 9.Hayreh SS, Zimmerman B. Management of giant cell arteritis: our 27-year clinical study: new light on old controversies. Ophthalmologica. 2003;217:239–259. doi: 10.1159/000070631. [DOI] [PubMed] [Google Scholar]
- 10.Hayreh SS, Fraterrigo L, Jonas J. Central retinal vein occlusion associated with cilioretinal artery occlusion. Retina. 2008;28:581–594. doi: 10.1097/IAE.0b013e31815ec29b. [DOI] [PubMed] [Google Scholar]
- 11.Hayreh SS, Podhajsky PA, Raman R, Zimmerman B. Giant cell arteritis: validity and reliability of various diagnostic criteria. Am J Ophthalmol. 1997;123:285–296. doi: 10.1016/s0002-9394(14)70123-0. [DOI] [PubMed] [Google Scholar]
- 12.Hayreh SS, Zimmerman MB. Non-arteritic anterior ischemic optic neuropathy - Natural history of visual outcome. Ophthalmology. 2008;115:298–305. doi: 10.1016/j.ophtha.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown GC, Moffat K, Cruess A, et al. Cilioretinal artery obstruction. Retina. 1983;3:182–187. doi: 10.1097/00006982-198300330-00007. [DOI] [PubMed] [Google Scholar]
- 14.Mason JO, Nixon PA, Albert MA. Trans-luminal nd:YAG laser embolysis for branch retinal artery occlusion. Retina. 2007;27:573–577. doi: 10.1097/IAE.0b013e3180546c4d. [DOI] [PubMed] [Google Scholar]
- 15.Opremcak E, Rehmar AJ, Ridenour CD, et al. Restoration of retinal blood flow via traslumenal Nd:YAG embolysis/embolectomy (TYL/E) for central and branch retinal artery occlusion. Retina. 2008;28:226–235. doi: 10.1097/IAE.0b013e31814b1d6e. [DOI] [PubMed] [Google Scholar]
- 16.Hayreh SS. Restoration of retinal blood flow via translumenal Nd:YAG embolysis/embolectomy (TYL/E) for central and branch retinal artery occlusion. (Correspondence) Retina. 2008;28:1369–1372. doi: 10.1097/IAE.0b013e3181846640. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Arumi J, Martinez-Castillo V, Boixadera A, et al. Surgical embolus removal in retinal artery occlusion. Br J Ophthalmol. 2006;90:1252–1255. doi: 10.1136/bjo.2006.097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayreh SS. Surgical embolus removal in retinal artery occlusion. (Correspondence) Br J Ophthalmol. 2007;91:1096–1097. [PMC free article] [PubMed] [Google Scholar]
- 19.Richard G, Lerche R-C, Knospe V, Zeumer H. Treatment of retinal arterial occlusion with local fibrinolysis using rTPA. Ophthalmology. 1999;106:768–773. doi: 10.1016/S0161-6420(99)90165-3. [DOI] [PubMed] [Google Scholar]
- 20.Hayreh SS. Retinal arterial occlusion with LIF using rTPA. (Correspondence) Ophthalmology. 1999;106:1236–1238. 2044. doi: 10.1016/S0161-6420(99)10101-5. [DOI] [PubMed] [Google Scholar]
- 21.Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog Retin Eye Res. 2005;24:493–519. doi: 10.1016/j.preteyeres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Hayreh SS, Zimmerman MB, Kimura A, Sanon A. Central retinal artery occlusion: retinal survival time. Exp Eye Res. 2004;78:723–736. doi: 10.1016/s0014-4835(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 23.Hayreh SS, Jonas JB. Optic disk and retinal nerve fiber layer damage after transient central retinal artery occlusion: an experimental study in rhesus monkeys. Am J Ophthalmol. 2000;129:786–795. doi: 10.1016/s0002-9394(00)00384-6. [DOI] [PubMed] [Google Scholar]
- 24.Fineman MS, Savino PJ, Federman JL, Eagle RC. Branch retinal artery occlusion as the initial sign of giant cell arteritis. Am J Ophthalmol. 1996;122:428–430. doi: 10.1016/s0002-9394(14)72073-2. [DOI] [PubMed] [Google Scholar]
- 25.Hayreh SS. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma and oedema of the optic disc. Br J Ophthalmol. 1969;53:721–748. doi: 10.1136/bjo.53.11.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayreh SS. The cilio-retinal arteries. Br J Ophthalmol. 1963;47:71–89. doi: 10.1136/bjo.47.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]