Abstract
Brain insulin has widespread metabolic, neurotrophic, and neuromodulatory functions and is involved in the central regulation of food intake and body weight, learning and memory, neuronal development, neuronal apoptosis, and aging. To understand the neuromodulatory role of insulin, we aimed to characterize its yet undefined in vivo electrophysiological effects. We elected to record from the cerebellar cortex because as this region has average insulin concentration and insulin receptor content in relation to the whole brain, and has been previously shown to be a target for insulin signaling. We used in vivo microiontophoresis to apply insulin juxtaneuronally while simultaneously recording changes in spontaneous neuronal activity. The analysis included 553 significant neuronal responses to insulin and other related agents recorded from 47 cerebellar neurons of the rat. We found that (1) insulin stimulation produced instant and reversible electrophysiological effects on all of the recorded neurons, and that (2) these effects were mostly dependent on prior or simultaneous GABA-stimulation (94–96%). Specifically, (a) inhibitory responses to insulin were the most common (58–62%), and were dose-dependent with respect to GABA pretreatments and blocked by co-administration of the insulin receptor inhibitor HNMPA. (b) In the second largest neuronal population (32–38%) insulin decreased the magnitude of GABA inhibitions when co-applied. (c) In contrast, only a small number of neurons showed GABA-independent responses to insulin application (4–6%), which were exclusively neuronal excitations. The present findings demonstrate that insulin has direct electrophysiological effects on central neurons in vivo and these effects are highly influenced by GABA-ergic inputs.
Keywords: GABA, cerebellum, excitability, neuropeptide, microiontophoresis
1. Introduction
Pancreatic insulin crosses the blood-brain barrier via a saturable transport mechanism (Banks et al. 1996). In contrast to its saturable profile, this transport is not static but dynamically regulated by various factors (Banks 2004). For example, several physiological and pathological conditions such as fasting, hyperglycemia, aging, obesity, diabetes, and Alzheimer’s disease have been shown to affect the insulin concentration in the brain.
The effects of insulin and its widely expressed receptors in the brain have been implicated in various metabolic, neurotrophic and neuromodulatory functions (Schulingkamp et al. 2000). The neuromodulatory effects of insulin can influence the regulation of food intake, food intake-related dopamine reward (Figlewicz et al. 2007), learning and memory (Moosavi et al. 2007), aging (Bartke 2007), and neurological disorders such as Alzheimer’s disease (Craft et al. 1999) and depression (Lustman et al. 1986). Recent studies have also implicated insulin in cognitive impairments and dementia associated with type 2 diabetes (Whitmer 2007).
However, little is known about the possible electrophysiological effects of insulin in the brain. Recordings from in vitro hypothalamus preparations showed insulin’s main electrophysiological action is neuronal hyperpolarization, which occurs via activation of ATP-dependent potassium channels (Plum et al. 2005). Other electrophysiological findings from hippocampal slices also support the theory that insulin inhibits neuronal activity (Palovcik and Phillips 1986). Given the particular relevance of altered insulin signaling to human pathology, it is imperative to expand this investigation, focusing on the direct in vivo effects of insulin (Gao and Horvath 2007; Needleman and McAllister 2008).
In relation to the whole brain, the cerebellum has an average insulin and insulin receptor content (Schulingkamp et al. 2000), and has localized tyrosine phosphorylation of the insulin receptor in response to insulin administration (Fernandes et al. 1999). The cerebellum has also been implied in the regulation of feeding behavior via direct cerebellar-hypothalamic pathways (Zhu and Wang 2008). In addition, an important connection between insulin and gamma-aminobutyric acid (GABA) signaling has been described in the cerebellum. Specifically, an in vivo microdialysis study demonstrated that glutamate-induced GABA release can be greatly depressed by the administration of insulin-like growth factor 1 (IGF-I) (Castro-Alamancos and Torres-Aleman 1993).
Several additional in vitro studies have provided evidence for a link between GABA and insulin-related signaling in other brain regions. Specifically, they proved that insulin interacts with GABAA receptors, affecting (1) synaptic plasticity in the hippocampus (Wan et al. 1997); (2) neuroprotection against ischemia in the cerebral cortex (Mielke and Wang 2005); (3) cholinergic signaling in the prefrontal cortex (Ma et al. 2003); and (4) hypothalamic inhibition of feeding (Sheng et al. 2006). Also, there is evidence that a connection exists between GABAA and insulin pathways in the periphery (Xu et al. 2006). Although one recent study in Xenopus oocytes revealed that insulin has a rapid inhibitory effect on GABAA receptor currents (Williams 2008), there is no in vivo electrophysiological description of the details of this insulin-GABA interaction in the mammalian brain.
On this basis, the present study was designed to fill an important gap in our understanding of how insulin modifies neuronal firing activity in the mammalian brain. Therefore, we investigated the effects of in vivo juxtaneuronal insulin application on the spontaneous firing rate of cerebellar neurons in anesthetized rats, combined with treatments of the GABA and insulin receptor inhibitor Hydroxy-2-naphthalenylmethylphosphonic acid (HNMPA).
2. Research design and Methods
2.1. Subjects
A total of 11 adult (20 ± 1.1 wks of age) male Sprague-Dawley rats with initial body weight of 568 ± 34 g were used. All procedures were approved by the Local Review Committee of the Pennsylvania State University based upon National Institutes of Health guidelines.
2.2 Surgical preparation for in vivo recordings
For anesthesia, 50 mg / kg Nembutal sodium solution (Abbott Laboratories, North Chicago, IL, USA) was administered intraperitoneally. The head of the rat was fixed into a stereotaxic frame. After skull trepanation and removal of the dura mater, the microiontophoretic electrode was lowered into the cerebellum tilted in a 20° angle (tipped to anterior). The target area for recording neuronal activity was the rostral lobules of the anterior lobe of the vermis (coordinates according to (Paxinos and Watson 1998): AP, −12.0–14.0 mm; ML, 1.0–3.0 mm; V, 0.8–5.3 mm).
2.3. Microiontophoretic experimental setup overview
For extracellular single unit recording and neurochemical stimulation, commercially available seven barreled microiontophoretic electrodes (Carbostar-7S, Kation Scientific, MN, USA) were used. These electrodes have a 7µm diameter carbon fiber tip for recording and 6 additional glass pipettes for drug delivery. The recorded extracellular action potentials were passed through a pre- and a main AC amplifier (A-M Systems, USA), than an A/D converter (CED Micro, Cambridge, UK). The digitalized signals were then collected and analyzed by Spike2 software (CED, Cambridge, UK).
Neurochemical stimulation routines were controlled by an iontophoresis pump module (NeuroPhore BH-2 System, Harvard Apparatus, USA) that delivers positive or negative DC currents between 1 and 500 nA to eject the desired compound into the brain. Our applied drug solutions and their microiontophoretic properties were the following: Insulin, 500 U / mL ≅ 3.59 mmol / L (Eli Lilly Co.) ejected as an anion with cathodal (-) currents; HNMPA (a membrane impermeable inhibitor of insulin receptor tyrosine kinase that inhibits serine and tyrosine autophosphorylation by the human insulin receptor (Baltensperger et al. 1992)), 21 mmol / L dissolved in Dimethyl sulfoxide (BIOMOL Research Laboratories Inc.) ejected as anion with cathodal (-) currents. We used glutamic acid (150 mmol / L, Sigma-Aldrich) ejected as anion with cathodal (-) currents and GABA (150 mmol / L, Sigma-Aldrich) ejected as cation with anodal (+) currents for excitatory and inhibitory controls, respectively. One channel filled with a 2% Pontamine Sky Blue (PSB) solution (dissolved in 0.5 M Na-acetate) served to deliver a balancing current (to avoid electric stimulation of the recorded neurons during microiontophoresis) and also to label the recording site after the sessions with an unbalanced ejection of PSB (500nA, for 3–10 minutes). The impedances of the drug-, control- and balancing channels were kept between 10–200 MOhm, to avoid leaking or clogging.
2.4. Spike sorting and frequency histograms
Sorting of the recorded neurons into single unit classes was accomplished using off line wavemark analyses (Spike2 software). To ensure correct separation of single units overdrawn waveform comparisons and principal component analyses were performed. After the single units were separated, frequency histograms (spikes / seconds) for each recorded neuron were built.
2.5. Statistical analyses
Neuronal responses to the applied drugs were analyzed with Statistica software (Statsoft Inc., Tulsa, OK, USA). Significant effects were determined by paired t-tests (p < 0.05) comparing the −10 s mean prestimulus baseline firing rate (spikes/seconds) with the +10 s mean poststimulus rate. Further ANOVAs were used to determine overall between-group differences for the various drug effects.
2.6. Histology
After the experiments, the rats were perfused with 4% paraformaldehyde, their brains removed and processed for histology. Parallel to the 20° tilted electrode penetrations, 40 µm slices were cut and stained with cresyl violet. Sections were studied under a light microscope to verify the placement of the electrode tracks and the recording areas that had been labeled with microiontophoretic PSB directly after the recording sessions.
3. Results
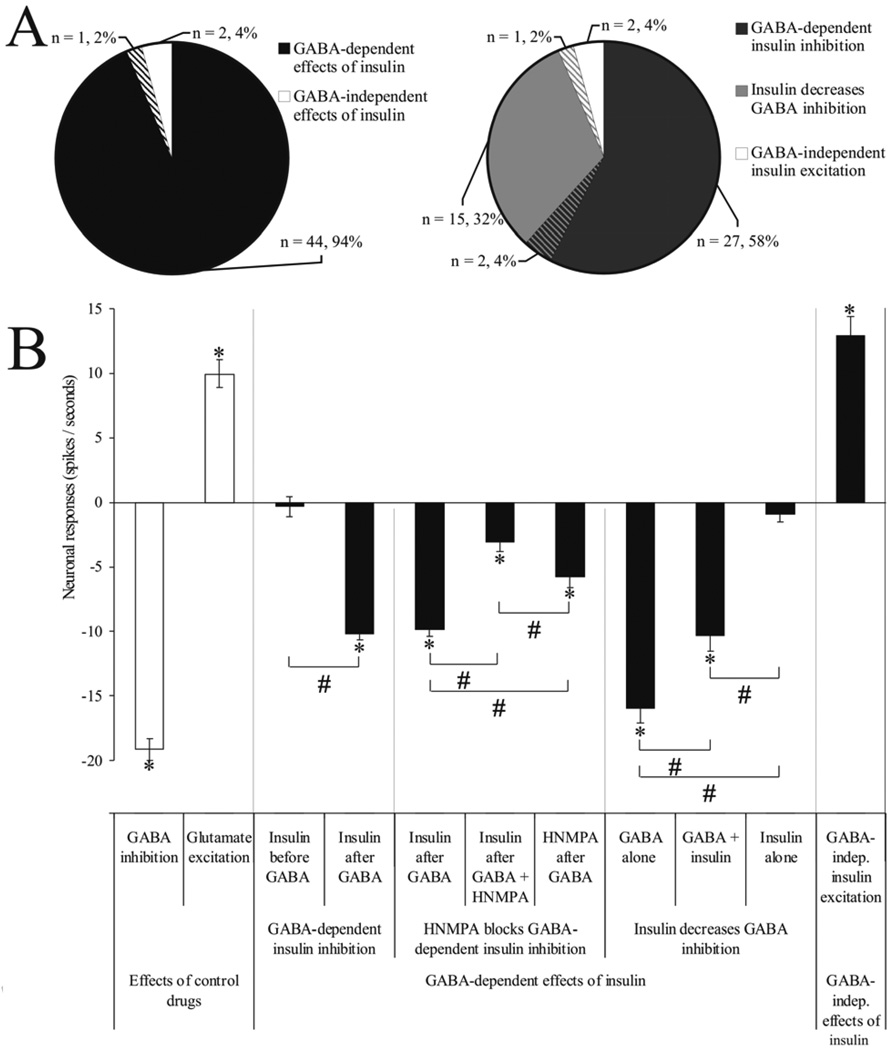
We analyzed 563 significant cerebellar neuronal responses (from a total of 885 trials) to different microiontophoretic drug ejections (GABA, glutamate, insulin, HNMPA), recorded from 47 neurons (32 recording sites) in 11 rats. The electrophysiological effects were categorized as “Effects of control drugs”, “GABA-dependent effects of insulin” and “GABA-independent effects of insulin” groups (Table 1). We found a significant main effect in the overall comparison for drug treatments (F11,873 = 43.605, p < 0.001, n = 885).
Table 1.
Effects of insulin on the single cell unit activity of cerebellar neurons.
| Electrophysiological effects | Number of neurons tested | Number of significant neuronal responses | Number and percent of neurons showing significant effects |
|---|---|---|---|
| Effects of control drugs: | |||
| 1. GABA inhibition | 46 | 285 | 46/46 (100%) |
| 2. Glutamate excitation | 5 | 21 | 5/5 (100%) |
| GABA-dependent effects of insulin: | |||
| 1. GABA-dependent insulin inhibition | 47 | 148 | 29/47 (62%) |
| 2. HNMPA blocks GABA-dependent insulin inhibition | 13 | 35 | 13/13 (100%) |
| 3. Insulin decreases GABA inhibition | 47 | 59 | 18/47 (38%) |
| GABA-independent effects of insulin: | |||
| 1. GABA-independent insulin excitation | 47 | 15 | 3/47 (6%) |
| Total number of neurons / microiontophoretic trials | 47 | 563 | |
3.1. Effects of control drugs
At the beginning of every recording session, the electrophysiological responsiveness of neurons was characterized by ejecting the inhibitory control GABA (46/46 neurons inhibited, 100%), and/or the excitatory control glutamate (5/5 neurons excited, 100%). However, in most recording sessions we applied only GABA controls and omitted glutamate applications, because the former is neuroprotective while the latter can be neurotoxic. Particularly, glutamate control was applied when we compared its excitatory effects with the relatively rare GABA-independent insulin excitations (3/47 neurons, 6%; detailed later).
3.2. GABA-dependent effects of insulin
We found that the majority of cerebellar neurons did not show any electrophysiological effects to microiontophoretically-applied insulin when GABA was not present (45/47 neurons, 96%). However, when we combined the insulin stimulations with pre- or simultaneous application of GABA, all of these previously insulin-insensitive neurons became insulin-sensitive, and showed significant electrophysiological responses to insulin application.
The larger GABA-dependent neuron population showed GABA-dependent insulin inhibitions (29/47 neurons, 62%). To test whether this action was mediated directly by the cell surface insulin receptor, we tested the effectiveness of insulin receptor inhibitor HNMPA on a subpopulation of neurons. We found that on all tested cells (13/13 neurons, 100%) HNMPA blocks GABA-dependent insulin inhibitions.
The smaller population of neurons showed another type of GABA-dependent insulin response, where insulin decreased GABA inhibitions (18/47 neurons, 38%). These GABA-dependent effects of insulin are detailed below (3.2.1.-3.2.3.).
3.2.1. GABA-dependent insulin inhibition
The largest population of the recorded cerebellar neurons (29/47 neurons, 62%) showed GABA-dependent inhibitory responses (F1,167 = 55.104, p < 0.001, n = 169; −10.2 spikes/seconds ± 0.5 S.E.M.). This inhibitory insulin-response was analyzed at a specific time point when the neurons were first hyperpolarized and then fully recovered (30 s) from a GABA pretreatment (Fig. 1). Before the GABA pretreatment, these neurons did not respond significantly to insulin (t = 0.403, df = 20, p = 0.691, n = 21, 21; −0.3 ± 0.8 spikes/seconds). Consequently, we found a significant difference between the magnitude of insulin-responses before and after the GABA treatments (t = −7.423, df = 167, p < 0.001, n = 21, 148).
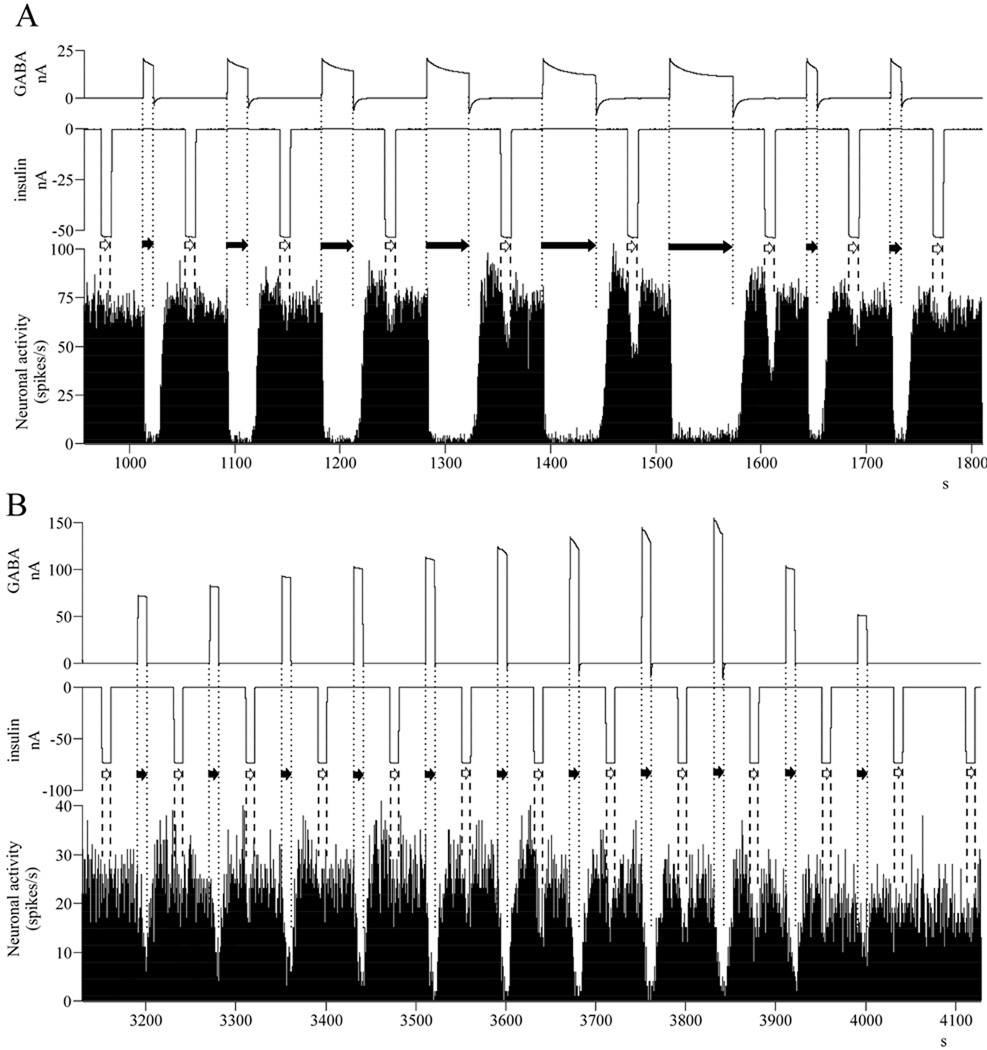
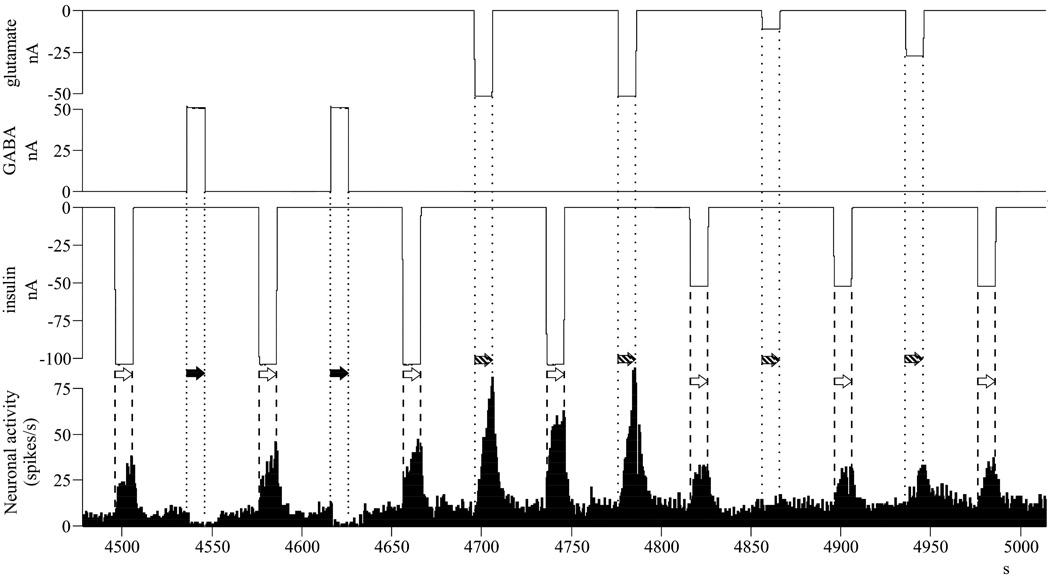
Fig. 1.
GABA pretreatment dose-dependently regulates the magnitude of the subsequent insulin inhibition – single neuronal recordings of typical neurons. A/ Dependence on GABA application time: as the GABA-inhibitions are getting longer in time, the magnitude of the following insulin inhibitions are becoming larger; B/ Dependence on current intensity of GABA: as the GABA-inhibitions are getting more intense, the magnitude of the following insulin inhibitions are becoming larger. Abbreviations: nA, nanoamperes (current intensity); s, seconds (time); insulin application: white arrow, between dashed lines; GABA application: black arrow, between dotted lines.
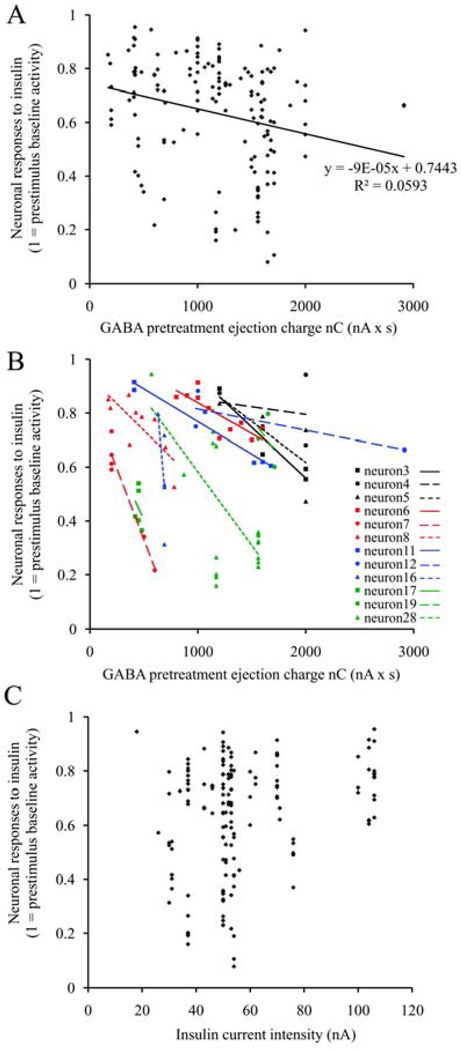
The GABA pretreatment was not only a prerequisite for the inhibitory action of insulin, but it also determined the magnitude of the insulin-response in a dose-dependent manner. The applied GABA concentrations were altered by modifying the microiontophoretic application time or the current intensity, based on a linear connection between the two parameters (Herr et al. 2008). Increasing the application time increased the latency (Fig. 1/A), while enhancing the current intensity raised the magnitude (Fig. 1/B) of the GABA inhibitions. Accordingly, the magnitude of the subsequent insulin inhibitions were higher (larger inhibitions), irrespective of whether the GABA concentration was increased by larger application time (Fig. 1/A) or larger current intensity (Fig. 1/B). As a next step in our analyses, the neuronal responses to insulin were described as a function of the GABA pretreatment ejection charge (Fig. 2), based on Coulomb’s equation (= GABA current intensity [nanoAmperes] x GABA application time [seconds]). We found a significant correlation between the magnitude of insulin inhibition and the GABA pretreatment ejection charge (Multiple Regression, r = 0.244, r2 = 0.059, F1,146 = 9.210, p = 0.003, n = 148). Parallel to the increasing GABA pretreatment ejection charge, both the matched linear regression line of all individual inhibitory responses to insulin (Fig. 2/A) and the linear regression lines of single neurons (Fig. 2/B) were descending (the x-factors were negative in all equations). However, in contrast to the applied GABA concentration, we did not find a significant correlation between the magnitude of the insulin inhibitions and the current intensity of insulin application (Fig. 2/C; Multiple Regression, r = 0.145, r2 = 0.021, F1,146 = 3.131, p = 0.079, n = 148). These results strongly suggest that the magnitude of the insulin inhibitions depend more on extracellular GABA than the insulin concentration itself, and show that increasing GABA pretreatment concentrations lead to increasing insulin inhibitions on the neurons.
Fig. 2.
GABA pretreatment dose-dependently regulates the magnitude of the subsequent insulin inhibition – neuronal response magnitude analysis to microiontophoretic insulin as a function of GABA pretreatment ejection charge or insulin current intensity. A, Neuronal response magnitudes to insulin are significantly correlating with the GABA pretreatment ejection charge (148 insulin applications recorded from 29 neurons); B, Neuronal responses of 12 neurons with individual GABA-dose-dependence tests and their linear trendlines show the same correlation; C, Neuronal response magnitudes to insulin are not correlating with the current intensity of insulin application (148 insulin applications recorded from 29 neurons). Abbreviations: nC, nanoCoulombs; nA, nanoAmperes; s, seconds.
3.2.2. HNMPA blocks GABA-dependent insulin inhibition
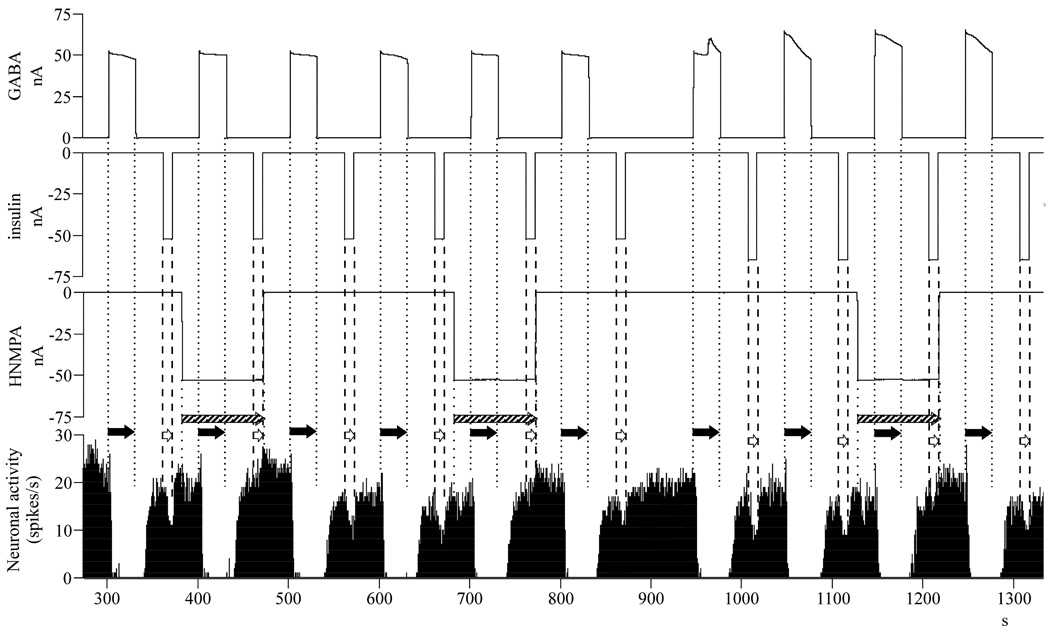
We also investigated whether the observed GABA-dependent electrophysiological effects of insulin are primary or secondary to the cell surface insulin receptor activation. As it is demonstrated on a single neuronal recording in Fig. 3, the insulin inhibitions vanished when HNMPA was simultaneously applied (-50 nA for 90 seconds), while GABA inhibitions were unaffected. All 13 neurons (100%) investigated showed the same profile. The analysis of the neuronal effects demonstrated that there was an overall difference between the effects of different drug applications (F3,173 = 20.024, p < 0.001, n = 177). Paired t-tests comparing pre- vs. poststimulus neuronal responses demonstrated that (a) insulin has an inhibitory response after GABA pretreatment but before the application of HNMPA (t = 16.836, df = 72, p < 0.001, n = 73, 73; −9.8 ± 0.6 spikes/seconds); (b) insulin remained inhibitory but with a reduced magnitude when applied together with HNMPA (t = 3.920, df = 34, p < 0.001, n = 35, 35; −3.1 ± 0.8 spikes/seconds); (c) the effects of GABA remained unaffected during the HNMPA ejection (t = 6.807, df = 33, p < 0.001, n = 34, 34; −15.8 ± 2.3 spikes/seconds); (d) similar to insulin, after GABA application, HNMPA alone showed significant but smaller inhibitory effects (t = 7.440, df = 34, p < 0.001, n = 35, 35; −5.8 ± 0.8 spikes/seconds).
Fig. 3.
Treatment with insulin receptor inhibitor HNMPA blocks the GABA pretreatment dependent insulin inhibitions, when co-applied – single neuronal recording of a typical neuron. Abbreviations: nA, nanoamperes (current intensity); s, seconds (time); insulin application: white arrow, between dashed lines; GABA application: black arrow, between dotted lines, HNMPA application: striped arrow, between rare dotted lines.
When we compared these drug effects (a-d) we found that the insulin inhibitions were 68% smaller when HNMPA was co-applied (t = −6.763, df = 106, p < 0.001, n = 73, 35). We also noticed that the magnitude of the GABA-dependent insulin inhibitions were significantly larger than the HNMPA inhibitions (t = 4.030, df = 106, p < 0.001, n = 73, 35). Finally, the co-applied insulin and HNMPA-induced inhibitions were smaller than HNMPA-inhibitions alone (t = −2.485, df = 68, p = 0.015, n = 35, 35).
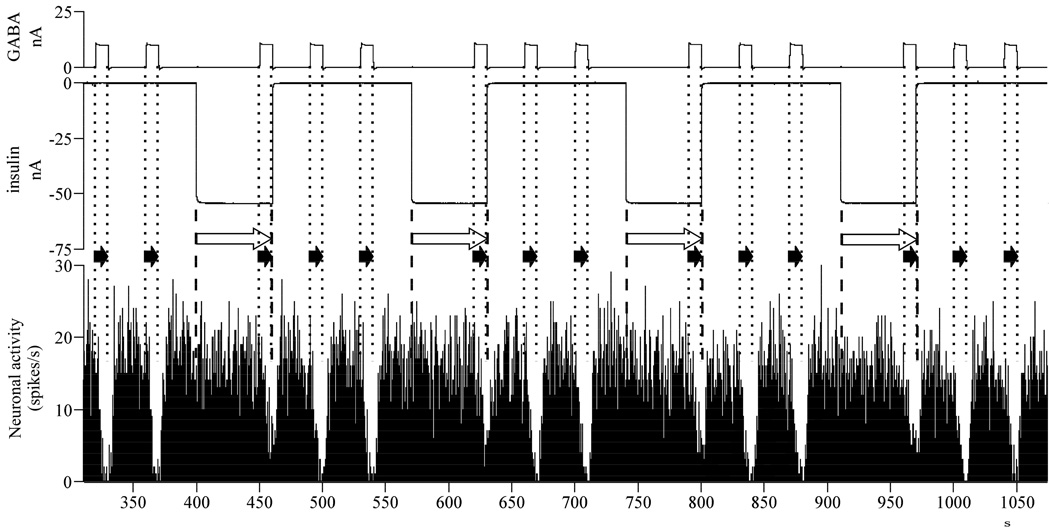
3.2.3. Insulin decreases GABA inhibition
Microiontophoretic application of insulin combined with simultaneous GABA ejection reduced the efficiency of GABA to inhibit neuronal firing in 38% (18/47) of total investigated neurons (for sample single neuronal responses see Fig. 4). When insulin was co-applied with GABA, the original significant GABA inhibition (t = 13.209, df = 102, p < 0.001, n = 103, 103; −15.9 ± 1.5 spikes/seconds) decreased by 35% (F1,160 = 9.143, p = 0.003, n = 162; t = −3.024, df = 160, p = 0.003, n = 103, 59). However, GABA remained significantly inhibitory even when its efficiency was decreased by insulin (t = 8.163, df = 58, p < 0.001, n = 59, 59; −10.3 ± 1.3 spikes/seconds). In these cases, insulin alone did not have significant effects on neuronal activity (t = 1.458, df = 55, p = 0.151, n = 56, 56; −0.9 ± 0.6 spikes/seconds).
Fig. 4.
Insulin decreases the magnitude of GABA inhibitions, when co-applied – single neuronal recording of a typical neuron‥ Abbreviations: nA, nanoamperes (current intensity); s, seconds (time); insulin application: white arrow, between dashed lines; GABA application: black arrow, between rare dotted lines.
3.3. GABA-independent effects of insulin
The GABA-independent effects of insulin were rare (3/47 neurons, 6%) compared to other GABA-dependent effects (45/47 neurons, 96%). All GABA-independent effects were excitations (Fig. 5; t = −8.770, df = 14, p < 0.001, n = 15, 15), resulting in firing rate elevations of +12.9 spikes/seconds ± 1.5 (S.E.M.) above the baseline. We compared the magnitudes of excitatory responses exerted by insulin and glutamate on the same neurons and did not find significant differences (t = −1.642, df = 34, p = 0.110, n = 15, 21).
Fig. 5.
Insulin is able to exert excitatory effects on neurons, irrespectively to GABA-application – single neuronal recording of a typical neuron. Abbreviations: nA, nanoamperes (current intensity); s, seconds (time); insulin application: white arrow, between dashed lines; GABA application: black arrow, between dotted lines, glutamate application: striped arrow, between rare dotted lines.
3.4. Histological localization of the recording areas
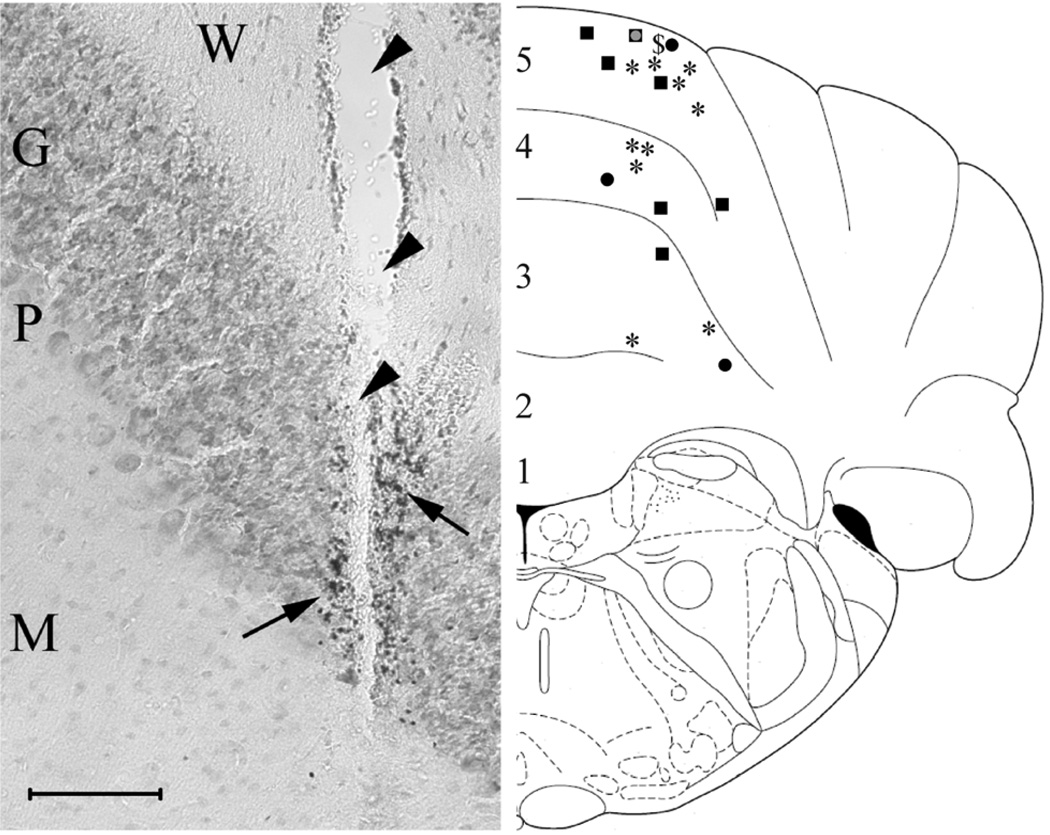
After completion of the recording sessions, 66% (21/32) of all recording areas were successfully labeled microiontophoretically by Pontamine Sky Blue (PSB) and located by light microscopy. PSB was picked up by granule cells that were located near the tip of the recording electrode (Fig. 6, left panel), evidence that the recording sites were in the granule cell layer of the cerebellar cortex. One may assume that the labeled cells may not be identical to those recorded because the ejected PSB potentially marked adjacent neurons as well. The reconstruction of the placement of these recording areas can be seen in Fig. 6/right panel. All labeled recording sites were in the cerebellar cortex, in the rostral lobules 3–4–5 of the anterior lobe of the vermis (Paxinos 1985; Paxinos and Watson 1998). Within these three lobules, 57% (12/21 neurons) of the verified recording areas were in the 5th lobule, 24% (5/21 neurons) in the 4th lobule, and 19% (4/21 neurons) in the 3rd lobule. No significant differences were found between the area location (lobule numbers) and the distribution of the different neuronal effects of insulin (paired t-tests’ p-values: lobule 5th vs. 4th, p = 0.46; lobule 5th vs. 3rd, p = 0.07; lobule 4th vs. 3rd, p = 0.17).
Fig. 6.
Histological localization of the recording areas. Left panel: sample image showing the electrode track (arrowheads) and a PSB-labeled recording area next to it. PSB was picked up by granule cells which were located nearby the recording site (arrows). The background is stained with cresyl violet; the insert at the left bottom is 100 µm. Abbreviations: W, white matter; G, granule cell layer; P, Purkinje cell layer; M, molecular layer. Right panel: schematic reconstruction of the placement of 21 recording areas and the effects of insulin recorded in them. Symbols: square, GABA-dependent insulin inhibition; star, HNMPA blocks GABA-dependent insulin inhibition; circle, insulin decreases GABA inhibition; dollar sign, insulin excitation. Numbers 1–5 refer to the rostral lobules 1–5 of the anterior lobe of the cerebellar vermis.
4. Discussion
The present findings constitute the first in vivo evidence that neurons in the mammalian brain change firing rate in response to insulin receptor stimulation. These data also suggest an important temporal connection between insulin and GABA signaling, since the electrophysiological effects of insulin were dependent on prior or simultaneous GABA application (Fig. 7/A) in the majority of the recorded neurons (94–96%). Specifically, the response characteristics of insulin on the spontaneous neuronal firing rate (Fig. 7/B) showed mostly inhibitory effects of insulin (58–62%) that only occurred following a pretreatment with GABA, which also seemed to regulate the magnitudes of the subsequent insulin inhibitions in a concentration-dependent manner. These GABA-dependent inhibitory actions of insulin were blocked by the co-application of cell-impermeable insulin receptor tyrosine kinase inhibitor HNMPA, demonstrating the dependency of these effects on insulin receptor activation. The other large neuron population showed a different GABA-dependent effect, where the application of insulin reduced GABA inhibitions (32–38%). In contrast to these GABA-dependent effects, the GABA-independent (excitatory) effects of insulin were much less frequent (4–6%).
Fig. 7.
Summary of the main findings. A, The distribution of the in vivo electrophysiological effects of insulin. A/left, The distribution of the GABA-dependent and GABA-independent effects (the cross-sections with transversal stripes show bi-functional neurons that belong to both groups); A/right, The distribution of the three main electrophysiological effects of insulin. B, Effects of insulin on the in vivo neuronal activity. The “Effects of control drugs” were detected as (1) GABA inhibition and (2) glutamate excitation; the “GABA-dependent effects of insulin” had been described as (1) GABA-dependent insulin inhibition, (2) HNMPA blocks GABA-dependent insulin inhibition, and (3) insulin decreases GABA inhibition; the “GABA-independent effects of insulin” were all (1) GABA-independent insulin excitations. Neuronal responses are displayed as mean (± S.E.M.) poststimulus 10 s firing rates (spikes / seconds), corrected to the prestimulus 10 s spontaneous firing rates (y = 0). * = p < 0.05 paired t-tests comparing mean pre- and poststimulus neuronal activity; # = p < 0.05 two-sample unequal variance t-tests between mean poststimulus firing rates.
4.1. GABA-dependent effects of insulin
We showed that the in vivo local administration of insulin mostly inhibits neurons, similarly, as it was demonstrated in vitro in the hypothalamus (Plum et al. 2005) and hippocampus (Palovcik and Phillips 1986). Our results demonstrated that, similar to previous in vitro findings (Ma et al. 2003; Mielke and Wang 2005; Sheng et al. 2006; Wan et al. 1997) and peripheral mechanisms (Xu et al. 2006), central insulin signaling is influenced by GABAA-receptor mediated mechanisms. The present data also supports previous electrophysiological findings (Williams 2008) demonstrating that insulin is capable of decreasing the magnitude of GABA inhibitions in vivo. In addition, our data provide novel evidence showing that the inhibitory effects of insulin in vivo are highly dependent on the prior GABAergic effects on the neuron. This observation reveals a plausible temporal connection between insulin and GABA-signaling. Future studies investigating the details of this connection and the underlying mechanisms are warranted. For example, the specific contribution of GABAA- and GABAB-receptors, and the role of various other GABA-related mechanisms (e.g. intracellular Cl− concentration, intracellular pH, transmembrane Cl− - and H+ -ion transporters) must be investigated. The subsequent discussion addresses potential mechanisms.
The neuromodulatory effects of insulin on neurotransmitter release such as norepinephrine, serotonin, N-methyl-D-aspartic acid (NMDA) and GABA (Schulingkamp et al. 2000; Zhao and Alkon 2001), as well as its effect on ATP-dependent potassium channels (Plum et al. 2005) have been previously demonstrated. Such effects of insulin, however, do not explain why its electrophysiological actions are dependent on pre- or co-administration of GABA, as was demonstrated in this study. The main effect of the primary GABAA receptor activation is the opening of Cl− channels, which causes an increase in the intracellular Cl− concentration ([Cl−]i) and a decrease in the intracellular pH (pHi) (Luckermann et al. 1997). However, this action only occurs in mature neurons as juvenile neurons have an opposite, excitatory, response to GABA that is based on the extrusion of intracellular Cl−. The difference is explained by the negative shift in the reversal potential of GABA, regulated by the late appearance of K+ - Cl−cotransporters (KCCs), particularly by KCC2, the neuronal isoform extruding K+ and Cl− ions from the intracellular space and hence decreasing the [Cl−]i (Kakazu et al. 1999; Shen et al. 2001). This developmental switch of GABA from excitatory to inhibitory can be accelerated by stimulation of IGF-I receptors (Kelsch et al. 2001), suggesting that insulin-related signaling mechanisms are connected to KCC2 mechanisms. Based on our current results, the most frequent effects of insulin require specific biochemical mechanisms that are only active in the presence of GABA. These mechanisms could reduce the elevated [Cl−]i and/or increase the pHi caused by GABA stimulation. The related transport mechanisms are (a) the KCC2 transporters (facilitating Cl− outward); (b) the Na+-driven chloride/bicarbonate exchanger (NCBE) that extrudes intracellular Cl− ions and increases pHi (Wang et al. 2000); and (c) the sodium–hydrogen exchangers (NHEs) that also increase the pHi by exchanging intracellular H+ for extracellular Na+ (Ali et al. 2004). Similar to insulin and insulin receptors (Schulingkamp et al. 2000), all three ion-transporters densely populate the cerebellum (Gagnon et al. 2007; Giffard et al. 2003; Schneider et al. 2004). In this context, there is a considerable chance that the GABA-related changes in [Cl−]i and/or pHi could effect insulin signaling. Therefore, one of the main effects of neuronal insulin signaling could be the inhibition of KCC2, NCBE and/or NHE transporters, accounting for the strong GABA dependency of the inhibitory actions of insulin in the cerebellum. In concert with this hypothesis, intracellular acidification caused by NHE- or NCBE-blockers has a long-lasting inhibitory effect on spontaneous neuronal activity on hippocampal CA3 neurons (Bonnet et al. 2000a; Bonnet et al. 2000b). In addition, insulin has been suggested to affect larger molecule transport mechanisms as well, such as the dopamine-, the serotonin-, the glutamic acid-, or the GABA molecule transport (Figlewicz 1999; Figlewicz et al. 1999; Rhoads et al. 1984).
Additionally, recent neurochemical measures find certain pH changes in the brain due to food rewards, i.e. intraoral sucrose infusions (Roitman et al. 2008). According to these observations, insulin-induced neuronal hyperpolarization may occur due to (a) prolonged intracellular acidification, (b) decreased membrane potential caused by increased [Cl−]i, and (c) decreased Na+-depolarization. Either of these processes may result from the inhibition of KCC2, NCBE and/or NHE transporters.
4.2. Insulin receptor inhibitor HNMPA blocks GABA-dependent insulin inhibitions
HNMPA and its cell-permeable analog HNMPA-(AM)3 have been employed in recent studies to demonstarte insulin-receptor dependent effects (Diaz et al. 2007; Melendez et al. 2000; Rose et al. 2007; Stella et al. 2001; Yu et al. 2004). In the present study, HNMPA blocked the inhibitory effects of insulin (Fig. 3), demonstrating that the GABA-dependent actions of insulin were primarily downstream effects of cell-membrane insulin receptors. This finding also supports previous findings that insulin induces tyrosine phosphorylation of the insulin receptor in the cerebellum (Fernandes et al. 1999). Furthermore, similarly but less effectively than insulin, HNMPA reduced neuronal firing after GABA application. This suggests that HNMPA may not only act as a cell membrane impermeable insulin receptor antagonist but may also have a small but significant partial insulin receptor agonist potential in vivo. For further investigation on the signaling mechanisms underlying the in vivo electrophysiological effects of insulin, other types of insulin-signaling inhibitors (e.g. cell-membrane permeable Akt inhibitors) should also be tested.
4.3. GABA-independent excitatory effects of insulin
The GABA-independent excitatory effects of microiontophoretically-applied insulin were relatively rare (6%) compared to the GABA-dependent effects (94%) and can be explained in two different ways. First, insulin could be excitatory based on the known glutamate-potentiating action of insulin and IGF-I that has been described in vitro in hippocampal neurons (Liu et al. 1995), and cerebellar granule cells (Calissano et al. 1993). Alternatively, insulin-evoked excitations could occur as reduced tonic inhibitions (disinhibition), if the neurons have strong tonic inhibitory GABA inputs, such as those that Golgi cells exert on cerebellar granule cells (Brickley et al. 1996). If the recorded excitatory insulin responses were actually reduced tonic inhibitions, then these neurons theoretically would also belong to the ‘insulin decreases GABA-inhibition’ group. The only difference between the two is that, in the ‘insulin decreases GABA-inhibition’ group, GABA was provided extrinsically by microiontophoresis, while in the ‘insulin excitation’ group there must have been a substantial intrinsic tonic GABA input. However, this explanation could suggest that even the smallest (6%) - seemingly GABA-independent - group can be dependent on intrinsic GABA signaling, similar to the previously described responses.
4.4. The role of insulin in cerebellar sensory-motor integration
Histology revealed (Fig. 6) that the neurons investigated in this study were cerebellar cortical granule cells within the granule layer of the rostral lobules 3–4–5 of the anterior lobe of the vermis. This raises the question: what specific effects could insulin exert by acting on this set of neurons. Cerebellar granule cells account for nearly half of the neurons in the central nervous system. It has been proposed that cerebellar granule cells are critical to the ascending spinocerebellar somatosensory synaptic integration by afferent excitatory mossy fibers (Jorntell and Ekerot 2006). Also, descending corticopontine somatosensory and somatomotor information is forwarded here using mossy fiber inputs (Paxinos 1985). Amongst somatosensory inputs, taste is also represented in the cerebellum of frogs (Hanamori and Ishiko 1987) and humans (Gautier et al. 1999). Besides the sensory and motor mossy fiber excitatory inputs, cerebellar granule cells have a local strong tonic GABA-ergic inhibitory input, exerted by the Golgi cells (Brickley et al. 1996). On the efferent side, granule cells give excitatory inputs to Purkinje cells that are inhibiting the deep cerebellar nuclei which finally project to various brain areas including the premotor cortex. With these currently investigated functions (Huang 2008), cerebellar granule cells seem to have an important role in the regulation of sensory-motor integration.
In connection with insulin signaling in this area, it has been demonstrated that the insulin-regulated glucose transporter, GLUT4 is mainly expressed on granule cells in the cerebellum (Vannucci et al. 1998). The same study also showed that GLUT4 is increased in the genetically diabetic and hyperinsulinemic db/db mouse, and that the expression of GLUT4 in the cerebellum responds to the level of circulating insulin.
Based on these properties of cerebellar granule cells and our data, insulin may be able to decrease or increase the firing rate of granule cells depending on the activity of GABA-ergic Golgi cell inputs. In this way, amongst other yet unknown functions, the cerebellar somatomotor outputs that are regulated by various sensory inputs might undergo an insulin-dependent modification at the granule cell level. For example, sensory inputs stimulating certain motor processes could be inhibited by elevated insulin levels. To further characterize these effects, future studies on how physiological and pathological phasic (meal-related) and tonic (obesity, diabetes) insulin level changes affecting central insulin sensitivity and signaling are needed.
4.5. The general significance of the electrophysiological effects of insulin
The above discussed direct GABA-dependent electrophysiological effects of insulin are likely to have an impact on other brain areas as well outside the cerebellum, which merits further investigation. Probably the two most important non-cerebellar related functions are food-reward and memory functions.
The possible role of insulin in food intake and dopamine-reward
Additionally to the well described anorexigenic effects of insulin in the hypothalamus (Schwartz et al. 2000), that have been explained by ATP-dependent K+-channel activation and neuronal hyperpolarization in vitro (Spanswick et al. 2000), it has been also established that insulin (along with leptin) works as an adiposity signal and regulates food intake by acting on the dopaminergic reward pathways. The effects on insulin within the ventral tegmental area may be of particular importance (Figlewicz et al. 2007). Insulin can act in the arcuate nucleus through GABA-ergic systems (Sato et al. 2005) and striatal neuronal spiking and dopamine release are regulated by a very potent interneuronal GABA-ergic regulation (Goto et al. 2007). However, no electrophysiological evidence has been provided describing the effects of insulin on hypothalamic or reward related neuronal activity in relation to GABA signaling. Based on these and our current results that indicate that the neuronal action of insulin is regulated by GABA signaling, it is possible that the anorexigenic effects of insulin on hypothalamic and striatal reward-related signaling might be based on an inhibitory effect of insulin which is regulated by interneuronal GABA-ergic inputs. This theory needs further attention for a more complete understanding of insulin’s role in reward within the striatum.
Long term memory and Alzheimer’s desease
It was shown that brain insulin is able to improve long term memory consolidation and retrieval by acting in the hippocampus (Babri et al. 2007; Moosavi et al. 2007). It is also suggested that intracellular pH changes can regulate hippocampal excitability by NHE- and NCBE-driven H+-extrusion (Bonnet et al. 2000a; Bonnet et al. 2000b), and hippocampal long-term potentiation (LTP) is supported by tyrosine receptor kinase B-mediated signaling (Gartner et al. 2006). Interestingly insulin seems to affect memory related protein functions such as CPEB (Sarkissian et al. 2004) or β-amyloid induced LTP-impairment (Lee et al. 2009). The conformation structure of the latter protein is also known to be sensitive to pH changes and regulating the development in Alzheimer’s disease (Fraser et al. 1991; Petkova et al. 2004). Based upon these data, we suggest that the mechanisms of brain insulin that regulate long term memory and neurodegenerative disorders might be influenced by pH.
4.6. Conclusions
The present study reinforces previous data showing that insulin has various direct electrophysiological neuromodulatory effects and also provides the first evidence for such effects in the mammalian brain in vivo. Insulin mostly inhibits neuronal activity; however, it can also decrease GABA inhibitions when co-applied, or less frequently, it can have GABA-independent excitatory effects. The inhibitory effects of insulin appear to be dependent on preceding GABA release that reveals a new temporal connection between insulin and GABA signaling.
Despite that insulin transport into the brain has been demonstrated to be affected by various physiological factors such as fasting or hyperglycemia (Banks 2004), there is no direct evidence for corresponding changes in brain insulin levels. Nevertheless, based on the available indirect evidences and our current findings, it is plausible that changes in brain insulin concentrations related either to physiological states (fasting, hyperglycemia, aging) or to pathological conditions (obesity, diabetes, Alzheimer’s disease) may directly influence the firing activity of neurons in brain regions where insulin receptors are available.
Acknowledgements
The authors thank Dr Kristy J. Bruno, Jennifer E. Nyland and Carolyn E. Pritchett for their useful comments on an earlier version of this manuscript.
Sources of support:
This research was supported by National Institute of Diabetes & Digestive & Kidney Diseases Grant DK080899, The Penn State Institute for Diabetes and Obesity, and The Pennsylvania Tobacco Settlement Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali A, Ahmad FJ, Pillai KK, Vohora D. Evidence of the antiepileptic potential of amiloride with neuropharmacological benefits in rodent models of epilepsy and behavior. Epilepsy Behav. 2004;5:322–328. doi: 10.1016/j.yebeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Babri S, Badie HG, Khamenei S, Seyedlar MO. Intrahippocampal insulin improves memory in a passive-avoidance task in male wistar rats. Brain and cognition. 2007;64:86–91. doi: 10.1016/j.bandc.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Baltensperger K, Lewis RE, Woon CW, Vissavajjhala P, Ross AH, Czech MP. Catalysis of serine and tyrosine autophosphorylation by the human insulin receptor. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:7885–7889. doi: 10.1073/pnas.89.17.7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA. The source of cerebral insulin. European journal of pharmacology. 2004;490:5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Bartke A. Aging: all in the head? Cell Metabolism. 2007;6:153–154. doi: 10.1016/j.cmet.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Bonnet U, Bingmann D, Wiemann M. Intracellular pH modulates spontaneous and epileptiform bioelectric activity of hippocampal CA3-neurones. Eur Neuropsychopharmacol. 2000a;10:97–103. doi: 10.1016/s0924-977x(99)00063-2. [DOI] [PubMed] [Google Scholar]
- Bonnet U, Leniger T, Wiemann M. Alteration of intracellular pH and activity of CA3-pyramidal cells in guinea pig hippocampal slices by inhibition of transmembrane acid extrusion. Brain research. 2000b;872:116–124. doi: 10.1016/s0006-8993(00)02350-7. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. The Journal of physiology. 1996;497(Pt 3):753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calissano P, Ciotti MT, Battistini L, Zona C, Angelini A, Merlo D, Mercanti D. Recombinant human insulin-like growth factor I exerts a trophic action and confers glutamate sensitivity on glutamate-resistant cerebellar granule cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8752–8756. doi: 10.1073/pnas.90.18.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Torres-Aleman I. Long-term depression of glutamate-induced gamma-aminobutyric acid release in cerebellum by insulin-like growth factor I. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:7386–7390. doi: 10.1073/pnas.90.15.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, Asthana S, Newcomer JW, Wilkinson CW, Matos IT, Baker LD, Cherrier M, Lofgreen C, Latendresse S, Petrova A, Plymate S, Raskind M, Grimwood K, Veith RC. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Archives of general psychiatry. 1999;56:1135–1140. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- Diaz LE, Chuan YC, Lewitt M, Fernandez-Perez L, Carrasco-Rodriguez S, Sanchez-Gomez M, Flores-Morales A. IGF-II regulates metastatic properties of choriocarcinoma cells through the activation of the insulin receptor. Mol Hum Reprod. 2007;13:567–576. doi: 10.1093/molehr/gam039. [DOI] [PubMed] [Google Scholar]
- Fernandes ML, Saad MJ, Velloso LA. Insulin induces tyrosine phosphorylation of the insulin receptor and SHC, and SHC/GRB2 association in cerebellum but not in forebrain cortex of rats. Brain research. 1999;826:74–82. doi: 10.1016/s0006-8993(99)01118-x. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP. Endocrine regulation of neurotransmitter transporters. Epilepsy Res. 1999;37:203–210. doi: 10.1016/s0920-1211(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, MacDonald Naleid A, Sipols AJ. Modulation of food reward by adiposity signals. Physiology & behavior. 2007;91:473–478. doi: 10.1016/j.physbeh.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Patterson TA, Zavosh A, Brot MD, Roitman M, Szot P. Neurotransmitter transporters: target for endocrine regulation. Horm Metab Res. 1999;31:335–339. doi: 10.1055/s-2007-978749. [DOI] [PubMed] [Google Scholar]
- Fraser PE, Nguyen JT, Surewicz WK, Kirschner DA. pH-dependent structural transitions of Alzheimer amyloid peptides. Biophysical journal. 1991;60:1190–1201. doi: 10.1016/S0006-3495(91)82154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon KB, Fyffe RE, Adragna NC, Lauf PK. Characterization of an extracellular epitope antibody to the neuronal K-Cl cotransporter, KCC2. Clinical and experimental pharmacology & physiology. 2007;34:566–573. doi: 10.1111/j.1440-1681.2007.04621.x. [DOI] [PubMed] [Google Scholar]
- Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annual review of neuroscience. 2007;30:367–398. doi: 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- Gartner A, Polnau DG, Staiger V, Sciarretta C, Minichiello L, Thoenen H, Bonhoeffer T, Korte M. Hippocampal long-term potentiation is supported by presynaptic and postsynaptic tyrosine receptor kinase B-mediated phospholipase Cgamma signaling. J Neurosci. 2006;26:3496–3504. doi: 10.1523/JNEUROSCI.3792-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier JF, Chen K, Uecker A, Bandy D, Frost J, Salbe AD, Pratley RE, Lawson M, Ravussin E, Reiman EM, Tataranni PA. Regions of the human brain affected during a liquid-meal taste perception in the fasting state: a positron emission tomography study. The American journal of clinical nutrition. 1999;70:806–810. doi: 10.1093/ajcn/70.5.806. [DOI] [PubMed] [Google Scholar]
- Giffard RG, Lee YS, Ouyang YB, Murphy SL, Monyer H. Two variants of the rat brain sodium-driven chloride bicarbonate exchanger (NCBE): developmental expression and addition of a PDZ motif. The European journal of neuroscience. 2003;18:2935–2945. doi: 10.1046/j.1460-9568.2003.03053.x. [DOI] [PubMed] [Google Scholar]
- Goto Y, Otani S, Grace AA. The Yin and Yang of dopamine release: a new perspective. Neuropharmacology. 2007;53:583–587. doi: 10.1016/j.neuropharm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamori T, Ishiko N. Taste responses of Purkinje cells in the frog cerebellum. Neuroscience letters. 1987;76:285–290. doi: 10.1016/0304-3940(87)90416-2. [DOI] [PubMed] [Google Scholar]
- Herr NR, Kile BM, Carelli RM, Wightman RM. Electroosmotic flow and its contribution to iontophoretic delivery. Anal Chem. 2008;80:8635–8641. doi: 10.1021/ac801547a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. Implications on cerebellar function from information coding. Cerebellum (London, England) 2008 doi: 10.1007/s12311-008-0032-1. [DOI] [PubMed] [Google Scholar]
- Jorntell H, Ekerot CF. Properties of somatosensory synaptic integration in cerebellar granule cells in vivo. J Neurosci. 2006;26:11786–11797. doi: 10.1523/JNEUROSCI.2939-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakazu Y, Akaike N, Komiyama S, Nabekura J. Regulation of intracellular chloride by cotransporters in developing lateral superior olive neurons. J Neurosci. 1999;19:2843–2851. doi: 10.1523/JNEUROSCI.19-08-02843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsch W, Hormuzdi S, Straube E, Lewen A, Monyer H, Misgeld U. Insulin-like growth factor 1 and a cytosolic tyrosine kinase activate chloride outward transport during maturation of hippocampal neurons. J Neurosci. 2001;21:8339–8347. doi: 10.1523/JNEUROSCI.21-21-08339.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Kuo YM, Huang CC, Hsu KS. Insulin rescues amyloid beta-induced impairment of hippocampal long-term potentiation. Neurobiol Aging. 2009;30:377–387. doi: 10.1016/j.neurobiolaging.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Liu L, Brown JC, 3rd, Webster WW, Morrisett RA, Monaghan DT. Insulin potentiates N-methyl-D-aspartate receptor activity in Xenopus oocytes and rat hippocampus. Neuroscience letters. 1995;192:5–8. doi: 10.1016/0304-3940(95)11593-l. [DOI] [PubMed] [Google Scholar]
- Luckermann M, Trapp S, Ballanyi K. GABA-and glycine-mediated fall of intracellular pH in rat medullary neurons in situ. Journal of neurophysiology. 1997;77:1844–1852. doi: 10.1152/jn.1997.77.4.1844. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Griffith LS, Clouse RE, Cryer PE. Psychiatric illness in diabetes mellitus. Relationship to symptoms and glucose control. The Journal of nervous and mental disease. 1986;174:736–742. doi: 10.1097/00005053-198612000-00005. [DOI] [PubMed] [Google Scholar]
- Ma XH, Zhong P, Gu Z, Feng J, Yan Z. Muscarinic potentiation of GABA(A) receptor currents is gated by insulin signaling in the prefrontal cortex. J Neurosci. 2003;23:1159–1168. doi: 10.1523/JNEUROSCI.23-04-01159.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez PA, Longo N, Jimenez BD, Cadilla CL. Insulin-induced gene 33 mRNA expression in Chinese hamster ovary cells is insulin receptor dependent. J Cell Biochem. 2000;77:432–444. [PubMed] [Google Scholar]
- Mielke JG, Wang YT. Insulin exerts neuroprotection by counteracting the decrease in cell-surface GABA receptors following oxygen-glucose deprivation in cultured cortical neurons. Journal of neurochemistry. 2005;92:103–113. doi: 10.1111/j.1471-4159.2004.02841.x. [DOI] [PubMed] [Google Scholar]
- Moosavi M, Naghdi N, Choopani S. Intra CA1 insulin microinjection improves memory consolidation and retrieval. Peptides. 2007;28:1029–1034. doi: 10.1016/j.peptides.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Needleman LA, McAllister AK. Seeing the light: insulin receptors and the CNS. Neuron. 2008;58:653–655. doi: 10.1016/j.neuron.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Palovcik RA, Phillips MI. A constant perfusion slice chamber for stable recording during the addition of drugs. Journal of neuroscience methods. 1986;17:129–139. doi: 10.1016/0165-0270(86)90066-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G. The Rat Nervous System. North Ride: Academic Press Australia; 1985. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Petkova AT, Buntkowsky G, Dyda F, Leapman RD, Yau WM, Tycko R. Solid state NMR reveals a pH-dependent antiparallel beta-sheet registry in fibrils formed by a beta-amyloid peptide. Journal of molecular biology. 2004;335:247–260. doi: 10.1016/j.jmb.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Plum L, Schubert M, Bruning JC. The role of insulin receptor signaling in the brain. Trends in endocrinology and metabolism: TEM. 2005;16:59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Rhoads DE, DiRocco RJ, Osburn LD, Peterson NA, Raghupathy E. Stimulation of synaptosomal uptake of neurotransmitter amino acids by insulin: possible role of insulin as a neuromodulator. Biochemical and biophysical research communications. 1984;119:1198–1204. doi: 10.1016/0006-291x(84)90903-3. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nature neuroscience. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose PP, Carroll JM, Carroll PA, DeFilippis VR, Lagunoff M, Moses AV, Roberts CT, Jr, Fruh K. The insulin receptor is essential for virus-induced tumorigenesis of Kaposi's sarcoma. Oncogene. 2007;26:1995–2005. doi: 10.1038/sj.onc.1210006. [DOI] [PubMed] [Google Scholar]
- Sarkissian M, Mendez R, Richter JD. Progesterone and insulin stimulation of CPEB-dependent polyadenylation is regulated by Aurora A and glycogen synthase kinase-3. Genes & development. 2004;18:48–61. doi: 10.1101/gad.1136004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato I, Arima H, Ozaki N, Watanabe M, Goto M, Hayashi M, Banno R, Nagasaki H, Oiso Y. Insulin inhibits neuropeptide Y gene expression in the arcuate nucleus through GABAergic systems. J Neurosci. 2005;25:8657–8664. doi: 10.1523/JNEUROSCI.2739-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D, Gerhardt E, Bock J, Muller MM, Wolburg H, Lang F, Schulz JB. Intracellular acidification by inhibition of the Na+/H+-exchanger leads to caspase-independent death of cerebellar granule neurons resembling paraptosis. Cell death and differentiation. 2004;11:760–770. doi: 10.1038/sj.cdd.4401377. [DOI] [PubMed] [Google Scholar]
- Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: review and clinical implications. Neuroscience and biobehavioral reviews. 2000;24:855–872. doi: 10.1016/s0149-7634(00)00040-3. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Shen MR, Chou CY, Hsu KF, Liu HS, Dunham PB, Holtzman EJ, Ellory JC. The KCl cotransporter isoform KCC3 can play an important role in cell growth regulation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14714–14719. doi: 10.1073/pnas.251388798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng G, Chang GQ, Lin JY, Yu ZX, Fang ZH, Rong J, Lipton SA, Li SH, Tong G, Leibowitz SF, Li XJ. Hypothalamic huntingtin-associated protein 1 as a mediator of feeding behavior. Nature medicine. 2006;12:526–533. doi: 10.1038/nm1382. [DOI] [PubMed] [Google Scholar]
- Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nature neuroscience. 2000;3:757–758. doi: 10.1038/77660. [DOI] [PubMed] [Google Scholar]
- Stella SL, Jr, Bryson EJ, Thoreson WB. Insulin inhibits voltage-dependent calcium influx into rod photoreceptors. Neuroreport. 2001;12:947–951. doi: 10.1097/00001756-200104170-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci SJ, Koehler-Stec EM, Li K, Reynolds TH, Clark R, Simpson IA. GLUT4 glucose transporter expression in rodent brain: effect of diabetes. Brain research. 1998;797:1–11. doi: 10.1016/s0006-8993(98)00103-6. [DOI] [PubMed] [Google Scholar]
- Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature. 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Yano H, Nagashima K, Seino S. The Na+-driven Cl-/HCO3- exchanger. Cloning, tissue distribution, and functional characterization. The Journal of biological chemistry. 2000;275:35486–35490. doi: 10.1074/jbc.C000456200. [DOI] [PubMed] [Google Scholar]
- Whitmer RA. Type 2 diabetes and risk of cognitive impairment and dementia. Current neurology and neuroscience reports. 2007;7:373–380. doi: 10.1007/s11910-007-0058-7. [DOI] [PubMed] [Google Scholar]
- Williams DB. A novel, rapid, inhibitory effect of insulin on alpha1beta2gamma2s gamma-aminobutyric acid type A receptors. Neuroscience letters. 2008;443:27–31. doi: 10.1016/j.neulet.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, Wendt A, Deng S, Ebina Y, Wheeler MB, Braun M, Wang Q. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 2006;3:47–58. doi: 10.1016/j.cmet.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Yu X, Rajala RV, McGinnis JF, Li F, Anderson RE, Yan X, Li S, Elias RV, Knapp RR, Zhou X, Cao W. Involvement of insulin/phosphoinositide 3-kinase/Akt signal pathway in 17 beta-estradiol-mediated neuroprotection. The Journal of biological chemistry. 2004;279:13086–13094. doi: 10.1074/jbc.M313283200. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Molecular and cellular endocrinology. 2001;177:125–134. doi: 10.1016/s0303-7207(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Zhu JN, Wang JJ. The cerebellum in feeding control: possible function and mechanism. Cell Mol Neurobiol. 2008;28:469–478. doi: 10.1007/s10571-007-9236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]