Abstract
HIV fusion and entry into CD4 T cells are mediated by two receptors, CD4 and CXCR4. This receptor requirement can be abrogated by pseudotyping the virion with the vesicular stomatitis virus glycoprotein (VSV-G) that mediates viral entry through endocytosis. The VSV-G-pseudotyped HIV is highly infectious for transformed cells, although the virus circumvents the viral receptors and the actin cortex. In HIV infection, gp120 binding to the receptors also transduces signals. Recently, we demonstrated a unique requirement for CXCR4 signaling in HIV latent infection of blood resting CD4 T cells. Thus, we performed parallel studies in which the VSV-G-pseudotyped HIV was used to infect both transformed and resting T cells in the absence of coreceptor signaling. Our results indicate that in transformed T cells, the VSV-G-pseudotyping results in lower viral DNA synthesis but a higher rate of nuclear migration. However, in resting CD4 T cells, only the HIV envelope-mediated entry, but not the VSV-G-mediated endocytosis, can lead to viral DNA synthesis and nuclear migration. The viral particles entering through the endocytotic pathway were destroyed within 1–2 days. These results indicate that the VSV-G-mediated endocytotic pathway, although active in transformed cells, is defective and is not a pathway that can establish HIV latent infection of primary resting T cells. Our results highlight the importance of the genuine HIV envelope and its signaling capacity in the latent infection of blood resting T cells. These results also call for caution on the endocytotic entry model of HIV-1, and on data interpretation where the VSV-G-pseudotyped HIV was used for identifying HIV restriction factors in resting T cells.
Author Summary
While receptor-mediated viral endocytosis or fusion with the cell membrane can be achieved through multiple surface molecules, the repetitious selection of two chemokine receptors, CCR5 or CXCR4, as the main HIV entry coreceptor implies an urgent viral need to exploit the chemotactic process in the immune system. Cytoskeletal rearrangement and cell migration are the primary consequences of chemotactic signaling. Nevertheless, previously published data demonstrated that depriving the virus of its signaling ability conferred higher infectivity through VSV-G-mediated endocytotic entry in transformed cells. We revisited the issue of chemokine coreceptor signaling and the role of cortical actin in HIV-1 latent infection of resting CD4 T cells, in which the virus can establish latency with a potential for productive replication upon T cell activation. Our results confirmed that only the genuine HIV-1 envelope protein, but not VSV-G, is capable of mediating latent infection of resting CD4 T cells. These findings highlight the importance of the HIV envelope and its signaling capacity in HIV infection of its natural target cells.
Introduction
Binding of the HIV envelope to its receptors, CD4 and the chemokine coreceptor, CCR5 or CXCR4, triggers sequential fusion and entry events [1],[2],[3],[4]. Fusion is believed to occur directly at the plasma membrane [5],[6],[7],[8],[9], but fusion in endosomes has also been proposed recently [10]. It has been known that HIV can enter cells through endocytosis, but the virion particles entering through this pathway appear to be trapped and subsequently destroyed [11],[12]. This endosomal degradation can be rescued either by blocking the acidification of the endosomal compartments [11],[12] or by pseudotyping the HIV virion with the vesicular stomatitis virus glycoprotein (VSV-G) [13],[14]. The VSV-G-pseudotyped HIV escapes from endosomes and is highly infectious, giving the virus 20- to 130-fold higher infectivity [15],[16]. The ease of producing high-titer virus through VSV-G pseudotyping has made the method very popular for manufacturing viral stock used for gene delivery, drug screening, and the identification of cellular genes and factors involved in HIV replication [13],[17],[18],[19].
Nevertheless, the VSV-G-pseudotyped viruses are not identical to the genuine HIV particles. For example, the HIV Nef protein, a critical factor involved in viral pathogenesis [20], no longer plays an important role in the infection by the VSV-G-pseudotyped virus [15]. Nef has been known to enhance viral infectivity by a factor of 4 to 40 [21],[22]. This positive effect of Nef on viral infectivity appears to be at an early step post entry, such as uncoating or reverse transcription [23],[24],[25]. Nef itself does not directly affect reverse transcription, since Nef-defective virions display normal levels of endogenous reverse transcriptase activity [25]. It is likely that this early activity of Nef is connected to cortical actin in some way. For example, when cells were treated with actin inhibitors, the effect of Nef on viral replication was lost [26]. This is also consistent with the fact that the VSV-G-pseudotyped virus circumvents the cortical actin; thus, the impact of Nef on viral infectivity is forfeited most likely because of the lack of interaction with the actin cortex [15].
The VSV-G-pseudotyped HIV also does not engage CD4 and CCR5 or CXCR4, and is deprived of the ability to transduce signals through these receptors [27],[28],[29]. These intracellular signaling cascades, particularly those transduced from the chemokine coreceptors, have been suggested to be unnecessary for viral fusion, entry, or the subsequent steps of viral replication in transformed cell lines [30],[31],[32],[33],[34],[35],[36],[37],[38],[39]. However, recently, several reports have suggested a requirement for CD4 receptor signaling to mediate viral fusion and entry [40],[41],[42],[43]. We have also observed an absolute requirement for CXCR4 signaling in HIV-1 latent infection of resting CD4 T cells [44] and demonstrated that HIV-1 relies on viral envelope and the Gαi-dependent signaling from CXCR4 to activate a cellular actin-depolymerizing factor, cofilin, to increase the cortical actin dynamics for viral intracellular migration [44]. Given that the VSV-G-pseudotyped HIV infects cells in the absence of receptor signaling, we performed parallel studies in which the VSV-G-pseudotyped HIV was used to infect both transformed and resting CD4 T cells to understand possible alternative pathways that the VSV-G-pseudotyped HIV-1 may employ to establish latent infection of resting CD4 T cells. Surprisingly, the VSV-G-pseudotyped HIV-1 exhibited a highly diminished ability to initiate viral DNA synthesis and nuclear migration in resting T cells, which is in striking contrast to the high efficiency of VSV-G to mediate HIV infection of transformed cells. The viral particles entering through the endocytotic pathway were destroyed within 1–2 days in resting T cells. These results indicate that the VSV-G-mediated endocytotic pathway, although active in transformed T cells, is defective and not a pathway that can establish HIV latent infection of primary CD4 T cells. These results highlight the importance of the genuine HIV envelope and its signaling capacity in the latent infection of primary resting T cells.
Results
Characterization of the VSV-G-pseudotyped HIV replication in transformed T cells
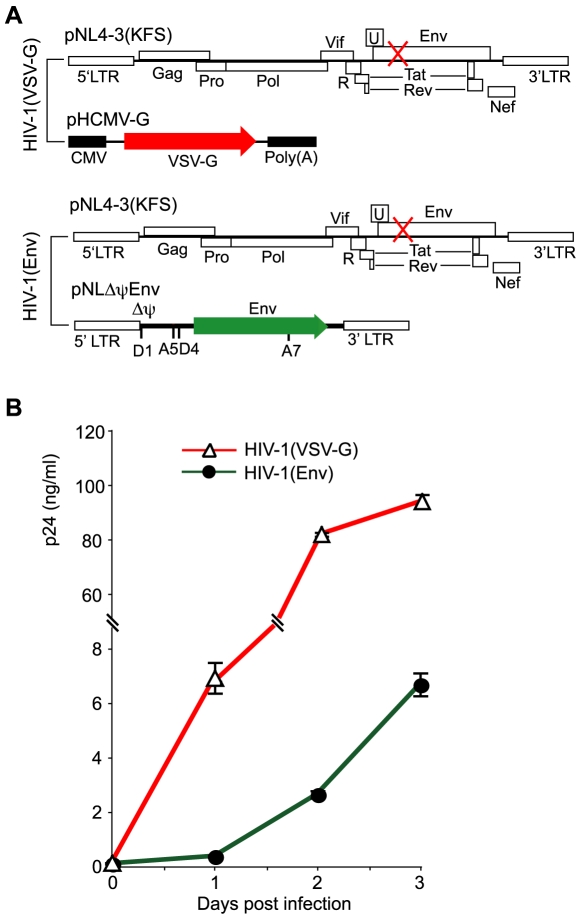
We compared the infectivity of HIV-1 carrying either the HIV envelope (Wt) or the VSV glycoprotein (VSV-G). Both viruses were produced in parallel using the same cell culture and transfection conditions (Figure 1A). Following harvesting of viral particles, an equal p24 level of both viruses was used to infect a transformed T cell line, CEM-SS. Viral replication was monitored by p24 release. As shown in Figure 1B, we observed faster and stronger replication of the VSV-G- pseudotyped virus, which reached a level approximately 30 fold higher (at 48 hours) than the wild-type HIV-1. Our result was consistent with previous reports showing that the VSV-G-pseudotyped virus was 20 to 130 times more infectious than wild-type HIV-1 [15],[16]. It is likely that without the limitation of HIV receptors, much more productive viral entry may occur through the VSV-G-mediated endocytosis, resulting in a much higher level of viral replication. In addition, the faster replication kinetics of the VSV-G-pseudotyped virus is likely a result of faster entry and nuclear migration.
Figure 1. Replication of HIV-1 carrying either VSV-G or the HIV envelope in a transformed T cell, CEM-SS.
(A) DNA constructs used to generate VSV-G-pseudotyped and HIV-1 Env-typed HIV-1. Viruses were generated by cotransfection and harvested at 48 hours. (B) An equal p24 level (260 ng) of HIV-1(VSV-G) or HIV-1(Env) was used to infect 1×106 cells. Following infection for 2 hours, cell-free viruses were washed away and cells were continuously incubated for 3 days. Viral replication was monitored by p24 release.
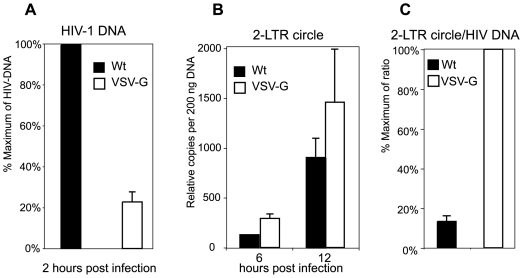
We also compared viral early processes after entry by following viral DNA synthesis and nuclear migration. We infected cells using an equal TCID50 dosage instead of an equal p24. Although more Wt particles were used (based on p24), infection with an equal TCID50 ensured that the productive viral processes such as viral DNA synthesis and nuclear migration would occur at comparable levels within the viral population in each case. The TCID50 of both viruses was measured on a Rev-dependent indicator cell, Rev-CEM, as previously described [45]. As shown in Figure 2, at 2 hours post infection, viral DNA synthesis was measured, and the VSV-G-pseudotyped HIV synthesized only approximately 20% of viral DNA in comparison with the wild-type virus (Figure 2A), probably either because fewer of the VSV-G-pseudotyped particles enter the cells or because these particles are less efficient at mediating viral DNA synthesis. We also followed viral nuclear migration at later time points using viral 2-LTR circles as a surrogate. As shown in Figure 2B, the VSV-G-pseudotyped virus produced slightly more 2-LTR circles than the wild-type HIV-1, and the relative ratio of 2-LTR circle to total viral DNA was approximately 7 times higher in the VSV-G-mediated infection (Figure 2C). These data suggest that in transformed T cells, the VSV-G-mediated endocytotic entry is much more efficient in delivering viral DNA into the nucleus. Even with a lower amount of viral DNA synthesized initially, a higher percentage of these DNA molecules entered the nucleus. On the other hand, in the wild-type infection, even with more viral DNA synthesis, a lower percentage of viral DNA molecules can enter the nucleus. These results are consistent with a model [46] in which the cortical actin plays an important role in viral reverse transcription [47], but the actin cortex also serves as a natural barrier for viral intracellular migration [44].
Figure 2. Comparison of viral DNA synthesis in CEM-SS cells infected with either HIV-1(VSV-G) or HIV-1NL4-3.
Cells were infected with an equal TCID50 dose of HIV-1(VSV-G) or HIV-1NL4-3 (Wt) (1.27×105 TCID50/Rev-CEM per million cells). Following infection for 2 hours, cell-free viruses were washed away. Total cellular DNA was extracted from cells, and then amplified by real-time PCR to measure the synthesis of full-length HIV-1 DNA (2 hours post infection) (A) and 2-LTR circles at later time points (6 and 12 hours post infection) (B). The relative ratios of 2-LTR circles at 12 hours and HIV-1 DNA at 2 hours were plotted (C).
Inability of the VSV-G-pseudotyped HIV-1 to establish latent infection of resting CD4 T cells
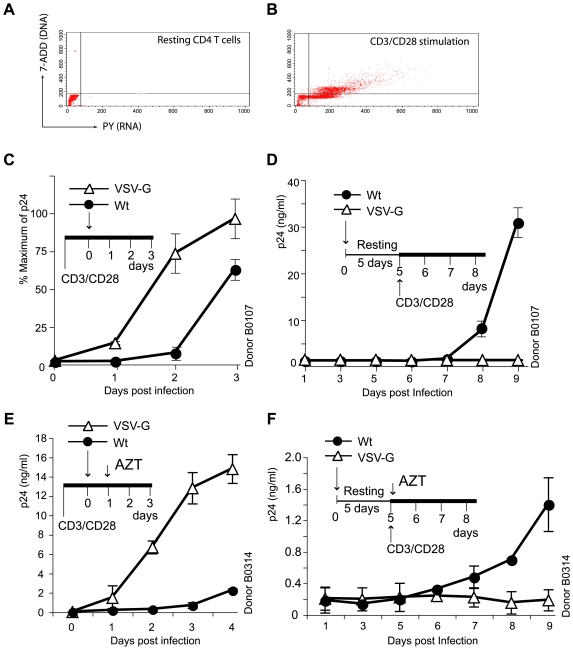
In contrast to the VSV-G-mediated endocytotic entry, the HIV envelope-mediated fusion and entry requires specific interaction with CD4 and the chemokine coreceptor, CCR5 or CXCR4. These receptors not only mediate fusion but also transduce signals upon gp120 binding [27],[48],[49]. In particular, signals transduced from the chemokine coreceptor CXCR4 have recently been shown to be essential for HIV-1 latent infection of resting CD4 T cells [44]. Thus, we examined the ability of VSV-G-pseudotyped HIV-1 to establish latent infection of resting CD4 T cells in the absence of HIV coreceptor signaling. Resting CD4 T cells were purified from the peripheral blood of healthy donors by negative depletion (Figure 3A). Cells were rested overnight, and then infected with an equal p24 level of the VSV-G-pseudotyped HIV-1 or the wild-type HIV-1. Following infection, cells were washed and incubated for 5 days in the absence of T cell activation. During this incubation, productive viral replication does not occur. However, viral replication remains inducible upon T cell activation [44]. As a control, cells were also pre-activated for 1 day with antibody stimulation of the CD3/CD28 receptors (Figure 3B) and then identically infected. As shown in Figure 3C, in CD3/CD28 pre-activated T cells, productive viral replication occurred, and the VSV-G-pseudotyped viral replication was approximately 10-fold greater (48 h.p.i) than that of the wild-type virus. This is similar to the VSV-G-pseudotyped viral replication in transformed T cells (Figure 1). However, in latently infected resting CD4 T cells, when cells were activated at day 5 post infection, only the wild-type virus but not the VSV-G-pseudotyped HIV-1 was induced to replicate (Figure 3D). This was strikingly different from the 10 to 30-fold higher replication capacity of the VSV-G- pseudotyped virus in pre-activated and transformed T cells (Figure 1 and Figure 3C). These results were repeated using CD4 T cells from another donor with AZT added to limit viral replication to a single cycle (Figure 3E and 3F). Reproducibly, only the wild-type virus but not the VSV-G-pseudotyped virus was able to replicate following activation of resting T cells at day 5 (Figure 3F), even though the VSV-G-pseudotyped virus replicated to a 30-fold higher level (48h.p.i) in pre-activated T cells (Figure 3E).
Figure 3. The HIV envelope but not VSV-G is capable of mediating latent infection of resting CD4 T cells.
Resting CD4 T cells were purified from the peripheral blood by negative depletion. Cells were unstimulated (A) or activated with magnetic beads conjugated with antibodies against the human CD3 and CD28 receptors (two beads per cell) for 1 day (B), and then analyzed for cell cycle progression using 7-AAD, PY staining to confirm sufficient T cell activation following stimulation. (C) In CD3/CD28 pre-stimulated CD4 T cells, a higher than Wt level of viral replication was observed in cells infected with HIV-1(VSV-G). One million cells were infected with 25 ng (p24) of HIV-1NL4-3 (wt) or the VSV-G pseudotyped virus (VSV-G). (D) In resting CD4 T cells that were not pre-stimulated, only the Wt but not the VSV-G pseudotyped HIV-1 replicated following T cell activation at day 5. Cells were infected with 25 ng (p24) of both viruses, incubated for 5 days, and then activated with anti-CD3/CD28 beads. (E) and (F) were a repeat of (C) and (D) in another donor, with AZT (50 µM) added at day 1 and day 5 post infection, respectively, to limit viral replication to a single cycle.
Inability of the VSV-G-pseudotyped HIV-1 to support viral DNA synthesis and nuclear migration in resting CD4 T cells
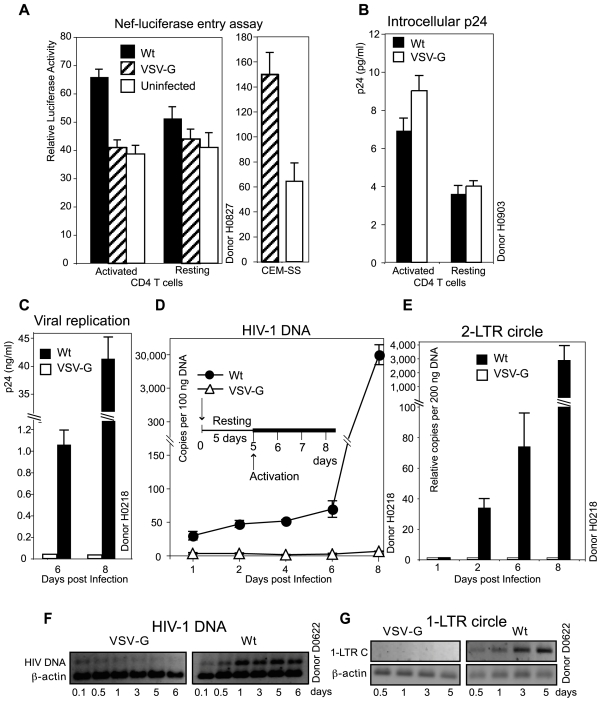
We followed the steps for viral infection of resting T cells. Using a sensitive Nef-luciferase-based entry assay [50], we detected Wt viral entry into both resting and activated T cells (Figure 4A), although the entry into resting T cells was significantly lower. However, we could not detect viral entry into both resting and activated T cells in the VSV-G-pseudotyped virus infection, although we detected the entry of the VSV-G-pseudotyped virus into CEM-SS cells using the identical infection condition (Figure 4A). Since the VSV-G-pseudotyped HIV-1 can replicate in activated T cells (Figure 4C and 4E), these results suggested that the Nef-luciferase-based entry assay may not have the sensitivity to measure the VSV-G-mediated entry in primary T cells, either resting or activated. Thus, we used an alternative method to detect viral entry by measuring intracellular p24 following infection. Cells were infected for 2 hours, trypsinized, washed, and then lysed for intracellular p24. As shown in Figure 4B, we observed a comparable level of intracellular p24 in resting T cells infected with the wild-type or the VSV-G-pseudotyped HIV-1. We also observed a higher level of intracellular p24 in HIV-1(VSV-G)-infected active T cells (Figure 4B). These results suggested that entry of virion particles was similar in resting T cells infected with the wild-type HIV-1 or HIV-1(VSV-G).
Figure 4. Measurement of viral entry and DNA synthesis following HIV-1(VSV-G) infection of resting CD4 T cells.
(A) Resting or pre-activated (CD3/CD28 beads, two beads per cell) CD4 T cells (1×106) were infected with 200 ng of Nef-luciferase-tagged HIV-1NL4-3 (Wt) or HIV-1(VSV-G) for 2 hours. Infected cells were washed three times and then used to measure luciferase activity in live cells. As a control, CEM-SS cells were identically infected with the Nef-luciferase-tagged HIV-1(VSV-G). Uninfected cells were identically treated and measured for luciferase activities. (B) Resting or pre-activated (overnight PHA plus IL-2 treatment) CD4 T cells (1×106) were infected with 200 ng of HIV-1NL4-3 (Wt) or HIV-1(VSV-G) in 0.5 ml for 2 hours. Following infection, cells were treated with TrypLE (Invitrogen) for 2 minutes at 37°C and then washed an additional three times with medium. Cells were pelleted and subsequently lysed for p24 ELISA. (C to E) Resting CD4 T cells were infected with an equal TCID50 dose of HIV-1NL4-3 (Wt) or HIV-1(VSV-G) (2.53×105 TCID50/Rev-CEM per million cells). Following infection for 2 hours, cell-free viruses were washed away. Cells were cultured for 5 days and then activated with anti-CD3/CD28 beads. (C) A measurement of p24 release confirmed that only the Wt virus but not HIV-1(VSV-G) replicated following CD3/CD28 stimulation. (D, E) Total cellular DNA from infected cells was extracted at different time points, and then amplified with real-time PCR for HIV late DNA (D) or 2-LTR-circles (E). (F, G) is a repeat of (D, E) on another donor using an equal p24 level of Wt and HIV-1(VSV-G) to infect resting T cells. Total cellular DNA was extracted from infected cells at different time points and PCR-amplified for HIV late DNA (F) or 1-LTR circles (G), along with the β-actin pseudogene as a control.
We then followed the course of viral DNA synthesis and nuclear migration in resting CD4 T cells after infection. Unstimulated resting CD4 T cells from another donor were infected with an equal TCID50 dose of the VSV-G-pseudotyped HIV-1 or the wild-type virus. After washing away free viruses at 2 hours post infection, cells were continuously incubated without activation for 5 days, and then activated at day 5 with CD3/CD28 stimulation to initiate viral replication (Figure 4C). When viral DNA synthesis was analyzed, we did not observe viral DNA synthesis above the initial background (Figure 4D) in cells infected with the VSV-G-pseudotyped virus at any time point post infection, whereas in cells infected with the wild-type virus, we observed the typical course of viral DNA synthesis in which viral DNA synthesis proceeds slowly and usually peaks at day 2, recedes at day 3, and then increases again following T cell activation [44]. When viral 2-LTR circles were measured, in cells infected with the VSV-G-pseudotyped virus, we also could not detect 2-LTR circles at any time point, even after CD3/CD28 stimulation at day 5, whereas in cells infected with the wild-type virus, 2-LTR circles were detected and the copy number increased with time (Figure 4E). We repeated these experiments using resting CD4 T cells from another donor. This time, resting cells were infected with an equal p24 level of both viruses. We observed similar results. As shown in Figure 4F, in cells infected with the VSV-G-pseudotyped virus, the initial viral DNA detected (0.1 to 0.5 day in Figure 4F) diminished with time, and no 1-LTR circles can be detected at any time point post infection, whereas in the wild-type infected cells, the syntheses of both viral DNA and 1-LTR circles were obvious (Figure 4F and 4G). Based on these results, we concluded that in resting CD4 T cells, only the HIV envelope-mediated entry but not the VSV-G-mediated endocytosis can lead to viral DNA synthesis and nuclear migration, which are a prerequisite for the establishment of HIV latent infection of resting CD4 T cells [44].
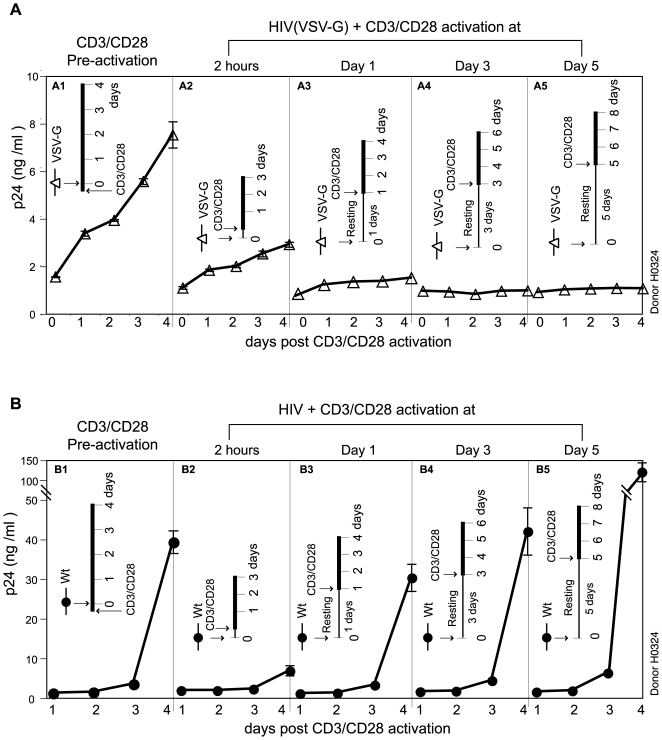
Decay of the VSV-G-pseudotyped HIV-1 in resting CD4 T cells
We also measured the decay kinetics of the VSV-G-pseudotyped HIV-1 in resting CD4 T cells. Unstimulated resting CD4 T cells were infected with an equal p24 level of both viruses. Following infection for 2 hours, cell-free viruses were washed away. Infected cells were then activated immediately or activated at day 1, 3, or 5 post infection. As a control, resting cells were also pre-activated with CD3/CD28 for 1 hour and then identically infected. As shown in Figure 5, in CD3/CD28 pre-activated CD4 T cells, both the VSV-G-pseudotyped HIV-1 and the wild-type virus replicated after infection (Figure 5A1 and 5B1). In resting T cells, when cells were activated immediately after infection and washing (2 hours post infection), viral replication was also initiated from both the VSV-G- pseudotyped HIV-1 and the wild-type virus (Figure 5A2 and 5B2). These results suggest that entry of the VSV-G-pseudotyped virus into resting T cells occurs, and viral replication can be rescued if cells are activated immediately. However, when resting cells were left unactivated, after 1 day, the replication of the VSV-G- pseudotyped virus following activation was greatly diminished (Figure 5A3), and no viral replication could be initiated after 3 days (Figure 5A4 and 5A5). This was in great contrast to the wild-type HIV infection of resting T cells, in which the capacity of HIV to replicate following activation increased with time (Figure 5B1 to 5B5). The highest viral replication occurred after 5 days of incubation. These data, in combination with the results in Figure 4, suggest that in resting CD4 T cells, the VSV-G-mediated endocytotic entry does not lead to a productive pathway, and the viral particles are trapped in cells and subsequently destroyed within 1–2 days. Our data are also consistent with a previous study demonstrating that the VSV-G-pseudotyped HIV-1 has a half-life of only 1–2 days in resting CD4 T cells [51]. The increasing ability of the wild-type HIV-1 to replicate following incubation has also been observed previously [52],[53],[54]. Although HIV does not directly replicate in resting CD4 T cells, the viral envelope-mediated entry establishes an active process that enhances the ability of HIV to replicate following T cell activation. This capacity has been attributed to the synthesis of Nef, which lowers the threshold required for the activation of resting CD4 T cells [52],[53],[54],[55],[56]. Certainly, our data confirmed these previous findings and further indicated that only the genuine HIV envelope protein but not the VSV-G can deliver the virus into the nucleus, where the subsequent action of Nef can occur.
Figure 5. Decay of the VSV-G-pseudotyped HIV-1 in resting CD4 T cells.
(A) Resting CD4 T cells were purified by negative depletion, rested overnight, and then pre-stimulated with anti-CD3/CD28 beads for 1 hour and infected with HIV-1(VSV-G) (552 ng p24 per million cells) (A1). Cells were also infected without pre-stimulation (A2 to A5) and then stimulated with anti-CD3/CD28 beads at 2 hours, day 1, day 3, or day 5 post infection to initiate viral replication. The p24 release was measured following anti-CD3/CD28 stimulation (marked as day “0” on the X-axis of each panel). (B) is a repeat of (A) in the same donor, using HIV-1NL4-3 (Wt) (552 ng p24 per million cells).
Differential inhibition of HIV-1 and the VSV-G-pseudotyped HIV-1 by dynasore
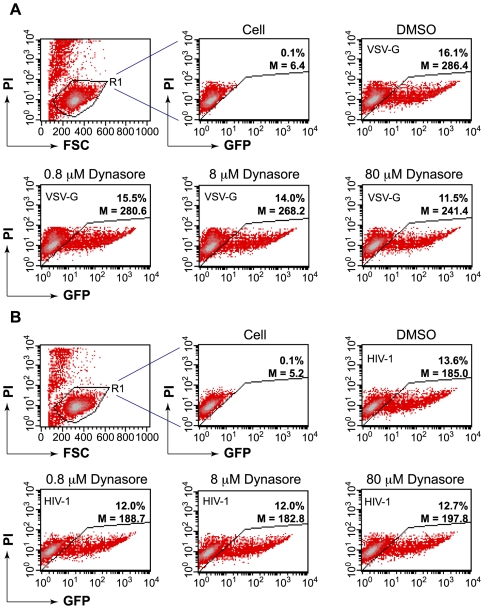
In contrast to resting T cells, in transformed cell lines, the VSV-G-mediated entry is very efficient in mediating HIV infection. This fact has prompted a major argument that HIV may predominately fuse in the endosome rather than at the plasma membrane [10]. Microscopic imaging tracking the behaviors of the majority of MLV particles suggested that the HIV-1 envelope-pseudotyped virus entered cells predominantly through endocytosis [10]. Indeed, dynasore, a dynamin-dependent endosomal scission inhibitor, was shown to inhibit viral replication [10], supporting the model that the endosomal fusion is associated with a productive pathway. Nevertheless, this mode of entry is in conflict with numerous previous observations suggesting that genuine HIV envelope-associated endocytotic entry, although occurring at a significant scale, does not naturally lead to productive infection [5],[6],[7],[9],[11],[12]. For example, inhibition of the endosomal/lysosomal functionality can spare HIV from degradation and enhance viral replication [11],[12], demonstrating that the endosomal viruses are normally directed for degradation. In addition, the rate of CD4 or CCR5 endocytosis does not appear to affect viral entry or replication [6],[8],[9], supporting direct viral fusion at the plasma membrane. Nevertheless, the endocytosis entry as proposed [10] is an attractive alternative pathway. If proven biologically, it would require significant remodeling of the role of the cortical actin in viral entry and early post-entry steps. The involvement of the cortical actin in early endocytosis is largely limited to membrane scission of clathrin-coated pits [57]. This process does not involve direct contact between the cortical actin and the viral particles. If there is any viral contact with actin, it would be in the cytoplasm following endosomal fusion. This interaction may also affect reverse transcription and nuclear migration, but such effects would occur at different levels. The issue of entry is so critical in the understanding of the role of the cortical actin in HIV biology that we felt compelled to revisit some of the key biological evidence - in particular, the inhibition of HIV replication by the dynamin-dependent endosomal fusion inhibitor, dynasore. Dynamins are a group of fundamental proteins involved in multiple cellular processes such as vesicle transport, cytokinesis, organelle division and cell signaling (for a review, see [58]). To minimize possible cytotoxicity from prolonged inhibition of fundamental cellular proteins, we treated cells only briefly with dynasore during viral infection. Viruses that failed to enter were subsequently washed away along with the drug. We also used the Rev-dependent indicator cell, Rev-CEM [45], to measure dynasore inhibition, instead of simply using p24 ELISA, which by itself is not capable of distinguishing between HIV-specific inhibition and general drug cytotoxicity. Additional advantages of using Rev-CEM are its high specificity and the ability to distinguish subpopulations of cells by flow cytometry so that non-specific cytotoxicity can be excluded [59]. As shown in Figure 6A, we first tested dynasore in the inhibition of the VSV-G-pseudotyped HIV-1 replication and observed dosage-dependent inhibition of viral replication. At 80 µM, dynasore moderately decreased the GFP+ population from 16.1% to 11.5%; at 8 µM, dynasore also slightly decreased the GFP+ population; at 0.8 µM, dynasore minimally affected viral infection. However, when dynasore was used on identically treated cells that were infected with HIV-1, we did not observe similar dosage-dependent inhibition. Even at 80 µM, dynasore minimally affected HIV-1 infection (Figure 6B). These results demonstrate a clear distinction between the VSV-G-mediated endocytotic entry and the HIV-1-envelope-mediated entry in mediating productive viral replication.
Figure 6. Different effects of dynasore on the replication of HIV-1(VSV-G) and Wt in a human T cell, Rev-CEM.
(A) Rev-CEM, a Rev-dependent GFP indicator cell, was pretreated for 30 minutes with 80 µM, 8 µM, or 0.8 µM dynasore, respectively, or treated with 0.1% DMSO as a control. Cells were subsequently infected with HIV-1(VSV-G) in the presence of dynasore for 2 hours. Following infection, cells were washed three times with medium, and then cultured in the absence of dynasore. Viral replication was monitored by flow cytometry analysis of HIV-dependent GFP expression at 48 hours (20,000 cells analyzed per sample). Propidum iodide (PI) was added into the cell suspension prior to flow cytometry. Viable cells were gated (R1) based on low PI staining and cell size (FSC). GFP expression within the viable cell population (R1) was measured. Both the GFP percentage (%) and mean intensity (M) were shown. (B) is an identical experiment using HIV-1NL4-3 (Wt). For the 80 µM dynasore treatment, another three-independent infections with each virus were performed. The averages from the three-independent experiments are: 15.05%±0.21 (VSV-G), 10.11%±0.06 (VSV-G plus 80 µM dynasore), p = 0.0003; 2.94%±0.39 (Wt), 2.84%±0.30 (Wt plus 80 µM dynasore), p = 0.38.
Discussion
In this report, we demonstrated a fundamental difference between the HIV-1 envelope and VSV-G in mediating HIV-1 latent infection of primary resting CD4 T cells, namely that only the HIV-1 envelope but not VSV-G is capable of supporting HIV latent infection of resting T cells. The block to the VSV-G-pseudotyped virus in resting T cells was most obvious at post-entry steps such as viral DNA synthesis and nuclear migration. The virion particles trapped in cells were subsequently destroyed within 1–2 days in resting T cells. These results demonstrated the importance of the genuine HIV envelope in mediating latent infection of resting T cells.
Previously, we demonstrated a critical function of the HIV-1 envelope in mediating CXCR4 signaling and promoting the cortical actin dynamics necessary for HIV latent infection of resting T cells [44]. We also proposed a dual function of F-actin in which the actin cortex serves as an anchorage for reverse transcription and as a vehicle for the delivery of the preintegration complex across the cortical actin through actin treadmilling [44],[46]. At least four HIV proteins in the preintegration complex are known to interact with actin; the viral nuclear capsid [60],[61],[62],[63], the large subunit of the reverse transcriptase [64], the integrase [65], and Nef [66]. We have also shown that blocking actin polymerization with Jasplakinolid (120 nM) or Latrunculin A (2.5 µM) inhibits viral DNA synthesis or HIV latent infection. Conversely, triggering actin polymerization through cofilin shRNA knockdown enhances viral DNA synthesis [44]. These previous results and other studies [47] are consistent with the findings in this study, in which the VSV-G-mediated entry that bypasses the cortical actin led to less viral DNA synthesis in transformed cells (Figure 2). The VSV-G-pseudotyping also resulted in a lack of the slow viral DNA synthesis that is normally seen in HIV-1 latent infection of resting T cells (Figure 4D and 4F). Viral nuclear DNA was also completely missing in the VSV-G-mediated entry in resting cells (Figure 4E and 4G). These results suggest that the VSV-G-pseudotyped particles may be delivered to a different cytoplasmic location and enter the nucleus by a different route, one that is normally highly effective in transformed or metabolically active cells but defective in resting T cells.
Our results are consistent with a recent independent study demonstrating that only the CXCR4-tropic HIV-1 envelope but not VSV-G can support lentiviral vectors to deliver genes into resting CD4 T cells [67]. In this study, Agosto and co-authors also found that viral DNA synthesis was greatly diminished in resting CD4 T cells infected with the VSV-G-pseudotyped lentiviral particles. Nevertheless, the limitation on viral infection was specifically attributed to the lack of viral entry and fusion in the VSV-G-mediated infection of resting T cells [67]. Our results suggested that the restriction was likely at an unknown post-entry step such as endosomal fusion, uncoating, or reverse transcription. The discrepancy in conclusions arises from different explanations of the data acquired from entry and fusion assays. Both Agosto and co-authors [67] and we observed an absolute lack of entry signals in HIV-1(VSV-G)-infected resting T cells, using two different entry assays. However, these assays, particularly the BlaM-Vpr-based fusion assay [68] may not be appropriate for the measurement of VSV-G-mediated fusion in resting T cells. It is possible that if the VSV-G-pseudotyped virus is trapped in a compartment, or is going through a degradation process with a half-life of only 1 day [51], the BlaM substrate which takes about 12–18 hours to load may not be able to access or sufficiently react with the enzyme. Given this lack of mechanistic clarity of how these enzyme-tagged particles are delivered through VSV-G in resting T cells, we did not feel confident that conclusions can be drawn based on a fusion assay. Thus, we drew our conclusions relying on multiple results. Firstly, we detected a comparable intracellular p24 level in resting T cells infected with Wt or HIV-1(VSV-G) (Figure 4B). Secondly, the VSV-G-pseudotyped virus can be partially rescued if resting T cells were activated within 1 day of infection, indicating some viral entry into the cells (Figure 5A2). Thirdly, low levels of viral DNA were also detected at early time (2 hours, Figure 4F), indicating again that there were some levels of entry. Given that the VSV-G-pseudotyped viruses are 20 to 130-fold more infectious than the wild-type HIV-1, these initial viral activities should give rise to a measurable level of viral replication, but they did not.
The failure of the VSV-G-mediated entry to establish latent infection of resting T cells is not currently understood. It is possible that the cellular environment in resting T cells may not permit viral fusion in endosomes. Alternatively, successful endosomal fusion may occur, but the quick delivery of viral particles into the cytosol may be detrimental [69], likely due to the possible restrictive environment of resting cells [17],[70] or a lack of cytosolic factors for uncoating [71] DNA synthesis, or nuclear localization. Our attempts to rescue the VSV-G-pseudotyped virus by changing the intracellular PH were not successful (data not shown). Pre-stimulation of the CD4 and CXCR4 receptors with gp120 or antibodies also could not rescue the VSV-G-pseudotyped virus in resting T cells (data not shown), although these pre-stimulations enhanced the wild-type HIV replication several fold following T cell activation [44]. These results are consistent with the fact that the positive benefits of viral receptor signaling are only associated with gp120-mediated entry but not with the VSV-G-mediated endocytosis that circumvents the cortical actin.
The high efficiency of VSV-G to mediate endosomal escape and HIV replication in transformed cells has led to the misconception that the VSV-G-pseudotyped HIV should be as effective as the wild-type HIV for latent infection of resting T cells [72],[73]. Several previous studies have also used the VSV-G-pseudotyped virus to identify restriction factors in resting T cells [17],[74]. Our results suggest that these data need to be interpreted cautiously. Apparently, the VSV-G-mediated entry does not experience the same intracellular environment as HIV does, and cannot lead to the establishment of latent infection in resting T cells. Thus, those previously identified cytoplasmic restriction factors may or may not directly affect HIV infection. Interestingly, a recent imaging study demonstrated a direct dependence of active viral nuclear migration on F-actin, since actin inhibitors diminished the nuclear concentration of the preintegration complex (PIC) (Dr. Thomas Hope, personal communication). This study raises the possibility that PIC may be associated with F-actin up to the nucleus [75],[76]. Given that viruses usually use F-actin for short-distance travel, and the cytoplasmic space between the cortical actin and the nucleus is relatively thin in T cells, it is possible that the cytosolic exposure of PIC in T cells is minimal.
Materials and Methods
Ethics statement
All protocols involving human subjects were reviewed and approved by the GMU IRB. Informed written consents from the human subjects were obtained in this study.
Plasmids and DNA cloning
Plasmid pNL4-3 was kindly provided by Dr. Malcolm Martin [77]. The env mutant, pNL4-3(KFS), was kindly provided by Dr. Eric Freed [78]. pHCMV-G that expresses the vesicular stomatitis virus glycoprotein has been described previously [79]. pNLΔΨEnv was constructed by inserting the env gene of HIV-1NL4-3 into the lentiviral vector pNL-RRE-SA [80]. The packaging signal was further deleted by cutting with KasI plus BssHII and re-ligating.
Viruses and cells
HIV-1NL4-3 was generated by transfection of plasmid pNL4-3 into HEK293T cells using lipofectamine 2000 (Invitrogen) as described previously [80]. The VSV-G-pseudotyped virus, HIV-1(VSV-G), was produced by cotransfection of HEK393T cells (3×106) with 10 µg of pHCMV-G and 10 µg of plasmid pNL4-3(KFS). The HIV-1 envelope-typed virus, HIV-1(Env), was produced by cotransfection of HEK293T cells with 10 µg of pNLΔΨEnv and 10 µg of pNL4-3(KFS). Viral supernatant was harvested at 48 hours post cotransfection, centrifuged for 15 minutes at 500×g to remove cellular debris, filtered through a 0.45 µm filter, treated with Benzonase (Novagen) (250 U/ml) at 37°C for 15 minutes, and then stored at -80°C. Levels of p24 in viral supernatant were measured using the Perkin Elmer Alliance p24 antigen ELISA Kit (Perkin Elmer). Plates were kinetically read using an ELx808 automatic microplate reader (Bio-Tek Instruments) at 630 nm. Viral titer (TCID50) was determined on the Rev-dependent GFP indicator cell, Rev-CEM [45],[81]. CEM-SS cells from Dr. Peter L. Nara [82] were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. All cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen), penicillin (50 U/ml), and streptomycin (50 mg/ml).
Isolation, culturing, and infection of resting CD4 T cells
Peripheral blood mononuclear cells (PBMC) were obtained from healthy donors at the Student Health Center, George Mason University (GMU), Fairfax, VA. Resting CD4 T cells were purified by two rounds of negative selection as previously described [54]. Purified cells were cultured in RPMI 1604 medium supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen), penicillin (50 U/ml), and streptomycin (50 µg/ml) overnight before infection or treatment. For activation of resting CD4 T cells with PHA (3 µg/ml) (Sigma) plus IL-2 (100 U/ml) (Roche Applied Science), cells were cultured in the presence of these agents for 12 hours. For infection, CD4 T cells were incubated with the virus for 2 hours and then washed twice with medium to remove unbound virus. Infected cells were resuspended in fresh RPMI 1604 medium supplemented with 10% heat-inactivated fetal bovine serum at a density of 106 per ml and incubated for 5 days without stimulation. Cells were activated at day 5 with anti-CD3/CD28 magnetic beads at 4 beads per cell. For the viral replication assay, 10% of infected cells were taken at days 1, 3, 5, 6, 7, 8, and 9 post infection. Cells were pelleted and the supernatant was saved for p24 ELISA.
CD3/CD28 bead conjugation and stimulation of resting CD4 T cells
Monoclonal antibodies against human CD3 (clone UCHT1) and CD28 (clone CD28.2) were purchased from BD Pharmingen (BD Biosciences). For conjugation, antibodies were conjugated with 4×108 Dynal beads (Invitrogen) for 30 minutes at room temperature. Free antibodies were washed away with PBS-0.5% BSA. The conjugated magnetic beads were resuspended in 1 ml of PBS-0.5% BSA. For stimulation of resting CD4 T cells, antibody-conjugated beads were washed twice and then added to cell culture and rocked for 5 minutes.
Cell cycle analysis by 7-AAD and PY staining
Resting CD4 T cells or CD3/CD28-stimulated cells (106) were used for the analysis. Before staining, magnetic beads were removed by incubating with DNase I releasing buffer as recommended by the manufacturer. Cells were suspended in 1 ml of 0.03% saponin in PBS and then incubated in 20 µM 7-amino-actinomycin D (Sigma) for 30 minutes at room temperature in the dark. Cells were kept on ice for at least 5 minutes, pyronin Y (Sigma) was added to a final concentration of 5 µM, and the cells were then incubated for 10 minutes on ice. Stained cells were directly analyzed by flow cytometry on a FACS (Becton Dickinson FACSCalibur).
Production of HIV-1 and VSV-G-pseudotyped HIV-1 containing Nef-luciferase fusion protein for entry assay
Plasmid pCDNA3-Nef-Luc was kindly provided by Dr. Robert Davey [50]. Viruses containing Nef-luciferase was produced as described previously [50]. Briefly, 293T cells cultured in a 10 cm petri dish were cotransfected with 10 µg pNL4-3 plus 10 µg of pcDNA3-Nef-luc, or with 10 µg pNL4-3(KFS) plus 7.5 µg pcDNA3-Nef-luc plus 2.5 µg pHCMV-G, using lipofectamine 2000 (Invitrogen) as recommended by the manufacturer. Viruses were harvested at 48 hours post cotransfection and filtered through a 0.45 µM filter. For entry assays, cells (1×106) were infected with 200 ng of Nef-luciferase containing viruses at 37°C for 2 hours, and then washed three times with medium. Cells were resuspended in 0.1 ml of luciferase assay buffer (Promega) and luciferase activity was measured in live cells using a GloMax-Multi Detection System (Promega).
PCR and Real-time PCR
Total cellular DNA was purified using the Wizard SV Genomic DNA Purification System as recommended by the manufacturer (Promega). The detection of viral late DNA and 1-LTR-circles by PCR was performed as described previously [83]. Briefly, for viral late DNA, forward primer: 5′ GGTTAGACCAGATCTGAGCCTG 3′ and reverse primer: 5′ TTAATACCGACGCTCTCGCACC 3′ were used. PCR was carried out in 1×Ambion PCR buffer, 125 µM dNTP, 50 pmol each primer, 1 U SuperTaq Plus (Ambion) with 30 cycles at 94°C for 20 seconds, 68°C for 40 seconds. For detection of 1-LTR circle, primers LTR-nef2 (5′ TGGGTTTTCCAGTCACACCTCAG 3′) and LTR-gag (5′ GATTAACTGCGAATCGTTCTAGC 3′) were used. The reaction was carried out in 1×Ambion PCR buffer, 1.5 nM Mg2+, 125 µM dNTP, 50 pmol each primer, 1 U SuperTaq Plus (Ambion) with 35 cycles at 94°C for 20 seconds, 68°C for 90 seconds. Real-time PCR quantification of viral late DNA and 2-LTR circles was also performed as described previously [44],[84], using 300 nM primers and 200 nM probes. The DNA standard used for both late DNA and 2-LTR circle quantification was constructed using a plasmid containing a complete 2 LTR region (pLTR-2C); the plasmid was cloned by amplification of infected cells with 5′-TGGGTTTTCCAGTCACACCTCAG-3′ and 5′-GATTAACTGCGAATCGTTCTAGC-3′. Measurement was run in triplicate ranging from 1 to 106 copies of pLTR-2C mixed with DNA from uninfected cells.
Confocal microscopy
FITC-phalloidin staining of F-actin has been described previously [44]. Stained cells were imaged using a Zeiss Laser Scanning Microscope, LSM 510 META, with a 40 NA 1.3 or 60 NA 1.4 oil DIC Plan-Neofluar objective. Images were processed and analyzed by LSM 510 META software.
Flow cytometry
Dynasore monohydrate (Sigma) was dissolved in DMSO. Following dynasore treatment, infection, and washing, cells were incubated for 48 hours, and then 500 µl cells were removed and stained with 2 µg/ml propidium iodide solution (Fluka) for 5 minutes at room temperature. Following incubation, cells were analyzed using the FACSCalibur (BD Biosciences). Data analysis was performed using CellQuest (BD Biosciences).
Acknowledgments
We thank the George Mason University (GMU) Student Health Center for blood donations; the NIH AIDS Research and Reference Reagent Program, NIAID, NIH for reagents; Z. Li for help on experiments; M. Martin, R. A. Davey and E. Freed for plasmids; J. W. Marsh and H. A. Nash for discussions; and J. Guernsey for editorial assistance.
Footnotes
The authors have declared that no competing interests exist.
The study was supported by NIH Public Health Service Grant AI069981 and 1R01AI081568 from NIAID to Y. Wu, and by the Intramural Program of NIMH/NIH. W. Wang and M. Spear were supported in part by the generous donation of the 2008 NYCDC AIDS Ride organized by M. Rosen and Day2 Inc. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, et al. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 2.Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, et al. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 3.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 4.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, et al. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 5.Stein BS, Gowda SD, Lifson JD, Penhallow RC, Bensch KG, et al. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987;49:659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- 6.Maddon PJ, McDougal JS, Clapham PR, Dalgleish AG, Jamal S, et al. HIV infection does not require endocytosis of its receptor, CD4. Cell. 1988;54:865–874. doi: 10.1016/s0092-8674(88)91241-x. [DOI] [PubMed] [Google Scholar]
- 7.McClure MO, Marsh M, Weiss RA. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. Embo J. 1988;7:513–518. doi: 10.1002/j.1460-2075.1988.tb02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandt SM, Mariani R, Holland AU, Hope TJ, Landau NR. Association of chemokine-mediated block to HIV entry with coreceptor internalization. J Biol Chem. 2002;277:17291–17299. doi: 10.1074/jbc.M108232200. [DOI] [PubMed] [Google Scholar]
- 9.Pelchen-Matthews A, Clapham P, Marsh M. Role of CD4 endocytosis in human immunodeficiency virus infection. J Virol. 1995;69:8164–8168. doi: 10.1128/jvi.69.12.8164-8168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell. 2009;137:433–444. doi: 10.1016/j.cell.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fredericksen BL, Wei BL, Yao J, Luo T, Garcia JV. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J Virol. 2002;76:11440–11446. doi: 10.1128/JVI.76.22.11440-11446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei BL, Denton PW, O'Neill E, Luo T, Foster JL, et al. Inhibition of lysosome and proteasome function enhances human immunodeficiency virus type 1 infection. J Virol. 2005;79:5705–5712. doi: 10.1128/JVI.79.9.5705-5712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 14.Reiser J, Harmison G, Kluepfel-Stahl S, Brady RO, Karlsson S, et al. Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc Natl Acad Sci U S A. 1996;93:15266–15271. doi: 10.1073/pnas.93.26.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aiken C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J Virol. 1997;71:5871–5877. doi: 10.1128/jvi.71.8.5871-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo T, Douglas JL, Livingston RL, Garcia JV. Infectivity enhancement by HIV-1 Nef is dependent on the pathway of virus entry: implications for HIV-based gene transfer systems. Virology. 1998;241:224–233. doi: 10.1006/viro.1997.8966. [DOI] [PubMed] [Google Scholar]
- 17.Chiu YL, Soros VB, Kreisberg JF, Stopak K, Yonemoto W, et al. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature. 2005;435:108–114. doi: 10.1038/nature03493. [DOI] [PubMed] [Google Scholar]
- 18.Konig R, Zhou Y, Elleder D, Diamond TL, Bonamy GM, et al. Global analysis of host-pathogen interactions that regulate early-stage HIV-1 replication. Cell. 2008;135:49–60. doi: 10.1016/j.cell.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, et al. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319:921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- 20.Kestler HWd, Ringler DJ, Mori K, Panicali DL, Sehgal PK, et al. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 21.Chowers MY, Spina CA, Kwoh TJ, Fitch NJ, Richman DD, et al. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller MD, Warmerdam MT, Gaston I, Greene WC, Feinberg MB. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chowers MY, Pandori MW, Spina CA, Richman DD, Guatelli JC. The growth advantage conferred by HIV-1 nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology. 1995;212:451–457. doi: 10.1006/viro.1995.1502. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz O, Marechal V, Danos O, Heard JM. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell EM, Nunez R, Hope TJ. Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity. Journal of Virology. 2004;78:5745–5755. doi: 10.1128/JVI.78.11.5745-5755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weissman D, Rabin RL, Arthos J, Rubbert A, Dybul M, et al. Macrophage-tropic HIV and SIV envelope proteins induce a signal through the CCR5 chemokine receptor. Nature. 1997;389:981–985. doi: 10.1038/40173. [DOI] [PubMed] [Google Scholar]
- 28.Popik W, Pitha PM. Binding of human immunodeficiency virus type 1 to CD4 induces association of Lck and Raf-1 and activates Raf-1 by a Ras-independent pathway. Molecular & Cellular Biology. 1996;16:6532–6541. doi: 10.1128/mcb.16.11.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melar M, Ott DE, Hope TJ. Physiological levels of virion-associated human immunodeficiency virus type 1 envelope induce coreceptor-dependent calcium flux. J Virol. 2007;81:1773–1785. doi: 10.1128/JVI.01316-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cocchi F, DeVico AL, Garzino-Demo A, Cara A, Gallo RC, et al. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 31.Farzan M, Choe H, Martin KA, Sun Y, Sidelko M, et al. HIV-1 entry and macrophage inflammatory protein-1beta-mediated signaling are independent functions of the chemokine receptor CCR5. Journal of Biological Chemistry. 1997;272:6854–6857. doi: 10.1074/jbc.272.11.6854. [DOI] [PubMed] [Google Scholar]
- 32.Alkhatib G, Locati M, Kennedy PE, Murphy PM, Berger EA. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology. 1997;234:340–348. doi: 10.1006/viro.1997.8673. [DOI] [PubMed] [Google Scholar]
- 33.Gosling J, Monteclaro FS, Atchison RE, Arai H, Tsou CL, et al. Molecular uncoupling of C-C chemokine receptor 5-induced chemotaxis and signal transduction from HIV-1 coreceptor activity. Proc Natl Acad Sci U S A. 1997;94:5061–5066. doi: 10.1073/pnas.94.10.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aramori I, Ferguson SS, Bieniasz PD, Zhang J, Cullen B, et al. Molecular mechanism of desensitization of the chemokine receptor CCR-5: receptor signaling and internalization are dissociable from its role as an HIV-1 co-receptor. Embo J. 1997;16:4606–4616. doi: 10.1093/emboj/16.15.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amara A, Gall SL, Schwartz O, Salamero J, Montes M, et al. HIV coreceptor downregulation as antiviral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Z, Berson JF, Chen Y, Turner JD, Zhang T, et al. Evolution of HIV-1 coreceptor usage through interactions with distinct CCR5 and CXCR4 domains. Proc Natl Acad Sci U S A. 1997;94:6426–6431. doi: 10.1073/pnas.94.12.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doranz BJ, Orsini MJ, Turner JD, Hoffman TL, Berson JF, et al. Identification of CXCR4 domains that support coreceptor and chemokine receptor functions. J Virol. 1999;73:2752–2761. doi: 10.1128/jvi.73.4.2752-2761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brelot A, Heveker N, Montes M, Alizon M. Identification of residues of CXCR4 critical for human immunodeficiency virus coreceptor and chemokine receptor activities. J Biol Chem. 2000;275:23736–23744. doi: 10.1074/jbc.M000776200. [DOI] [PubMed] [Google Scholar]
- 39.Amara A, Vidy A, Boulla G, Mollier K, Garcia-Perez J, et al. G protein-dependent CCR5 signaling is not required for efficient infection of primary T lymphocytes and macrophages by R5 human immunodeficiency virus type 1 isolates. Journal of Virology. 2003;77:2550–2558. doi: 10.1128/JVI.77.4.2550-2558.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pontow SE, Heyden NV, Wei S, Ratner L. Actin cytoskeletal reorganizations and coreceptor-mediated activation of rac during human immunodeficiency virus-induced cell fusion. Journal of Virology. 2004;78:7138–7147. doi: 10.1128/JVI.78.13.7138-7147.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naghavi MH, Valente S, Hatziioannou T, de Los Santos K, Wen Y, et al. Moesin regulates stable microtubule formation and limits retroviral infection in cultured cells. Embo J. 2007;26:41–52. doi: 10.1038/sj.emboj.7601475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barrero-Villar M, Cabrero JR, Gordon-Alonso M, Barroso-Gonzalez J, Alvarez-Losada S, et al. Moesin is required for HIV-1-induced CD4-CXCR4 interaction, F-actin redistribution, membrane fusion and viral infection in lymphocytes. J Cell Sci. 2009;122:103–113. doi: 10.1242/jcs.035873. [DOI] [PubMed] [Google Scholar]
- 43.Jimenez-Baranda S, Gomez-Mouton C, Rojas A, Martinez-Prats L, Mira E, et al. Filamin-A regulates actin-dependent clustering of HIV receptors. Nat Cell Biol. 2007;9:838–846. doi: 10.1038/ncb1610. [DOI] [PubMed] [Google Scholar]
- 44.Yoder A, Yu D, Dong L, Iyer SR, Xu X, et al. HIV envelope-CXCR4 signaling activates cofilin to overcome cortical actin restriction in resting CD4 T cells. Cell. 2008;134:782–792. doi: 10.1016/j.cell.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Y, Beddall MH, Marsh JW. Rev-dependent indicator T cell line. Current HIV Research. 2007;5:395–403. doi: 10.2174/157016207781024018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Y, Yoder A. Chemokine coreceptor signaling in HIV-1 infection and pathogenesis. PLoS Pathog. 2009 doi: 10.1371/journal.ppat.1000520. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bukrinskaya A, Brichacek B, Mann A, Stevenson M. Establishment of a functional human immunodeficiency virus type 1 (HIV-1) reverse transcription complex involves the cytoskeleton. J Exp Med. 1998;188:2113–2125. doi: 10.1084/jem.188.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davis CB, Dikic I, Unutmaz D, Hill CM, Arthos J, et al. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. Journal of Experimental Medicine. 1997;186:1793–1798. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Briant L, Signoret N, Gaubin M, Robert-Hebmann V, Zhang X, et al. Transduction of activation signal that follows HIV-1 binding to CD4 and CD4 dimerization involves the immunoglobulin CDR3-like region in domain 1 of CD4. J Biol Chem. 1997;272:19441–19450. doi: 10.1074/jbc.272.31.19441. [DOI] [PubMed] [Google Scholar]
- 50.Saeed MF, Kolokoltsov AA, Davey RA. Novel, rapid assay for measuring entry of diverse enveloped viruses, including HIV and rabies. J Virol Methods. 2006;135:143–150. doi: 10.1016/j.jviromet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Pierson TC, Zhou Y, Kieffer TL, Ruff CT, Buck C, et al. Molecular characterization of preintegration latency in human immunodeficiency virus type 1 infection. J Virol. 2002;76:8518–8531. doi: 10.1128/JVI.76.17.8518-8531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spina CA, Kwoh TJ, Chowers MY, Guatelli JC, Richman DD. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spina CA, Guatelli JC, Richman DD. Establishment of a stable, inducible form of human immunodeficiency virus type 1 DNA in quiescent CD4 lymphocytes in vitro. J Virol. 1995;69:2977–2988. doi: 10.1128/jvi.69.5.2977-2988.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Y, Marsh JW. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science. 2001;293:1503–1506. doi: 10.1126/science.1061548. [DOI] [PubMed] [Google Scholar]
- 55.Schrager JA, Marsh JW. HIV-1 Nef increases T cell activation in a stimulus-dependent manner. Proc Natl Acad Sci U S A. 1999;96:8167–8172. doi: 10.1073/pnas.96.14.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fenard D, Yonemoto W, de Noronha C, Cavrois M, Williams SA, et al. Nef is physically recruited into the immunological synapse and potentiates T cell activation early after TCR engagement. J Immunol. 2005;175:6050–6057. doi: 10.4049/jimmunol.175.9.6050. [DOI] [PubMed] [Google Scholar]
- 57.Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell. 2005;121:593–606. doi: 10.1016/j.cell.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 58.Praefcke GJ, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- 59.Iyer SR, Yu D, Biancotto A, Margolis LB, Wu Y. Measurement of human immunodeficiency virus type 1 preintegration transcription by using Rev-dependent Rev-CEM cells reveals a sizable transcribing DNA population comparable to that from proviral templates. J Virol. 2009;83:8662–8673. doi: 10.1128/JVI.00874-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rey O, Canon J, Krogstad P. HIV-1 Gag protein associates with F-actin present in microfilaments. Virology. 1996;220:530–534. doi: 10.1006/viro.1996.0343. [DOI] [PubMed] [Google Scholar]
- 61.Wilk T, Gowen B, Fuller SD. Actin associates with the nucleocapsid domain of the human immunodeficiency virus Gag polyprotein. J Virol. 1999;73:1931–1940. doi: 10.1128/jvi.73.3.1931-1940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu B, Dai R, Tian CJ, Dawson L, Gorelick R, et al. Interaction of the human immunodeficiency virus type 1 nucleocapsid with actin. J Virol. 1999;73:2901–2908. doi: 10.1128/jvi.73.4.2901-2908.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ibarrondo FJ, Choi R, Geng YZ, Canon J, Rey O, et al. HIV type 1 Gag and nucleocapsid proteins: cytoskeletal localization and effects on cell motility. AIDS Res Hum Retroviruses. 2001;17:1489–1500. doi: 10.1089/08892220152644197. [DOI] [PubMed] [Google Scholar]
- 64.Hottiger M, Gramatikoff K, Georgiev O, Chaponnier C, Schaffner W, et al. The large subunit of HIV-1 reverse transcriptase interacts with beta-actin. Nucleic Acids Res. 1995;23:736–741. doi: 10.1093/nar/23.5.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turlure F, Devroe E, Silver PA, Engelman A. Human cell proteins and human immunodeficiency virus DNA integration. Front Biosci. 2004;9:3187–3208. doi: 10.2741/1472. [DOI] [PubMed] [Google Scholar]
- 66.Fackler OT, Kienzle N, Kremmer E, Boese A, Schramm B, et al. Association of human immunodeficiency virus Nef protein with actin is myristoylation dependent and influences its subcellular localization. Eur J Biochem. 1997;247:843–851. doi: 10.1111/j.1432-1033.1997.00843.x. [DOI] [PubMed] [Google Scholar]
- 67.Agosto LM, Yu JJ, Liszewski MK, Baytop C, Korokhov N, et al. The CXCR4-tropic human immunodeficiency virus envelope promotes more-efficient gene delivery to resting CD4+ T cells than the vesicular stomatitis virus glycoprotein G envelope. J Virol. 2009;83:8153–8162. doi: 10.1128/JVI.00220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cavrois M, De Noronha C, Greene WC. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat Biotechnol. 2002;20:1151–1154. doi: 10.1038/nbt745. [DOI] [PubMed] [Google Scholar]
- 69.Dismuke DJ, Aiken C. Evidence for a functional link between uncoating of the human immunodeficiency virus type 1 core and nuclear import of the viral preintegration complex. J Virol. 2006;80:3712–3720. doi: 10.1128/JVI.80.8.3712-3720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ganesh L, Burstein E, Guha-Niyogi A, Louder MK, Mascola JR, et al. The gene product Murr1 restricts HIV-1 replication in resting CD4+ lymphocytes. Nature. 2003;426:853–857. doi: 10.1038/nature02171. [DOI] [PubMed] [Google Scholar]
- 71.Auewarakul P, Wacharapornin P, Srichatrapimuk S, Chutipongtanate S, Puthavathana P. Uncoating of HIV-1 requires cellular activation. Virology. 2005;337:93–101. doi: 10.1016/j.virol.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 72.Klages N, Zufferey R, Trono D. A stable system for the high-titer production of multiply attenuated lentiviral vectors. Mol Ther. 2000;2:170–176. doi: 10.1006/mthe.2000.0103. [DOI] [PubMed] [Google Scholar]
- 73.Ducrey-Rundquist O, Guyader M, Trono D. Modalities of interleukin-7-induced human immunodeficiency virus permissiveness in quiescent T lymphocytes. J Virol. 2002;76:9103–9111. doi: 10.1128/JVI.76.18.9103-9111.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kamata M, Nagaoka Y, Chen IS. Reassessing the role of APOBEC3G in human immunodeficiency virus type 1 infection of quiescent CD4+ T-cells. PLoS Pathog. 2009;5:e1000342. doi: 10.1371/journal.ppat.1000342. doi: 10.1371/journal.ppat.1000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bettinger BT, Gilbert DM, Amberg DC. Actin up in the nucleus. Nat Rev Mol Cell Biol. 2004;5:410–415. doi: 10.1038/nrm1370. [DOI] [PubMed] [Google Scholar]
- 76.Forest T, Barnard S, Baines JD. Active intranuclear movement of herpesvirus capsids. Nat Cell Biol. 2005;7:429–431. doi: 10.1038/ncb1243. [DOI] [PubMed] [Google Scholar]
- 77.Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, et al. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Freed EO, Delwart EL, Buchschacher GL, Jr, Panganiban AT. A mutation in the human immunodeficiency virus type 1 transmembrane glycoprotein gp41 dominantly interferes with fusion and infectivity. Proc Natl Acad Sci U S A. 1992;89:70–74. doi: 10.1073/pnas.89.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yee JK, Miyanohara A, LaPorte P, Bouic K, Burns JC, et al. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci U S A. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Young J, Tang Z, Yu Q, Yu D, Wu Y. Selective killing of HIV-1-positive macrophages and T Cells by the Rev-dependent lentivirus carrying anthrolysin O from Bacillus anthracis. Retrovirology. 2008;5:36. doi: 10.1186/1742-4690-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu Y, Beddall MH, Marsh JW. Rev-dependent lentiviral expression vector. Retrovirology. 2007;4:12. doi: 10.1186/1742-4690-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nara PL, Hatch WC, Dunlop NM, Robey WG, Arthur LO, et al. Simple, rapid, quantitative, syncytium-forming microassay for the detection of human immunodeficiency virus neutralizing antibody. AIDS Res Hum Retroviruses. 1987;3:283–302. doi: 10.1089/aid.1987.3.283. [DOI] [PubMed] [Google Scholar]
- 83.Kelly J, Beddall MH, Yu D, Iyer SR, Marsh JW, et al. Human macrophages support persistent transcription from unintegrated HIV-1 DNA. Virology. 2008;372:300–312. doi: 10.1016/j.virol.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu Y, Marsh JW. Early transcription from nonintegrated DNA in human immunodeficiency virus infection. J Virol. 2003;77:10376–10382. doi: 10.1128/JVI.77.19.10376-10382.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]