Abstract
The skin epidermis and its array of appendages undergo ongoing renewal by a process called homeostasis. Stem cells in the epidermis have a crucial role in maintaining tissue homeostasis by providing new cells to replace those that are constantly lost during tissue turnover or following injury. Different resident skin stem cell pools contribute to the maintenance and repair of the various epidermal tissues of the skin, including interfollicular epidermis, hair follicles and sebaceous glands. Interestingly, the basic mechanisms and signalling pathways that orchestrate epithelial morphogenesis in the skin are reused during adult life to regulate skin homeostasis.
Although barely thicker than paper, the skin epidermis protects animals against major environmental stresses, such as water loss and microorganism infection. The epidermis also has the remarkable ability to elaborate the body surface with appendages, which range from hair follicles, nails, oil and sweat glands in mammals to scales and feathers in lower vertebrates. These adornments not only contribute to thermal regulation and to protection against environmental irradiation, but also function in camouflage, social interactions between animals and reproductive behaviour. The body surface is an important tissue because it provides animals with valuable information about their species, gender and social status.
The physiological process that maintains a constant number of cells in renewing organs is called tissue homeostasis. Stem cells (SCs) that are located in these organs are responsible for the maintenance of tissue homeostasis and repair following injuries. SCs are defined by their characteristic ability to self-renew and to give rise to the different cell lineages that form mature adult tissues.
Homeostasis in skin is fuelled by SCs in epithelial tissues, which replace the keratinocytes that are lost either through normal differentiation and tissue turnover, or through cell death owing to the damaged incurred following injury1. Recent studies have begun to explain some of the mysteries of these special ‘fountains of youth’, which reside in different compartments of the skin and underlie the remarkable resilience of the skin. Here, we discuss how the different SC compartments of the skin epidermis are established during embryonic development and how they are maintained thereafter during adult homeostasis. We present the basic mechanisms and signalling pathways that orchestrate the morphogenesis of the epidermis and its appendages, and explore how SCs can balance states of dormancy, activity and lineage commitment to regulate homeostasis.
Homeostasis of the skin barrier
The skin barrier is essential throughout life for animals to survive in an external environment, and therefore it must be established before the animal leaves the protective surroundings of the womb.
Skin development in the embryo
The skin epidermis originates from the ectoderm during embryonic development. Soon after gastrulation, a single layer of epidermal cells forms and persists from embryonic day 9.5 (E9.5) to E12.5. As mesenchymal cells populate the skin, they transmit signals that instruct the stratification of the epidermis and dictate the positioning of downgrowths that mark the initiation of hair follicle (HF) morphogenesis2,3. In alliance with the mesenchyme, the innermost, or basal, layer of the stratifying epidermis produces and organizes an underlying basement membrane that is rich in extracellular matrix proteins and growth factors. The epidermis adheres to this basement membrane, which serves not only as a growth-promoting platform but also as a physical boundary between the epithelium and the dermis.
During the initial stages of stratification (E12.5–E15.5), cell division is occasionally seen suprabasally, perhaps as a means of rapidly expanding the stratifying layers that are built from scratch at this time. However, these suprabasal cells soon differentiate. Although regional variation exists, stratification is largely completed by E17.5, by which point the epidermis consists of an inner layer of basal cells with proliferative potential and layers of terminally differentiating, suprabasal cells. During transit to the skin surface, suprabasal cells undergo several discrete transcriptional stages as they form the spinous layers, granular layers and finally dead, flattened stratum corneum cells (BOX 1).
Box 1 Homeostasis in the epidermis and hair follicle.
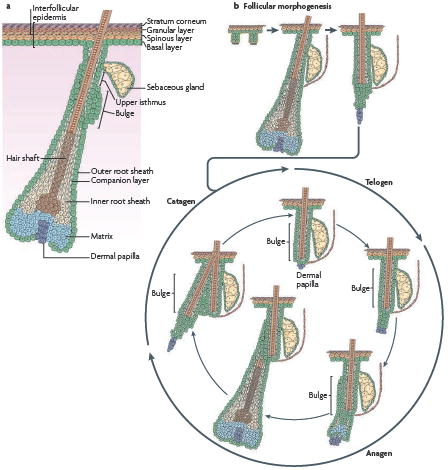
As a result of the physico-chemical assaults that are delivered to the skin surface, the epidermis continuously renews throughout the life of animals by a process that is referred to as tissue homeostasis. During this process, the number of epidermal cells remains constant, such that the number of new cells generated by each cell division must exactly compensate for the number lost by differentiation or cell death. Mammalian haired skin is composed of a pilosebaceous unit that contains a hair follicle (HF) and its surrounding interfollicular epidermis (IFE) (see the figure, part a). Stem cells (SCs) that are present in the skin ensure the continual turnover of the skin epidermis during tissue homeostasis and repair. The IFE, which is responsible for skin barrier function, is composed of an inner basal layer of proliferative cells and suprabasal layers of differentiating progeny. It takes about 2 to 3 weeks before basal cells have completed their migration and are shed from the skin surface. HFs are composed of different concentric layers of cells. At the base of the HF, the matrix cells proliferate transiently and then embark on the seven concentric terminal differentiation programmes that generate the mature follicle. HF SCs reside in a niche that is located at the bottom of the non-cycling portion of the outer root sheath (ORS), called the bulge. HFs also contain sebaceous glands, which form from and are replenished by resident SCs located in the ORS. These SCs differentiate to form the sebocytes, which release oils that lubricate the hair channel and skin surface following degeneration. As matrix cells exhaust their proliferative capacity, hair growth stops (see the figure, part b). At this time, the follicle enters a destructive phase (known as catagen), which leads to the degeneration of the lower two-thirds of the follicle, whereas the bulge region that contains the HF SCs remains intact. Following a quiescent stage (telogen), HF SCs are activated and initiate a new growth phase (anagen).
Renewing the skin barrier during adult life
Once mature, the epidermis undergoes homeostatic regulation as basal cells periodically execute their programme of terminal differentiation and move outwards in a columnar fashion. The basal to suprabasal (or spinous) transition is a key first step in the programme of terminal differentiation. As cells enter the spinous layer, they switch off the expression of genes encoding keratin 5 (KRT5; also known as K5) and KRT14. These abundant intermediate filament (IF) proteins mark the stratified squamous epithelial cells that possess proliferative potential4. Concomitantly, spinous cells switch on the expression of genes encoding KRT1 and KRT10 to form an even more robust IF network that is interlinked with desmosomes4. This expansive cytoskeleton reinforces cell–cell junctions and provides resistance against mechanical stresses at the body surface.
Granular cells express additional structural proteins that are deposited beneath the plasma membrane. In the late stages of terminal differentiation, these proteins become enzymatically crosslinked, which results in an indestructible proteinaceous sac. This sac serves as a scaffold for specialized lipid bilayers that are extruded from intracellular lamellar granules into the extra-cellular space between squames (dead flattened stratum corneum cells), thereby waterproofing the skin surface. When terminal differentiation is complete, the squames exist as dead cellular ghosts that are sandwiched by lipids on the outside and filled with an indestructible fibrous mass of keratins that is encased by the cornified envelope. Although squamous cells are eventually shed from the skin surface and are replaced by differentiating cells from below, they briefly serve as the barrier that keeps harmful microbes out and essential body fluids in2,3,5.
Signals that regulate skin differentiation
Whereas the morphogenetic changes that are associated with stratification have been well studied, the molecular mechanisms that orchestrate skin differentiation remain poorly understood3. Mouse genetics have identified multiple signalling pathways that are essential for proper epidermal stratification and the acquisition of the skin barrier function2,3. These pathways involve Notch, mitogen-activated protein kinase (MAPK), nuclear factor-κB (NF-κB) and the transcriptional regulators p63 (which is related to p53), the AP2 family, the CCAAT/enhancer-binding protein (C/EBP) transcriptional regulators, interferon regulatory factor 6 (IRF6), grainyhead-like 3 (GRHL3) and Kruppel-like factor 4 (KLF4). The interplay between these signalling pathways and transcription factors is beginning to emerge.
At the heart of the decision-making process is the basal to spinous switch, which is controlled by p63 and the canonical Notch pathway. In the absence of p63 in mice, the epidermis fails to stratify and only a few poorly differentiated cells remain attached to the embryo surface. Gain- and loss-of-function studies in vertebrates show that p63 is required for initiating the skin stratification programme and for maintaining the renewal potential of different epithelial SCs3,6-10. The canonical Notch pathway is also crucial during the early step of basal cell commitment to spinous cells11-13. Specification of spinous cell fate is completely blocked on conditional ablation of RBPJ11, a DNA-binding protein that forms a bipartite transcription factor with the Notch intracellular domain to relay active Notch signalling to the nucleus. Specification of spinous fate is also altered following the loss of Hes1 (REF. 14), which is one of the main Notch target genes in the skin epidermis. Conversely, excessive Notch signalling converts basal cells into spinous cells11,12. Notch signalling seems to act in part by influencing the expression of the C/EBP DNA-binding proteins, which work in concert with the AP2 family of transcription factors to regulate the commitment to terminally differentiate15.
The involvement of microRNAs (miRNAs) provides an additional layer of complexity to the transcriptional regulatory switches. miRNAs seem to function in the fine-tuning of the signalling transcription factor circuitry, which prompts a basal epidermal SC to terminally differentiate16-18. miR-203 is an abundant and evolutionarily conserved miRNA that is expressed suprabasally at the same time as epidermal stratification and differentiation17. The precocious expression of miR-203 in basal cells induces their premature differentiation and diminishes their proliferative potential17,19. When miR-203 is absent (following conditional Dicer ablation) or decreased (by a miR-203 antagonist), cell proliferation is no longer restricted to the basal layer17. Intriguingly, one of the targets of miR-203 is p63 mRNA, the translation of which increases in suprabasal cells when miR-203 is absent and repressed basally when miR-203 is precociously expressed. These studies suggest that miR-203 functions in part by preventing the expression of key basal targets in suprabasal cells, and sharpening the commitment to epidermal differentiation.
In addition to miRNAs, histone modifications have recently emerged as epigenetic regulators of epidermal differentiation20. However, the mechanisms and actions that are involved in this process are unknown21,22. In the epidermis, MYC is required for the departure of SCs from their SC niche and their subsequent proliferation and differentiation23. MYC might regulate the transition from quiescent SCs to transit-amplifying (TA) cells by inducing global histone modifications that are typically associated with an activate chromatin state22. In another study, however, removal of a different chromatin repressive mark, namely trimethylated Lys27 of histone H3, was associated with the switch from epidermal basal cell proliferation to differentiation21. Overexpression of the specific histone demethylase involved caused the precocious activation of the terminal differentiation programme in cultured human epidermal cells, whereas RNA interference against the demethylase suppressed differentiation21. Future studies will be needed to further characterize the epigenetic switches and their associated target genes, which govern the transition between proliferation and differentiation in the epidermis.
Asymmetric cell division in the skin
During normal homeostasis, the pool of SCs in a tissue remains constant. Although other models are possible, the maintenance of SC equilibrium is most easily explained by asymmetric divisions (BOX 2). Two types of asymmetric cell division have been described in skin epidermis: one that positions the mitotic spindle parallel to the basement membrane, and another that places the plane of cell division perpendicular to the basement membrane. The second type of asymmetric cell division, which occurs during embryonic mouse skin development, provides a simple mechanism for the asymmetrical partitioning of two daughter cells with different fate determinants. In this scenario, the basal cell remains attached by integrins to the basement membrane and maintains close contact to the pro-survival and growth-promoting factors and their receptors, whereas the suprabasal daughter cell that is positioned away from the SC niche begins to terminally differentiate.
Box 2 Homeostasis through asymmetric and symmetric SC divisions.
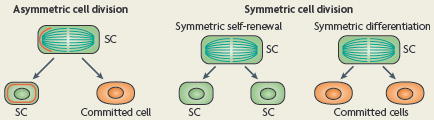
A priori, the maintenance of a constant pool of stem cells (SCs) can be accomplished by one of two distinct types of cell division during tissue homeostasis (see the figure): in an asymmetric division, one daughter remains a SC throughout self-renewal, and the other daughter becomes committed to enter a programme of terminal differentiation. This asymmetrical cell fate specification can be achieved either by the unequal segregation of a cell fate determinant, either proteins or RNAs (red line), or by positioning one of the daughters away from the SC niche27,28,102. By contrast, symmetrical cell divisions result in both daughters adopting the same fate, which for SCs would result in the generation of two SCs (a process that is called symmetric self-renewal) or two differentiated cells (a process that is called symmetric differentiation).
By generating a daughter cell that is placed away from the SC niche, this mode of division might combine an intrinsic asymmetric segregation of cell fate determinants with an extrinsic mode of cell fate specification24,25 (FIG. 1). By contrast, when the division plane is parallel to the basement membrane, as occurs in adult tail skin26, both daughters remain at least transiently inside the basal layer. One daughter might receive an asymmetric amount of a signal that leads to the downregulation of integrin expression and to the subsequent detachment of the cell from the basal layer (FIG. 1). Numb, which is an inhibitor of Notch, is an attractive candidate for such a signal, and could facilitate the generation of one daughter that is attached and one daughter cell that is detached from the basement membrane26. As Notch signalling down-regulates integrin expression11,12 and has been proposed to function in asymmetric cell divisions of Drosophila melanogaster neuroblasts27, differential inhibition of this pathway might result in differential detachment of daughter cells.
Figure 1. Asymmetrical SC division during development and homeostasis.
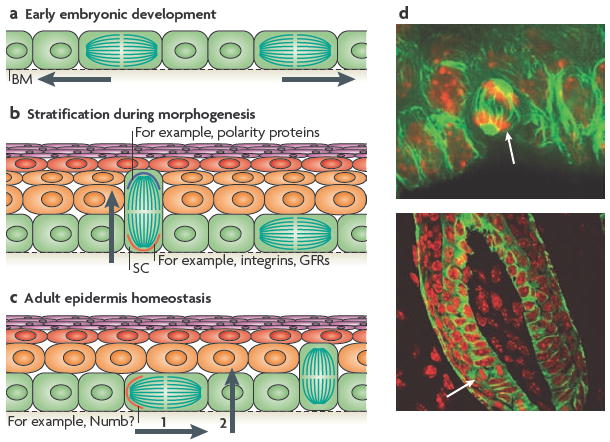
a During the early stages of embryonic skin development, most cell divisions are symmetric and parallel to the basement membrane (BM), which ensures the growth of the surface of the developing embryo and maintains the epithelium as a single layer. b During epidermal stratification, ~70% of the cell divisions become asymmetric, such that the mitotic spindle is perpendicular to the basement membrane. This allows the development of suprabasal cells that terminally differentiate and establish the skin barrier (indicated by the arrow). In this mode of asymmetric cell division, different cell fate determinants are segregated unequally between the two daughter cells. The basal cell segregates the integrins and growth factor receptors (GFRs), which provide survival and proliferative cues to the stem cell (SC). Polarity proteins are concentrated to the apical surface and presumably become preferentially distributed to the suprabasal cell25. c During epidermal homeostasis in the adult tail skin, asymmetric cell divisions occur with the plane of division parallel to the BM, such that only one daughter cell inherits a cell fate determinant, such as Numb26, and remains a SC (step 1), whereas the other becomes committed to terminal differentiation, and probably undergoes delamination to reach the suprabasal layers (step 2). d Examples of asymmetric spindle orientations (white arrow) in the oral epithelium, which are similar to those seen in the embryonic epidermis (upper panel) and in the adult hair bulb (lower panel). Nuclei are shown in red and microtubules in green. Images in part d are courtesy of T. Lechler and E.F., Rockefeller University, New York, USA.
It is tempting to speculate that the mammalian epidermis might use a mechanism that involves evolutionarily conserved apical–basal polarity determinants, such as partitioning defective 3 (PAR3), PAR6 and atypical protein kinase C (aPKC), to set up the orientation of the spindle pole (FIG. 1). These proteins are preferentially localized along the apical domain of the basal cells of the developing skin epidermis25. During mitosis, these polarity proteins seem to recruit LGN (also known as GPSM2), inscuteable (INSC) and components of the mitotic spindle pole, such as nuclear and mitotic apparatus 1 (NUMA1), all of which in lower eukaryotes have been genetically involved with asymmetric cell divisions27,28. Although not directly implicated in the segregation of cell fate determinants in the mammalian epidermis, this proteinaceous crescent provides a potential docking site for astral microtubules that might facilitate the asymmetric alignment of one of the spindle poles to the apical domain of basal cells25.
It remains to be determined whether mitotic spindle orientation is linked to asymmetric fate determination in the skin, and, if so, how. However, the ability of spindle orientation to change temporally during epidermal development is well documented. Basal progenitors begin as a single layer in which divisions are uniformly parallel to the basement membrane. During stratification, basal cells must balance the generation of all suprabasal layers from scratch with lateral skin expansion to accommodate the increased size of the embryo. By contrast, in normal homeostasis in the adult, basal progenitors only need to replace the dead protective stratum corneum cells that are shed from the skin surface. Correspondingly, the proportion of perpendicular cell divisions rises from 0% at E12.5 to 70% between E14.5 and E17.5 (REFS 24,25). Thereafter, the proportion of such divisions is thought to decrease significantly in accordance with the reduced need to generate suprabasal cells24,26.
Models of epidermal homeostasis
Another key issue in adult epidermal homeostasis is whether, in an asymmetric division that occurs parallel to the basement membrane, the committed daughter cell is a terminally differentiating cell or a TA cell. TA cells undergo a number of cell divisions before they differentiate. Lineage-tracing experiments have documented the organization of interfollicular epidermis (IFE) into discrete proliferative units by revealing the presence of long-term clones of marked cells. These are arranged as independent stacks of cells that extend from the basal layer to the stratum corneum29-32. Interestingly, some labelled epidermal clones continue to increase in size over time, a feature that is not expected from a hierarchical model in which rare SCs in the basal layer give rise to TA cells in the basal layer26,29,33. One possible explanation is that epidermal SCs migrate laterally and colonize the adjacent proliferative unit29.
More recently, lineage tracing in adult tail epidermis points to a model that explains epidermal homeostasis on the basis of two parameters: the fraction of proliferating cells in the basal layer and the probability of an asymmetric cell division26. In this study, the authors posit that all proliferative cells in the basal layer might be functionally equivalent, such that SCs give rise directly to committed spinous cells without entering a TA phase. Although this model is attractive for its simplicity, a TA population has long provided a means of understanding how tissues are generated from a small number of SCs and how tissues can adapt to a sudden imbalance in homeostasis, as occurs during injury. Moreover, studies with human and mouse epidermal and HF SC cultures reveal the existence of keratinocytes with different long-term proliferative potentials34-38, which suggests the presence of both SC and TA cells in the epidermis. Further research is needed to resolve this apparent discrepancy.
Multiple SCs ensure skin homeostasis
The different compartments of skin epidermis, including the HF, IFE and sebaceous glands (SGs), are maintained during adult homeostasis by the presence of different resident SCs or progenitor cells.
The battles of the bulge
Mammalian skin with hair can be partitioned into small molecular regions, each composed of a pilosebaceous unit (which consists of a HF and its SG) and its surrounding IFE. Two-thirds of the adult pilosebaceous unit undergoes cyclical bouts of degeneration (known as catagen), rest (telogen) and growth (anagen), which are together called the hair cycle. Residing in a region at or near the base of the non-cycling portion of each HF, SCs generate and maintain the cycling portion of the HF during the growth phase of the hair cycle. During this time, HF SCs fuel the production of TA matrix cells that rapidly divide in the hair bulb and then terminally differentiate to produce both the hair shaft and its inner root sheath (IRS) channel (BOX 1).
HF SCs reside in a specialized microenvironment called the bulge. These cells cycle slowly, as revealed by their ability to retain a pulse of nucleotide label following weeks of chase39,40. By adapting the pulse-chase concept to a tetracycline-regulatable, stable, fluorescently tagged histone H2B in mice, it is now possible to isolate viable bulge cells based on their slow-cycling behaviour in vivo41. Although relatively quiescent, most bulge cells divide multiple times during each hair cycle34. Regional and temporal variation in cycling behaviour has been described among bulge SCs35,41-44, but the reason for this heterogeneity remains unclear. Bulge SCs also seem to randomly partition their newly synthesized DNA to their daughters43,45, which contradicts the immortal strand hypothesis that has been proposed for SCs46. Intriguingly, this slow-cycling niche, which later gives rise to the adult bulge, is formed early during skin development47.
The involvement of bulge cells in maintaining HF homeostasis has been well documented by a series of lineage-tracing experiments in mice41,44,48-51 (FIG. 2). Mice that have fluorescently tagged histone H2B were used to show that the matrix progenitor cells of HF are derived from previously slow-cycling bulge cells that proliferate as they exit from the base of the SC niche41. Transgenic mice that express an inducible Cre recombinase under the control of the promoters of Krt15 or Leu-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) have been used to genetically mark the region of the follicle that encompasses both the bulge and the secondary hair germ, a small cluster of cells at the base of the bulge that directly abuts the dermal papilla (DP) in the resting stage of the hair cycle. These studies suggest that the bulge and possibly hair germ cells contain multipotent HF SCs that have the ability to differentiate into all of the lineages of the pilosebaceous unit44,48-51. Recent studies on LGR5 (REF. 44), although similar to those reported earlier on KRT15 (REF. 48), traced progeny over several months and thereby documented the ability of bulge or HG cells to contribute in the long term to the HF. By contrast, under normal homeostatic conditions, these cells seem to contribute only minimally, if at all, to the maintenance of IFE. This underscores the existence of a separate SC pool in the IFE48,49.
Figure 2. Different SC niches ensure epidermal homeostasis.
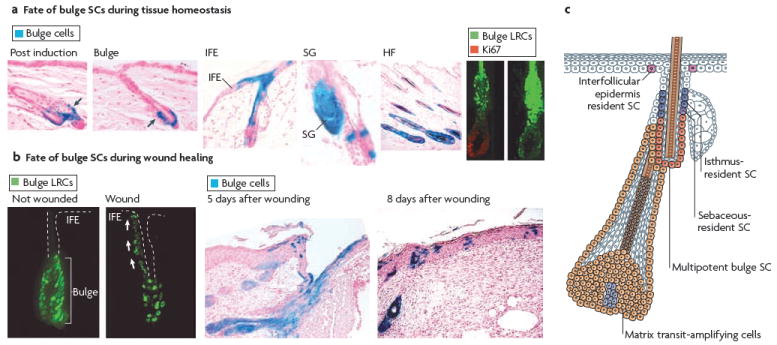
a During telogen, bulge stem cells (SCs) are specifically marked by β-galactosidase (LacZ; blue) by inducing the activity of an inducible Cre recombinase expressed under the control of a bulge promoter. When hair follicles (HFs) undergo cycling, all cells of newly formed HFs are marked, demonstrating that bulge SCs fuel normal follicle homeostasis. Bulge SCs can also contribute to the formation of interfollicular epidermis (IFE) and sebaceous glands (SGs), although this phenomenon is rare and, in most cases, IFE cells are not derived from bulge cells48. This demonstrates that the IFE can be maintained independently of bulge SCs during tissue homeostasis. The bulge SCs, which are shown as green histone H2B–green fluorescent protein-labelled retaining cells (LRCs), exit the SC niche and actively proliferate (as shown here by Ki67 immunoreactivity) to provide cells that initiate HF regeneration41. b Following wounding, the bulge SCs41,49 become activated and migrate upward to repair the IFE. c Schematic representation of the skin epidermis with the different resident SC compartments and transit-amplifying progeny identified. Bulge SCs are multipotent, residing in the permanent portion of the HF. IFE SCs reside in the basal layer of the epidermis. Resident progenitors of the isthmus and SG reside in the outer root sheath that is above the bulge and below the SG. It is not clear whether these two resident progenitors are equivalent. Figure part a is reproduced, with permission, from REF. 41 © (2004) American Association for the Advancement of Science, and from Nature Biotechnology REF. 48 © (2004) Macmillan Publishers Ltd. All rights reserved. Figure part b, is reproduced, with permission, from REF. 41 © (2004) American Association for the Advancement of Science, and from Nature Medicine REF. 49 © (2005) Macmillan Publishers Ltd. All rights reserved.
The ability of bulge cells to possess SC potential has been further suggested by in vitro studies and clonal analyses of the progeny of purified bulge cells35,52. The importance of bulge SCs to skin development and homeostasis has been shown by skin-specific ablation of the gene that encodes the transcription factor SOX9. When Sox9 is conditionally targeted postnatally, the expression of the adult bulge cell surface marker CD34 is lost and HFs fail to cycle, which is suggestive of an SC defect53. When Sox9 is conditionally targeted in the embryo, slow-cycling bulge cells fail to form during development, HF morphogenesis is arrested and SG formation is blocked altogether47. These findings provide compelling evidence that the bulge is a residence of SCs that are essential not only for hair cycling but also for HF development.
One of the major questions in the field is: what is the point of no return at which a SC becomes irreversibly fated to terminally differentiate? Because Krt15 and Lgr5 are expressed in the bulge as well as the secondary hair germ, and during the HF growing stage in the outer root sheath (ORS)44,48, the extent to which these early bulge progeny might retain their stem cell characteristics (stemness) after leaving their niche is not known. However, the bulge seems to be repopulated by cells of the secondary hair germ when hairs are plucked, suggesting that early progeny that have left their niche might be able to reacquire SC potential, at least under circumstances of stress54. By contrast, the rapidly proliferating TA matrix cells seem to be more committed to a fate of terminal differentiation. Lineage tracing suggests that the differentiation potential of matrix cells is spatially organized into different zones of unipotent progenitors, one for each lineage of the six concentric layers of the mature HF55 (BOX 3).
Box 3 current model of SC migration during skin homeostasis and repair.
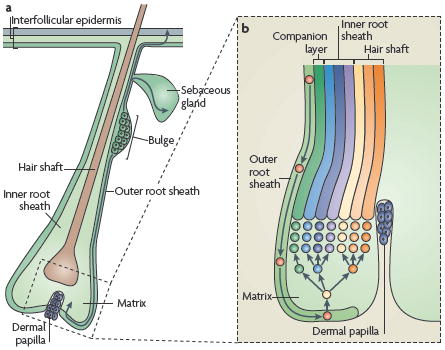
During normal homeostasis, bulge stem cells (SCs) are periodically activated to form a new hair follicle (HF; see the figure, part a). During the hair follicle growth period (known as anagen), bulge cell progeny populate the lower outer root sheath (ORS) towards the matrix, a specialized population of highly proliferative transit-amplifying cells that are responsible for producing a new hair. Under conditions of injury in which the resident interfollicular epidermal or sebaceous gland SCs are damaged, bulge SCs become mobilized and migrate to and differentiate along these lineages.
As bulge SC progeny move down the ORS to the base of the follicle, they subsequently become matrix cells, which, after proliferation, differentiate along one of seven hair lineages (see the figure, part b). Intimate contact with the dermal papilla is essential for maintaining the high proliferative capacity of the matrix and driving lineage decisions. Recent lineage-tracing experiments suggest that midstream in HF morphogenesis, once the early bulge forms, its progeny progressively replace the previously generated lower ORS and matrix. As morphogenesis is completed, the entire pilosebaceous unit consists of SOX9-marked progeny47. These findings provide support for previous observations that suggest that bulge cells periodically exit the SC niche during anagen and transit along the follicle to fuel the TA matrix cells, to sustain the differentiation and production of the cells that form the hair shaft and its channel, the inner root sheath55,103. In SOX9-deficient neonatal mouse skin, the matrix, which normally expands during morphogenesis as it does during early anagen, diminishes in size until no proliferative matrix cells remain at the base47. Together, these studies provide compelling evidence that migration of bulge SC progeny to the base of the HF is important to sustain the growth of regenerating follicles.
In response to injury, SCs in the follicle rapidly adopt differentiation programmes that do not occur in normal homeostasis. Genetically marked bulge SCs can be rapidly mobilized to migrate upward and repair damaged epidermis in vivo41,49,51 (FIG. 2). Moreover, the flux of bulge cells towards the epidermis seems to be tightly regulated, as it stops once the wound is healed.
Long-term lineage tracing suggests that bulge-cell-mediated repair of the skin surface is transient in adult skin44,47-51. Interestingly, however, there are SCs inside the neonatal HF that possess longer-term repair activity51. Initially, it was thought that perhaps these SCs resided in the upper ORS rather than the bulge. More recent studies show that neonatal skin that lacks Sox9 cannot repair wounds efficiently47, which raises the intriguing possibility that bulge SC potential for wound repair might decline with age. Overall, whether young or old, skin SCs seem to be able to restore and/or maintain homeostasis using any combination of intrinsic and extrinsic resources.
Although the ability of skin SCs to respond to injury shows their capacity to become activated, their features of stemness seem to be ingrained in their intrinsic behaviour. The remarkable ability of epidermal SCs to be removed from their niche and to be cultured over long periods of time without losing their stemness was first shown by Green and co-workers, who exploited this intrinsic behaviour for the use of human epidermal SCs in burn therapy56,57. When placed in culture, adult rodent bulge cells also exhibit much higher proliferative potential than keratinocytes that originate from other locations34-36,52,58,59. Intriguingly, adult bulge SCs that are passaged in culture and then engrafted onto the backs of immunocompromised mice can generate not only SGs and cycling HFs, but also epidermis35. Whether multipotency is a feature of bulge SCs that is lost with age and then regained when adult bulge cells are cultured and transplanted into a wounded area is not clear. Additionally, although adult mouse basal epidermal cells in culture have not been shown to possess intrinsic features of multipotent SCs, studies will be needed to further define the intrinsic distinctions between IFE and bulge SC populations and the importance of the niche in dictating HF fate determination.
Making and maintaining SGs
In addition to bulge and IFE SCs, evidence for other types of epidermal progenitors has begun to emerge. Transplantation experiments of genetically marked cells have long postulated the existence of unipotent sebaceous lineage progenitors31. Recent studies have identified a few resident cells in the SGs that are marked by BLIMP1 (also known as PRDM1) expression and located at the juncture of the gland and the HF. Blimp1–Cre-lineage tracing confirmed that these are bona fide sebocyte progenitors that can give rise to the entire gland60. These findings suggest that, like the epidermis, SG homeostasis can be maintained by its own resident progenitors (FIG. 2).
BLIMP1 is a transcriptional repressor of MYC, which has been implicated in the conversion of epidermal SCs to committed TA cells, which then terminally differentiate23. Intriguingly, SG hyperplasia arises when Blimp1 is conditionally ablated60. This feature is attributable in part to the activation of MYC, which stimulates SG proliferation61,62. However, it also seems that bulge SCs provide cells to maintain SG homeostasis when cell turnover in the gland is aberrantly accelerated60. These findings suggested that resident progenitors ensure homeostasis, but that bulge SCs can regenerate SGs when their resident progenitors are damaged or lost.
A niche outside the epidermis and the bulge
Another population of putative SCs was recently identified in a region between the bulge and the SG of the HF. Known as the upper isthmus (UI), cells in this zone of the HF are marked by their expression of the cell surface marker MTS24 (REF. 63). In vitro, MTS24-positive cells were found to possess higher clonogenic potential than MTS24-negative cells. Moreover, transplanted UI cells reformed all three epidermal lineages64, which suggests that they might even be multipotent63,64 (FIG. 2). New lineage-tracing experiments are now needed to determine whether UI progenitors are activated bulge cell progeny or whether they are independent, long-term, self-renewing SCs that can maintain the long-term homeostasis of this discrete compartment.
Signalling in development and homeostasis
Among the molecular signals that govern the complex epithelial–mesenchymal interactions that underlie epithelial morphogenesis and homeostasis (BOX 4), Wnt and bone morphogenetic protein (BMP) signalling have substantial roles in the specification and activation of the HF SCs during development and in the adult.
Box 4 epithelial–mesenchymal interactions in the epidermis.
Hair follicle (HF) morphogenesis begins during embryonic development through complex and reciprocal epithelial–mesechymal interactions. The first recognizable feature of HF morphogenesis is the formation of the placode, an epidermal invagination that occurs at the site of an underlying dermal condensation of mesenchymal cells. The dermal condensate is the precursor of the dermal papilla (DP), which provides potent inductive signals that drive the hair cycle and produce the hair shaft in the adult. The production of the hair shaft is fuelled by the matrix cells of the bulb. These are transit-amplifying cells that surround and receive signals from the DP, although the precise nature of these signals remain to be determined.
It has always been a mystery as to why the growth phase of the hair cycle suddenly comes to a halt and the lower two-thirds of the follicle degenerates rapidly by a mechanism that resembles programmed cell death. The DP is essential for follicle formation in the embryo and for initiating the next period of hair growth in the adult104. Exactly how the DP imposes its powers to initiate the new cycle of hair follicle regeneration is not clear. It is possible that the mesenchymal–epithelial crosstalk is constant during the resting stage and leads to the temporal accumulation of stimulatory factors that induce stem cell (SC) activation following a certain threshold level. Alternatively, it could be that DP–epithelial interactions trigger dynamic cellular changes that culminate in the temporal expression of the growth stimulus towards the end of the resting phase. This could be accomplished by either a decrease in the expression of growth inhibitors, by an increase of growth promoting factors or by both mechanisms, as recently proposed79.
Wnt signalling at the root of follicle specification
In the embryo, Wnt/β-catenin signalling is the earliest molecular signal that is required to instruct epithelial cells to adopt a HF fate65. Placodes, which are epidermal invaginations that occur at the sites of underlying dermal condensations of mesenchymal cells, fail to form when the gene that encodes β-catenin is mutated66. β-catenin is the adhesion molecule that is required to transmit the Wnt signal from the epidermal membrane to the nucleus. A similar block in HF morphogenesis occurs following the overexpression of the soluble Wnt inhibitor Dickopff-related protein 1 (DKK1)67. Conversely, a constitutively stabilized form of β-catenin triggers the skin epithelium to undergo spontaneous de novo HF morphogenesis in various ectopic locations, such as the IFE, the SG and the follicle ORS68,69 (FIG. 3). Importantly, these ectopic HFs are replete with all of the other non-epithelial cells of the HF, including the DP and melanocytes. This emphasizes the dominant role of the epithelium in organizing the HF SC niche68-71. The early discovery of perturbed HF spacing following the superstabilization of β-catenin69 has provided the foundation for an intriguing model, in which a putative reaction–diffusion mechanism in normal skin identifies sites in which Wnts and Wnt inhibitors are balanced. These sites, in turn, establish the locations in which HFs will emerge72.
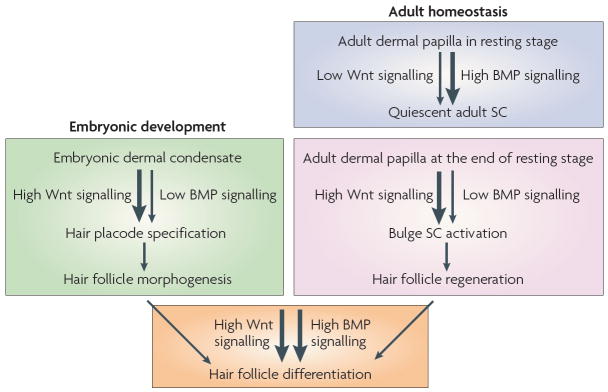
Figure 3. Similar mechanisms regulate morphogenesis and homeostasis.
Loss- and gain-of-function studies in mice highlight the different functions of Wnt/β-catenin and bone morphogenetic protein (BMP) signalling during morphogenesis and adult skin homeostasis. During hair follicle development, Wnt/β-catenin signalling is required to specify the hair follicle fate in the undifferentiated basal epidermis. During the adult hair cycle, increased Wnt signalling promotes stem cell (SC) activation to initiate the growth of a new hair during the transition from resting to growing stage. An even more robust response to Wnt signalling is involved later as matrix cells specifically commit to terminal differentiation along the hair shaft lineage. BMP signals are transmitted from the underlying mesenchyme to the epidermis. As dermal condensates form, the prospective dermal papilla expresses the BMP inhibitor noggin. Noggin is required for normal follicle development and stimulates lymphoid enhancer-binding factor 1 (LEF1) expression and Wnt signalling. During the adult hair cycle, active BMP signalling propels bulge SC quiescence during the resting stage. During the transition from resting to growing stage of the hair cycle, the levels of dermal BMPs and BMP inhibitors change, resulting in a net inhibition of BMP signalling and SC activation. As the new follicle matures, activation of BMP receptor signalling is essential for the differentiation of transit-amplifying matrix cells to form the hair shaft and its channel.
In adult follicles, when stabilized β-catenin is artificially elevated in resting SCs, HFs are precociously induced to begin a new round of hair growth68,73,74. This is consistent with the detected accumulation of nuclear β-catenin in the secondary hair germ at the transition from the resting to growing phase of normal HFs (FIG. 3). Wnt/β-catenin signalling also acts at a later step in the hair cycle, when matrix cells are stimulated to differentiate to make the hair shaft65,75. During severe injury, a Wnt-dependent wound signal can induce local de novo HF formation from the IFE, thereby recapitulating embryonic HF development76. Collectively, these results underscore the major influence of Wnt signalling in promoting HF progenitor specification. They also highlight the multipotency of IFE SCs, which, through an often underused mechanism, can adopt an HF fate in response to Wnt signals during physiopathological conditions76.
Although the post-translational regulatory mechanisms that are involved are likely to be complex, the stabilization of β-catenin enables it to function as a nuclear transcription cofactor for the lymphoid enhancer-binding factor 1 (LEF1)/T-cell factor (TCF) family of DNA-binding proteins77. In the quiescent bulge, in which nuclear β-catenin is either low or absent, TCF3 seems to be a transcriptional repressor of genes that govern all three programmes of terminal differentiation that are afforded to bulge SCs75,78. By contrast, as β-catenin becomes stabilized and localized to the nucleus in the activated hair germ73,75, a number of transcriptional changes occur, which include those that regulate cell proliferation, extracellular matrix remodelling and HF fate specification73. Matrix cells differ from bulge cells in that they express LEF1 rather than TCF3. Additionally, matrix cells respond to Wnt/β-catenin signalling by activating hair-specific keratin genes and subsequently undergo terminal differentiation75. The mechanisms by which Wnt/β-catenin signals govern these distinct stages of HF homeostasis are being revealed.
Despite the importance of Wnt signalling in follicle SC activation and fate specification, ectopic levels of stabilized β-catenin do not cause bulge SCs to lose their slow-cycling characteristics73. The relative resistance of bulge SCs to elevated β-catenin signalling is also shown by the rarity of de novo follicle formation originating from bulge SCs68, as well as the ability of the bulge to maintain its constant cell number73. These findings imply that additional factors are required to promote bulge SC activation of Wnt target genes.
BMP signalling regulates SC quiescence
Increasing evidence has pointed to the view that an equilibrium between secreted BMPs and their soluble inhibitors, for example, noggin and gremlin, has an important role not only in HF morphogenesis but also in HF SC activation during adult homeostasis. BMP2 and BMP4 are expressed by embryonic mesenchyme79 and noggin is expressed in the embryonic DP80. Loss-of-function mutations in noggin results in a profound decrease in the specification and developmental progression of HFs in embryonic skin. The morphogenetic effect of noggin is mediated in part by promoting the expression of LEF1 (REFS 80,81), thereby enabling the cells to respond to Wnt/β-catenin signalling and change the programme of gene expression that is necessary for follicle fate specification80-82.
In the adult, gain- and loss-of-function studies in mice suggest that BMP signalling stimulates bulge SC quiescence35,83-85 (FIG. 3). The resting stage of the hair cycle can be subdivided into two stages, during which bulge SCs are either refractory or sensitive to activation86. Interestingly, and somewhat surprisingly, cyclic BMP2 and BMP4 expression in the dermis seems to dictate the competence of bulge SCs for activation and HF regeneration79 (FIG. 3). During the refractory stage of telogen, BMPs are highly expressed by a range of dermal cells, including fibroblasts, adipocytes and DP. This results in the nuclear localization of phosphorylated SMAD1, 5 and 8 — the transcriptional effectors of BMP signalling — in the bulge SCs.
Recent studies suggest that bulge SC quiescence that is induced by BMP signalling is governed in part by its ability to regulate the transcriptional repressor nuclear factor of activated T cells C1 (NFATC1)87.
Dermal BMP signals progressively diminish during the resting period of the HF cycle. This promotes the switch from quiescent to activated HF SCs79 (FIG. 3). The nuclear localization of NFATC1 is lost following follicle SC activation, which coincides with the activation of genes that are involved in cell cycle regulation, including cyclin dependent kinase 4 (Cdk4), which regulates progression into the DNA-synthesis phase of the cell cycle73. Interestingly, Cdk4 is a direct target of NFATC1 in the quiescent bulge87. These studies provide new insights into how BMPs might participate in controlling bulge SC quiescence and how this molecular brace is released when BMP signalling wanes.
In addition to regulating bulge SC behaviour, BMP signalling also maintains the hair-inducing activity of DP35,88,89. Moreover, in response to BMPs, DP cells activate a feedback mechanism that leads to increased expression of BMP inhibitors79,90. If timed correctly, this spike could provide a stimulus to further restrict BMP signalling in the sensitive phase of telogen35,88 and promote bulge SC activation and hair regeneration79,90,91. In this regard, it is notable that the cyclic expression of BMP in the dermis is out of phase with the follicle SC Wnt/β-catenin expression cycle, and could provide a potential means to govern the hair cycle79.
Once bulge SCs have been activated, downstream signals are required to maintain the growth and differentiation phase of the hair cycle. Sonic hedgehog (SHH) has long been known to be a key signalling pathway that operates downstream of Wnts and is essential for maintaining the hair proliferative phase70,92,93. However, Notch signalling functions in promoting the early steps of differentiation in the HF, as it does in the epidermis11,13,94. Finally, like Wnts, BMPs also resurface at later stages of the hair cycle, where they influence the decision of matrix cells to choose between the hair shaft and IRS fates. Thus, whereas Wnts act through LEF1 to stimulate matrix cells to form the hair shaft75,95, BMPs stimulate matrix cells to express the transcription factor GATA3 and induce the IRS differentiation pathway84,85,96.
SCs in skin cancers
An important question for the future is whether adult skin SCs are the only agents that sufficiently accumulate mutations to cause cancer. This hypothesis is attractive because adult SCs exist and proliferate for a long time. Also, they do not protect their genome by asymmetric chromosome segregation43,45, providing further opportunities to accumulate oncogenic mutations and potentially induce cancer formation. Superimposed on the issue of the origin of cancer SCs is how many of them exist in a tumour. For most cancers, the initial target cell of oncogenic mutations is unknown, as are the numbers of so-called cancer SCs in a tumour. This is also true for skin cancers, which are the most prevalent cancers worldwide.
The recent identification of markers to specifically isolate normal epithelial SCs has enabled researchers to identify populations of skin cancer cells that share biochemical and behavioural characteristics of SCs97. In one recent case, it was found that a large number of CD34-expressing cells that have tumour-initiating capacity reside in squamous cell carcinomas97. Additionally, the researchers found that β-catenin was essential for maintaining the tumorigenic potential of the CD34-positive cancer SCs.
These results are intriguing given earlier findings that mutations that stabilize β-catenin in an active form do not form squamous cell carcinomas, but rather cause pilomatricomas, a type of hair tumour98. Moreover, recent studies have shown that oncogenic SHH signalling, which can lead to basal cell carcinomas, is also dependent on canonical Wnt/β-catenin signalling for its maintenance99. Together, these findings underscore the role of Wnt signalling as the primary instigator of regulating SC behaviour and cell fate commitment during epidermal homeostasis and skin cancers, as first suggested over a decade ago69,95. As the mechanisms of skin epithelial homeostasis continue to unfold, the molecular explanations that underlie these fascinating results are likely to surface in the future.
Unsolved pieces of the homeostatic puzzle
This Review shows the similarities between epidermal and HF specification during embryonic development and the processes of SC activation and lineage commitment during adult homeostasis in the skin. The ability to genetically mark HF skin SCs and their progeny at different stages of lineage determination and differentiation has enabled researchers to purify and transcriptionally profile developmental progenitors and adult HF SCs35,41,48,100, and paves the way for the characterization of additional skin epithelial SC populations in the future. Studies have unveiled many new pieces of the complex puzzle of epidermal, SG and HF homeostasis. This Review has outlined some of these pieces, such as Wnt/β-catenin and BMP signalling, and explained their functions in the overall pathways involved, including the regulation of SC quiescence and the transition from SCs to TA and differentiating cells. Other components, such as the transcription factor LHX2 (REF. 100), MYC61,62, RUNX1 (REF. 101) and GATA3 (REF. 96), have been shown genetically to be important for SC maintenance and/or the commitment to differentiate. Although essential to the programme of homeostasis, it remains a mystery as to how they function in this way.
The existence of multiple pools of long-term SCs and progenitors that reside in the skin epithelium has raised many new fascinating questions. How many more skin SCs or progenitors are lurking in undiscovered niches? What is the hierarchical relation between multipotent bulge SCs and resident progenitors? How is the flux of bulge cells orchestrated such that these different resident niches are appropriately fuelled during skin homeostasis and/or physio-pathological conditions? Does ageing influence the number or the function of these different skin SCs?
Further studies are also needed to fully understand how different regulators are integrated at the molecular level to prompt SCs to embark on a specific lineage. What is the hierarchical organization of the transcriptional networks that are required to specify the epidermal, SG and HF cell fates? How does the surrounding microenvironment influence SC behaviour during normal homeostasis and in response to tissue injury? What other mechanisms regulate SC quiescence and stimulate SC activation during tissue regeneration? What are the target genes of these crucial transcription factors required for skin epithelial homeostasis? Do these transcription factors act in a specific or in a cooperative manner? Genome-wide chromatin immunoprecipitation coupled with transcriptional analyses will help to better understand how the regulatory gene network that underlies epidermal homeostasis and HF regeneration is integrated.
Acknowledgments
We thank our many colleagues in the field who contributed to our understanding of skin homeostasis. We apologize to those whose papers are not cited owing to space constraints. We thank W. Lowry, J. Nowak, Y. Hsu, P. Chi, M. Hack, G. Guasch, R. Yi, G. Lapouge and A. Vankeymeulen for their thoughtful comments and critical reading of the manuscript. C.B. is supported by the Human Frontiers in Science Program Organization (HFSPO), the Belgian Fund for Scientific Research (FRS/FNRS), Wallonia Region, the Schlumberger Foundation, and the European Research Council. E.F. is an investigator of the Howard Hughes Medical Institute and receives funding from the National Institutes of Health and the Starr Foundation.
- Hair follicle
A skin appendage that produces a hair.
- Gastrulation
A crucial stage during embryonic development that results in the formation of the three embryonic layers — ectoderm, endoderm and mesoderm — from which all tissues and organs arise.
- Dermis
The mesenchymal part of the skin that is rich in collagen fibres and contains fibroblasts, blood vessels and immune cells.
- Intermediate filament
(IF). A cytoskeletal filament that is 10 nm in diameter and exists in skin epithelium as a heteropolymer that is assembled from ~10,000 individual keratin proteins of two distinct types. The IF network provides the epidermal cells with resistance against mechanical stress.
- Desmosome
A robust cell–cell junction, which is composed of adhesive desmosomal transmembrane cadherins that associate intracellularly with two proteins — plakoglobin, which is a relative of β-catenin, and desmoplakin. These, in turn, link the structure to keratin intermediate filaments.
- Cornified
The transformation of cells into a horny-like material, such as horn and nails.
- SC niche
The specialized microenvironment that is necessary to support the maintenance and differentiation of stem cells (SCs).
- Mitotic spindle
The molecular apparatus that allows the segregation of chromosomes to daughter cells during cell division and is composed mainly of microtubules and proteins.
- Astral microtubule
A microtubule that is nucleated at the spindle pole and grows outwards towards the cell cortex it is involved in mitotic spindle positioning.
- Interfollicular epidermis
The skin epidermis located between the emergence of the periodically spaced hair follicles.
- Sebaceous gland
Attached to a hair follicle, this gland produces sebum, an oily substance that is made of lipids and cellular debris of sebaceous cells. The sebum helps to protect and waterproof the hair and the skin surface.
- Hair shaft
The structure that is composed of terminally differentiated keratinocytes and emerges from the skin surface as a hair.
- Inner root sheath
The inner channel of the hair shaft.
- Bulge
A specialized portion of the hair follicle that contains multipotent stem cells that give rise to all hair follicle cell lineages as well as sebocytes and cells of the interfollicular epidermis during physio-pathological conditions.
- Hair germ
An outgrowth emanating from the bulge that forms during the early stage of hair follicle regeneration and corresponds to early committed progenies of bulge stem cells.
- Dermal papilla
The dermal part of the hair follicle, which consists of a small cluster of specialized fibroblast cells that have an instructive role to epithelial cells during hair follicle specification and regeneration.
- Outer root sheath
The external layer of the hair follicle that is contiguous with the basal layer of the interfollicular epidermis.
- Upper isthmus
The part of the hair follicle between the top of the bulge and the infundibulum, the uppermost part of the hair follicle that contacts the interfollicular epidermis.
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
Cdk4 ∣ Hes1 ∣ Lgr5
The miRNA Registry: http://microrna.sanger.ac.uk/sequences/
miR-203
UniProtKB: http://www.uniprot.org
BLIMP1 ∣ BMP2 ∣ BMP4 ∣ DKK1 ∣ INSC ∣ KRT1 ∣ KRT5 ∣ KRT10 ∣ KRT14 ∣ LEF1 ∣ LGN ∣ NFATC1 ∣ Noggin ∣ NUMA1 ∣ Numb ∣ PAR3 ∣ PAR6 ∣ p63 ∣ SHH ∣ SOX9
FURTHER INFORMATION
Cédric Blanpain’s homepage: http://blanpainlab.ulb.ac.be/index.htm
Elaine Fuch’s homepage: http://www.rockefeller.edu/labheads/fuchs/intro.php
References
- 1.Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–458. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koster MI, Roop DR. Mechanisms regulating epithelial stratification. Annu Rev Cell Dev Biol. 2007;23:93–113. doi: 10.1146/annurev.cellbio.23.090506.123357. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs E, Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980;19:1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- 5.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nature Rev Mol Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 6.Senoo M, Pinto F, Crum CP, McKeon F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 7.Truong AB, Kretz M, Ridky TW, Kimmel R, Khavari PA. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev. 2006;20:3185–3197. doi: 10.1101/gad.1463206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mills AA, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 9.Yang A, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 10.Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004;18:126–131. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanpain C, Lowry WE, Pasolli HA, Fuchs E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006;20:3022–3035. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rangarajan A, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watt FM, Estrach S, Ambler CA. Epidermal Notch signalling: differentiation, cancer and adhesion. Curr Opin Cell Biol. 2008;20:171–179. doi: 10.1016/j.ceb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moriyama M, et al. Multiple roles of Notch signaling in the regulation of epidermal development. Dev Cell. 2008;14:594–604. doi: 10.1016/j.devcel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Pasolli HA, Williams T, Fuchs E. AP-2 factors act in concert with Notch to orchestrate terminal differentiation in skin epidermis. J Cell Biol. 2008;183:37–48. doi: 10.1083/jcb.200804030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi R, et al. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nature Genet. 2006;38:356–362. doi: 10.1038/ng1744. [DOI] [PubMed] [Google Scholar]
- 17.Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature. 2008;452:225–229. doi: 10.1038/nature06642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andl T, et al. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol. 2006;16:1041–1049. doi: 10.1016/j.cub.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lena AM, et al. miR-203 represses ‘stemness’ by repressing ΔNp63. Cell Death Differ. 2008;15:1187–1195. doi: 10.1038/cdd.2008.69. [DOI] [PubMed] [Google Scholar]
- 20.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Sen GL, Webster DE, Barragan DI, Chang HY, Khavari PA. Control of differentiation in a self-renewing mammalian tissue by the histone demethylase JMJD3. Genes Dev. 2008;22:1865–1870. doi: 10.1101/gad.1673508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frye M, Fisher AG, Watt FM. Epidermal stem cells are defined by global histone modifications that are altered by Myc-induced differentiation. PLoS ONE. 2007;2:e763. doi: 10.1371/journal.pone.0000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watt FM, Frye M, Benitah SA. MYC in mammalian epidermis: how can an oncogene stimulate differentiation? Nature Rev Cancer. 2008;8:234–242. doi: 10.1038/nrc2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smart IH. Variation in the plane of cell cleavage during the process of stratification in the mouse epidermis. Br J Dermatol. 1970;82:276–282. doi: 10.1111/j.1365-2133.1970.tb12437.x. [DOI] [PubMed] [Google Scholar]
- 25.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922.. Describes how the temporal regulation of spindle pole orientation controls epidermal stratification during embryonic development.
- 26.Clayton E, et al. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574.. Combines lineage tracing experiments and mathematical modelling and suggests that epidermal tail homeostasis does not require the existence of TA cells.
- 27.Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Gönczy P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nature Rev Mol Cell Biol. 2008;9:355–366. doi: 10.1038/nrm2388. [DOI] [PubMed] [Google Scholar]
- 29.Ro S, Rannala B. Evidence from the stop-EGFP mouse supports a niche-sharing model of epidermal proliferative units. Exp Dermatol. 2005;14:838–843. doi: 10.1111/j.1600-0625.2005.00366.x. [DOI] [PubMed] [Google Scholar]
- 30.Kolodka TM, Garlick JA, Taichman LB. Evidence for keratinocyte stem cells in vitro: long term engraftment and persistence of transgene expression from retrovirus-transduced keratinocytes. Proc Natl Acad Sci USA. 1998;95:4356–4361. doi: 10.1073/pnas.95.8.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghazizadeh S, Taichman LB. Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin. EMBO J. 2001;20:1215–1222. doi: 10.1093/emboj/20.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackenzie IC. Retroviral transduction of murine epidermal stem cells demonstrates clonal units of epidermal structure. J Invest Dermatol. 1997;109:377–383. doi: 10.1111/1523-1747.ep12336255. [DOI] [PubMed] [Google Scholar]
- 33.Ro S, Rannala B. A stop-EGFP transgenic mouse to detect clonal cell lineages generated by mutation. EMBO Rep. 2004;5:914–920. doi: 10.1038/sj.embor.7400218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rochat A, Kobayashi K, Barrandon Y. Location of stem cells of human hair follicles by clonal analysis. Cell. 1994;76:1063–1073. doi: 10.1016/0092-8674(94)90383-2.. The first paper to demonstrate that HF bulge SCs present a greater clonogenic potential and can reconstitute the IFE on transplantation.
- 35.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012.. This work, together with reference 52, show that the progeny of a single cultured bulge SC can differentiate in all cell lineages of the skin epidermis.
- 36.Trempus CS, et al. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120:501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- 37.Li A, Simmons PJ, Kaur P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci USA. 1998;95:3902–3907. doi: 10.1073/pnas.95.7.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k.. The first study to isolate and functionally characterize SCs and TA cells of the human skin epidermis.
- 39.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c.. The first study to suggest that HF SCs are slow-cycling cells that reside in the bulge region.
- 40.Braun KM, et al. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130:5241–5255. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 41.Tumbar T, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436.. Introduced a transgenic mouse model to fluorescently tag, isolate and functionally characterize slow-cycling cells in mice.
- 42.Wilson C, et al. Cells within the bulge region of mouse hair follicle transiently proliferate during early anagen: heterogeneity and functional differences of various hair cycles. Differentiation. 1994;55:127–136. doi: 10.1046/j.1432-0436.1994.5520127.x. [DOI] [PubMed] [Google Scholar]
- 43.Waghmare SK, et al. Quantitative proliferation dynamics and random chromosome segregation of hair follicle stem cells. EMBO J. 2008;27:1309–1320. doi: 10.1038/emboj.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaks V, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nature Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 45.Sotiropoulou PA, Candi A, Blanpain C. The majority of multipotent epidermal stem cells do not protect their genome by asymmetrical chromosome segregation. Stem Cells. 2008;26:2964–2973. doi: 10.1634/stemcells.2008-0634. [DOI] [PubMed] [Google Scholar]
- 46.Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- 47.Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009.. Shows that the slow-cycling bulge SCs are specified during embryogenesis, in which they function to make the SG, complete HF morphogenesis and efficiently repair epidermal wounds.
- 48.Morris RJ, et al. Capturing and profiling adult hair follicle stem cells. Nature Biotech. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 49.Ito M, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nature Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 50.Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358–1366. doi: 10.1096/fj.06-6926com.. References 48–51 show that during homeostasis, the IFE is maintained independently of HF SCs, but that during wound repair, HF cells contribute to the epidermis.
- 52.Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc Natl Acad Sci USA. 2005;102:14677–14682. doi: 10.1073/pnas.0507250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vidal VP, et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. 2005;15:1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 54.Ito M, Kizawa K, Hamada K, Cotsarelis G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation. 2004;72:548–557. doi: 10.1111/j.1432-0436.2004.07209008.x. [DOI] [PubMed] [Google Scholar]
- 55.Legue E, Nicolas JF. Hair follicle renewal: organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development. 2005;132:4143–4154. doi: 10.1242/dev.01975. [DOI] [PubMed] [Google Scholar]
- 56.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 57.Gallico GG, 3rd, O’Connor NE, Compton CC, Kehinde O, Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984;311:448–451. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- 58.Kobayashi K, Rochat A, Barrandon Y. Segregation of keratinocyte colony-forming cells in the bulge of the rat vibrissa. Proc Natl Acad Sci USA. 1993;90:7391–7395. doi: 10.1073/pnas.90.15.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barrandon Y, Green H. Cell size as a determinant of the clone-forming ability of human keratinocytes. Proc Natl Acad Sci USA. 1985;82:5390–5394. doi: 10.1073/pnas.82.16.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horsley V, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated expression of c-Myc depletes epidermal stem cells. Nature Genet. 2001;28:165–168. doi: 10.1038/88889. [DOI] [PubMed] [Google Scholar]
- 62.Arnold I, Watt FM. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol. 2001;11:558–568. doi: 10.1016/s0960-9822(01)00154-3. [DOI] [PubMed] [Google Scholar]
- 63.Nijhof JG, et al. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development. 2006;133:3027–3037. doi: 10.1242/dev.02443. [DOI] [PubMed] [Google Scholar]
- 64.Jensen UB, et al. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J Cell Sci. 2008;121:609–617. doi: 10.1242/jcs.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 66.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 67.Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- 68.Lo Celso C, Prowse DM, Watt FM. Transient activation of β-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131:1787–1799. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- 69.Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1.. The first study to point to the key role of Wnt signalling in HF specification and in inducing HF derived tumours.
- 70.Silva-Vargas V, et al. β-catenin and hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev Cell. 2005;9:121–131. doi: 10.1016/j.devcel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y, et al. Activation of β-catenin signaling programs embryonic epidermis to hair follicle fate. Development. 2008;135:2161–2172. doi: 10.1242/dev.017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science. 2006;314:1447–1450. doi: 10.1126/science.1130088. [DOI] [PubMed] [Google Scholar]
- 73.Lowry WE, et al. Defining the impact of β-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Mater D, Kolligs FT, Dlugosz AA, Fearon ER. Transient activation of β-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 2003;17:1219–1224. doi: 10.1101/gad.1076103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ito M, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 77.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen H, Rendl M, Fuchs E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell. 2006;127:171–183. doi: 10.1016/j.cell.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 79.Plikus MV, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Botchkarev VA, et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nature Cell Biol. 1999;1:158–164. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- 81.Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muller-Rover S, et al. E- and P-cadherin expression during murine hair follicle morphogenesis and cycling. Exp Dermatol. 1999;8:237–246. doi: 10.1111/j.1600-0625.1999.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 83.Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci USA. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol. 2003;163:609–623. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Andl T, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- 86.Paus R, Foitzik K. In search of the “hair cycle clock”: a guided tour. Differentiation. 2004;72:489–511. doi: 10.1111/j.1432-0436.2004.07209004.x. [DOI] [PubMed] [Google Scholar]
- 87.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal–epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–557. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Botchkarev VA, et al. Noggin is required for induction of the hair follicle growth phase in postnatal skin. FASEB J. 2001;15:2205–2214. doi: 10.1096/fj.01-0207com. [DOI] [PubMed] [Google Scholar]
- 91.Zhang J, et al. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells. 2006;24:2826–2839. doi: 10.1634/stemcells.2005-0544. [DOI] [PubMed] [Google Scholar]
- 92.St-Jacques B, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- 93.Oro AE, Higgins K. Hair cycle regulation of Hedgehog signal reception. Dev Biol. 2003;255:238–248. doi: 10.1016/s0012-1606(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 94.Pan Y, et al. γ-secretase functions through Notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Dev Cell. 2004;7:731–743. doi: 10.1016/j.devcel.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 95.Zhou P, Byrne C, Jacobs J, Fuchs E. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev. 1995;9:700–713. doi: 10.1101/gad.9.6.700. [DOI] [PubMed] [Google Scholar]
- 96.Kaufman CK, et al. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev. 2003;17:2108–2122. doi: 10.1101/gad.1115203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Malanchi I, et al. Cutaneous cancer stem cell maintenance is dependent on β-catenin signalling. Nature. 2008;452:650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- 98.Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in β-catenin. Nature Genet. 1999;21:410–413. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- 99.Hoseong Yang S, et al. Pathological responses to oncogenic Hedgehog signaling in skin are dependent on canonical Wnt/β-catenin signaling. Nature Genet. 2008;40:1130–1135. doi: 10.1038/ng.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rhee H, Polak L, Fuchs E. Lhx2 maintains stem cell character in hair follicles. Science. 2006;312:1946–1949. doi: 10.1126/science.1128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Osorio KM, et al. Runx1 modulates developmental, but not injury-driven, hair follicle stem cell activation. Development. 2008;135:1059–1068. doi: 10.1242/dev.012799. [DOI] [PubMed] [Google Scholar]
- 102.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 103.Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2.. The first study to suggest that the bulge region contains multipotent epidermal stem cells.
- 104.Hardy MH. The secret life of the hair follicle. Trends Genet. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]