Abstract
HIV-1 patients who abuse opiate-based drugs, including herion and morphine, are at a higher risk of developing HIV dementia. The effects of opiates are mediated predominantly through opioid receptors, which are expressed on glial cells. As HIV-1 infection in the CNS is restricted to glial cells, experiments were designed to measure the cell-specific effects of HIV Tat and morphine exposure on opioid receptor expression in both astrocytes and microglia. Specifically, the cell-type specific pattern of mu opioid receptor (MOR), delta opioid receptor (DOR), and kappa opioid receptor (KOR) localization (surface vs. intracellular) and levels of opioid receptor mRNA was determined following exposure to morphine in the presence and absence of Tat in primary cultured microglia and astrocytes. Data show that morphine treatment causes significantly decreased cell surface expression of opioid receptors in microglia, but not in astrocytes. However, morphine treatment in the presence of Tat significantly increased the intracellular expression opioid receptors and prevented morphine-induced cell surface opioid receptor down-regulation in microglia. These findings document that cell surface opioid receptor expression is divergently regulated by morphine in microglia compared to astrocytes, and further suggest that HIV-Tat could exacerbate opioid receptor signaling in microglia by increasing receptor expression and/or altering the ligand-induced trafficking of opioid receptors.
Keywords: HIV dementia, neuroinflammation, opioid receptors, glia
Introduction
Neurological abnormalities including dementia occur in a significant number of HIV-1 infected individuals, but the mechanism(s) by which HIV-1 infection perturbs brain homeostasis is not fully understood. The gross pathology of HIV dementia (HIVD) generally includes sulcal widening and ventricular dilation (Ensoli et al., 1999), while histologically, HIVD brains exhibit extensive microglial and astrocyte activation, decreased synaptic density, and in some cases neuronal loss (Navia et al., 1986; Everall et al., 1993; Spargo et al., 1993; Fox et al., 1997; Masliah et al., 1997). Despite changes in neuronal morphology and function in many HIVD brains, neurons are not infected by HIV. Microglia and astrocytes are the only brain-resident cells with the proper pattern of cell surface chemokine receptors to support productive HIV infection, and extensive glial activation is a characteristic feature of HIVD brain. Furthermore, markers of glial cell activation and inflammation have been reported to correlate well with HIVD (Glass et al., 1995; Tyor et al., 1995; Gray et al., 2001). However, the physiologic mechanisms mediating the generally widespread glial activation in HIVD brains are not completely understood.
HIV-Tat is a trans-activating regulatory protein of 72 to 101 amino acids encoded by 2 exons of the HIV genome (Sodroski et al., 1985). As Tat is actively secreted by HIV-1-infected cells and can interact with adjacent cells (Tardieu et al., 1992; Ensoli et al., 1993; Chirmule et al., 1995; Chang et al., 1997), it has been suggested that Tat could participate in the progression of HIVD. Indeed, Tat expression is elevated in brain tissue of patients with HIV dementia (Wesselingh et al., 1993; Hofman et al., 1994; Wiley et al., 1996), and is neurotoxic in vitro and in vivo (Hayman et al., 1993; Magnuson et al., 1995; Jones et al., 1998; Turchan et al., 2003; Turchan-Cholewo et al., 2006). Furthermore, Tat has been shown to be potently pro-inflammatory (Philippon et al., 1994; Chen et al., 1997; Albini et al., 1998; Bruce-Keller et al., 2001; Bruce-Keller et al., 2003; Pu et al., 2003), and immunocytochemical studies have localized Tat protein to microglia and astrocytes in the brains of AIDS patients (Kruman et al., 1998; Bonwetsch et al., 1999), suggesting that Tat may be an important mediator of HIV-mediated glial inflammatory responses in the brain.
While it is still not understood why only selected AIDS patients develop HIVD, it has been established that HIV infected individuals who also abuse opiate-based drugs have an accelerated rate of AIDS disease progression (Donahoe and Vlahov, 1998; Bell et al., 2002). Furthermore, opiate-addicted individuals, which may account for nearly one-third of HIV patients (UNAIDS, 2006), have a higher incidence of HIVD and have more severe neurocognitive and pathological abnormalities (Bell et al., 1996; Davies et al., 1997; Nath et al., 2002). It is not yet clear how opiate abuse exascerbates HIVD, but brains from opiate-abusing HIV patients appear to have increased numbers of macrophages/microglia (Bell et al., 2002; Anthony et al., 2005) which may help to accelerate HIVD. The biological effects of opiates are mediated primarily through the activation of opioid receptors, which have been well documented in the brain, particularly in terms of their analgesic effects on neurons and neuronal signaling (Pasternak, 2005). However, inflammatory brain responses, particularly to HIV infection, are very likely to be mediated by brain-resident glial cells, and opioid receptors are present on both astrocytes and microglia (Ruzicka et al., 1995; Chao et al., 1997; Stiene-Martin et al., 1998). Activation of glial opioid receptors by agonists such as morphine has been shown to dramatically alter immune responses (Rojavin et al., 1993; Chao et al., 1997; El-Hage et al., 2005), (Mahajan et al., 2005; El-Hage et al., 2006), which could impair local brain anti-viral responses and accelerate HIV-associated neurological dysfunction. However, opiate-HIV interactions are still poorly understood and the degree to which opiate abuse per se contributes to the progression to HIVD is controversial (Donahoe, 2001; Everall, 2004). Thus, to better understand opioid receptor signaling in glia, the modulatory effects of Tat and morphine on opioid receptor expression was measured in cultured glial cells. Specifically, the cell-type specific pattern of mu opioid receptor (MOR), delta opioid receptor (DOR), and kappa opioid receptor (KOR) localization (surface vs. intracellular) and levels of opioid receptor mRNA were determined following exposure to morphine in the presence and absence of HIV-Tat in N9 microglial cells, primary rodent microglia, and primary rodent astrocytes.
Materials and Experimental Procedures
Materials
All cell culture media and serum were obtained from Invitrogen Sciences (Carlsbad, CA). Morphine was purchased from Sigma (St. Louis, MO). The following primary antibodies were used for immunocytochemistry and FACS analyses: anti-MOR (AB1580) and anti-DOR (AB1560) from Chemicon, Inc (Temecula, CA), anti-KOR (AB10283) from ABCAM Inc. (Cambridge, UK), anti-glial fibrillary acidic protein (MAB360) from Chemicon, anti-F4/80 (MCA497) from Serotec (Raleigh, NC) and anti–L1 (MAC387) from Serotec.
Recombinant Tat 1-72 was produced using the Tat gene sequence from HIV-1BRU (obtained from Dr. Richard Graynor, through the AIDS repository at the NIH) and purified as described previously (Chen et al., 1997; Turchan-Cholewo et al., 2006).
Cell culture
N9 microglial cells were grown in Iscove's Modified Dulbecco's Medium with 10% heat-inactivated FBS. Primary microglia and astrocytes were established from the forebrains of 1-day old Sprague Dawley rats as previously described (Bruce-Keller et al., 2000). In brief, forebrains were isolated under sterile conditions, the meninges removed, and the tissue cut into 0.5 mm pieces. The cells were dissociated by mild trypsinization, followed by trituration. Mixed glial cells were grown to confluence in 75 cm flasks at 37°C/5%CO2 in Dulbecco's Modified Eagle Medium containing 10% heat-inactivated FBS and 2 mM glutamine. Microglial cells were removed from confluent astroglial layers flasks by differential panning (flasks rotated on an orbital shaker at 180 rpm for 20-30 min), and plated into PEI-coated 48 well plates or 35mm dishes at a density of 5×104 cells/cm2. After 1-2 h, the medium was changed to remove nonadherent cells, and experiments were initiated 24 h after plating. Following removal of the microglia, the astrocytes were removed from the flasks by trypsonization, and the purified astrocytes were plated into PEI-coated 48 well plates or 35mm dishes at a density of 5×104 cells/cm2. The purity of the cell cultures (90-95%) was confirmed using antisera to either GFAP for astrocyte cultures or CD11b (OX-42, Serotec) and/or L1 (MAB387) for microglial cultures. All cells were treated in phenol red-free medium supplemented with 1% FBS.
Immunocytochemistry
For immunostaining, cultured microglia or astrocytes were fixed for 30 min with 4% paraformaldehyde at room temperature, and blocked for an additional 30 min with 1.5% goat serum and 0.1% Triton x-100 in PBS (pH 7.4). Immunocytochemistry for opioid receptors and cell markers was conducted as previously described (Bruce-Keller et al., 2000) (Turchan et al., 2001). Cells were incubated in primary antibody diluted in blocking buffer for 24 h at 4°C, followed by fluorochrome-conjugated secondary antibodies. After the final wash, the cells were air dried in the dark and mounted on glass slides with ProLong™ Antifade mounting media containing DAPI (Sakura Finitek USA, Torrence, CA) to stain nuclei. Non-specific staining was determined by omission of the primary antibody from the immunostaining procedure, while double-staining procedures were conducted sequentially and employed secondary antibodies from different species. Images of immunostained cells were acquired using a fluorescence microscope equipped with a digital camera (Zeiss).
FACS analysis of opioid receptor-positive microglia and astrocytes
Cells were harvested by incubation with 0.5 mM EDTA in PBS at 37 °C for 5 min. For cell surface staining, cells were then washed once, resuspended at 1×107 cells/ml and incubated with 1–2 μg primary antibody for 1 h at 4°C. For intracellular staining, cells were first fixed and permeabilized using Leucoperm, following the manufacturer's instructions (Serotec). Briefly, cells were fixed using a proprietary formulation (Reagent A) for 25 minutes at 4°C, and then permeabilized with Reagent B for 30 min at 25°C. All cells were then washed and exposed to primary antibody for 1 h at 4°C, then washed twice, and incubated with RPE-conjugated goat F(ab')2 secondary antibody (10 μg/ml; Leinco Technologies, St. Louis, MO). The cells were then again washed by centrifugation and resuspended. Data were immediately acquired on a FACSCalibur cytometer (BD Biosciences, San Jose, CA) with detection of PE fluorescence at 585 nm and flow cytometric analysis performed using CellQuest Pro software (BD Biosciences, Mountain View, CA). Data represent the % of the total cell population gated positive for each respective receptor antibody, with each treatment performed in triplicate and with each data point acquired from at least 1×106 cells. Background autofluorescence was measured in cells treated with 100 μl buffer in place of fluorescent antibodies, and live cells were gated using a propidium iodide-stained control at 1 μg/106 cells. Flow cytometric analysis was performed using CellQuest Pro software.
PCR methods
Quantitative real time PCR was directed as described in pervious reports (Ding et al., 2003), with PCR reactions conducted using one-step (Invitrogen) methods with cycle number monitored using SYBR® Green. The procedure started with reverse transcription of total RNA, and the cDNA was used as template for real time PCR with gene specific primers. All data were normalized to 18S mRNA (for murine N9 cells) or GAPDH mRNA (for rat primary microglia and astrocytes).
For quantitative real time PCR analysis of opioidergic receptor expression, total RNA was collected from cells, and 150 ng of total RNA was amplified according to the manufacturer's instructions using SYBR® Green PCR Master Mix reagent. Primers used for amplification were as follows; rat 5′(279bp); rat DOR: 5′-AGA GCG CCA AGT ACC TGA TGG AAA-3′,3′-AGG AAC ACG CAG ATC TTG GTC ACA-5′ (342bp); rat KOR : 5′-GGG CAA TTC CCT GGT CAT GTT TGT-3′,3′-AAT CCA AAG CTT TCA CAG GGT GGC-5′ (280bp); rat GAPDH: 5′-ATG CCA GTG AGC TTC CCG TT-3′,3′-GAA CAT CAT CCC TGC ATC CA-5′ (85 bp); mouse MOR: 5′-AGT TCT GCA TCC CAA CTT CCT CCA-3′,3′-ACA CTC ACC TGT CCA TGC AAC TCT-5′(209bp); mouse DOR: 5′-AGC TCA GTG GTA GGG TGT TTG TGT-3′,3′-ACT ATG TGG GTG CTG GGA ATC GAA-5′ (360bp); mouse KOR : 5′-TGC CTT TGT GAT CCC AGT CCT CAT-3′,3′-ACA GGA TTC AGG CTG CTG TTG GTA-5′ (284bp) and mouse 18S: 5′-CAC TTG TCC CTC TAA GAA GTT G-3′,3′-GAC AGG ATT GAC AGA TTG ATA G-5′(174bp). Following amplification one major band was detected by gel electrophoresis, with all amplifications adhering to the SYBR® Green dissociation protocol. Data were collected and analyzed using ABI Prism 7000 sequence detector system (Applied Biosystems, Warrington, UK).
Statistical analyses
All data were analyzed using one-way ANOVA, followed by Tukey's post-hoc analysis to determine statistical significance; p values < 0.05 were designated as statistically significant, and are indicated as *, **, or *** corresponding to p values < 0.05, < 0.01, or < 0.001, respectively.
Results
1. Immunocytochemical evaluation of opioid receptors in cultured glia
Opioid receptors are expressed on glial cells (Ruzicka et al., 1995; Chao et al., 1997; Stiene-Martin et al., 1998; Thorlin et al., 1999; Dimitrijevic et al., 2000), but systematic comparisons of opioid receptor expression and regulation on microglia versus astrocytes have not been carried out. To visually evaluate the basal pattern of MOR, DOR, and KOR expression on cultured glial cells, opioid receptor expression was qualitatively evaluated in untreated N9 microglia, primary microglia, and primary astrocytes using immunocytochemistry as described in Methods. N9 microglial cells were used in addition to the primary microglial cells in this study because they represent a homogenous population of pure cells, and they also have the advantage of a reliably high yield. Additionally, this particular cell line has been used in the past to study many aspects of microglial activation, inlcuding the roles of tyrosine phophorylation (Lockhart et al., 1998), NFκB activation (Boniauto et al., 1997), and MAPK pathways (Bruce-Keller et al., 2000; Bruce-Keller et al., 2001). N9 cells, which were all F4/80 positive, displayed prominent but diffuse non-nuclear MOR immunoreactivity (Fig. 1 A). In cultures of primary microglia, cell specificity was determined using an antibody to L1 antigen, an intracellular, formalin-resistant marker of microglia and other tissue macrophages (Brandtzaeg et al., 1988; Foster et al., 2006). Cultured primary microglia were 90-95% positive for L1 antigen, and also expressed MOR more variably, but also in a diffuse, cytosolic pattern (Fig. 1B). The purity of the primary astrocyte cultures was verified using GFAP, and likewise, the cultured astrocytes displayed prominent cytosolic MOR immunoreactivity (Fig. 1C). Prominent DOR immunoreactivity was also observed in both primary microglia and astrocytes (Fig. 2), with DOR expression in some astrocytes forming distinct punctuate deposits around the membranous edges of the cells (Fig. 2B). Finally, KOR immunoreactivity was detected in both primary microglia and astrocytes (Fig. 3). KOR expression microglia was diffuse (Fig. 3A), while more punctuate in astrocytes (Fig. 3B). It is noteworthy that the astrocyte culture procedure outlined herein appeared to yield high proportions of MOR, DOR, and KOR expressing astrocytes. Opioid receptor expression among astrocytes is quite variable (Ruzicka et al., 1995; Stiene-Martin et al., 1998; Thorlin et al., 1999). The astrocyte culture method outlined herein may provide a means of propagating large numbers of relatively homogenous opioid receptor expressing cells.
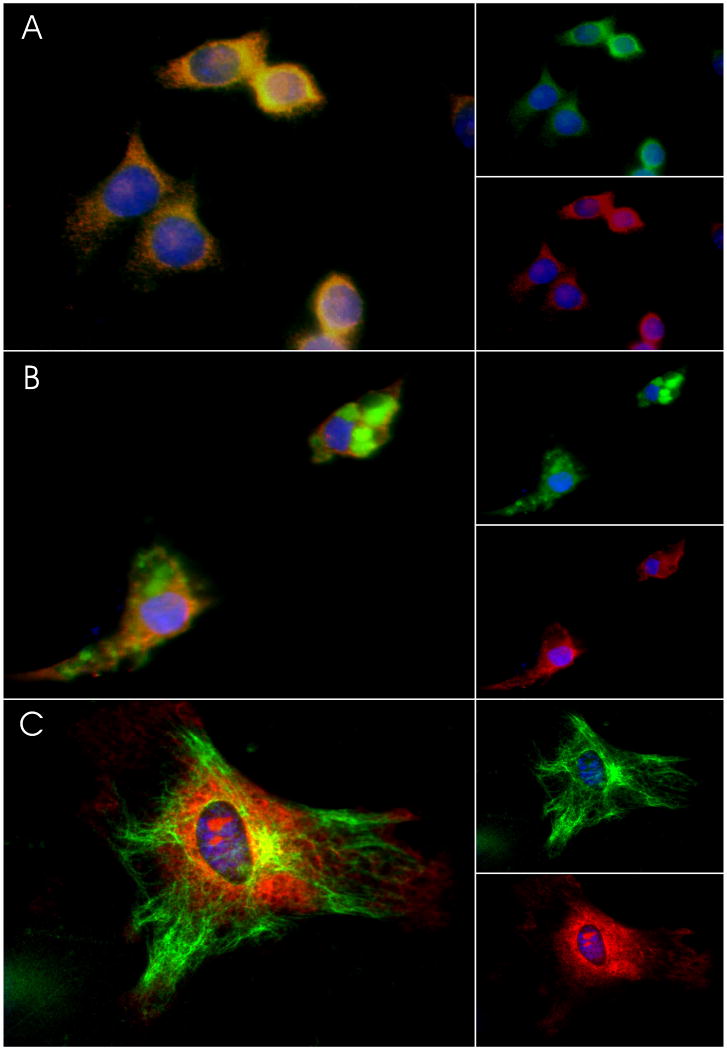
Figure 1. MOR Immunoreactivity in cultured glia.
Cultured glial cells were fixed and sequentially double-labeled with antibodies to cell-markers and to MOR as described in methods. Cells were then exposed to DAPI to label all nuclei. (A) Representative images of N9 cells depict the pattern of co-localization (left panel) of immunostaining with antibodies against F4/80 (green, top right panel) and against MOR (red, lower right panel) (B) Primary microglia were stained with antibodies against anti-L1 (green, top right panel) and against MOR (red, lower right panel), also showing significant co-localization (left panel). (C) Primary astrocytes were double-labeled (left panel) with antibodies against GFAP (green, top right panel) and against MOR (red, lower right panel).
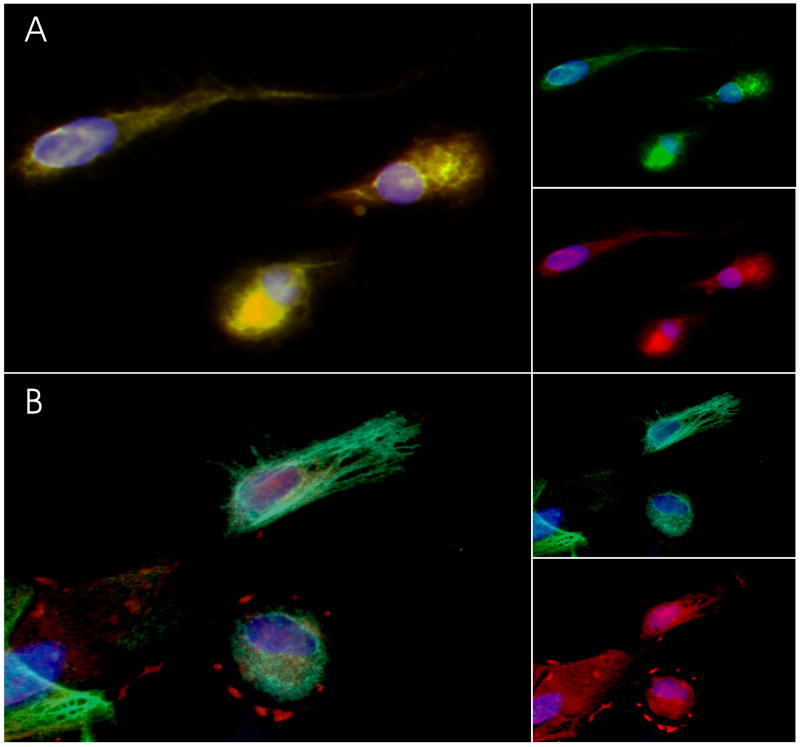
Figure 2. DOR Immunoreactivity in cultured glia.
Cultured glial cells were fixed and sequentially double-labeled with antibodies to cell-markers and to DOR as described in methods. Cells were then exposed to DAPI to label all nuclei. (A) Representative images of primary microglia depict the pattern of co-localization (left panel) of immunostaining with antibodies against anti-L1 (green, top right panel) and against DOR (red, lower right panel). (B) Primary astrocytes were double-labeled (left panel) with antibodies against GFAP (green, top right panel) and against DOR (red, lower right panel).
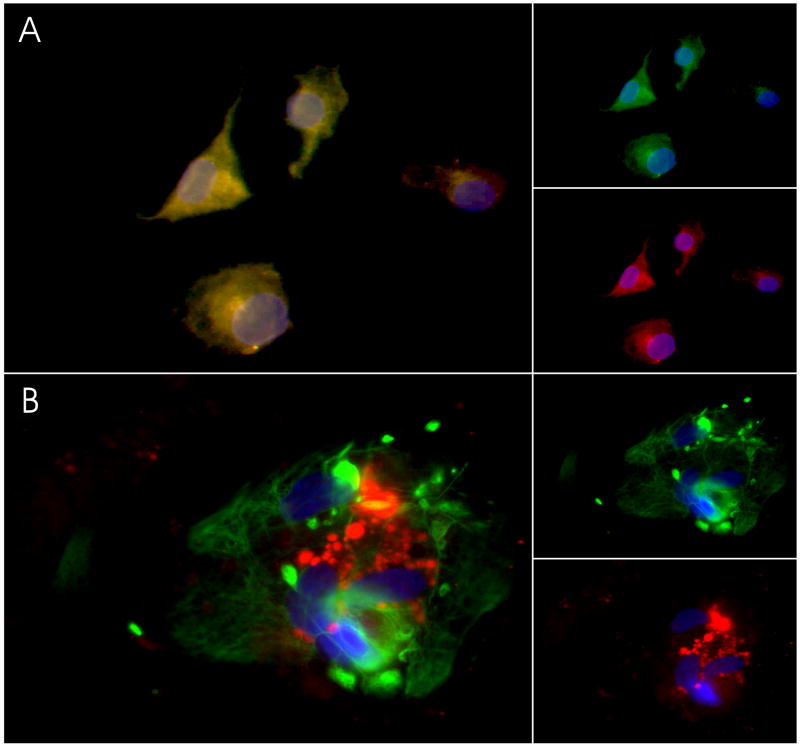
Figure 3. KOR Immunoreactivity in cultured glia.
Cultured glial cells were fixed and sequentially double-labeled with antibodies to cell-markers and to KOR as described in methods. Cells were then exposed to DAPI to label all nuclei. (A) Representative images of primary microglia depict the pattern of co-localization (left panel) of immunostaining with antibodies against anti-L1 (green, top right panel) and against KOR (red, lower right panel). (B) Primary astrocytes were double-labeled (left panel) with antibodies against GFAP (green, top right panel) and against KOR (red, lower right panel).
2. Effects of Tat and morphine on regulation of opioid receptor expression in microglia
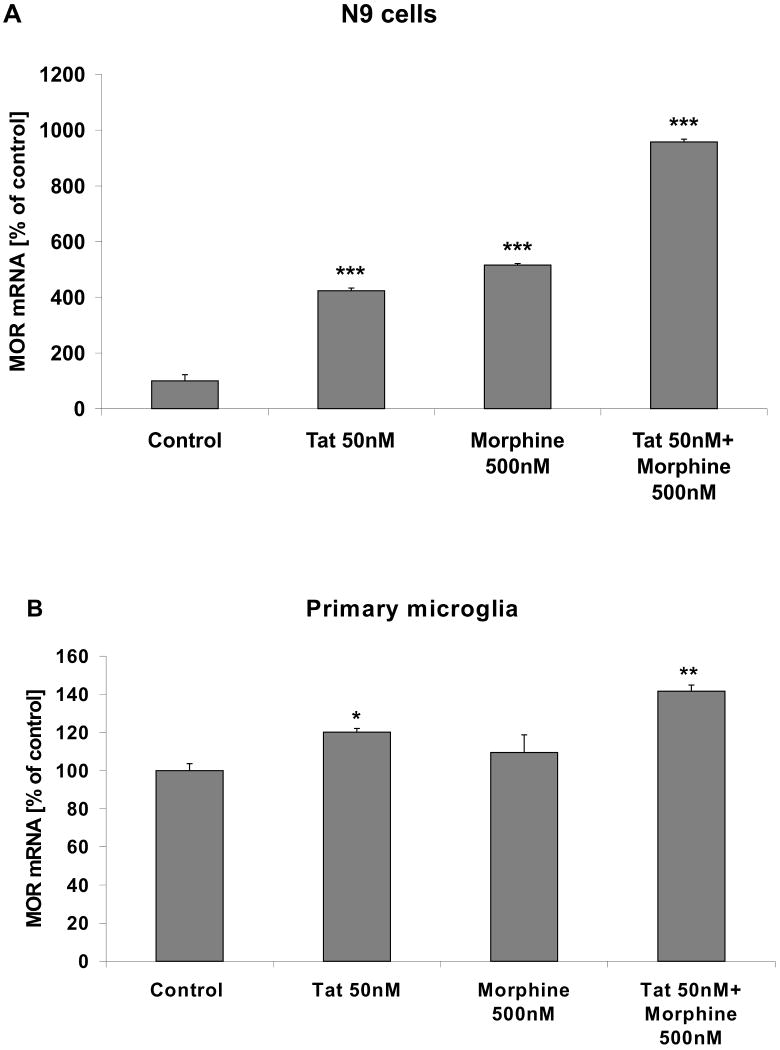
While there were some observable gross differences in the immunocytochemical patterns of opioid receptor immunoreactivity observed in cultured glia, opioid receptors are well known to be subject to complex ligand-dependent regulation (reviewed in (Connor et al., 2004; von Zastrow, 2004)). To thus determine if ligand-induced regulation of opioid receptors was different in microglia versus astrocytes, cell surface and intracellular expression of opioid receptors was determined in microglia using sensitive and quantitative FACS techniques as described in Methods. Experiments were designed to also evaluate if the presence of HIV viral proteins could alter ligand-induced changes in opioid receptor expression. To this end, cultured N9 microglial cells and primary microglia were grown as described in Methods and treated with 500 nM morphine, 50 nM Tat, or combined Tat and morphine and for 24 h. Drug doses were chosen based on preliminary experiments designed to determine optimal concentrations of both Tat and morphine to alter microglial inflammatory signaling without affecting viability (data not shown). Cells were then harvested and both cell surface and intracellular MOR expression was analyzed using FACS. Data show that exposure to 500 nM morphine for 24 h significantly decreased the proportion of N9 cells (Fig. 4A) and primary microglia (Fig. 4B) positive for cell surface MOR localization. The effects of morphine on surface MOR expresion in microglia were not apparent at earlier timepoints (3-6 h, data not shown), and appeared to be dependent on ligation of the receptors, as morphine-induced decreases in surface MOR were prevented when cells were pretreated with the mu-specific receptor antagonist β-FNA (3μM, data not shown). Treatment of N9 cells or primary microglia with 50 nM Tat did not significantly affect surface MOR expression on its own, but attenuated the effects of morphine on cell surface MOR expression (Fig. 4A, B). Additionally, combined Tat/morphine exposure significantly increased intracellular expression of MOR in microglia (Fig 4A, B), suggesting that combined Tat/morphine exposure caused an increase in the total pool of microglial MOR. Furthermore, evaluation of MOR mRNA 24 h after application of 50 nM Tat, 500 nM morphine, or combined Tat/morphine showed that in both N9 cells (Fig. 5A) and in primary microglia (Fig. 5B), combined Tat/morphine treatment caused significant increases in MOR mRNA, suggesting that Tat increases surface MOR via increases in receptor synthesis. Furthermore, experiments in which cells were treated for 24 hrs with DAMGO ([D-Ala2, N-MePhe4. Gly-ol5] enkephalin), which is well-known to induce MOR internalization (Xu et al., 2007) did not suggest that Tat blocks internalization, as Tat did not affect DAMGO-induced loss of surface MOR expression (500 nM DAMGO: 82.1 ± 7.84 % of control; 50 nM Tat/500 nM DAMGO: 89.5 ± 4.2 % of control). Finally, when N9 cells were treated for 24 h with brefeldin A (5 μM) and Monensin (50 μM) to block the trafficking of nascent proteins from endoplasmic reticulum to the golgi to the membrane (Mollenhauer et al., 1990; Donaldson et al., 1992) cell surface expression of MOR was decreased by over 35% (data not shown), indicating that a continuous supply of newly synthesized proteins is necessary to maintain stable expression of cell surface opioid receptors. Thus, these data collectively indicate that Tat increases MOR expression via increases in receptor synthesis rather than interfering with internalization and/or trafficking.
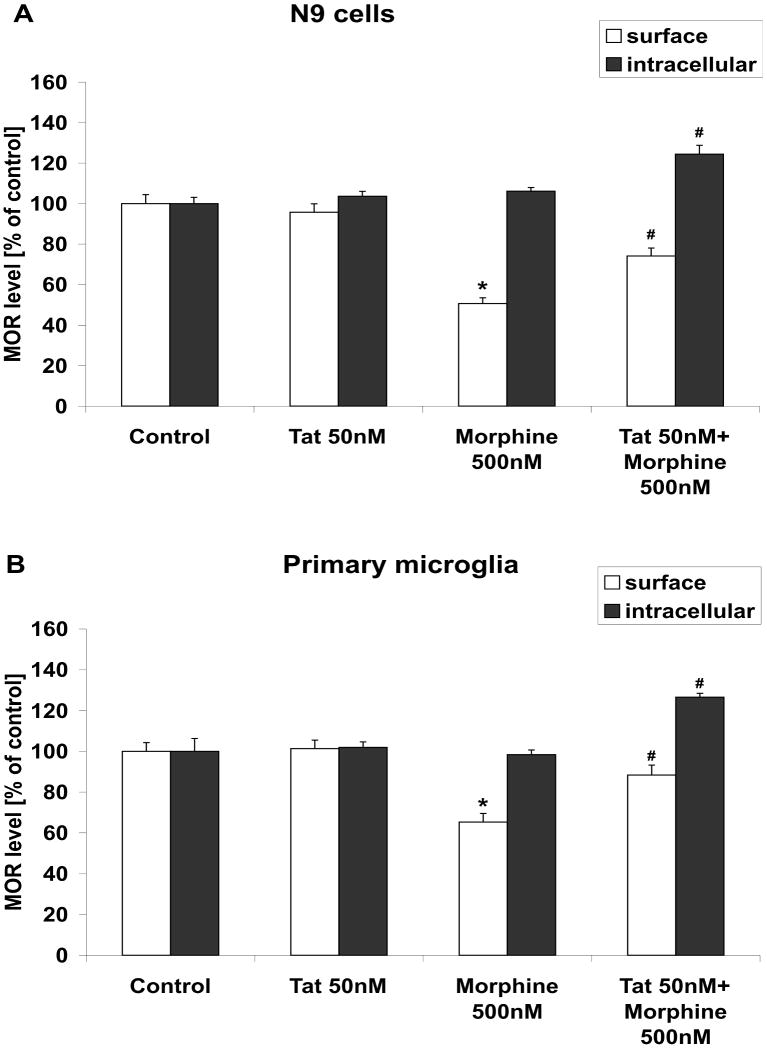
Figure 4. Effects of Tat and morphine on cell surface and intracellular MOR expression in microglia.
Cultured (A) N9 microglial cells and (B) primary microglia were grown as described in Methods, and treated with vehicle, 50 nM Tat and/or 500 nM morphine for 24 h as described in Methods. Cells were then harvested and the percentage of cells positive for cell surface and intracellular MOR expression was analyzed using FACS as described in Methods. All data were analyzed by ANOVA, and are presented as means ± SEM in relation to control cells. Numerical values for the percentage of control N9 and primary microglial cells positive for cell surface MOR were 48% and 44%, respectively; while numerical values for the percentage of control cells positive for intracellular MOR in N9 cells and in primary microglia were 70% and 53%, respectively. * indicates the statistically significant decrease (p<0.05) in cell surface MOR expression in morphine-treated cells compared to control cells, while # indicate statistically significant increases (p<0.05) in cell surface or intracellular MOR expression in Tat/morphine-treated cells compared to microglial treated with morphine alone.
Figure 5. Effects of Tat and morphine on MOR mRNA expression in microglia.
Cultured (A) N9 microglial cells and (B) primary microglia were grown as described in Methods, and treated with vehicle, 50 nM Tat and/or 500 nM morphine for 24 h as described in Methods. Cells were then harvested, mRNA extracted, and MOR expression was analyzed by real-time PCR as described in Methods. Data were analyzed by ANOVA, and *, **, *** indicate statistically significant differences (p<0.05, p<0.01, and p<0.001, respectively) in mRNA expression compared to vehicle-treated (control) microglia.
Similar effects were observed when surface, intracellular, and mRNA levels of DOR and KOR were analyzed in N9 cells following treatment with morphine in the presence or absence of Tat. Specifically, 500 nM morphine significantly decreased cell surface levels of DOR and KOR, but co-administration of 50 nM Tat prevented morphine-induced downregulation of DOR and KOR in N9 cells (Table 1). Likewise, administration of 500 nM morphine to primary microglia decreased cell surface levels of DOR and KOR, with co-administration of 50 nM Tat reversing morphine-induced decreases in surface receptors (Table 2). Finally, while Tat treatment did prevent morphine-induced decreases in cell surface expression of DOR and KOR and primary microglia, it was also noted that treatment with Tat treatment in the absence of morphine caused a decrease in KOR and DOR cell surface expression in primary microglia (Table 2).
Table 1. Protein and mRNA expression of DOR and KOR in N9 cells.
N9 microglial cell lines were treated for 24 h with 50nMTat, 500nM Morphine, or combined Tat/Morphine, after which the cells were either fixed and stained with antibodies to DOR or KOR using flow cytometry, or processed for real-time PCR analyses as described in Methods. All data were analyzed by ANOVA, and are presented as means ± SEM in relation to control cells. Numerical values for the percentage of control cells positive for cell surface DOR and KOR were 38% and 41%, respectively; while numerical values for the percentage of control cells positive for intracellular DOR and KOR were 68% and 80%, respectively. * and ** indicate statistically significant (p<0.05 and p<0.01, respectively) differences in opioid receptor expression in treated cells compared to control cells, while # and ## indicate statistically significant (p<0.05 and p<0.01, respectively) differences in cell surface opioid receptor expression in Tat/morphine treated cells as compared to cells treated with morphine alone.
| Treatment | DOR | KOR | |
|---|---|---|---|
|
Cell surface expression [% of control] |
Control | 100.00±13.13 | 100.00±9.99 |
| Tat 50nM | 103.11±3.34 | 100.90±7.52 | |
| Morphine 500nM | 70.29±12.42 * | 62.28±6.18 ** | |
| Tat 50nM+Morphine 500nM | 125.92±4.45 ## | 100.54±15.26 # | |
|
intracellular expression [% of control] |
Control | 100.00±6.68 | 100.00±4.52 |
| Tat 50nM | 97.50±4.86 | 101.92±2.65 | |
| Morphine 500nM | 124.13±5.80 * | 99.95±10.87 | |
| Tat 50nM+Morphine 500nM | 117.00±6.02 * | 90.16±7.94 | |
|
mRNA [% of control] |
Control | 100.00±7.16 | 100.00±7.87 |
| Tat 50nM | 151.35±21.77 * | 92.48±7.33 | |
| Morphine 500nM | 117.49±22.03 | 110.05±9.75 | |
| Tat 50nM+Morphine 500nM | 78.28±15.77 * | 64.79±10.42 ** |
Table 2. Protein and mRNA expression of DOR and KOR in primary microglial cells.
Primary rat microglial cells were isolated as described in Methods, and were treated for 24 h with 50nMTat, 500nM Morphine, or combined Tat/Morphine, after which the cells were either fixed and stained with antibodies to DOR or KOR cells using flow cytometry, or processed for real-time PCR analyses as described in Methods. All data were analyzed by ANOVA, and are presented as means ± SEM in relation to control cells. Numerical values for the percentage of control cells positive for cell surface DOR and KOR were 28% and 25%, respectively; while numerical values for the percentage of control cells positive for intracellular DOR and KOR were 33% and 39%, respectively. *, **, and *** indicate statistically significant (p<0.05, p<0.01, and p<0.001, respectively) differences in opioid receptor expression in treated cells compared to control cells, while # and ## indicate statistically significant (p<0.05 and p<0.01, respectively) differences in opioid receptor expression in Tat/morphine treated cells as compared to cells treated with morphine alone.
| Treatment | DOR | KOR | |
|---|---|---|---|
|
Cell surface expression [% of control] |
Control | 100.00±7.99 | 100.00±11.60 |
| Tat 50nM | 48.24±12.42 * | 72.65±12.49 | |
| Morphine 500nM | 63.17±7.01 ** | 81.14±13.57 | |
| Tat 50nM+Morphine 500nM | 89.61±17.47 | 155.52±4.03 ## | |
|
intracellular expression [% of control] |
Control | 100.00±6.93 | 100.00±3.78 |
| Tat 50nM | 43.63±4.49 *** | 84.03±10.00 | |
| Morphine 500nM | 74.10±10.73 *** | 124.25±2.32 | |
| Tat 50nM+Morphine 500nM | 107.65±2.32 # | 170.00±3.32 # | |
|
mRNA [% of control] |
Control | 100.00±1.96 | 100.00±2.04 |
| Tat 50nM | 137.84±7.07 * | 109.64±2.91 | |
| Morphine 500nM | 96.73±3.80 | 98.93±10.96 | |
| Tat 50nM+Morphine 500nM | 109.77±7.17 | 121.41±10.09 |
3. Effects of Tat and morphine on regulation of opioid receptors in astrocytes
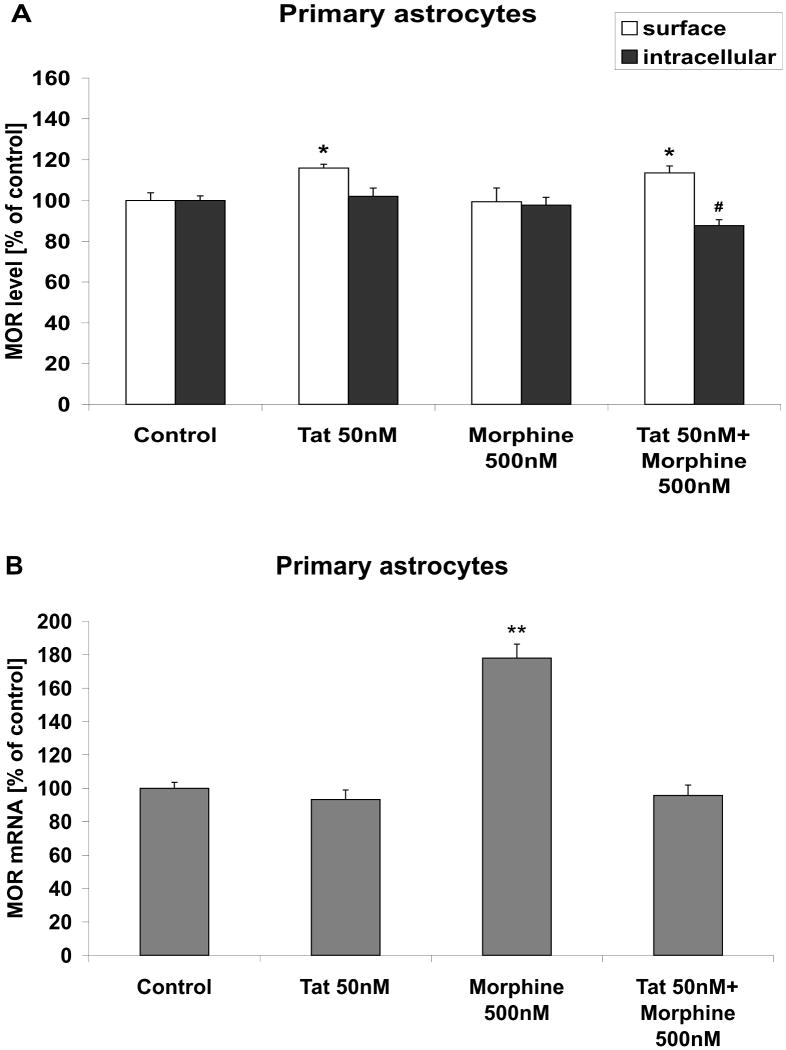
To determine the effects of morphine exposure on cell surface expression of opioid receptors in cultured astrocytes, rat primary astrocytes were grown and treated with 50 nM Tat and 500 nM morphine for 24 h as described in Methods. As was done with microglia, the cells were then harvested and both cell surface and intracellular MOR expression was analyzed using FACS. Unlike what was observed in microglia, data show that exposure to 500 nM morphine for 24 h did not affect the percentage of astrocytes positive for cell surface MOR (Fig. 6A). However, 50 nM Tat, applied either alone or in combination with morphine, elicited a modest but statistically significantly increase in cell surface MOR expression (Fig. 6A). However, the intracellular pool of MOR was not significantly affected by Tat or morphine exposure in astrocytes, although a modest decrease was observed after combined Tat/morphine treatment (Fig. 6A). Finally, evaluation of mRNA showed that in astrocytes, MOR mRNA levels were significantly increased by morphine treatment (Fig. 6B), but not by Tat or combined Tat/morphine treatment. Interestingly, when surface, intracellular, and mRNA levels of DOR and KOR were analyzed in cultured astrocytes, data indicated that cell surface expression of these receptors was significantly increased by both Tat and morphine, whether applied separate or together (Table 3). The patterns of surface DOR and KOR expression were not mirrored by increases in intracellular expression or mRNA, suggesting that receptor trafficking or degradation was effected.
Figure 6. Effects of Tat and morphine on MOR protein and mRNA expression in astrocytes.
Cultured primary astrocytes were grown and treated with vehicle, 50 nM Tat and/or 500 nM morphine for 24 h as described in Methods. (A) Cells were then harvested and processed for evaluation of cell surface and intracellular MOR expression using FACS. All data were analyzed by ANOVA, and are presented as means ± SEM in relation to control cells. The numerical value for the percentage of control astrocytes positive for cell surface MOR was 41%; while the numerical value for the percentage of control cells positive for intracellular MOR was 89%. * indicates the statistically significant increase (p<0.05) in cell surface MOR expression in morphine-treated and Tat/morphine treated astrocytes compared to control cells, while # indicates a statistically significant decrease (p<0.05) in intracellular MOR expression in Tat/morphine-treated cells as compared to control astrocytes. (B) Cultured astrocytes were treated vehicle, 50 nM Tat and/or 500 nM morphine for 24 h, harvested, and MOR mRNA expression was analyzed by real-time PCR as described in Methods. Data were analyzed by ANOVA, and ** indicates the statistically significant increase (p<0.01) in MOR mRNA expression in morphine-treated astrocytes compared to control cells.
Table 3. Protein and mRNA expression of DOR and KOR in primary astrocytes.
Primary rat astrocytes were isolated as described in Methods, and were treated for 24h with 50nMTat, 500nM Morphine, or combined Tat/Morphine, after which the cells were either fixed and stained with antibodies to DOR or KOR cells using flow cytometry, or processed for real-time PCR analyses as described in Methods. All data were analyzed by ANOVA, and are presented as means ± SEM in relation to control cells. Numerical values for the percentage of control cells positive for cell surface DOR and KOR were 38% and 36%, respectively; while numerical values for the percentage of control cells positive for intracellular DOR and KOR were 91% and 83%, respectively * and ** indicate statistically significant p<0.05 and p<0.01 differences in opioid receptor expression in treated cells compared to control cells.
| Treatment | DOR | KOR | |
|---|---|---|---|
|
Cell surface expression [% of control] |
Control | 100.00±9.27 | 100.00±1.83 |
| Tat 50nM | 138.29±6.99 *** | 120.96±9.26 ** | |
| Morphine 500nM | 123.84±9.14 ** | 113.07±1.79 * | |
| Tat 50nM+Morphine 500nM | 126.09±7.08 ** | 121.13±8.35 ** | |
|
intracellular expression [% of control] |
Control | 100.00±1.76 | 100.00±1.79 |
| Tat 50nM | 95.60±3.61 | 95.25±6.38 | |
| Morphine 500nM | 87.81±7.78 | 94.95±3.75 | |
| Tat 50nM+Morphine 500nM | 80.62±5.76 * | 76.87±4.76 *** | |
|
mRNA [% of control] |
Control | 100.00±3.18 | 100.00±1.77 |
| Tat 50nM | 89.67±4.83 * | 108.71±5.09 | |
| Morphine 500nM | 153.89±5.84 *** | 103.87±1.27 | |
| Tat 50nM+Morphine 500nM | 99.11±5.13 | 101.58±7.92 |
Discussion
Opioid receptors are expressed on glial cells and have been shown to modulate immune responses both in the brain and periphery. As HIV-1 infection in the brain is restricted to glia, and as opiate abuse can exacerbate the progression of HIV, this study was undertaken to better understand glial cell-type specific regulation of opioid receptor expression in response to morphine and HIV viral proteins. While both astrocytes and microglia express MOR, DOR, and KOR, evaluation of the effects of morphine treatment on opioid receptor regulation showed that morphine caused a significant decrease in cell surface opioid receptors in microglia, but did not affect cell surface receptor localization in astrocytes. Furthermore, co-administration of Tat attenuated morphine-induced cell surface opioid receptor down-regulation in microglia, and combined Tat/morphine treatment induced significant increases in both MOR mRNA and in intracellular levels of MOR. Hence, these data suggest that opioid receptor regulation in the brain is cell-type specific, supporting a highly specialized role for opioid receptors in brain inflammatory signaling in response to morphine and HIV viral proteins. However, these data also underscore that pharmacological and inflammatory signaling in the brain is complex and involves multiple cell types, and suggest that to fully understand the physiological consequences of opiate-based drug abuse in the context of HIV, investigators are obliged to evaluate the individual responses of neurons, microglia, and astrocytes both separately and together.
Three major classes of seven transmembrane, G protein-coupled receptors have been identified as opioid receptors, and indeed, MOR, DOR, and KOR have been identified in cells of both the brain and immune system. Data in this manuscript confirm that cultured microglial and astrocytes express MOR, DOR, and KOR, and further document that opioid receptors are differentially regulated in these two cell types. Opioid receptors are well known to be subject to complex ligand-dependent regulation, and opioid receptor desensitization, internalization, and degradation are all established effects of opiate administration. Indeed, the phenomena of opioid tolerance, in which increasing levels of agonists are needed to elicit a clinical response, is very well known, and there are a number of studies linking acute receptor desensitization to tolerance and dependence (Bohn et al., 2000; Finn and Whistler, 2001; Arttamangkul et al., 2006). It has been proposed that MOR desensitization begins with receptor phosphorylation by G-protein receptor kinases, with phosphorylation increasing the affinity of the receptor for β-arrestin, which then drives the ligand-β-arrestin-receptor complex into the endocytic pathway. From this point, opioid receptors can either be recycled from the endosomal compartment back to the plasma membrane, or be degraded via lysosome and proteasome pathways (Hicke, 1999; Chaturvedi et al., 2001). However, the relationship between desensitization, internalization, and degradation are still under intense investigation. While internalization was not directly addressed in this study, data in this manuscript show that chronic (24 h) treatment of microglia with morphine leads to significant decreases in cell surface opioid receptor expression in microglia, which is in agreement with other reports suggesting that long-term activation of opioid receptors leads to receptor downregulation (Chaturvedi et al., 2001; Horner and Zadina, 2004). Interestingly, these data indicate that such effects are not observed in astrocytes, suggesting that the typical progression of ligand-dependent opioid receptor trafficking does not occur in astrocytes. However, it has been shown that MOR actions in astrocytes are contextual and can differ depending on the duration of agonist exposure or presence/absence of other factors. For example, EGF can diametrically shift the ability of the selective MOR agonist [D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (DAMGO) to activate ERK, EGF receptor downregulation and internalization in immortalized rat cortical astrocytes (Belcheva et al., 2003). Thus, further experimentation is needed to understand why morphine decreases cell surface MOR in microglia but not in astrocytes, and how such alterations (or their absence) in cell surface MOR contribute to the effects of morphine on brain homeostasis.
Morphine is a potent narcotic analgesic and is most commonly used in clinical settings as pain management. However, morphine has also been shown to possess potent immunomodulatory properties, and there have been a number of studies showing that the use or abuse of morphine can have adverse effects on immune function (McCarthy et al., 2001; Walker, 2003). For example, several studies have shown that exposure to opioids can compromise immune responses to bacterial infection and lead to immunosupression (Rojavin et al., 1993; Risdahl et al., 1998; Bhaskaran et al., 2001; Ocasio et al., 2004). As microglia are the major immunocompetent cells in the brain, morphine-mediated suppression of microglial immune responses to severely predispose the brain to infection. This suggests that one potential mechanism whereby opiates increase the neurological complications of HIV could be through the facilitation of brain-resident opportunistic infections, which could aggravate the progression of AIDS (Quang-Cantagrel et al., 2001). Conversely, morphine exposure has also been shown to directly facilitate HIV infection and accelerate HIV replication (Peterson et al., 1990; Peterson et al., 1994; Kumar et al., 2006; Wang et al., 2006). Thus, activation of opioid receptors in the brain of AIDS patients could precipitate neurological abnormalities vie increases in CNS levels of HIV, and/or increases in opportunistic infections. It is very important to note that co-application of HIV protein Tat attenuates morphine-induced downregulation of cell surface mu opioid receptors in microglia. These data suggest that the presence of HIV viral proteins such as Tat could exacerbate MOR-based signaling by effectively increasing cell surface receptors. Thus, if the typical actions of morphine in the brain include suppression of innate anti-bacterial and anti-viral microglial immunity, and if Tat acts to increase the availability of surface opioid receptors to morphine, then these data raise the possibility that the combination of Tat and morphine could synergistically accelerate the already detrimental actions of morphine. Since many HIV-infected individuals may be treated with MOR agonists for pain, these data raise troubling implications of pain management in AIDS patients.
While the mechanisms whereby Tat offsets the effects of morphine on opioid receptor expression in microglia are not yet known, PCR data illustrates that combined Tat and morphine causes striking increases in microglial MOR mRNA in microglia. This data is in agreement with previous published reports documenting that MOR mRNA in macrophages is upregulated in the presence of HIV viral proteins in novel HIV-1 transgenic rats (Chang et al., 2007). However, the patterns of opioid receptor cell surface expression were not always in congruence with mRNA, particularly in astrocytes. Hence, these data raise the possibility of cell-type specific modulation of degradation pathways involved in regulation of opioid receptors. Thus, one could speculate that differential regulation of cell surface opioid receptors by the proteasomal (or lysosomal) degradation system could potentially affect morphine-induced signaling, and thus could even underlie in part the differential effects of morphine in astrocytes as compared to microglia. Alternatively, there is widespread evidence that opioid and chemokine receptors can undergo bidirectional, heterologous cross-sensitization (Rogers and Peterson, 2003), suggesting that cell-specific responses to Tat-induced chemokine release might modulate the response of glia to opioids. Although chemokine-induced modulation of mu opioid receptor signaling in glia has not been assessed, in dorsal root ganglion neurons, CCL3 desensitizes mu opioid receptors and this reportedly contributes to increased pain with inflammation (Zhang et al., 2004). Thus the differential induction of chemokines by Tat and morphine in astrocytes and microglia, and/or the differential consequences of chemokine receptor activation in the two cell types could lead to differential regulation of surface opiod receptors. However, future investigations are clearly required to thoroughly elucidate the complex interplay between the regulation of opioid receptor trafficking and the cell-type specific biological consequences of morphine exposure in neurodegenerative conditions such as HIV/AIDS.
Acknowledgments
The authors are grateful to Dr. Greg Bauman and Jennifer Strange in the Flow Cytometry Facility of the University of Kentucky College of Medicine for their help in FACS analysis. Additional gratitude goes to Dr. P. Ricciardi-Castagnoli of the Department of Biotechnology and Bioscience, Piazza della Scienza 2, University of Milano-Bicocca, Milan, Italy for the N9 cell line. Finally, the authors benefited tremendously from the assistance of Dr. Chris Norris and Irina Artiushin with regards to assistance with primary cultures.
This work was supported by grants from the NIH (NS46267, DA19398, and RR15592).
References
- Albini A, Ferrini S, Benelli R, Sforzini S, Giunciuglio D, Aluigi MG, Proudfoot AE, Alouani S, Wells TN, Mariani G, Rabin RL, Farber JM, Noonan DM. HIV-1 Tat protein mimicry of chemokines. Proc Natl Acad Sci U S A. 1998;95:13153–8. doi: 10.1073/pnas.95.22.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Does drug abuse alter microglial phenotype and cell turnover in the context of advancing HIV infection? Neuropathol Appl Neurobiol. 2005;31:325–38. doi: 10.1111/j.1365-2990.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Torrecilla M, Kobayashi K, Okano H, Williams JT. Separation of mu-opioid receptor desensitization and internalization: endogenous receptors in primary neuronal cultures. J Neurosci. 2006;15:4118–25. doi: 10.1523/JNEUROSCI.0303-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcheva MM, Tan Y, Heaton VM, Clark AL, Coscia CJ. Mu opioid transactivation and down-regulation of the epidermal growth factor receptor in astrocytes: implications for mitogen-activated protein kinase signaling. Mol Pharmacol. 2003;64:1391–1401. doi: 10.1124/mol.64.6.1391. [DOI] [PubMed] [Google Scholar]
- Bell JE, Arango JC, Robertson R, Brettle RP, Leen C, Simmonds P. HIV and drug misuse in the Edinburgh cohort. J Acquir Immune Defic Syndr. 2002;31 2:S35–42. doi: 10.1097/00126334-200210012-00003. [DOI] [PubMed] [Google Scholar]
- Bell JE, Donaldson YK, Lowrie S, McKenzie CA, Elton RA, Chiswick A, Brettle RP, Ironside JW, Simmonds P. Influence of risk group and zidovudine therapy on the development of HIV encephalitis and cognitive impairment in AIDS patients. AIDS. 1996;10:493–9. doi: 10.1097/00002030-199605000-00007. [DOI] [PubMed] [Google Scholar]
- Bhaskaran M, Reddy K, Sharma S, Singh J, Radhakrishnan N, Kapasi A, Singhal PC. Morphine-induced degradation of the host defense barrier: role of macrophage injury. J Infect Dis. 2001;184:1524–31. doi: 10.1086/324667. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–3. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Bonwetsch R, Croul S, Richardson MW, Lorenzana C, Valle LD, Sverstiuk AE, Amini S, Morgello S, Khalili K, Rappaport J. Role of HIV-1 Tat and CC chemokine MIP-1alpha in the pathogenesis of HIV associated central nervous system disorders. J Neurovirol. 1999;5:685–94. doi: 10.3109/13550289909021297. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Jones DB, Flavell DJ, Fagerhol MK. Mac 387 antibody and detection of formalin resistant myelomonocytic L1 antigen. J Clin Pathol. 1988;41:963–70. doi: 10.1136/jcp.41.9.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Barger SW, Moss NI, Pham JT, Keller JN, Nath A. Pro-inflammatory and pro-oxidant properties of the HIV protein Tat in a microglial cell line: attenuation by 17beta-estradiol. J Neurochem. 2001;78:1315–1324. doi: 10.1046/j.1471-4159.2001.00511.x. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A. Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J Neurosci. 2003;23:8417–22. doi: 10.1523/JNEUROSCI.23-23-08417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Anti-inflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141:3646–3656. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS. 1997;11:1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Chang SL, Beltran JA, Swarup S. Expression of the mu opioid receptor in the human immunodeficiency virus type 1 transgenic rat model. J Virol. 2007;81:8406–11. doi: 10.1128/JVI.00155-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Hu S, Shark KB, Sheng WS, Gekker G, Peterson PK. Activation of mu opioid receptors inhibits microglial cell chemotaxis. J Pharmacol Exp Ther. 1997;281:998–1004. [PubMed] [Google Scholar]
- Chaturvedi K, Bandari P, Chinen N, Howells RD. Proteasome involvement in agonist-induced down-regulation of mu and delta opioid receptors. J Bio l Chem. 2001;276:12345–55. doi: 10.1074/jbc.M008054200. [DOI] [PubMed] [Google Scholar]
- Chen P, Mayne M, Power C, Nath A. The Tat protein of HIV-1 induces tumor necrosis factor-alpha production. J Biol Chem. 1997;272:22385–22388. doi: 10.1074/jbc.272.36.22385. [DOI] [PubMed] [Google Scholar]
- Chirmule N, Than S, Khan SA, Pahwa S. Human immunodeficiency virus Tat induces functional unresponsiveness in T cells. J Virol. 1995;69:492–498. doi: 10.1128/jvi.69.1.492-498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Osborne PB, Christie MJ. Mu-opioid receptor desensitization: is morphine different? Br J Pharmacol. 2004;143:685–96. doi: 10.1038/sj.bjp.0705938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J, Everall IP, Weich S, McLaughlin J, Scaravilli F, Lantos P. HIV-associated brain pathology in the United Kingdom: an epidemiological study. AIDS. 1997;11:1145–50. doi: 10.1097/00002030-199709000-00010. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic M, Stanojevic S, Kovacevic-Jovanovic V, Miletic T, Vujic-Redzic V, Radulovic J. Modulation of humoral immune responses in the rat by centrally applied Met-Enk and opioid receptor antagonists: functional interactions of brain OP1, OP2 and OP3 receptors. Immunopharmacology. 2000;49:255–62. doi: 10.1016/s0162-3109(00)00213-7. [DOI] [PubMed] [Google Scholar]
- Ding Q, Dimayuga E, Martin S, Bruce-Keller AJ, Nukala V, Cuervo AM, Keller JN. Characterization of chronic low-level proteasome inhibition on neural homeostasis. J Neurochem. 2003;86:489–98. doi: 10.1046/j.1471-4159.2003.01885.x. [DOI] [PubMed] [Google Scholar]
- Donahoe RM. Multiple ways that drug abuse might influence AIDS progression: clues from a monkey model. J Neuroimmunol. 2001;147:28–32. doi: 10.1016/j.jneuroim.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infections to AIDS. J Neuroimmunol. 1998;83:77–87. doi: 10.1016/s0165-5728(97)00224-5. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Finazzi D, Klausner RD. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–2. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Wu G, Ambati J, Bruce-Keller AJ, Knapp PE, Hauser KF. CCR2 mediates increases in glial activation caused by exposure to HIV-1 Tat and opiates. J Neuroimmunol. 2006;178:9–16. doi: 10.1016/j.jneuroim.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan R, Wingfield P, Gallo RC. Release, uptake, and affects of extracellular human immunodeficiency virus type-1 Tat protein on cell growth and viral replication. J Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli F, Fiorelli V, Muratori S, De Cristofaro M, Vincenzi L, Tpoino S, Novi A, Luzi G, Sirianni MC. Immune-derived cytokines in the nervous system: epigenic instructive signals or neuropathic mediators? Crit Rev Immunol. 1999;19:97–116. [PubMed] [Google Scholar]
- Everall I, Luthert P, Lantos P. A review of neuronal damage in human immunodeficiency virus infection: its assessment, possible mechanisms and relationship to dementia. J Neuropath Exp Neurol. 1993;52:561–566. doi: 10.1097/00005072-199311000-00002. [DOI] [PubMed] [Google Scholar]
- Everall IP. Interaction between HIV and intravenous heroin abuse? J Neuroimmunol. 2004;147:13–5. doi: 10.1016/j.jneuroim.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Finn AK, Whistler JL. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron. 2001;32:829–39. doi: 10.1016/s0896-6273(01)00517-7. [DOI] [PubMed] [Google Scholar]
- Foster R, Kandanearatchi A, Beasley C, Williams B, Khan N, Fagerhol MK, Everall IP. Calprotectin in microglia from frontal cortex is up-regulated in schizophrenia: evidence for an inflammatory process? Eur J Neurosci. 2006;24:3561–6. doi: 10.1111/j.1460-9568.2006.05219.x. [DOI] [PubMed] [Google Scholar]
- Fox L, Alford M, Achim C, Mallory M, Masliaah E. Neurodegeneration of somatostatinimmunoreactive neurons in HIV encephalitis. J Neuropathol Exp Neurol. 1997;56:360–8. doi: 10.1097/00005072-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Glass J, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantification of human immunodeficiency virus in the brain; correlation with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Gray F, Adle-Biassette H, Chretien F, Lorin de la Grandmaison G, Force G, Keohane C. Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin Neuropathol. 2001;20:146–55. [PubMed] [Google Scholar]
- Hayman M, Arbuthnott G, Harkiss G, Brace H, Filippi P. Neurotoxicity of peptide analogues of the transactivating protein Tat from Maedi-Visna virus in human immunodeficiency virus. Neuroscience. 1993;53:1–6. doi: 10.1016/0306-4522(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Hicke L. Gettin' down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol. 1999;9:107–112. doi: 10.1016/s0962-8924(98)01491-3. [DOI] [PubMed] [Google Scholar]
- Hofman FM, Dohadwala MM, Wright AD, Hinton DR, Walker SM. Exogenous tat protein activates central nervous system-derived endothelial cells. J Neuroimmunol. 1994;54:19–28. doi: 10.1016/0165-5728(94)90226-7. [DOI] [PubMed] [Google Scholar]
- Horner KA, Zadina JE. Internalization and down-regulation of mu opioid receptors by endomorphins and morphine in SH-SY5Y human neuroblastoma cells. Brain Res. 2004;1028:121–32. doi: 10.1016/j.brainres.2004.07.055. [DOI] [PubMed] [Google Scholar]
- Jones M, Olafson K, Del Begio MR, Peeling J, Nath A. Inventricular administration of human immunodeficiency virus 1 Tat protein causes inflammation, gliosis, apoptosis, and ventricular enlargement. J Neuropath Exp Neurol. 1998;57:563–570. doi: 10.1097/00005072-199806000-00004. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Kumar R, Orsoni S, Norman L, Verma AS, Tirado G, Giavedoni LD, Staprans S, Miller GM, Buch SJ, Kumar A. Chronic morphine exposure causes pronounced virus replication in cerebral compartment and accelerated onset of AIDS in SIV/SHIV-infected Indian rhesus macaques. Virology. 2006;354:192–206. doi: 10.1016/j.virol.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Knudsen BE, Geiger JD, Brownstone RM, Nath A. Human immunodeficiency virus type 1 Tat activates non-N-methyl-D-asparate excitatory amino acid receptors and causes neurotoxicity. Ann Neurol. 1995;37:373–380. doi: 10.1002/ana.410370314. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Aalinkeel R, Reynolds JL, Nair BB, Fernandez SF, Schwartz SA, Nair MP. Morphine exacerbates HIV-1 viral protein gp120 induced modulation of chemokine gene expression in U373 astrocytoma cells. Curr HIV Res. 2005;3:277–288. doi: 10.2174/1570162054368048. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. Ann Neurol. 1997;42:963–72. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ. Opioids, opioid receptors, and the immune response. Drug Alcohol Depend. 2001;62:111–23. doi: 10.1016/s0376-8716(00)00181-2. [DOI] [PubMed] [Google Scholar]
- Mollenhauer HH, Morre DJ, Rowe LD. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim Biophys Acta. 1990;1031:225–46. doi: 10.1016/0304-4157(90)90008-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31 2:S62–9. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petiio CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Ocasio FM, Jiang Y, House SD, Chang SL. Chronic morphine accelerates the progression of lipopolysaccharide-induced sepsis to septic shock. J Neuroimmunol. 2004;149:90–100. doi: 10.1016/j.jneuroim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Anderson WR, Kravitz F, Portoghese PS, Balfour HHJ, Chao CC. Morphine amplifies HIV-1 expression in chronically infected promonocytes cocultured with human brain cells. J Neuroimmunol. 1994;50:167–75. doi: 10.1016/0165-5728(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HHJ. Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS. 1990;4:869–73. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- Philippon V, Vellutini C, Gambraelli D, Harkiss G, Arbuthnutt G. The basic domain of lentiviral Tat protein is responsible for damage in mouse brain: involvement of cytokines. Virology. 1994;205:519–529. doi: 10.1006/viro.1994.1673. [DOI] [PubMed] [Google Scholar]
- Pu H, Tian J, Flora G, Lee YW, Nath A, Hennig B, Toborek M. HIV-1 Tat protein upregulates inflammatory mediators and induces monocyte invasion into the brain. Mol Cell Neurosci. 2003;24:224–37. doi: 10.1016/s1044-7431(03)00171-4. [DOI] [PubMed] [Google Scholar]
- Quang-Cantagrel ND, Wallace MS, Ashar N, Mathews C. Long-term methadone treatment: effect on CD4+ lymphocyte counts and HIV-1 plasma RNA level in patients with HIV infection. Eur J Pain. 2001;5:415–420. doi: 10.1053/eujp.2001.0262. [DOI] [PubMed] [Google Scholar]
- Risdahl JM, Khanna KV, Peterson PK, Molitor TW. Opiates and infection. J Neuroimmunol. 1998;83:4–18. doi: 10.1016/s0165-5728(97)00216-6. [DOI] [PubMed] [Google Scholar]
- Rogers TJ, Peterson PK. Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol. 2003;24:116–121. doi: 10.1016/s1471-4906(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Rojavin M, Szabo I, Bussiere JL, Rogers TJ, Adler MW, Eisenstein TK. Morphine treatment in vitro or in vivo decreases phagocytic functions of murine macrophages. Life Sci. 1993;53:997–1006. doi: 10.1016/0024-3205(93)90122-j. [DOI] [PubMed] [Google Scholar]
- Ruzicka BB, Fox CA, Thompson RC, Meng F, Watson SJ, Akil H. Primary astroglial cultures derived from several rat brain regions differentially express m, d and kappa opioid receptor mRNA. Mol Brain Res. 1995;34:209–220. doi: 10.1016/0169-328x(95)00165-o. [DOI] [PubMed] [Google Scholar]
- Sodroski J, Patarca R, Rosen C, Wong-Staal F, Haseltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985;229:74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- Spargo E, Everall IP, Lantos PL. Neuronal loss in the hippocampus in Huntington's disease; a comparison with HIV infection. J Neurol Neurosurg Psychiatry. 1993;56:487–91. doi: 10.1136/jnnp.56.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiene-Martin A, Zhou R, Hauser KF. Regional, developmental, and cell cycle-dependent differences in mu, delta, and kappa-opioid receptor expression among cultured mouse astrocytes. Glia. 1998;22:249–259. [PMC free article] [PubMed] [Google Scholar]
- Tardieu M, Hery C, Peudenier S, Boespflug O, Montagnier L. Human immunodeficiency virus type 1- infected monocytic cells can destroy human neural cells after cell- to-cell adhesion. Ann Neurol. 1992;132:11–17. doi: 10.1002/ana.410320104. [DOI] [PubMed] [Google Scholar]
- Thorlin T, Persson PA, Eriksson PS, Hansson E, Ronnback L. Delta-opioid receptor immunoreactivity on astrocytes is upregulated during mitosis. Glia. 1999;25:370–378. doi: 10.1002/(sici)1098-1136(19990215)25:4<370::aid-glia6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Turchan J, Anderson C, Hauser KF, Sun Q, Zhang J, Liu Y, Wise PM, Kruman I, Maragos W, Mattson MP, Booze R, Nath A. Estrogen protects against the synergistic toxicity by HIV proteins, methamphetamine and cocaine. BMC Neurosci. 2001;2:3–11. doi: 10.1186/1471-2202-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan J, Pocernich CB, Gairola C, Chauhan A, Schifitto G, Butterfield DA, Buch S, Narayan O, Sinai A, Geiger J, Berger JR, Elford H, Nath A. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology. 2003;60:307–14. doi: 10.1212/01.wnl.0000042048.85204.3d. [DOI] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Liu Y, Gartner S, Reid R, Jie C, Peng X, Chen KC, Chauhan A, Haughey N, Cutler R, Mattson MP, Pardo C, Conant K, Sacktor N, McArthur JC, Hauser KF, Gairola C, Nath A. Increased vulnerability of ApoE4 neurons to HIV proteins and opiates: protection by diosgenin and L-deprenyl. Neurobiol Dis. 2006;23:109–19. doi: 10.1016/j.nbd.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Tyor WR, Wesselingh W, Griffith SW, McArther JC, Griffen DE. Unifying hypothesis of the pathogenesis of HIV-associated dementia complex, vacuolar myelopathy, and sensory neuropathy. J AIDS Human Ret. 1995;9:379–388. [PubMed] [Google Scholar]
- UNAIDS. 2006 Report on the global AIDS epidemic. Geneva: United Nations; 2006. [Google Scholar]
- von Zastrow M. Opioid receptor regulation. Neuromolecular Med. 2004;5:1–8. doi: 10.1385/NMM:5:1:051. [DOI] [PubMed] [Google Scholar]
- Walker JS. Anti-inflammatory effects of opioids. Adv Exp Med Biol. 2003;521:148–60. [PubMed] [Google Scholar]
- Wang X, Douglas SD, Peng JS, Zhou DJ, Wan Q, Ho WZ. An in vitro model of morphine withdrawal manifests the enhancing effect on human immunodeficiency virus infection of human T lymphocytes through the induction of substance P. Am J Pathol. 2006;169:1663–70. doi: 10.2353/ajpath.2006.060358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselingh SL, Power C, Glass JD, Tyor WR, McArthur J, Farber JM, Griffin JW, Griffin DE. Intracerebral cytokine messenger RNA expression in acquired immunodefiency syndrome dementia. Ann Neurol. 1993;33:576–582. doi: 10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Baldwin M, Achim CL. Expression of HIV regulatory and structural mRNA in the central nervous system. AIDS. 1996;10:843–847. doi: 10.1097/00002030-199607000-00007. [DOI] [PubMed] [Google Scholar]
- Xu H, Partilla JS, Wang X, Rutherford JM, Tidgewell K, Prisinzano TE, Bohn LM, Rothman RB. A comparison of noninternalizing (herkinorin) and internalizing (DAMGO) mu-opioid agonists on cellular markers related to opioid tolerance and dependence. Synapse. 2007;61:166–75. doi: 10.1002/syn.20356. [DOI] [PubMed] [Google Scholar]
- Zhang N, Rogers TJ, Caterina M, Oppenheim JJ. Proinflammatory chemokines, such as C-C chemokine ligand 3, desensitize mu-opioid receptors on dorsal root ganglia neurons. J Immunol. 2004;173:594–599. doi: 10.4049/jimmunol.173.1.594. [DOI] [PubMed] [Google Scholar]