Abstract
Standard protocols for the generation of murine dendritic cells (DCs) employ medium supplemented with heat-inactivated fetal calf serum (FCS). Recently, several attempts have been made to avoid serum exposure during DC culture. The impetus for these efforts has been a desire to generate DCs for clinical use, as preclinical data have demonstrated their efficacy in immune activation and in immune suppression both in vitro and in vivo. However, these protocols have resulted in contradictory outcomes with respect to DC survival in culture and activation status. In this report, we compared several serum-free culture conditions with respect to survival, differentiation, activation, and cytokine profile of murine DC progenitors. DC progenitors can survive only in some serum-free conditions. Surprisingly, DCs grown in serum-free medium display a higher expression of activation markers upon stimulation. They produce increased IL-12 and decreased IL-6 following stimulation. Furthermore, DCs derived under serum-free conditions may express unusual surface markers, B220 and Ly6C/G, implying an increased differentiation to plamacytoid DCs (pDCs).
Keywords: dendritic cell, serum-free, plasmacytoid DC, myeloid DC, tolerance
Introduction
Dendritic cells (DC) are the most potent antigen-presenting cells in the immune system (Banchereau and Steinman, 1998). They take up both self and foreign antigens, process these to peptides which are then presented on the cell surface in peptide/MHC complexes. Upon encountering antigen and inflammatory cytokines (Cella et al., 1997), DCs migrate from the periphery to local lymphoid organs, up-regulate expression of the major histocompatability complex (MHC) and costimulatory molecules and lose their capacity to take up additional antigen. Once in lymphoid organs, the phenotypically mature DCs interact with naïve T cells to generate either antigen-specific tolerance or immunity. The choice between immune tolerance and activation is complex. Several factors are known to be critical in this decision: the type of antigen presented by DC, the functional maturity of the DC, as determined by its ability to release pro-inflammatory cytokines, and the DC subtype (Lutz and Schuler, 2002).
Since the initial description of DCs by Steinmann and Cohn (Steinman and Cohn, 1973), it has become evident that there are many distinct DC subtypes, each having a particular location and specialized functions in the immune system. Although there are many descriptions of DC subtypes, three types are most commonly accepted: conventional DCs (cDCs), plasmacytoid DCs (pDCs), and inflammatory DCs (iDCs). The cDC subset has a dendritic form, antigen-presentation function, and includes both migratory DCs and lymphoid tissue-resident DCs. Migratory cDCs can be further subdivided based on the peripheral tissue of origin. cDCs resident in lymphoid tissue also can be subdivided based on surface expression of CD8 and/or CD4. The pDC subset is relatively newly identified and is also termed interferon-producing DC, owing to its ability to secrete type I IFNs upon activation. They express the conventional DC marker, CD11c, as well as CD123 in humans (Olweus et al., 1997) and B220, Ly6C/G in mice (Bjorck, 2001). The iDC subset is a novel DC population, not found in steady state, but appearing after infection or inflammation.
In general, DCs are tolerogenic when immature and immunogenic when mature. A more refined analysis has revealed that expression of cytokines and costimulatory molecules are crucial in determining the functional outcome of T cell encounter with DCs. DCs with a moderate expression of costimulatory molecules without secretion of adequate proinflammatory cytokines lead to immune tolerance rather than immune activation (Akbari et al., 2001; Menges et al., 2002). This type of DC is termed ‘semi-mature DC’ and its generation may depend on exposure to tumor necrosis factor (TNF)-α.
DCs are sparsely but widely distributed throughout the body. Because of the relative infrequency of steady-state DCs in vivo, most studies have been performed with in vitro generated DCs using DC progenitors from peripheral blood monocytes in humans or bone marrow in mice. Inaba and others reported the successful generation of a large number of murine bone marrow derived DCs with xenologous sera such as fetal bovine serum (FBS) (Inaba et al., 1992). However, when DCs are used as vehicles to induce antigen-specific immunity or tolerance, those cultured in vitro generated unwanted immune responses against molecules present in the FBS (Toldbod et al., 2003). Several attempts have been made to avoid this activation. Although there are successful reports of serum-free culture for human monocyte-derived DCs (Luft et al., 1998; Thurner et al., 1999; Zheng et al., 2000), the efficacy and consistency of serum-free culture for murine bone marrow-derived DCs has been controversial.
In this report, we describe reproducible serum-free culture conditions for murine bone marrow-derived DCs. We found that serum-free culture of murine DCs requires specialized medium, and the resulting DCs expressed higher levels of activation markers than those grown in serum-containing media. They also secrete more IL-12 but less IL-6 following stimulation with LPS, CpG, and anti-CD40 antibody. Phenotypically, DCs grown in serum-free culture display features of plasmacytoid DCs (pDC).
Material and Methods
Animals
BALB/c mice were purchased from Taconic (Germantown, NY) and housed in the specific pathogen-free (SPF) facility of the Columbia University Medical Center.
Media and Reagents
RPMI 1640 supplemented with 100 IU penicillin and streptomycin/ml, 2mM glutamate, and 10% heat-inactivated fetal bovine serum (FBS) and Ultralow IgG FBS were used as serum-containing culture conditions. For serum-free culture of DCs, CellGro GMP serum-free media (CellGenix, Antioch, IL), X-VIVO 15, X-VIVO20 (Bio-Whittaker, Cambrex Charles City, MD) were used. RPMI 1640 was supplemented with 10% heat-inactivated Ultralow IgG serum (GibcoBRL, Carlsbad, CA), 100 IU penicillin and streptomycin/ml, 2mM glutamate for initial experiments. LPS from E.Coli, B5:55 (Sigma-Aldrich, St.Louis, MO), control CpG: 5′-ggGGGAGCATGCTGCggggG-3′, and stimulatory CpG: 5′-ggGGGACGATCGTCGggggG-3′(Gene Link, Hawthorne, NY) were used for DC maturation, anti-CD40 antibody was purchased from BD Pharmingen. The protocol for CpG activation was by previously reported (Boule et al., 2004)
Generation of bone marrow-derived DC
DC generation from bone marrow progenitors was performed as described (Inaba et al., 1992). Briefly, 6-8wk old mice were sacrificed and hind limbs were collected. Bone marrow cells were flushed from the tibia and femur with PBS and red blood cells were removed by ammonium chloride treatment. To deplete non-DC progenitors, bone marrow cells were incubated with supernatant from the following cell cultures, TIB120 (I-A), TIB 211 (CD8), TIB146 (B220), GK1.5 (CD4), and rabbit complement for 1h in a 37 °C water bath. The remaining cells were washed with PBS and cultured at 1×106 cells/ml in 6 well plates in either serum-containing or serum-free medium. RPMI 1640 containing serum was supplemented with J558L-GM-CSF conditioning media or recombinant murine GM-CSF (1000U/ml, Peprotech, Rocky Hill, NJ) alone or GM-CSF and recombinant murine IL-4 (500U/ml, Peprotech) as indicated. All other serum-free culture media were supplemented with recombinant murine GM-CSF alone or together with IL-4. Cytokine supplemented culture media were replenished on days 3 and 6.
Cytokine analysis
Supernatant from DCs was collected at 18h after activation with LPS or CpG DNA. Cellular debris was removed by centrifugation, and supernatants were transferred to new tubes and frozen at -20 °C until analyzed. Murine IL-12 p70, IL-12 p40, IL-6, and IL-10 in supernatants were analyzed by ELISA using the OptEIA ELISA Set (BD Pharmingen, San Jose, CA) following the manufacturer’s instructions.
Flow Cytometry
Cells were washed with staining buffer (PBS with 2% FBS) and incubated with fluorochrome-conjugated antibodies (PE-CD40, APC-CD11c, FITC-CD86, PerCP-I-Ad, Pharmingen) for 20min on ice. Cells were analyzed on a Becton Dickinson LSR II flow cytometer.
Statistical Analysis
A paired t-test (two tailed) was used to establish differences between groups. A P value of less than 0.05 was considered significant.
Results
DC culture in different serum-free conditions
Before investigating serum-free conditions for the growth of DCs, we compared three different serum-containing culture systems: RPMI 1640 containing 10% FBS with either GM-CSF derived from J558L conditioned medium or recombinant GM-CSF or RPMI 1640 with 10% Ultralow IgG FBS. There were no significant differences in the number of viable cells after 6 days by Trypan Blue exclusion assay (1.198±0.13, 1.213±0.15, 1.583±0.227, respectively). Since only recombinant GM-CSF is suitable for culture systems that avoid any serum factor, we decided to use the second culture condition (RPMI supplemented with 10% FBS and recombinant GM-CSF) as the control for subsequent experiments.
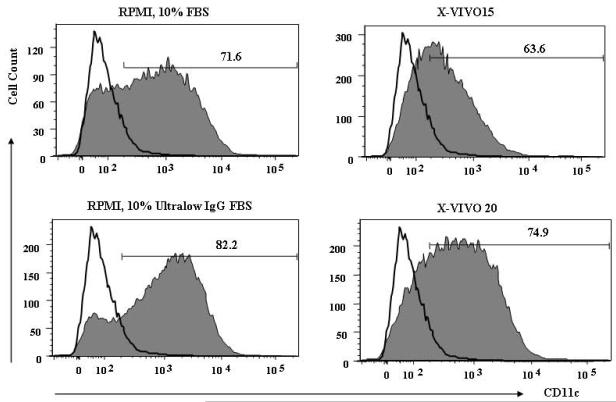
Table 1 shows that serum-free RPMI and CellGro GMP (SF-DC) medium could not support DC survival. Two serum-free media from Bio Whittaker, X-VIVO15 and X-VIVO20, led to a reasonable number of DCs, compared to the serum-containing culture condition. DCs generated in both serum-free media have low level intensity of CD11c expression (Fig.1), although the percentage of cells in the culture expressing CD11c is similar.
Table. 1.
DC yield under different culture conditiona
| Culture conditions | CD11c-positive cells (%) | DC yield / 106 progenitor |
|---|---|---|
| RPMI, 10% FBS | 78.25 ± 2.75 | 1.2 ± 0.24 |
| RPMI, 10% Ultralow IgGFBS | 70.83 ± 4.23 | 1.18 ± 0.11 |
| SF Media | ND | ND |
| SF DC Media | ND | 0.08 ± 0.02 |
| X-VIVO20 | 68.13 ± 5.33 | 0.72 ± 0.21 |
| X-VIVO15 | 77.33 ± 0.43 | 0.75 ± 0.06 |
106 purified DC progenitors were cultured in medium supplemented with 1000U/ml of GM-CSF. Media and sera used are shown above and described in the Material and Methods. On day 6 of culture nonadherent and loosely attached cells were gently rinsed off the plates and DC numbers were determined by Trypan Blue exclusion. The data are expressed as mean ± SD of five independent experiments.
Figure 1. CD11c expression of DCs grown in different culture media.
Bone marrow cells were collected from 6-8 wk old female Balb/c, and non-DC precursor cells were removed as described in Material and Methods. 107 DC precursor cells were placed in each culture medium supplemented with 1,000 U/ml of GM-CSF. Fresh medium was replaced on days 2, 4, and 6. On day 6, viable cell number was counted by the Trypan-Blue exclusion method, and cells were stained with FITC-conjugated anti-CD11c antibody. CD11c positive cells were analyzed by flow cytometry. The thin line represents control staining, and filled line represents DC population. The picture is a representative of 4 individual experiments.
Increased expression of maturation markers
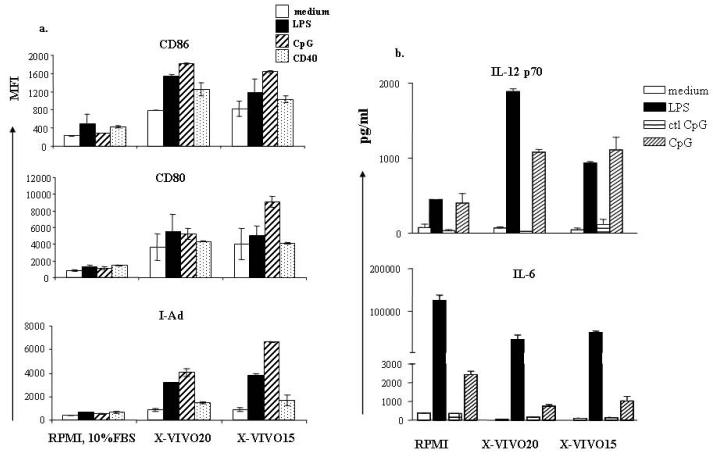
To characterize the phenotype of DCs cultured in serum-free conditions, we first measured the expression of several activation markers, MHC Class II, CD80, and CD86. Consistent with previous reports, there was an increased expression of all these activation markers in unstimulated DCs grown in both serum-free conditions. Moreover, all these markers were further increased upon stimulation with LPS, CpG, and anti-CD40 antibody (Fig.2a), and were increased to a much higher level on DCs grown in serum-free conditions.
Figure 2. Expression of costimulatory molecules and pro-inflammatory cytokine of serum-free cultured DCs.
DCs were grown in different culture media as described. DCs were further stimulated as indicated on day 6 for 48 hours (a) or 16-18 hours (b). (a) DCs were exposed to inflammatory stimuli, washed with PBS, and stained with fluorescence conjugated antibodies to CD11c, CD80, CD86, and I-Ad. MFI of CD80, CD86, and I-Ad was calculated from CD11c-positive cells exclusively. (b) DCs were plated with inflammatory stimuli indicated in 24 well plates at 106/ml for 16-18 hours. Supernatants were harvested and cytokines were measured by Sandwich ELISA. Results represent mean concentration ± SD of 5 individual experiments.
Next, we measured expression of the proinflammatory cytokines, IL-12 and IL-6. A number of reports have shown that DCs can be induced to display increased expression of activation markers with no increase in production of proinflammatory cytokines; these DCs have been termed semi-mature and induce immune tolerance rather than immune activation. We wished, therefore, to determine if DCs grown under serum-free conditions might display the phenotype of tolerogenic DC or of fully mature, activating DC following stimulation. The DCs grown in serum-free culture media produced more IL-12 upon stimulation with LPS, CpG, and anti-CD40 antibody. Interestingly, production of another proinflammatory cytokine, IL-6, was severely decreased in serum-free cultured DCs (about 10 times less). The decreased expression of IL-6 is also found in non-stimulated DCs. (Fig.2b). We did not find any significant changes in IL-10 expression (data not shown).
Different subtype of DCs were generated under the serum-free condition
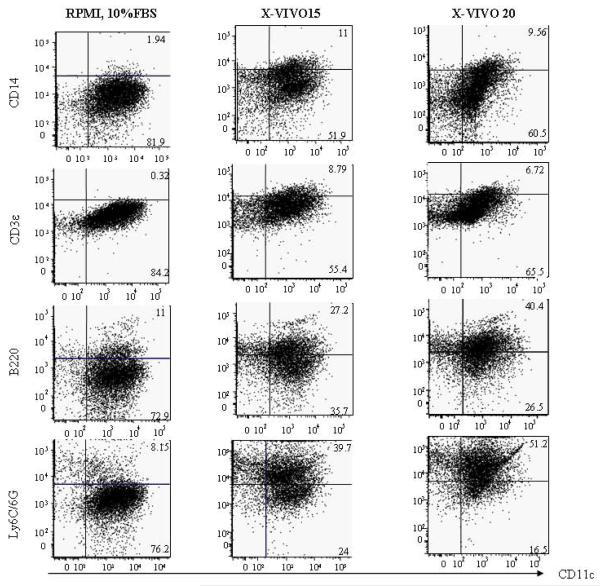
When CD11c positive DCs were analyzed by flow cytometry for expression of additional surface markers, we consistently noted a decreased size of DCs cultured in serum-free conditions. Cells were stained with antibodies to the following molecules: CD14, CD3e, B220, Ly6C/G. As Fig.3 shows, there was very low expression of CD14 and CD3e on cells from the control culture, and minor population of DCs are B220 and Ly6C/6G positive (~10%). DCs cultured in serum-free media composed of about 10% of less differentiated DCs (CD14-positive cells). Interestingly, in these cultures the majority of CD11c positive DCs co-expressed B220 and Ly6C/6G.
Figure 3. Surface expression of plasmacytoid DC markers in serum-free cultured DCs.
DCs grown in various culture conditions were harvested and stained with antibody to CD11c, CD14, CD3e, B220, and -Ly6C/G. Dead cell debris was excluded by size and size matched populations were analyzed. The figure is a representative of 3 individual experiments.
Discussion
Dendritic cells (DCs) are critical antigen presenting cells in immune system, and form the interface between the innate and the adaptive immune response. They perform the critical function of determining whether there is a tolerogenic or immunogenic response to antigen. They reside throughout the body and continually collect, process, and present antigens both self and foreign (Mellman and Steinman, 2001; Shortman and Liu, 2002).
Because of their potent function in antigen presentation, DCs have been explored as vaccine vehicles. They have also been explored as a potential therapeutic strategy in autoimmune disease, where inoculation with antigen-pulsed DC can prevent or ameliorate tissue inflammation. The use of DC to induce antigen specific immune responses in vivo, however, led to an inappropriate activation of immune cells reactive with antigens other than the vaccine antigens (Toldbod et al., 2003). Careful examination revealed that this non-specificity was a consequence of the use of fetal bovine serum (FBS) in the in vitro culture of DCs. Because it is crucially important to generate antigen-specific DCs and avoid any nonspecific activation in clinical applications, several attempts have been made to generate DCs under serum-free condition. Several laboratories have successfully generated human monocyte-derived DCs in serum-free culture condition (Luft et al., 1998) and shown that these DCs are able to migrate to local lymphoid organs, present antigens, and activate antigen-specific T cells. In contrast, there are few studies of serum-free culture of murine bone marrow-derived DCs. Muller et al replaced FBS with mouse serum; DCs cultured in mouse serum showed increased expression of costimulatory molecules, CD80, CD86, MHC Class II, and DEC-205, and successful antigen-specific T cell activation (Muller et al., 2000). Others have suggested that exposures IL-4 is critical for the up-regulation of expression of co-stimulatory molecules on DCs grown in serum-free conditions (Wells et al., 2005).
In this study, we compared different serum-free media for DC propagation with respect to yield, purity of CD11c+ cells, and activation. Murine bone marrow-derived DCs grew only in X-VIVO serum-free media with a slightly lower yield than grown in serum-containing medium. Although serum-free culture resulted in low number of cells, the purity of DCs, based on CD11c-positive cells, was equivalent. CD11c+ cells from serum-free culture displayed phenotypic maturity in the absence of IL-4. Functional activation was measured by secretion of IL-12. As we expected, serum-free cultured DCs secreted 2-4 times more IL-12 measured by ELISA (Fig.2b). Interestingly, the same DCs displayed severely decreased secretion of another proinflammatory cytokine, IL-6.
In addition to the difference in cytokine expression, we found that the cell size of DCs grown in serum-free is more homogenous and small in comparison to DCs in serum-containing culture. To address whether serum-free culture condition drives DC progenitors to differentiate into the same DC subtype, we stained cells with additional markers. Serum-free cultured DCs have a high expression of B220 and Ly6C/G together with CD11c, implying the generation of plasmacytoid DCs (pDCs).
Since the initial description of immature and mature DC, we have come to appreciate a much greater complexity of DC phenotype. The development of DCs from DC progenitors to fully differentiated DCs is a delicate process, affected by many factors. For example, bone marrow derived DC precursors can develop into pDC, CD8+ DC and CD8-DC in the presence of GM-CSF and FMS-related tyrosine kinase 3 ligand (FLT3L) (Brasel et al., 2000; Brawand et al., 2002; Gilliet et al., 2002; Naik et al., 2005), but develop to interstitial DCs, LCs, and inflammatory DCs in the presence of GM-CSF only (Inaba et al., 1992; Nikolic et al., 2003). Our study shows that FBS dramatically affects DC maturation presumably because ligands for DC receptors, such as pattern recognition and toll-like receptors or Fc receptors, are present in xenologous serum. The generation of pDC has particular implication in immune non-responsiveness (Liang et al., 2007). pDC can activate IL-10 producing regulatory T cells (Ito et al., 2007). Thus, the ability to pulse pDC with particular antigen in vitro represents a strategy for inducing antigen specific non-responsiveness.
We demonstrate that murine bone marrow-derived DCs cultured with specific serum-free media exhibit a more mature phenotype and secrete increased amounts of IL-12 and decreased amounts of IL-6. Most importantly, serum-free cultured DCs exhibit an altered differentiation pathway, maturing to pDCs rather than conventional DCs. Clearly, the careful analysis of culture conditions for DC is of critical importance in generating population of DCs that differ in functional capacity. This analysis is crucial in bringing this technology to clinical application.
Acknowledgement
These studies are supported by grants of the NIH, Sung Jung Kim recipient of Fellowship from SLE Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–31. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bjorck P. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 2001;98:3520–6. doi: 10.1182/blood.v98.13.3520. [DOI] [PubMed] [Google Scholar]
- Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–40. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasel K, De Smedt T, Smith JL, Maliszewski CR. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 2000;96:3029–39. [PubMed] [Google Scholar]
- Brawand P, Fitzpatrick DR, Greenfield BW, Brasel K, Maliszewski CR, De Smedt T. Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand-supplemented bone marrow cultures are immature APCs. J Immunol. 2002;169:6711–9. doi: 10.4049/jimmunol.169.12.6711. [DOI] [PubMed] [Google Scholar]
- Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–6. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, O’Garra A, Liu YJ. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–8. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–15. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Ma L, Thai NL, Fung JJ, Qian S, Lu L. The role of liver-derived regulatory dendritic cells in prevention of type 1 diabetes. Immunology. 2007;120:251–60. doi: 10.1111/j.1365-2567.2006.02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft T, Pang KC, Thomas E, Bradley CJ, Savoia H, Trapani J, Cebon J. A serum-free culture model for studying the differentiation of human dendritic cells from adult CD34+ progenitor cells. Exp Hematol. 1998;26:489–500. [PubMed] [Google Scholar]
- Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Menges M, Rossner S, Voigtlander C, Schindler H, Kukutsch NA, Bogdan C, Erb K, Schuler G, Lutz MB. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller G, Muller A, Jonuleit H, Steinbrink K, Szalma C, Paragnik L, Lingnau K, Schmidt E, Knop J, Enk AH. Fetal calf serum-free generation of functionally active murine dendritic cells suitable for in vivo therapeutic approaches. J Invest Dermatol. 2000;114:142–9. doi: 10.1046/j.1523-1747.2000.00832.x. [DOI] [PubMed] [Google Scholar]
- Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, Lahoud MH, O’Keeffe M, Shao QX, Chen WF, Villadangos JA, Shortman K, Wu L. Cutting edge: generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 2005;174:6592–7. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- Nikolic T, de Bruijn MF, Lutz MB, Leenen PJ. Developmental stages of myeloid dendritic cells in mouse bone marrow. Int Immunol. 2003;15:515–24. doi: 10.1093/intimm/dxg050. [DOI] [PubMed] [Google Scholar]
- Olweus J, BitMansour A, Warnke R, Thompson PA, Carballido J, Picker LJ, Lund-Johansen F. Dendritic cell ontogeny: a human dendritic cell lineage of myeloid origin. Proc Natl Acad Sci U S A. 1997;94:12551–6. doi: 10.1073/pnas.94.23.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–62. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurner B, Roder C, Dieckmann D, Heuer M, Kruse M, Glaser A, Keikavoussi P, Kampgen E, Bender A, Schuler G. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J Immunol Methods. 1999;223:1–15. doi: 10.1016/s0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]
- Toldbod HE, Agger R, Bolund L, Hokland M. Potent influence of bovine serum proteins in experimental dendritic cell-based vaccination protocols. Scand J Immunol. 2003;58:43–50. doi: 10.1046/j.1365-3083.2003.01267.x. [DOI] [PubMed] [Google Scholar]
- Wells JW, Darling D, Farzaneh F, Galea-Lauri J. Influence of interleukin-4 on the phenotype and function of bone marrow-derived murine dendritic cells generated under serum-free conditions. Scand J Immunol. 2005;61:251–9. doi: 10.1111/j.1365-3083.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Takahashi M, Narita M, Toba K, Liu A, Furukawa T, Koike T, Aizawa Y. Generation of dendritic cells from adherent cells of cord blood by culture with granulocyte-macrophage colony-stimulating factor, interleukin-4, and tumor necrosis factor-alpha. J Hematother Stem Cell Res. 2000;9:453–64. doi: 10.1089/152581600419116. [DOI] [PubMed] [Google Scholar]