Abstract
Cavities within proteins that are strictly apolar typically appear to be empty. It has been suggested, however, that water molecules may be present within such cavities but are too disordered to be seen in conventional crystallographic analyses. In contrast, it is argued here that solvent mobility will be limited by the size of the cavity and for this reason high-occupancy solvent in cavities of typical volume should be readily detectable using X-ray crystallography. Recent experimental studies of cavity hydration are reviewed. Such studies are consistent with theoretical predictions that it is energetically unfavorable to have a single water molecule in an apolar cavity. As apolar cavities become larger, a point is reached where it is favorable to have the cavity occupied by a cluster of mutually H-bonded water molecules. The exact size of such a cavity in a protein is yet to be verified.
Keywords: cavity, water, T4 lysozyme, interleukin-1β, hydration, empty
Introduction
Protein cores are tightly but not perfectly packed. Typically 1% or so of the interior of a protein forms cavities which may or may not be occupied by solvent.1,2 Ninety-four percent of water molecules observed in such cavities form three or four hydrogen bonds and the survey of Hubbard et al.2 did not reveal a single example of a nonhydrogen bonded internal solvent molecule. This implies that there was no example of a single water molecule in a strictly apolar cavity. (The parallel survey of Williams et al.1 gave similar results. They did find that 3% of the interior water molecules make no hydrogen bonds to the protein, but it is possible that some if not all of these were hydrogen-bonded by other solvent molecules.)
Whether apolar cavities in proteins are truly empty, however, has remained contentious.3–8 On one hand, it is known that the free energy of removing a single water molecule from bulk solvent is very unfavorable (about 6.4 kcal/mol) due to the loss of hydrogen bonding interactions.9,10 Unless these favorable interactions can be restored, it would be expected that placing a water molecule in a protein cavity will be energetically unfavorable. On the other hand, it is argued that “Nature abhors a vacuum,” and that a water molecule in a nonpolar cavity is likely to be very mobile and not detectable in a conventional crystallographic refinement. In other words, water molecules that form hydrogen bonds will have well-defined positions and these are the only waters that can be seen by crystallography.3,4,11 The purpose of this report is in part to rebut this argument.
Whether water molecules in proteins are hydrogen bonded or not, it should be emphasized that they are by no means static. Halle and coworkers11,12 have shown in the case of bovine trypsin inhibitor that internal waters exchange with bulk water on the time scale of 15 ns to 1 μs. They also found that the internal solvent molecules had root-mean-square (rms) liberation amplitudes in the range ∼10°–30° (for water in ice the amplitude is 8°). It bespeaks the dynamic nature of protein structures.
Locating “Mobile” water molecules
As noted in the Introduction, it is frequently stated that solvent molecules in apolar cavities will be very mobile and therefore undetectable in conventional crystallographic refinement. It needs to be kept in mind, however, that the mobility of a water molecule will be restricted by the size and shape of the cavity in which it is contained.
Crudely, if an atom is undergoing translational displacements r around an average position, its crystallographic thermal factor (or B-factor) is given by B ∼ 80 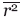 where the bar indicates an average value (see e.g., Levitt and Park3 for a more complete analysis). For a well-ordered atom in a protein, the typical B-factor might be 10–20 Å2, which corresponds to rms positional fluctuations of around ±0.4 Å. Typically, water molecules with B-factors up to about 50 Å2; can be located fairly reliably in a crystallographic refinement (e.g., see Ref.3). This corresponds to rms fluctuations of about ±0.8 Å. A water molecule (radius 1.4 Å) undergoing fluctuations of this magnitude would be expected to occupy a volume of about 44 Å3 [Fig. 1(A)].13 Conversely, if a water molecule were restricted to a cavity of this volume, or smaller, it should be located by the refinement procedure.
where the bar indicates an average value (see e.g., Levitt and Park3 for a more complete analysis). For a well-ordered atom in a protein, the typical B-factor might be 10–20 Å2, which corresponds to rms positional fluctuations of around ±0.4 Å. Typically, water molecules with B-factors up to about 50 Å2; can be located fairly reliably in a crystallographic refinement (e.g., see Ref.3). This corresponds to rms fluctuations of about ±0.8 Å. A water molecule (radius 1.4 Å) undergoing fluctuations of this magnitude would be expected to occupy a volume of about 44 Å3 [Fig. 1(A)].13 Conversely, if a water molecule were restricted to a cavity of this volume, or smaller, it should be located by the refinement procedure.
Figure 1.
A: Sketch comparing, from left to right, the volume occupied by a water molecule at rest, a water molecule with a thermal factor of B = 50 Å2, a krypton atom at rest and an apolar cavity present in wildtype T4 lysozyme. At a pressure of 8bar, krypton is seen to bind within the lysozyme cavity (occupancy ∼0.4; Quillin et al., 2000), even though there are no direction-specific interactions to help localize the krypton atom. Crystallographic analysis suggests that the occupancy of water in this cavity is very low. B: Stereo pair showing the apolar cavity present in pseudo-wildtype lysozyme (WT*; C54T/C97A; PDB code 1L63). The “chicken wire” grid shows not only the internal cavity (cyan arrow) but also the external (solvent-exposed) surface of the protein. If, for example, there is an oxygen atom (red) close to the cavity, the surface grid at that location is also shaded red. This figure as well as Figures 2(B) and 3 were prepared with PyMOL (DeLano, 2008).
As a specific example, consider the cavity in wildtype T4 lysozyme that is surrounded by Leu84, Leu99, Val111, Phe114, Leu121, Leu133, and Phe153 [(Fig. 1(A,B)].14 It is fully nonpolar in character and has a calculated volume of 48 Å2. (Cavity volumes are estimated by rolling a test probe over the surface of the cavity. The calculation is sensitive to the radius of the probe, the atomic radii assumed for the protein atoms, and relatively small changes in the coordinates of the protein. For these reasons there can be variations in stated cavity volumes.13) Crystallographic refinement shows no evidence of water inside this cavity. However, under fairly low pressure of 8 bar (equivalent to ∼0.3 M) an atom of krypton or xenon is seen to bind within this cavity with occupancy of 0.4 or 0.6, respectively.13,15 Xenon has a van der Waals radius of 2.2 Å and a volume of 45 Å3. Although it makes no hydrogen-bonding or directional interactions with the walls of the cavity its position is sufficiently localized that it is readily visible in the electron density map. Indeed, the B-factor of the xenon atom is 18 Å2 which is as well ordered as the atoms that define the walls of the cavity. The van der Waals radius of krypton (2.0 Å) [Fig. 1(A)] is somewhat less than that of xenon, but it is localized just as well (B = 15 Å2). A krypton atom with occupancy of 0.4 corresponds to 14 electrons, which is not that much more than water (10 electrons). Therefore, one cannot argue that the krypton or xenon atoms are only seen because they have much higher electron density than water.
For both the xenon and krypton atoms, it appears that the total extent of their movement is proscribed by the atoms that form the walls of cavity. A water molecule has a smaller radius than that of xenon (1.4 Å vs 2.2 Å), and therefore would be expected to have some freedom to move independently within the confines of the cavity. For the reasons given earlier, however, its overall movement should still be sufficiently restricted that its B-factor would not exceed about 50 Å2. As such, it should be detectable in a crystallographic electron density map.
The argument that water molecules in apolar cavities will be too mobile to be seen crystallographically may hold for larger cavities, but is not justified for those of volume less than 40 Å3 or thereabouts. Furthermore, the large majority of cavities in proteins that appear to be empty have volumes less than 40 Å3.2,16 There are many of these cavities with volumes in the range 30–40 Å2 which would be large enough to accommodate a water molecule without steric hindrance. For the reasons given earlier, if these cavities contained high-occupancy solvent it should be readily detectable by X-ray crystallography. Conversely, the overwhelming lack of visible electron density in such cavities strongly indicates that they are empty.
The apolar cavity in interleukin-1β
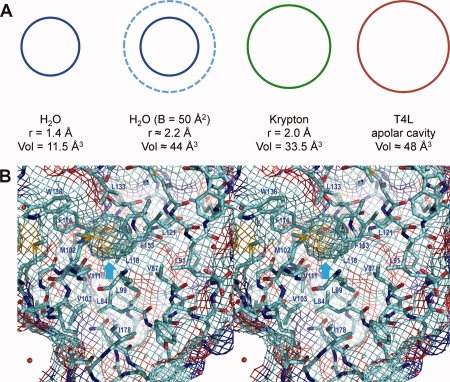
Interleukin-1β (IL-1β) is a hormone-like protein produced in response to infection or injury. Its 153-residue polypeptide chain has a so-called β-trefoil fold made up of a series of curved antiparallel β-strands.17 At four different locations where the β-strands separate there are water molecules located within the protein structure [Fig. 2(A)]. These water molecules make multiple hydrogen bonds with the surrounding protein. At the center of the protein there is a cavity of volume about 40 Å3 surrounded by nonpolar side-chains [Fig. 2(A,B)]. Much of the controversy concerning solvent in proteins has focused on this cavity. Is the IL-1β cavity empty or does it contain disordered solvent? We will first review the NMR evidence.
Figure 2.
A: Backbone structure of Il-1β. The four cavities toward the top and bottom of the figure each contain one or two water molecules which are well ordered and hydrogen-bonded by surrounding backbone amides and carbonyl groups. The fifth cavity, located in the center of the molecule, is surrounded by nonpolar side-chains and its occupancy by solvent is contentious. B: Stereo pair with the cyan arrow showing the central apolar cavity in Il-1β [PDB code 2NVH; cf. Fig. 1(B)]. The red arrow at the top shows an internal cavity containing a single water molecule (red); the red arrow at the bottom shows a cavity containing two solvent molecules. The cavities that contain solvent have partial dark-blue and red coloring indicating their polar nature. The view direction is essentially the same as in Figure 2(A) and the two solvent-filled cavities correspond to those at the top left and bottom left in Figure 2(A).
NMR studies of IL-1β
The solution structure of IL-1β was determined by Clore et al.18 and shown to be very similar to that in the crystal. The rms difference between the NMR structure and the X-ray structure of Finzel et al.17 was 0.85 Å for the backbone atoms and 1.3 Å for all atoms.18 The structure is composed of three topologically similar units, each of which consists of five antiparallel strands. By observing through-space rotating frame Overhauser interactions it was possible to identify 15 NH protons that are within about 3.5 Å of water molecules. As noted by Clore et al.19 the bound water resonances are degenerate (i.e., the NMR data show that there is a water molecule within 3.5 Å of a given NH proton, but the specific location of the water is not defined). By cross-referencing with the water locations seen in the X-ray structure,17 Clore et al.19 inferred that all 15 of the NH protons were close (<3 Å) and/or hydrogen-bonded to one of these water molecules. The water molecules are well ordered in the crystal structure and, as noted earlier, make multiple hydrogen bonds with the protein backbone. None of these solvent molecules is within the central apolar cavity of IL-1β.
In a further NMR study, Ernst et al.4 identified methyl and/or methylene protons of Leu10, Leu18, Leu26, Leu60, Leu69, Leu80, Ile122, and Val132 that were within about 5 Å of a water molecule. They inferred that this water was within the apolar cavity of IL-1β. It has been pointed out, however, that at least 18 of the 26 protons identified by Ernst et al.4 are within 5 Å of water molecules that are within the Il-1β structure, but not in the apolar cavity.5,6 The correct interpretation of the experiments of Ernst et al.4 remains unresolved. Given that the central cavity of IL-1β is apolar, it could well bind a hydrophobic ligand such as methane, or one of the noble gases. It would be instructive to repeat the experiments of Ernst et al.4 in the presence of such a ligand. The ability of a bound ligand to displace putative solvent from the cavity and to eliminate the relevant NOE cross-peaks would be strong evidence that the cavity of IL-1β is indeed hydrated.
X-ray studies of IL-1β
There are at least four independent determinations of the crystal structure of IL-1β by X-ray diffraction.17–22 Conventional crystallographic refinement consistently shows no evidence for solvent with the central apolar cavity.
In contrast, an X-ray analysis by Yu et al.23 presented evidence for about two water molecules in the cavity region. The analysis of Yu et al. was based on an iterative density modeling procedure developed for the purpose. A number of ad hoc assumptions were made including truncation of the data, resolution-dependent damping and damping to smooth out high spatial frequency ripples. No attempt was made to validate these ad hoc factors, e.g., by using known solvent-binding sites in the protein to check the results. Also, rather than calculating the putative amount of water within the actual cavity, Yu et al. determined the density within a sphere of radius 6 Å. Such a sphere has a volume of 905 Å3, 23 times larger than the observed cavity. The use of such a large sphere is difficult to justify physically, i.e., much of the 905 Å3 is presumably occupied by the protein and as such cannot be simultaneously occupied by solvent. Also the use of an artificial volume of this magnitude makes the calculation very sensitive to experimental and computational uncertainties. For example, the crystallographic F000 term cannot be measured experimentally and has to be inferred from the modeling algorithm. An error of 1% in F000, which is likely to be optimistic, would change the occupancy of the cavity by almost half a water molecule. The procedure of Yu et al.23 does not provide controls whereby the results can be tested independently.
More recently, Quillin et al.24 carried out a further X-ray analysis of the cavity in the IL-1β crystal structure using a method that is strictly experimental in nature. In this procedure the X-ray phases for all the reflections are determined as accurately as possible and conventional crystallographic refinement, which can introduce model-dependent artifacts, is avoided.25 The objective is to obtain an electron density map of the protein and any associated solvent which is as error-free as possible. By using known solvent-binding cavities within the protein it was possible to check the results. Technical factors limited the study to a resolution of 2.1 Å,24 at which individual atoms are not fully resolved. This means that electron density from surrounding protein atoms can “spill over” into the cavity and, likewise, density from atoms within the cavity can extend outside. Taking this effect into account it was estimated that the solvent occupancy of the apolar cavity in IL-1β is close or equal to zero.
It might be noted that the NMR analysis of IL-1β was carried out a room temperature,4 whereas the X-ray data were collected at ∼100 K. Because of the reduced entropy cost, solvent molecules should be more readily localized at the lower temperature. The difference between the NMR and X-ray analyses are therefore the opposite of what might have been expected based on the difference in temperature of the respective studies.
Hydration of cavities in hen egg-white lysozyme
Otting et al.8 have used NMR to analyze the binding of both water and small gas molecules to cavities in hen egg-white lysozyme (HEWL). This well-studied protein has three apolar cavities (I, II, and III) of volumes 40, 11, and 13 Å3 which appear to be empty in crystallographic analyses.8 At pressures up to 200 bar, hydrogen, methane, ethylene, and cyclopropane bind in Cavities I and II. For methane at 170 bar the occupancy of Cavity I was estimated to be between 10 and 50%. In the NMR spectra, NOESY cross peaks to water were also observed for protons surrounding Cavities I and II. These were attributed to water molecules within these cavities. At the same time, the intensities of these peaks were approximately an order of magnitude smaller than NOEs typically observed for solvent at classical hydrogen-bonded sites. This was taken to indicate that Cavities I and II are populated by water, but with only partial occupancy.8
Cavity analysis in T4 lysozyme
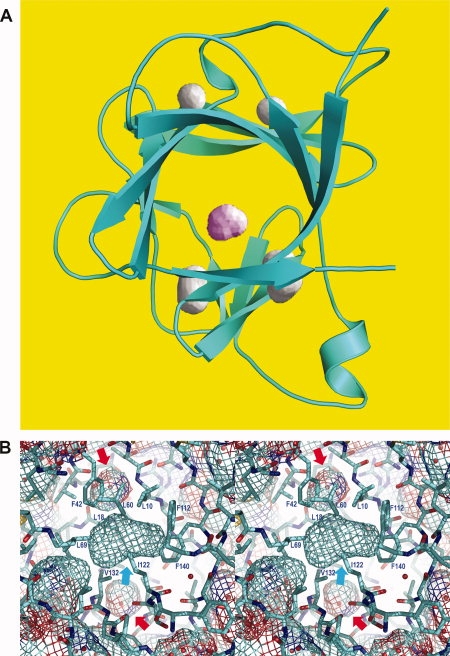
The L99A cavity in T4 lysozyme (see Fig. 3) has also been examined by direct experimental phasing of the X-ray reflections.26 By using selenomethionine substitution and measuring the X-ray amplitudes at different wavelengths it was possible to obtain a high-resolution electron density map free of the bias that can occur in conventional refinement procedures.
Figure 3.
Stereo pair showing the cavity in the mutant T4 lysozyme L99A/M102L (PDB code 3DKE). As in Figure 1(B), the “chicken wire” grid shows both the internal cavity (cyan arrow) and the external solvent-exposed surface of the protein. The site of the Leu99 to Ala substitutions is shown in red. Most of the cavity wall is colored turquoise, indicating its nonpolar nature. Close to the carbonyl group of Ala99, however, the red color indicates some polar character. There is also some dark blue and red in the vicinity of Val87.
The L99A/M102L mutant used for these studies includes a total of four cavities and the electron density associated with each of these is shown in Figure 4.26,27 Cavity 1 contains two hydrogen-bonded water molecules and the density for these is clearly seen in Figure 4(A). Likewise, density for a single hydrogen-bonded solvent molecule is seen in Cavity 2 [Fig. 4(B)]. The third cavity is nonpolar and of volume 21 Å3. Conventional crystallographic refinement shows no evidence for solvent in this cavity and this is confirmed by the experimental map [Fig. 4(C)].
Figure 4.
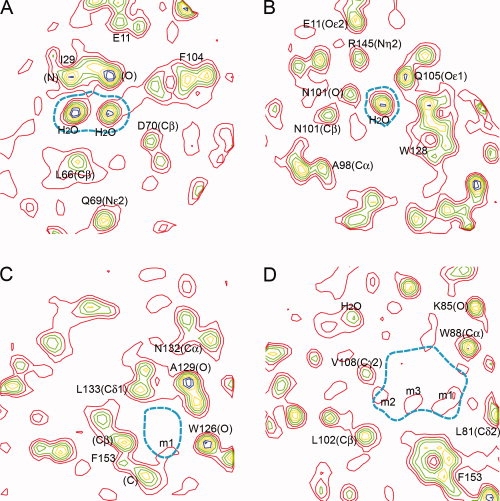
Electron density based on experimental phases to 1.2 Å resolution for the cavity-containing lysozyme mutant L99A/M102L (Liu et al., 2008). The four panels show, respectively, sections of electron density passing through the four cavities in this lysozyme mutant. The boundary of the cavity in question is indicated in dashed cyan. The electron density, starting in red and continuing through blue, is contoured at 0.55, 1.0, 1.45, 1.9, 2.35, 2.8, 3.25, and 3.7 e/Å3. Density corresponding to selected atoms is labeled. A: The density confirms the presence of two well-ordered solvent molecules in this polar cavity. B: Second polar cavity with a single water molecule bound. C: The very weak density in this apolar cavity (labeled m1) indicates that the occupancy by solvent is close to zero. D: The engineered L99A cavity contains weak electron density indicating that the overall occupancy of this cavity is about 1.5 water molecules. The features labeled m1–m3 could indicate a chain of weakly occupied solvent molecules transiently H-bonded to each other and to the carbonyl oxygen of Ala99 in the wall of the cavity. Figure prepared by Mapman (Kleywegt and Jones, 1996).
The fourth cavity is the one created by the L99A substitution and is substantially larger than the other three (volume 133.5 Å3). The experimental density in this cavity [Fig. 4(D)] is much weaker than in Cavities 1 and 2, but not zero. Integrating over the whole cavity the density corresponds to about 1.5 water molecules. Although weak, the density has some structure, suggesting that the solvent, when present, may tend to form a mutually hydrogen-bonded cluster. Although the cavity was created in the non-polar core of T4 lysozyme by the Leu99 to Ala replacement, the walls of the cavity do include a polar region and it appears that solvent in the cavity takes advantage of this hydrogen-bonding potential. An example of the same type was seen for the Ile76 to Ala mutant of barnase.7 In this case, the resultant cavity contained a fairly well ordered water molecule hydrogen bonded to a backbone carbonyl and perhaps more weakly to a backbone amide group. Also the cavities resulting from the I59A, I59G, and I106A mutants of human lysozyme contained one or two water molecules each making two or three hydrogen bonds.28
Effect of pressure on cavities
When a large hydrophobic residue such as leucine within the core of a protein is replaced with a small one such as alanine a cavity will usually be created.7,28,29 Sometimes the protein structure will hardly change at all, but more typically there will be some relaxation such that surrounding residues move in to partially occupy the vacated space. The larger the cavity that is created the greater will be the loss of stability in the mutant protein.29
Given this behavior, one might expect that the exposure of a cavity-containing protein to pressure might cause the structure to collapse. Experience to date suggests that this is not the case.
Collins et al.30,31 exposed T4 lysozyme and its L99A mutant, which contains a cavity of 150 Å3, to pressures up to 2000 bar. It was found that the presence of the cavity had almost no effect on the response to pressure, with essentially the same changes in structure being observed in the WT* and L99A proteins. In the vicinity of the cavity the protein structure was remarkably rigid such that the volume of the L99A cavity hardly decreased (possibly 3%).
Starting at about 1000 bar, however, electron density corresponding to apparent water molecules could be seen in the cavity. At 2000 bar, the limiting pressure, density corresponding to the entry of two water molecules could be seen. Parallel molecular dynamics simulations30 suggested that the application of pressure causes ∼4 water molecules to enter the cavity in a highly cooperative manner. A corollary of both the experimental result, and the theoretical calculations, is that the cavity at ambient pressure is empty, or nearly so.
Studies at high pressure have also been made of the cavity-containing c-Myb R2 subdomain, in this case using NMR to probe structural changes.32 C-Myb binds DNA via a “tryptophan-sliding mechanism” in which DNA binding is associated with the movement of the indole of Trp95 into a putatively empty cavity.33 Mutation of Val103 to leucine fills the cavity, reduces conformational fluctuations and reduces DNA binding. For the wildtype protein, with its cavity of 33.1 Å3, a pressure up to 3700 bar is sufficient to unfold the protein almost fully. The point mutant V103L, which nearly fills the cavity, dramatically increases the stability against pressure.32 A corollary of these experiments is that the cavity in wildtype c-Myb is indeed empty and not prefilled with solvent.
Theoretical considerations
Most theoretical studies have concluded that the entry of a water molecule into a small apolar cavity is energetically unfavorable.10,34,35 Wolfenden and Radzicka estimated that the chance of finding a water molecule in an apolar cavity just large enough to accommodate it is of the order of 1 in 20,000. Zhang and Hermans35 pointed out that the binding of water in larger apolar cavities becomes more favorable because the solvent molecules can make hydrogen bonds among themselves. This notwithstanding, they estimated that the binding of two solvent molecules within the nonpolar cavity of IL-1β and would be unfavorable.
Two models may be used to approximate the behavior of confined water molecules in nonpolar chemical environment. One is the infinite dilute solution of water in nonpolar solvents such as alkanes, alkenes, and their derivatives, where water molecules are dispersed in transient cavities formed in the solvent. In such a situation, water has been shown by far infrared spectroscopy (FIR) to dissolve mostly as monomers.36–40 Small clusters may also be present but with much lower population.41 FIR also demonstrated that these monomeric water molecules could rotate nearly freely along their main axis in alkanes, less freely in alkenes and tetrachlorocarbon and were significantly hindered in benzene, following the increase in strength of the solute-solvent interaction.36 In accordance with this model, a nonpolar protein cavity might bind a single water molecule. On the other hand, the cavity of a protein is much more rigid than in solvent. This could imply that a trapped water molecule would have less freedom to rotate. Moreover, such hydration in a nonpolar environment drives water molecules to be present with lower density and larger partial molar volume (tending to form normal ice),42 which lowers the chance of water being present in a small cavity. More importantly, this theoretical approximation is based on extremely low solubility of water in nonpolar solvents, which itself implies a very low and perhaps nondetectable occupancy of water in the nonpolar cavity.
The second model is that of water molecule(s) in a hydrophobic cage such as the interior of fullerenes. The wall of the nonpolar cavity is rigid and isolated from the outside, causing the water molecule(s) to be strictly confined. Most theoretical computations based on this approach43–48 support the existence of water clusters under particular conditions. For instance, Hummer and coworkers43 studied the structure and stability of water in nonpolar cavities of various sizes by Monte Carlo simulations. They found that when the size of a spherical cavity reaches 10.0 Å a stable situation is reached in which the cavity is occupied by a cluster of three mutually hydrogen-bonded water molecules. A diameter of 10.4 Å allows a cluster of four water molecules which has greater stability. Conversely, a sphere of diameter 9 Å accommodates two water molecules but this is not a thermodynamically stable arrangement. It should be noted, however, that a cavity diameter of 10 Å corresponds to a volume of about 500 Å3, which is over four times larger than the volume of the cavity in the L99A mutant of T4 lysozyme [Fig. 3]. If a 10 Å cavity was completely filled with solvent, it would contain about 17 water molecules.
The description of Vaitheeswaran et al.43 is essentially consistent with much of the experimental data reviewed earlier. It predicts that apolar cavities in proteins large enough to bind one or two solvent molecules will remain empty as supported by a wealth of crystallographic data. In contrast, water has been demonstrated experimentally to be present in open or semi-open hydrophobic “cavities” as in cyclodextrins,49 calixarenes50 and recently in a bowl-shaped fullerene.51,52
In the case of the L99A cavity in T4 lysozyme the volume of the cavity is large enough to accommodate about 4–5 water molecules. At the same time, the cavity is rather flat and disk-like in shape, which may restricted hydrogen bonding possibilities. Against this, the wall of the cavity has some polar character which will facilitate hydrogen bonding. The experimental electron density [Fig. 4(D)] shows that the cavity does not contain a fully-developed high-occupancy cluster of three or more water molecules, but it does suggest that such clusters may occupy the cavity on a transient basis.
Conclusions
Different theoretical approaches consistently predict that it is energetically unfavorable to locate a single water molecule in an apolar cavity. If the cavity is strictly hydrophobic, and is only large enough to accommodate the single water, then it is energetically more favorable for the cavity to remain empty.
This prediction has much experimental support. In particular, we are not aware of a single well-verified example in which a high occupancy, stand-alone water molecule has been shown to occupy a strictly nonpolar cavity in a protein. (We have also argued that the X-ray crystallographic method should be capable of identifying such examples, if they exist.)
Theory also predicts that as apolar cavities become larger they can accommodate clusters of water molecules that are stabilized by water-water hydrogen bonds. Vaitheeswaran et al.43 have suggested that the minimum size of such a “droplet” might be as small as three or four water molecules and can occur when the volume of the apolar cavity reaches about 500 Å3. This is substantially larger than apolar cavities that have been observed in known protein structures. How the exact number of solvent molecules depends on the geometry of the cavity is yet to be tested experimentally. Stetefeld et al.53 described the structure of a highly stable naturally occurring coiled-coil tetramer with hydrophobic cavities that contain up to nine water molecules. Molecular dynamics simulations of Yin et al.54 confirmed that hydrogen-bonded clusters of 7–9 water molecules were stable in this structure. This could provide an upper limit for the size of an empty cavity at ambient pressure.
References
- 1.Williams MA, Goodfellow JM, Thornton JM. Buried waters and internal cavities in monomeric proteins. Protein Sci. 1994;3:1224–1235. doi: 10.1002/pro.5560030808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hubbard SJ, Gross KH, Argos P. Intramolecular cavities in globular proteins. Protein Eng. 1994;7:613–626. doi: 10.1093/protein/7.5.613. [DOI] [PubMed] [Google Scholar]
- 3.Levitt M, Park BH. Water: now you see it, now you don't. Structure. 1993;1:223–226. doi: 10.1016/0969-2126(93)90011-5. [DOI] [PubMed] [Google Scholar]
- 4.Ernst JA, Clubb RT, Zhou H-X, Gronenborn AM, Clore GM. Demonstration of positionally disordered water within a protein hydrophobic cavity by NMR. Science. 1995;267:1813–1817. doi: 10.1126/science.7892604. [DOI] [PubMed] [Google Scholar]
- 5.Ernst JA, Clubb RT, Zhou H-X, Gronenborn AM, Clore GM. Use of NMR to detect water within nonpolar protein cavities. Science. 1995;270:1848–1849. doi: 10.1126/science.270.5243.1847. [DOI] [PubMed] [Google Scholar]
- 6.Matthews BW, Morton AG, Dahlquist FW. Use of NMR to detect water within non-polar cavities. Science. 1995;270:1847–1849. doi: 10.1126/science.270.5243.1847. [DOI] [PubMed] [Google Scholar]
- 7.Buckle AM, Cramer P, Fersht AR. Structural and energetic responses to cavity-creating mutations in hydrophobic cores: observation of a buried water molecule and the hydrophilic nature of such hydrophobic cavities. Biochemistry. 1996;35:4298–4305. doi: 10.1021/bi9524676. [DOI] [PubMed] [Google Scholar]
- 8.Otting G, Liepinsh E, Halle B, Frey U. NMR identification of hydrophobic cavities with low water occupancies in protein structures using small gas molecules. Nature Struct Biol. 1997;4:396–404. doi: 10.1038/nsb0597-396. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Naim A, Marcus Y. Solvation thermodynamics of nonionic solutes. J Chem Phys. 1984;81:2016–2027. [Google Scholar]
- 10.Wade RC, Mazor MH, McCammon JA, Quiocho FA. A molecular dynamics study of thermodynamic and structural aspects of the hydration of cavities in proteins. Biopolymers. 1991;31:919–931. doi: 10.1002/bip.360310802. [DOI] [PubMed] [Google Scholar]
- 11.Denisov VP, Venu K, Peters J, Hurlein HD, Halle B. Orientational disorder and entropy of water in protein cavities. J Phys Chem B. 1997;101:9380–9389. [Google Scholar]
- 12.Wiesner S, Kurian E, Prendergast FG, Halle B. Water molecules in the binding cavity of intestinal fatty acid binding protedynamic characterization by water 17O and 2H magnetic relaxation dispersion. J Mol Biol. 1999;286:233–246. doi: 10.1006/jmbi.1998.2490. [DOI] [PubMed] [Google Scholar]
- 13.Quillin ML, Breyer WA, Griswold IJ, Matthews BW. Size versus polarizability in protein-ligand interactions: binding of noble gases within engineered cavities in phage T4 lysozyme. J Mol Biol. 2000;302:955–977. doi: 10.1006/jmbi.2000.4063. [DOI] [PubMed] [Google Scholar]
- 14.DeLano WL, The PyMOL molecular graphics system. Palo Alto, CA, USA: DeLano Scientific LLC 2008. Available at http://222.pymol.org.
- 15.Desvaux H, Dubois L, Huber G, Quillin ML, Berthault P, Matthews BW. Dynamics of xenon binding inside the hydrophobic cavity of pseudo-wild-type bacteriophage T4 lysozyme explored through xenon-based NMR spectroscopy. J Am Chem Soc. 2005;127:11676–11683. doi: 10.1021/ja053074p. [DOI] [PubMed] [Google Scholar]
- 16.Rashin AA, Iofin M, Honig B. Internal cavities and buried waters in globular proteins. Biochemistry. 1986;25:3619–3625. doi: 10.1021/bi00360a021. [DOI] [PubMed] [Google Scholar]
- 17.Finzel BC, Clancy LL, Holland DR, Muchmore SW, Watenpaugh KD, Einspahr HM. Crystal structure of recombinant human interleukin-1β at 2.0Å resolution. J Mol Biol. 1989;209:779–791. doi: 10.1016/0022-2836(89)90606-2. [DOI] [PubMed] [Google Scholar]
- 18.Clore GM, Wingfield PT, Gronenborn AM. High-resolution three-dimensional structure of interleukin 1β in solution by three- and four-dimensional nuclear magnetic resonance spectroscopy. Biochemistry. 1991;30:2315–2323. doi: 10.1021/bi00223a005. [DOI] [PubMed] [Google Scholar]
- 19.Clore GM, Bax A, Wingfield PT, Gronenborn AM. Identification and localization of bound internal water in the solution structure of interleukin 1β by heteronuclear three-dimensional proton rotating-fram Overhauser nitrogen-15-proton multiple quantum coherence NMR spectroscopy. Biochemistry. 1990;29:5671–5676. doi: 10.1021/bi00476a004. [DOI] [PubMed] [Google Scholar]
- 20.Priestle JP, Schar HP, Grutter MG. Crystallographic refinement of interleukin 1β at 2.0Å resolution. Proc Natl Acad Sci USA. 1989;86:9667–9671. doi: 10.1073/pnas.86.24.9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treharne AC, Ohlendorf DH, Weber PC, Wendoloski JJ, Salemme FR. X-ray structural studies of the cytokine interleukin 1β. Prog Clin Biol Res. 1990;349:309–319. [PubMed] [Google Scholar]
- 22.Veerapandian B, Gilliland GL, Raag R, Svensson AL, Masui Y, Hirai Y, Poulos TL. Functional implications of interleukin-1β based on the three-dimensional structure. Proteins. 1992;12:10–23. doi: 10.1002/prot.340120103. [DOI] [PubMed] [Google Scholar]
- 23.Yu B, Blaber M, Gronenborn AM, Clore GM, Caspar DLD. Disordered water within a hydrophobic protein cavity visualized by X-ray crystallography. Proc Natl Acad Sci USA. 1999;96:103–108. doi: 10.1073/pnas.96.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quillin ML, Wingfield PT, Matthews BW. Determination of solvent content in cavities in IL-1β using experimentally phased electron density. Proc Natl Acad Sci USA. 2006;103:18148–18153. doi: 10.1073/pnas.0609442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burling FT, Weis WI, Flaherty KM, Brunger AT. Direct observation of protein solvation and discrete disorder with experimental crystallographic phases. Science. 1996;271:72–77. doi: 10.1126/science.271.5245.72. [DOI] [PubMed] [Google Scholar]
- 26.Liu L, Quillin ML, Matthews BW. Use of experimental crystallographic phases to examine the hydration of polar and nonpolar cavities in T4 lysozyme. Proc Natl Acad Sci USA. 2008;105:14406–14411. doi: 10.1073/pnas.0806307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleywegt GJ, Jones TA. xdlMAPMAN and xdlDATAMAN—programs for reformatting, analysis and manipulation of biomacromolecular electron-density maps and reflection data sets. Acta Cryst D. 1996;52:826–828. doi: 10.1107/S0907444995014983. [DOI] [PubMed] [Google Scholar]
- 28.Takano K, Funahashi J, Yamagata Y, Fuji S, Yutani K. Contribution of water molecules in the interior of a protein to the conformational stability. J Mol Biol. 1997;274:132–142. doi: 10.1006/jmbi.1997.1365. [DOI] [PubMed] [Google Scholar]
- 29.Eriksson AE, Baase WA, Zhang X-J, Heinz DW, Blaber M, Baldwin EP, Matthews BW. Response of a protein structure to cavity-creating mutations and its relation to the hydrophobic effect. Science. 1992;255:178–183. doi: 10.1126/science.1553543. [DOI] [PubMed] [Google Scholar]
- 30.Collins MD, Hummer G, Quillin ML, Matthews BW, Gruner SM. Cooperative water filling of a nonpolar protein cavity observed by high-pressure crystallography and simulation. Proc Natl Acad Sci USA. 2005;102:16668–16671. doi: 10.1073/pnas.0508224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins MD, Quillin ML, Hummer G, Matthews BW, Gruner SM. Structural rigidity of a large cavity-containing protein revealed by high-pressure crystallography. J Mol Biol. 2007;367:752–763. doi: 10.1016/j.jmb.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lassalle MW, Yamada H, Morii H, Ogata K, Sarai A, Akasaka K. Filling a cavity dramatically increases pressure stability of the c-Myb R2 subdomain. Proteins. 2001;45:96–101. doi: 10.1002/prot.1128. [DOI] [PubMed] [Google Scholar]
- 33.Ogata K, Kanei-Ishii C, Sasaki M, Hatanaka H, Nagadoi A, Enari M, Nakamura H, Nishimura Y, Ishii S, Sarai A. The cavity in the hydrophobic core of Myb DNA-binding domain is reserved for DNA recognition and trans-activation. Nature Struct Biol. 1996;3:178–187. doi: 10.1038/nsb0296-178. [DOI] [PubMed] [Google Scholar]
- 34.Wolfenden R, Radzicka A. On the probability of finding a water molecule in a nonpolar cavity. Science. 1994;265:936–937. doi: 10.1126/science.8052849. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Hermans J. Hydrophilicity of cavities in proteins. Proteins. 1996;24:433–438. doi: 10.1002/(SICI)1097-0134(199604)24:4<433::AID-PROT3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 36.Tassaing T, Danten Y, Besnard M, Zoidis E, Yarwood J, Guissani Y, Guillot B. A far infrared study of water diluted in hydrophobic solvents. Mol Phys. 1995;84:769–785. [Google Scholar]
- 37.Danten Y, Tassaing T, Besnard M. Molecular dynamics of monomeric water dissolved in very hydrophobic solvents: the current state of the art of vibrational spectroscopy analyzed from analytical model and MD simulations. J Phys Chem A. 2000;104:9415–9427. [Google Scholar]
- 38.Graziano G. Solvation of a water molecule in cyclohexane and water. Can J Chem. 2001;79:105–109. [Google Scholar]
- 39.Henn AR, Kauzmann W. New considerations of the Barclay-Butler rule and the behavior of water dissolved in organic solvents. Biophys Chem. 2003;100:205–220. doi: 10.1016/s0301-4622(02)00282-x. [DOI] [PubMed] [Google Scholar]
- 40.Graziano G. Solvation thermodynamics of water in nonpolar organic solvents indicate the occurrence of nontraditional hydrogen bonds. J Phys Chem B. 2005;109:981–985. doi: 10.1021/jp0456739. [DOI] [PubMed] [Google Scholar]
- 41.Koddermann T, Schulte F, Huelsekopf M, Ludwig R. Formation of water clusters in a hydrophobic solvent. Angew Chem Int Ed. 2003;42:4904–4908. doi: 10.1002/anie.200351438. [DOI] [PubMed] [Google Scholar]
- 42.Chalikian TV. Structural thermodynamics of hydration. J Phys Chem B. 2001;105:12566–12578. [Google Scholar]
- 43.Vaitheeswaran S, Yin H, Rasaiah JC, Hummer G. Water clusters in nonpolar cavities. Proc Natl Acad Sci USA. 2004;101:17002–17005. doi: 10.1073/pnas.0407968101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramachandran CN, Sathyamurthy N. Water clusters in a confined nonpolar environment. Chem Phys Lett. 2005;410:348–351. [Google Scholar]
- 45.Raschke TM, Levitt M. Nonpolar solutes enhance water structure within hydration shells while reducing interactions between them. Proc Natl Acad Sci USA. 2005;102:6777–6782. doi: 10.1073/pnas.0500225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kofinger J, Hummer G, Dellago C. Macroscopically ordered water in nanopores. Proc Natl Acad Sci USA. 2008;105:13218–13222. doi: 10.1073/pnas.0801448105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rasaiah JC, Garde S, Hummer G. Water in nonpolar confinement: from nanotubes to proteins and beyond. Ann Rev Phys Chem. 2008;59:713–740. doi: 10.1146/annurev.physchem.59.032607.093815. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Zhao J, Fang H. Water clusters confined in nonpolar cavities by ab initio calculations. J Phys Chem C. 2008;112:11779–11785. [Google Scholar]
- 49.Sabadini E, Cosgrovea T, do Carmo Egidio F. Solubility of cyclomaltooligosaccharides (cyclodextrins) in H2O and D2O: a comparative study. Carbohydr Res. 2006;341:270–274. doi: 10.1016/j.carres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Pochini A, Ungaro R. Calixarene and related hosts. In: Lehn JM, Vogtle F, editors. Vol. 2. Rugby: Pergamon Press; 1996. pp. 103–142. Comprehensive Supramolecular Chemistry. [Google Scholar]
- 51.Iwamatsu SI, Uozaki T, Kobayashi K, Re S, Nagase S, Murata S. A bowl-shaped fullerene encapsulates a water into the cage. J Am Chem Soc. 2004;126:2668–2669. doi: 10.1021/ja038537a. [DOI] [PubMed] [Google Scholar]
- 52.Iwamatsu SI, Murata S. H2O@open-cage fullerene C60: Control of the encapsulation property and the first mass spectroscopic identification. Tetrahedron Lett. 2004;45:6391–6394. [Google Scholar]
- 53.Stetefeld J, Jenny M, Schulthess T, Landwehr R, Engel J, Kammerer RA. Crystal structure of a naturally occurring parallel right-handed coiled coil tetramer. Nature Struct Biol. 2000;7:772–776. doi: 10.1038/79006. [DOI] [PubMed] [Google Scholar]
- 54.Yin H, Hummer G, Rasaiah JC. Metastable water clusters in the nonpolar cavities of the thermostable protein tetrabrachion. J Am Chem Soc. 2007;129:7369–7377. doi: 10.1021/ja070456h. [DOI] [PubMed] [Google Scholar]


