Abstract
Ethanol exerts its biological actions through multiple receptors, including ion channels. Ion channels that are sensitive to pharmacologically relevant ethanol concentrations constitute a heterogeneous set, including structurally unrelated proteins solely sharing the property that their gating is regulated by a ligand(s). Receptor desensitization is almost universal among these channels, and its modulation by ethanol may be a crucial aspect of alcohol pharmacology and effects in the body. We review the evidence documenting interactions between ethanol and ionotropic receptor desensitization, and the contribution of this interaction to overall ethanol action on channel function. In some cases, such as type 3 serotonin, nicotinic acetylcholine, GABA-A, and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors, ethanol actions on apparent desensitization play a significant role in acute drug action on receptor function. In a few cases, mutagenesis helped to identify different areas within a receptor protein that differentially sense n-alcohols, resulting in differential modulation of receptor desensitization. However, desensitization of a receptor is linked to a variety of biochemical processes that may alter protein conformation, such as the lipid microenvironment, post-translational channel modification, and channel subunit composition, the relative contribution of these processes to ethanol interactions with channel desensitization remains unclear. Understanding interactions between ethanol and ionotropic receptor desensitization may help to explain different ethanol actions 1) when ethanol is evaluated in vitro on cloned channel proteins, 2) under physiological or pathological conditions or in distinct cell domains with modified ligand concentration and/or receptor conformation. Finally, receptor desensitization is likely to participate in molecular and, possibly, behavioral tolerance to ethanol, which is thought to contribute to the risk of alcoholism.
I. Introduction
Application of allosteric theory to ligand-receptor interaction brought to pharmacology the fundamental concept that receptors exist in multiple functional states before ligand binding (Wyman and Gill, 1990; Colquhoun, 1998). Thus, differential actions of a given ligand or structurally related ligands that are mediated via a defined receptor could be attributed to differential interaction (initial association and/or receptor conformational change upon binding) between the ligand(s) and the different states of the receptor. These concepts have been successfully applied to the heterogeneous set of ligand-gated ion channels (LGICs).1 Indeed, for several LGICs, experimental data from binding assays and single-channel recordings complemented with kinetic modeling, seem to validate the concepts formulated above (Dilger et al., 1995; Spivak, 1995; Jones and Westbrook, 1996; Auerbach and Akk, 1998; Colquhoun, 1998; Bianchi and Macdonald, 2001; Hille, 2001).
Differential sensing of a ligand (or structurally related ligands) by different functional states of a receptor requires that each discernible state contribute with different chemical groups and/or forces to ligand-receptor interaction. Differences in chemical groups or forces may alter the ligand initial association, ligand-receptor dissociation equilibrium, and/or receptor activation upon ligand binding, leading to the different pharmacological responses. Disregarding the specific process contributing to the different pharmacological responses, the involvement of distinct chemical groups and forces paves the way for pharmacological selectivity.
However, the possibility that different channel receptor states are differentially targeted by a given (or even similar) modulating ligand(s) appears far-fetched when it comes to consideration of the so-called “promiscuous ligands.” These structurally simple molecules 1) are known to modulate the activity of a wide variety of proteins, including most LGICs, bringing into question the selectivity of drug action; 2) exert their effects by acting at the high micromolar to millimolar range in the aqueous medium, as opposed to most central nervous system (CNS)-targeted therapeutic drugs, neuropeptides, and some simple neurotransmitters, which primarily modulate ion channel proteins by acting at the picomolar to low micromolar range (although it must be noted that most small-molecule neurotransmitters, such as GABA and glutamate, activate LGICs at micromolar to millimolar concentrations); 3) their exact docking locus in the receptor-channel protein remains unknown. A prototypical example of these agents is ethyl alcohol (ethanol), a drug that alters several body functions and behaviors by modifying the activity of a wide variety of receptors, including LGICs within and outside the CNS) (Little, 1991; Harris, 1999; Woodward, 2000; Fleming et al., 2001; Narahashi et al., 2001; Lovinger and Crabbe, 2005). Considering ethanol's widespread targeting of structurally unrelated LGICs (Lovinger, 1997; Harris, 1999), the drug's failure to alter receptor function at aqueous concentrations that are normally reached by active ligands to modify LGIC function, and the difficulty in defining, using clear-cut structural methods, the ethanol-recognition site in the LGIC receptor protein, it sounds a bit paradoxical that this promiscuous ligand could differentially interact with different states of a given LGIC.
This short review will critically summarize the increasing evidence documenting that ethanol at concentrations that modify LGIC function and cell physiology can indeed differentially interact with distinct states of a given receptor channel. In particular, we focus our attention on interactions between ethanol and LGIC desensitization. We can obtain from classic pharmacology a definition of desensitization as the process(es) that leads to the transient character of a receptor-mediated effect in response to protracted or repeated exposure to a given ligand or ligands that bind to the same receptor (homologous desensitization), or in response to ligands that bind to different receptors yet share downstream signaling cascades (heterologous desensitization) (Taylor and Insel, 1990). It must be emphasized that these two forms of desensitization do not correspond to two unequivocal biophysical mechanisms. For the time being, however, operational definitions provide a good starting point to help explain our interest in the interactions between ethanol and the desensitized states of LGIC. First, for LGIC-mediated currents that are potentiated when first exposed to ethanol, an increased fraction of desensitized “receptors” in the overall population of “naive receptors” (i.e., that never experienced the drug before) will lead to reduced receptor-mediated current. Furthermore, as agonist and/or ethanol presence continues, the contribution of desensitized receptors to the overall channel population will increase. This mechanism can explain, or at least contribute to, the so-called “acute tolerance” to ethanol exposure, which is the reduced response to a dose of ethanol that develops within minutes of drug application to a naive system (Kalant, 1998). Thus, desensitization induced by either protracted or repeated ethanol exposure and a fast and significant desensitization process before ethanol exposure can both lead to reduced responsiveness of LGICs to alcohol. It is noteworthy that reduced responsiveness to ethanol is one of the characteristics or “endophenotypes” that are thought to contribute to the risk of developing alcoholism (Schuckit, 1985, 2000). Such a link between reduced alcohol responses and risk for alcoholism development, together with the involvement of LGICs in alcohol actions in the body (Little, 1991; Lovinger, 1997; Harris, 1999; Woodward, 2000; Fleming et al., 2001; Narahashi et al., 2001; Lovinger and Crabbe, 2005), provide toxicological and clinical relevance to the study of acute tolerance (LGIC desensitization in particular) and alcohol exposure.
Second, biomedical research on alcoholism relies heavily on animal and in vitro cell models. Reductionist approaches, such as studying ethanol action on recombinant LGICs expressed in isolated cells, in cell membranes, or, after channel protein reconstitution, into artificial lipid bilayers, are ideal to obtain information about ethanol site(s) of action and modus operandi on the receptor itself. These reductionist approaches can be followed by more integrative studies in which ethanol action can be studied in vivo, with the target under study being genetically or pharmacologically manipulated to assess the involvement of the target in alcohol actions. This knowledge will help to identify, among the myriad of macromolecules in the living organism that can interact with ethanol and other promiscuous ligands, the relevant targets of ethanol actions in the body. Moreover, in vitro and in vivo approaches (the latter incorporating pharmacokinetic considerations), will help to design pharmacological treatments that, by modulating relevant targets of ethanol action, may be of therapeutic benefit in alcohol intoxication or alcoholism. Unfortunately, the most basic studies suffer a premature setback when similar LGICs (even recombinant channels with similar subunit composition) expressed in a defined, isolated in vitro system, respond differently to short-term ethanol exposure (Borghese and Harris, 2007; Botta et al., 2007; Olsen et al., 2007).
Assessment of the receptor response to a modulator becomes critically dependent on the time course over which the response fades from its initial magnitude in the naive system. Moreover, if desensitization processes are fast enough, the response of the naive system to a modulator can be blunted before the experimenter records it. For most LGICs, desensitization occurs within milliseconds or seconds (Lovinger, 1997). Thus, slow drug application may cause the investigator to study an LGIC population that is largely desensitized and/or just recovering from desensitization. In addition, desensitization rate is often a monotonic function of ligand concentration. Thus, lower agonist concentrations would produce slower desensitization and ethanol would still be effective, despite slow agonist delivery. These considerations underscore the critical importance of rapid agonist application in the study of ethanol action on LGICs (Lovinger, 1997). Thus, different agonist perfusion rates and/or time of evaluation of ethanol action would likely result in different predominance of desensitized receptors. The relative predominance of desensitized receptors can explain or, at least contribute to, different results in studies of ethanol action, even when drug action on LGICs is evaluated in a highly simplified in vitro system, such as that defined by recombinant channel proteins reconstituted into a controlled lipid environment.
Finally, it has been speculated that in addition to their fast binding to LGICs and other postsynaptic receptors, neurotransmitters exert their biological actions via a slower process: disruption of the neuronal membrane lipids with consequent nonselective LGIC desensitization. This putative molecular basis of anesthesia could provide an explanation for the broadly conserved sensitivity to anesthetic agents found across different animal species (Sonner, 2002, 2008). Thus, neurotransmitters have been hypothesized as the endogenous anesthetics, “the survival advantage conferred by their proper membrane-mediated desensitization of receptors explaining the selection pressure for anesthetic sensitivity” (Cantor, 2003). Evaluation of channel desensitization of a given receptor by ethanol or anesthetic in the presence of different neurotransmitters will be critical to help to answer this fundamental problem of evolutionary biology.
Although ethanol is our focus, in a few cases we consider other simple molecules, including ethanol analogs, other n-alcohols, and small inhalational anesthetics, because results with these agents will help us to understand ethanol and desensitization of a particular LGIC receptor. Finally, we should note that the studies of ethanol action on LGICs have been performed using a wide range of concentrations, including levels that far exceed those reached in circulation that would kill a vertebrate. Considering that the blood alcohol level constituting legal intoxication in the United States is approximately 18 mM, and recent criteria proposed for the existence of a defined ethanol-recognizing site in a protein receptor (Harris et al., 2008), we focus on in vitro studies documenting modulation of LGICs by aqueous concentrations of ethanol smaller than 150 mM.
II. Of Desensitization and Receptors
Most studies assessing the role of LGIC desensitization during short-term ethanol exposure report drug action on macroscopic ionic current. Total (macroscopic) ionic current is determined by the independent contribution of channel unitary conductance (γ), individual open probability (Po), and the number of channels present in the membrane under study (N). Thus, changes in N, although distinct from modifications that lead to desensitization of the individual receptor, cannot be excluded from contributing to a reduced alcohol response, particularly when a decrease in total macroscopic current is observed. The molecular mechanisms that can lead to a decrease in N are numerous, from changes in gene expression to internalization of membrane channel protein into the cell via receptor-mediated endocytosis or other type of cell membrane internalization. However, the experimental system and conditions may help to sort out some of the mechanisms underlying ethanol and/or ligand-induced decrease in N. For example, changes in gene expression with modified expression of channel subunit isoforms are unlikely to occur during brief (seconds to several minutes) applications of ligand or ethanol and certainly will not contribute significantly to drug action when ligands are probed on channel proteins studied in isolated membrane patches. In addition, the use of cell-free membrane patches when studying changes in LGIC responses by electrophysiological methods makes it very unlikely that changes in N could be attributed to cell catabolism of channel protein. Moreover, membrane internalization of channels cannot contribute to a time-dependent decrease in channel steady-state activity (NPo) when the phenomenon is studied with channel proteins reconstituted into artificial, simple lipid bilayers. Finally, the rapid recovery from ethanol actions once the drug is removed from the preparation, seen in many studies, argues against a role for internalization or other mechanisms with slow rates of recovery.
In principle, block of the ionic conductance pathway or change in LGIC conformation leading to decreased γ could also contribute to decreased macroscopic current. Decreased unitary conductance, however, may be apparent only when transitions between alcohol-bound and alcohol-free receptor within bursts of activity are too fast to be resolved. This has been reported for long-chain alcohols and nicotinic acetylcholine receptors (nAchRs), which may result in a time-dependent block of macroscopic current (Dilger et al., 1994; Forman, 1998; Godden et al., 2001; Zuo et al., 2001, 2004). In addition, trichloroethanol at concentrations above 20 mM (as well as hexanol and octanol) causes a decrease in serotonin-mediated cation current amplitude that cannot be distinguished from channel desensitization (Zhou and Lovinger, 1996; Stevens et al., 2005). Ethanol inhibits the function of N-methyl-d-aspartate (NMDA) type glutamate receptor, as will be discussed in section IV, but this inhibition does not involve a decrease in γ (Lima-Landman and Albuquerque, 1989; Wright et al., 1996). To our knowledge, there is no evidence documenting that decreased γ contributes to the reduction in LGIC-mediated current in response to protracted or repeated exposure to clinically relevant ethanol concentrations.
Therefore, changes in Po are the main end product of ethanol- or ligand-induced modification of LGIC desensitization, the latter being a significant component of the channel “gating” processes [for practical considerations, we use the term “gating” to designate the whole set of channel dwelling states, and kinetic transitions among them, that determine channel activity; this broad definition engulfs the usual definition (i.e., the channel response to a specific stimulus, which leads to opening or closing of the channel pore; Hille, 2001)]. In the simplest possible case, when the LGIC has a single desensitized state, ethanol- or ligand-modification of gating leading to altered desensitization may encompass modification of entry into the desensitized state, modification of exit from the state (destabilization/stabilization of desensitized state), or a combination of both. This simplicity rarely applies. For example, 36Cl- determinations of GABA-activated chloride flux in native brain membranes shows, at a minimum, two desensitization kinetic components, each affected by drugs, including pentobarbital (Cash and Subbarao, 1987, 1988). High concentrations of activating internal calcium drive potassium channels of the BK type (see section V) into a low Po gating mode (Rothberg et al., 1996), with channel dwelling within this mode being favored by ethanol (Liu et al., 2008). Dwell-time analysis of the calcium-driven BK low Po mode, however, reveals three open-time and eight closed-time constants (Rothberg et al., 1996), suggesting the existence of at least 11 channel kinetic states.
For the vast majority of LGICs, whether inhibitory or excitatory (see sections III–VI), ethanol is considered a heterotropic ligand; that is, it interacts with the “receptor” (see next paragraph) at a site different from that of the receptor-activating ligand (Peoples et al., 1996; Mihic et al., 1997; Li et al., 1998; Harris et al., 2008; Liu et al., 2008). Thus, when considering modified LGIC desensitization in response to protracted or repeated presentation of ligand, the altered responsiveness can occur as consequence of molecular adaptations in distinct agonist-receptor sites, ethanol-receptor sites, or both. Indeed, fluorescent labeling of nAchRs shows that isoflurane at twice the half-maximal effective concentration (EC50) for general anesthesia in tadpoles (0.6 mM), as well as saturating concentrations of butanol, increases the fraction of receptors preexisting in the desensitized state before agonist-induced desensitization (Raines et al., 1995), demonstrating that anesthetics/n-alcohols can desensitize LGICs in the absence of agonist.
When trying to delineate possible biophysical mechanisms that underlie ethanol interactions with the desensitized states of a receptor, we immediately encounter the difficulty of defining what an ethanol receptor may be. In classic pharmacology, a receptor is usually defined as any biological recognition unit that confers a response or transduces a signal to a drug or a naturally occurring ligand. In addition, it is usually assumed that receptors of peptidic nature have at least a tertiary structure (Taylor and Insel, 1990; Kenakin, 2006). Thus, mechanisms of decreased response to an agonist are determined by protein conformational change secondary or not to post-translational modification (phosphorylation, lipidation, glycosylation, and the like), kinase membrane translocation, or channel-associated membrane protein interactions. In some cases, specific protein domains that primarily control LGIC desensitization but not other aspects of ligand-receptor interaction, such as initial binding, have been identified [e.g., the flip-flop cassette in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors; Partin et al., 1994]. LGICs, however, are homo- and heteromeric protein complexes, raising the possibility that differential subunit composition (and thus subunit replacement) or negative cooperativity among subunits could play a role in diminished responsiveness to ligand binding. One report made the interesting suggestion that nAchRs made of α3-β4 subunits, but not those of α3-β2, α4–1β4, or homomeric α7, display significant tolerance to short-term ethanol exposure (Covernton and Connolly, 1997). However, it should be noted that another study examining human α3-β4 nAchRs indicated little effect of ethanol on this receptor subtype (Cardoso et al., 1999). The ethanol preapplication protocol used in this study mimics continuous ethanol exposure during intoxication but may also bring about acute tolerance. Both of these studies were conducted using whole Xenopus laevis oocytes, a preparation in which desensitization cannot be accurately measured. Thus, it is not yet known whether ethanol effects on desensitization contribute to the different results obtained in the two studies.
In addition, LGIC channel complexes span the whole lipid bilayer and are often associated with chaperone proteins in a dynamic lipid environment (Boyd et al., 2002; Bollan et al., 2003; Millar and Harkness, 2008; Schwappach, 2008). Thus, LGIC desensitization can also involve changes in channel complex composition of, distribution of, or association with the LGIC immediate lipid microenvironment, as characteristically exemplified by nAchRs (Rankin et al., 1997; Baenziger et al., 2000; Barrantes, 2004; Nievas et al., 2007). Matters become more complicated for ethanol because several critical “ethanol recognition areas” that have been proposed in LGICs (nAchRs, GABAA, glycine receptors) are mapped to channel protein transmembrane areas (Forman et al., 1995; Mihic et al., 1997; Mascia et al., 2000; Ueno et al., 2000; Zhou et al., 2000; Harris et al., 2008) where both drug and receptor are in close contact with membrane lipids. In this case, it becomes a challenging task, if at all possible, to separate a “protein component” from a “lipid component” when trying to understand the structural bases of ethanol action on overall receptor activity in general and receptor desensitization in particular.
Following these generalizations, in the next two sections we consider the experimental evidence documenting ethanol differential interactions with desensitized LGIC states, analyzing each LGIC family, class, or subtype separately.
III. Ethanol and Desensitization of Receptor Channels of the Cys-Loop Family
Ethanol effects on desensitization play a prominent role in acute drug action on LGICs. Ethanol and some longer chain alcohols potentiate the function of several LGICs of the “Cys-loop” family, including the glycine and GABAA anion channels, and the type 3 serotonin (5-HT3) receptor and nAchR cation channels. These receptors are characterized by their similar structural features, including four transmembrane (TM4) segments per subunit, a pentameric holoprotein structure, a full membrane-spanning pore region (TM2), and a short loop defined by a disulfide bond in the amino terminal domain that gives this subfamily its name (Conley, 1996; Lovinger, 1997). At certain receptor subtypes, ethanol actions occur at concentrations relevant to behavioral intoxication, and there is now a large body of literature indicating that ethanol effects on LGICs contributes to intoxication and other actions of the drug (Lovinger, 1997; Harris et al., 2008).
A. Type 3 Serotonin Receptor
In discussing ethanol effects on cys-loop LGICs, our initial focus will be on 5-HT3 receptor, because alcohol effects on this LGIC are well characterized. Potentiation of 5-HT3 LGICs by ethanol and other alcohols is a robust form of allosteric modulation that has enabled investigators to examine alcohol interactions with desensitized and other functional channel states using a relatively simple LGIC. Functional 5-HT3 receptors can be formed by assembly of at least two subunits (5-HT3A and 5-HT3B; Davies et al., 1999; Jensen et al., 2008), but fully functional homopentameric channels can be made by 5-HT3A (Maricq et al., 1991). This LGIC shows robust expression in various heterologous expression systems, including mammalian cells (Lovinger and Zhou, 1994, 1998). Furthermore, several neuroblastoma cell lines originally developed in the Nirenberg laboratory express endogenous homomeric 5-HT3A receptors (Jensen et al., 2008). The kinetics of the 5-HT3A receptor-channel are generally slower than those exhibited by most other LGICs (Zhou et al., 1998; Solt et al., 2007). Desensitization, usually estimated from measurement of current decay in the continuous presence of agonist, occurs over a time course of seconds. The estimated forward rate constant for desensitization is 1 sec-1. We were surprised to find that a similar time course is found for 5-HT3A deactivation; that is, the process of agonist unbinding and channel closing, which is usually estimated by current decay after brief agonist exposure and rapid removal (Zhou et al., 1998; Solt et al., 2007). This behavior can be explained by a kinetic scheme in which channel closing is dominated by desensitization even after agonist removal (i.e., the “real” deactivation is much slower than desensitization) (Solt et al., 2007). The slow channel kinetics of 5-HT3A, along with other factors described above, have made it easy to apply electrophysiological and pharmacological techniques to the study of this LGIC.
Ethanol potentiates 5-HT3A function at concentrations obtained in circulation during intoxication (25–100 mM) (Lovinger, 1991; Lovinger and White, 1991; Machu and Harris, 1994). A variety of other alcohols and small-molecule volatile anesthetics have a similar action (Jenkins et al., 1996; Zhou and Lovinger, 1996; Stevens et al., 2005). Alcohols with chain lengths up to six carbons produce potentiation, whereas inhibition is observed with longer, more hydrophobic alcohols (Jenkins et al., 1996). The potentiating effect of ethanol and other alcohols is observed in neuroblastoma cells and neurons, as well as in all heterologous expression systems examined to date (Lovinger and White, 1991; Machu and Harris, 1994; Sung et al., 2000), facilitating the study of alcohol interactions with the kinetics of this LGIC. When the 5-HT3B subunit is added to the receptor to the receptor complex, ethanol-induced potentiation is lost and the efficacy of potentiation by other alcohols and anesthetics is greatly reduced (Hayrapetyan et al., 2005; Stevens et al., 2005). Inclusion of the B subunit increases the rate of most of the receptor-channel state transitions, indicating a possible loss of state-dependent alcohol effects in the heteropentameric 5-HT3 receptor complex (Stewart et al., 2003).
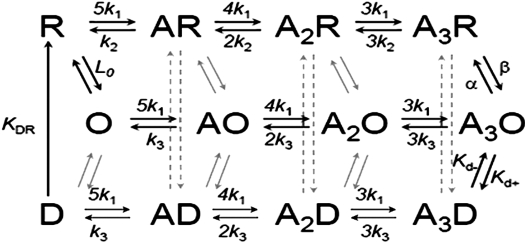
Ethanol potentiation of the function of 5-HT3A and other cys-loop LGICs is most commonly characterized by an increase in the magnitude of current produced by a given concentration of agonist (the so-called peak current). This increase is usually observable only at relatively low agonist concentrations, because ethanol increases Po and thus the alcohol effects are occluded at high agonist concentrations when Po is near maximum (Lovinger and White 1991; see also Eggers and Berger, 2004 for similar findings with glycine receptors). However, investigators examining 5-HT3 as well as nAchRs and glycine LGICs often observe that ethanol and other alcohols increase the rate of current decay in the continuous presence of agonist (Zhou et al., 1998). The first assumption that is often made from this result is that ethanol increases the rate of the desensitization process itself (i.e., the desensitization rate constant). However, the situation is more complex. For the 5-HT3 LGIC, the rate constants governing desensitization [i.e., the rate constants governing channel entry into (Kd+) and exit from (Kd-) the desensitized state] do not vary directly with agonist concentration (Solt et al., 2007; see kinetic model in Fig. 1). The rate of current decay, however, does exhibit a dependence on agonist concentration because increasing receptor occupancy by the agonist increases Po, with increased forward rate constant for channel activation. As channels open, they can transition into the desensitized state at a rate primarily determined by the forward rate constant Kd+, and the faster the channels open, the faster the whole process will occur. Thus, in the presence of drugs like ethanol, which increase Kd+ (and in some cases also decrease receptor deactivation; Zhou et al., 1998), current decay will increase in the presence of drug if applied in the presence of a nonsaturating agonist concentration.
Fig. 1.
A general kinetic scheme for the gating of the 5-HT3 ligand-gated ion channel. Transitions between channel states are governed by rate constants denoted above and below the arrows. Although the main pathway governing desensitization is expected to occur at full occupancy of the receptor by the ligand (A3O↔ A3D), entry into desensitized states may occur from partially ligated channel states (A2O, A1O) and even the unligated, open channel receptor; r = receptor, A = agonist, O = open channel state(s), D = desensitized state(s). [Adapted from Hu XQ and Lovinger DM (2008) The L293 residue in transmembrane domain 2 of the 5-HT3A receptor is a molecular determinant of allosteric modulation by 5-hydroxyindole. Neuropharmacology 54:1153–1165. Copyright © 2008 Elsevier, Ltd. Used with permission.]
The “true” effect of ethanol on desensitization (i.e., any possible drug modulation of Kd+ and/or Kd-) can be estimated using kinetic models of receptor channel function. Whole-cell or single-channel data can be simulated using a given kinetic scheme and rate constants can be adjusted to produce changes in the simulated current that adequately resemble the changes produced by a drug of interest. An accurate model can be developed by using the kinetic scheme to fit experimentally derived data using iterative nonlinear curve fitting and/or global analysis (Colquhoun and Hawkes, 1995; Cox et al., 1997; Zhou et al., 1998; Liu et al., 2008; Shelley and Magleby, 2008)
One way to observe the true alcohol effect on desensitization is to examine the current activated by a saturating agonist concentration, and experiments of this type have been performed on the 5-HT3A receptor. Under such conditions, ethanol and other alcohols produce a slowing of desensitization and an increase in steady-state current (Zhou et al., 1998; Hu et al., 2006), indicating that alcohols actually slow Kd+ or affect the Kd+/Kd- equilibrium. Kinetic models of receptor function and alcohol action indicate that decreased desensitization is necessary to fully explain alcohol actions on 5-HT3A receptors (Zhou et al., 1998). Moreover, recent data and refinement of kinetic models also suggest that measurements of 5-HT3 deactivation most likely reflect receptor desensitization, as the desensitization pathway seems to offer the fastest exit from the open state even after removal of extracellular agonist (Solt et al., 2007). Thus, the slowing of deactivation by alcohols probably reflects decreased desensitization as well.
B. Nicotinic Acetylcholine Receptor
As described for 5-HT3 receptors in the previous paragraphs, ethanol modulation of nAchRs is widespread and well characterized. Indeed, modulation of nAchR-mediated currents by ethanol has been reported with native nAchRs from Torpedo californica (Wu et al., 1994), PC12 cells (Nagata et al., 1996), and cultured neurons (Aistrup et al., 1999; Zuo et al., 2001), as well as with recombinant nAchRs expressed in X. laevis oocytes (Forman and Zhou, 1999, 2000; Borghese et al., 2002) or after transfection of cell lines (Zuo et al., 2002). However, modulation of nAchR currents by alcohols varies drastically, even rendering different qualitative outcomes: potentiation, refractoriness, and inhibition of current have all been reported, depending upon receptor subunit composition, agonist (nicotine, acetylcholine, etc.) concentration, alcohol species and concentration, and other experimental recording conditions.
In brief, nAchRs are made of a single, pentameric transmembrane protein, each subunit spanning the membrane bilayer (Conley, 1996). nAchRs in skeletal muscle and T. californica electric organ have an α12β1γδ stoichiometry (where subscripts denote number of a given subunit type), with an ε subunit substituting for γ in some adult skeletal muscles (Conley, 1996; Millar and Harkness, 2008). Neuronal nAchRs display high regional variety in subunit composition, heteromeric α42β23 and homomeric α7 being the two receptor types that predominate in vertebrate brain (Lindstrom, 2003; Nashmi and Lester, 2006). After expression in Xenopus laevis oocytes, heteromeric nAchRs resulting from combinations of α4 or α2 with β4 or β2 subunits elicit currents that are potentiated by ethanol, whereas most studies report that homomeric α7-mediated currents are inhibited (Cardoso et al., 1999; Narahashi et al., 1999; Zuo et al., 2002). These differences are not limited to heterologously expressed recombinant channels, because native neuronal nAchRs also show differential responses to ethanol, α4β2-mdiated currents being potentiated and α7-mediated currents being inhibited (Narahashi et al., 1999, 2001). It is noteworthy that α7-mediated currents display notoriously fast desensitization. Thus, perhaps the inhibitory effect of ethanol on these homomeric nAchRs reflects enhanced desensitization of the receptor-channel resulting indirectly from a potentiating effect of ethanol, as considered above for 5-HT3 receptors.
Like 5-HT3 receptors, and also reported with GABAA and glycine receptors (see section III.C), ethanol (≥100 mM) consistently potentiates nAchR currents that are evoked by low agonist concentrations, an effect due primarily to ethanol-induced stabilization of channel open states (Wu et al., 1994; Forman and Zhou, 1999; Zuo et al., 2004). It has also been observed that ethanol increases the rate of current decay in the continuous presence of agonist (Nagata et al., 1996), suggesting ethanol modulation of receptor desensitization. nAchR desensitization, however, involves not one but several distinguishable kinetic processes and is regulated by several biochemical mechanisms, their modulation by ethanol still being fully unresolved.
A classic, simple model of nAchR kinetics involves sequential binding of two molecules of agonist to the channel closed state, upon which the channel opens (Colquhoun and Sakmann, 1985; Lingle et al., 1992). Openings in the absence of ligand are possible yet highly infrequent (Grosman and Auerbach, 2000). From the very early studies, however, it became evident that continuous exposure to agonist leads to progressive decrement of nAchR-mediated macroscopic currents, leading to the pioneering conceptualization of LGIC desensitization (Katz and Thesleff, 1957). This desensitization of macroscopic current is a function of agonist concentration, reaching a maximum at saturating acetylcholine concentration (low millimolar), as reported in studies of BC3H-1 myocytes (Dilger and Liu, 1992). Later, fast-resolution single-channel recordings revealed that nAchRs can enter several desensitized states of varied average duration (Auerbach and Akk, 1998), leading to the standard distinction between “fast” and “slow” desensitized states.
Channel entry into desensitization varies, first and foremost, with nAchR subtype. Agonist (nicotine) evokes rapid (within a few milliseconds) desensitization of the current mediated by chick α7 or α8 homopentameric nAchRs expressed in X. laevis oocytes, as well as current passed by bungarotoxin-sensitive nAchRs in rat hippocampal neurons. This fast component of desensitization in the continuous presence of ligand is not observed with bungarotoxin-insensitive nAchRs in rat hippocampal neurons or adult rat cerebellum (reviewed in Conley, 1996). Ethanol, however, can potentiate channel responses to weak partial agonists such as suberyldicholine even when the receptor site is fully occupied, indicating that this short-chain alcohol most likely increases nAchR Po in the absence of changes in agonist affinity. This conclusion is also supported by the observation that the apparent dissociation constant for suberyldicholine self-inhibition is not altered in the presence of ethanol (Wu et al., 1994).
Results with longer n-alcohols bring additional insight into ethanol interactions with the desensitized nAchRs. Long-chain n-alcohols (C4 and above) do not activate but inhibit T. californica nAchRs expressed in X. laevis oocytes (Forman et al., 1995; Forman and Zhou, 2000), and human α4β2 receptors expressed in human embryonic kidney cells (Zuo et al., 2004). Furthermore, the dissociation constant for n-alcohol block of cultured rat myotubule nAchRs decreases with increasing chain length, from 8 to 0.15 mM for pentanol to octanol, respectively (Murrell et al., 1991). The affinity of nAchRs for agonist and thus a fluorescent signal from bound agonist increases when the receptor enters the desensitized state. Thus, dansyl-C6-choline fluorescence can be used as a measure of receptor desensitization. Using this technique, it was demonstrated that n-alcohols enhanced the apparent desensitization rate of nAchRs (Raines et al., 1995), corroborating ion flux studies indicative of enhanced receptor desensitization by alcohols (discussed in Lovinger, 1997).
Whole-cell recordings in the presence of long-chain n-alcohols show a time-dependent decrease in current amplitude after combined exposure to agonist and alcohol. This inhibition depends on the presence of agonist and the channel being in the open state (Murrell and Haydon, 1991; Forman et al., 1995; Wood et al., 1995), suggesting a use-dependent block of channels by n-alcohols, which bind within the conduction pore-lining region.
Differential overall effects of ethanol (potentiation of current) versus longer n-alcohols (inhibition of current) were also found when human embryonic kidney cells were transfected with identical human α4β2 combinations (Aistrup et al., 1999; Zuo et al., 2001). It is tempting to speculate that differential actions of n-alcohols on a given receptor are due to different alcohol locations within its target site. Ethanol and more hydrophobic n-alcohols, indeed, differ in depth of location at the lipid bilayer/channel complex (Forman and Zhou, 2000) and thus may interact with distinct nAchR amino acids and differentially modify overall channel activity. It is noteworthy that electrophysiological data after receptor cysteine-mutagenesis combined with sulfhydryl-specific labeling document the existence of at least two n-alcohol sensing sites in the nAchR α2 subunit: Leu263 (“excitatory”) and Leu262 (“inhibitory”), the final receptor response (e.g., activation by ethanol, inhibition by octanol) depending on the relative sensing of each n-alcohol by these two sites (Borghese et al., 2003).
It is likely that differential drug modification of channel de-/resensitization process(es) involving distinct channel sensing sites may lead to different n-alcohol actions on overall nAchR activity. For example, occupation of αGlu262 by 3-azioctanol (and probably by hydrophobic n-alcohols as well) specifically slows nAchR resensitization, which leads to stabilization of the nAchR desensitized state(s) and thus current inhibition (Forman et al., 2007). It is noteworthy that increasing the ethanol concentration from 0.2 to 1 M (admittedly high concentrations) linearly increases acetylcholine-induced fast desensitization maximal rate as a consequence of ethanol-induced shifting of the equilibrium between open and fast desensitization states toward the latter (Wu and Miller, 1994). Moreover, ethanol at concentrations higher than 300 mM usually inhibits mouse muscle nAchRs expressed in X. laevis oocytes (Forman and Zhou, 2000). As found with octanol (0.25–1 mM) and butanol (25–100 mM), high ethanol concentrations (≥250 mM) strongly favor the nAchR conversion to a high-affinity state that is indicative of receptor desensitization (Young and Sigman, 1981).
The simplest explanation for n-alcohol modulation of nAchR desensitized states is that such modulation is secondary to a direct alcohol-ion channel protein interaction. However, alcohol action secondary to drug interaction with bilayer lipids that control channel gating or to post-translational modification of the receptor that affects desensitization are also possible. In brief, nAchR TM segments are arranged in three concentric rings, the outer two (made of M1/M3 and M4, respectively) being in direct contact with the lipid environment. Among membrane lipid species that participate in nAchR gating, cholesterol, other steroids, and fatty acids deserve special attention (for review, see Barrantes, 2003). In particular, cholesterol at concentrations found in native membranes is needed for agonist activation of nAchR (Rankin et al., 1997). Fourier-transform infrared resonance spectroscopic analysis of T. californica nAchRs reconstituted in dioleoylphosphatidylcholine bilayers shows that cholesterol significantly increases the α-helical content, suggesting that this steroid causes an unordered-to-helix transition that is thought to be critical for stabilization of the nAchRs (Butler and McNamee, 1993). Moreover, cholesterol is needed for nAchRs to shift from low to high affinity states (Fong and McNamee, 1986), and recent data obtained with crystal violet, which rather selectively labels the nAchR desensitized state, document that both cholesterol and free fatty acids decrease the crystal violet KD for the receptor (Fernández Nievas et al., 2008). For years, it has been known that ethanol “disorders” the lipid bilayer core (Hitzemann et al., 1986), and ethanol interactions with bilayers are modulated by the bilayer cholesterol content (Barry and Gawrisch, 1995; Tierney et al., 2005). These interactions have been observed using 0.8 to 4.3 M ethanol in nonaqueous phases, so it is difficult to directly drive meaningful extrapolations from these findings to modulation of ion channel function by ethanol concentrations that are relevant to alcohol intoxication (i.e., below 100 mM in the aqueous phase). Whether ethanol targeting of nAchR desensitized states is dependent on cholesterol or other species conforming the lipid shell that surrounds the nAchR outer rings remains to be determined.
Finally, several biochemical processes known to be altered by ethanol, regulate nAchR desensitization, including cAMP-dependent phosphorylation (Huganir and Miles, 1989), protein kinase C activation, and phosphatase 2B inhibition (Marszalec et al., 2005).
C. Glycine and Type A γ-Aminobutyric Acid Receptors
Ethanol potentiation of GABAA receptor-mediated current has been demonstrated in a variety of neuronal preparations and in some studies using heterologous expression systems, most notably X. laevis oocytes (Celentano et al., 1988; Aguayo, 1990; Reynolds et al., 1992; Mihic et al., 1997; Tsujiyama et al., 1997; Sapp and Yeh, 1998; Homanics et al., 1999; Sundstrom-Poromaa et al., 2002; McCool et al., 2003; Wallner et al., 2003). However, the direct effects of ethanol on this receptor-channel remain highly controversial, and there are few demonstrations of receptor potentiation in mammalian heterologous systems (Sigel et al., 1993; Marszalec et al., 1994; Sapp and Yeh, 1998; Sundstrom-Poromaa et al., 2002; McCool et al., 2003). When potentiation is observed, increases in the rate of decay of current in the continuous presence of agonist have sometimes been observed (Tsujiyama et al., 1997; Homanics et al., 1999). Recent studies in some laboratories have demonstrated potent ethanol potentiation of current mediated by δ subunit-containing GABAA receptors (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003), whereas others have failed to observe such effects (Borghese et al., 2006; Korpi et al., 2007). It is worth noting in this context that δ-subunit-containing receptors show little or no agonist-induced desensitization, and thus it is unlikely that decreased desensitization contributes to potentiation of this GABAA receptor subtype. However, increased current decay is observed in the presence of ethanol (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003), indicating that desensitization may contribute to shaping responses mediated by these receptors when ethanol is present.
Potentiation associated with an increased rate of decay is definitely observed when longer-chain alcohols are applied to GABAA receptors (Marszalec et al., 1994). This increase in apparent desensitization seems similar to the alcohol effects on the 5-HT3 receptor noted above. No attempt has yet been made, however, to determine the effect of ethanol on the true desensitization or estimate changes in the desensitization rates constant for the GABAA receptor. Recent work, however, has shown that ethanol increases the potency with which neurosteroids potentiate the GABAA receptor, and this effect can be observed even at fairly low ethanol concentrations (Akk et al., 2007). In these experiments, ethanol did not seem to have any overt effects on receptor desensitization in the presence of potentiating neurosteroids. Finally, ethanol has also been shown to inhibit GABAA receptor function in some studies that examined receptor function in neurons and mammalian heterologous systems (Marszalec et al., 1994; McCool et al., 2003). In one such study, an increase in the decay rate of GABA-activated current in the presence of ethanol was found (Marszalec et al., 1994), perhaps indicative of an increase in receptor desensitization induced by the alcohol.
Ethanol potentiation of the glycine-gated LGICs has been consistently observed in both neurons and heterologous expression systems, particularly in receptors that contain only the α subunit (Celentano et al., 1988; Reynolds et al., 1992; Aguayo and Pancetti, 1994; Mihic et al., 1997; Valenzuela et al., 1998; Ye et al., 1998, 2001; Eggers et al., 2000; McCool et al., 2003). As described above for the 5-HT3 and GABAA receptors, alcohol potentiation of glycine receptors is sometimes accompanied by increased current decay (Aguayo and Pancetti, 1994; Ye et al., 2001). However, at the low concentrations generally used to assess ethanol effects on glycine receptors, there is often little or no detectable current decay in the absence or presence of the alcohol because of the relatively low forward rate constants for glycine receptor desensitization. Eggers and Berger (2004) examined ethanol-induced changes in glycine LGIC kinetics in hypoglossal motoneurons at both macroscopic and single-channel levels. Desensitization was not explicitly examined in this study or included in the kinetic model used to fit the experimental data, because desensitization does not seem to contribute to glycine receptor function during brief agonist exposure. Ethanol increased the rate of current activation, and increased the time constant of decay of current activated by brief glycine application, indicating a decrease in current deactivation. Potentiation by ethanol could be adequately described using a kinetic model that does not include desensitization (Eggers and Berger, 2004), suggesting that modified desensitization is not required to explain ethanol effects on glycine-activated LGICs.
IV. Ethanol and Desensitization of Glutamate-Activated Ligand-Gated Ion Channels
Alcohols have a predominant inhibitory effect on another major LGIC family, the ionotropic glutamate receptors. This receptor family includes the AMPA receptor, kainate, and NMDA subtypes, which are named for the exogenous agonist that best activates each receptor subtype (Collingridge et al., 2009). In contrast to the pentameric cys-loop receptors, glutamate-activated LGICs are tetramers that contain a “venus fly trap” type of agonist binding domain, three full TM segments, a re-entrant pore-loop ion conduction pathway, and an intracellular carboxyl terminus that is often quite long (Conley, 1996; Collingridge et al., 2009). Each of the three receptor subtypes can be formed by multiple subunits, including GluRs 1 to 4, also known as GluA1 to 4 (AMPARs), GluRs 5–7, also known as GluK1–3, as well as kainate receptors 1–2, also known as GluK4–5 (kainate receptors), and NMDARs 1–3, also known as GluN1–3 (NMDARs). Ethanol inhibition of current has been observed with members of all of these receptor subtypes. In most neuronal systems, however, ethanol inhibition of the NMDARs and kainate receptors is more robust than ethanol actions on AMPARs. However, thorough examination of ethanol effects on AMPARs and kainate receptors has revealed conditions under which these receptors are every bit as sensitive to ethanol inhibition as the NMDARs, as described in sections IV.A and C.
A. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid Receptor
As mentioned above, ethanol (and other n-alcohols) inhibits the function of all ionotropic glutamate-gated LGICs, including AMPA receptors (GluR 1–4). At the AMPARs, however, the alcohol inhibitory effect critically depends on the type of agonist used to activate the receptor and also appears to vary with channel kinetic state (Möykkynen et al., 2003). Moreover, increased desensitization of AMPARs seems to contribute to ethanol inhibition of receptor-channel function. AMPARs are among the most rapidly desensitizing LGICs in the nervous system: at high agonist concentrations, current decay proceeds within a few milliseconds after channel opening (Trussell and Fischbach, 1989). The initial peak current is unaltered in the presence of ethanol. However, a steady-state current can be observed in less than 100 ms, which is inhibited at concentrations of ethanol as low as 10 mM (Lovinger, 1993; Akinshola, 2001; Möykkynen et al., 2003). Ethanol also potently inhibits AMPAR-mediated current evoked by low agonist concentrations. Under this condition, the peak/steady-state current ratio is relatively small, reflecting the slower activation of the channel population. When the partial agonist kainate is used to activate the AMPAR, however, desensitization is so rapid that the experimenter is likely to measure only steady-state current. Under this condition, ethanol also inhibits ionic current potently. What accounts for this ethanol preferential inhibition of steady-state current? Ethanol does not increase the rate of current decay and thus does not seem to produce a more rapid entry into the desensitized state (Möykkynen et al., 2003). However, ethanol may stabilize the desensitized state by decreasing rate constants leading out of that state(s). Ethanol also produces a modest increase in the rate of AMPAR recovery from desensitization. Furthermore, inhibition of steady-state current is reduced by cyclothiazide, a compound that greatly slows receptor desensitization, and by the L497Y mutation in GluR1, which slows desensitization (Möykkynen et al., 2003). Collectively, these findings are consistent with the idea that stabilization of desensitization is an important mechanism of ethanol inhibition of AMPARs.
The preferential effect on desensitization seems to account for the relatively low potency of ethanol to inhibit most AMPAR-mediated synaptic responses. At most synapses, the excitatory postsynaptic responses mediated by AMPARs are rapid and transient, and there might not be sufficient time for receptors to desensitize during the brief period that glutamate is present in the synaptic cleft. Thus, it is not surprising that ethanol produces only a modest inhibition of this synaptic current, because the situation is analogous to the ethanol resistance of the peak current observed just after application of a high concentration of a full agonist (i.e., glutamate or AMPA). Nevertheless, it remains unclear whether and when desensitization contributes to intercellular communication involving AMPARs. At some synapses, the duration of the elevation in glutamate in the synaptic cleft during normal transmission is sufficiently long to allow for the onset of desensitization during the synaptic response (Jones and Westbrook, 1996).
B. N-Methyl-d-aspartate Receptor
As found for AMPAR, ethanol usually inhibits NMDAR function (Lovinger, 1997; Woodward, 2000). However, the consensus view at present is that ethanol inhibition of NMDA receptors does not involve altered desensitization. Ethanol does not alter the decay of macroscopic current in the continuous presence of agonist (Peoples et al., 1997; Woodward, 2000). However, this might be expected even when the desensitization rate constant is altered, given that initial current activation and peak current are reduced by ethanol, effects that would slow any observable decay. Under some conditions, ethanol does increase the ratio of steady-state-to-peak-current ratio, perhaps indicating a change in desensitization secondary to changes in current activation. These effects are most prominent with continuous ethanol application and intermittent agonist exposure (Popp et al., 1999). There is no strong evidence, however, implicating desensitization in this effect of ethanol.
Single-channel analysis reveals that ethanol inhibition of NMDAR involves decreases in burst frequency, burst duration, and intraburst open channel lifetime but no changes in closed-time distributions (Wright et al., 1996). The changes in burst frequency and intraburst open lifetime could be consistent with increased desensitization. Furthermore, mutations that alter ethanol inhibition also alter desensitization kinetics, and there is some correlation between the effects of individual mutations on ethanol actions and their effects on desensitization (Ren et al., 2008). In conclusion, although ethanol does not have overt effects on the decay kinetics of NMDAR-mediated macroscopic current, there may be some role for stabilization of channel desensitized state(s) in ethanol's inhibitory effects on this receptor-channel.
C. Kainate Receptor
As discussed for the other members of the family of glutamate-gated ion channels, kainate receptor-mediated currents are reduced in response to ethanol (Valenzuela and Cardoso, 1999; Weiner et al., 1999; Carta et al., 2003; Läck et al., 2008). Little is known at present about the effect of ethanol on kainate receptor kinetics. Indeed, there are limited data from experiments directly examining ethanol effects on receptor-channel function in isolated cells exposed to appropriate agonist application. Ethanol inhibits current activated by application of kainate and domoate to human embryonic kidney cells expressing different combinations of kainate receptor-forming subunits (Valenzuela and Cardoso, 1999). Ethanol potency was relatively low in this preparation. However, the currents examined in this study consisted only of a steady-state component; thus, it was not possible to assess ethanol effects on desensitization kinetics. Activation by glutamate or kainate normally produces rapid desensitization of kainate-preferring receptors (Zorumski and Thio, 1992; Cui and Mayer, 1999), but ethanol effects have not been examined under conditions in which this desensitization is observed.
Ethanol has been reported to produce a potent inhibition of kainate-receptor-mediated synaptic responses in projection neurons in hippocampus and basolateral amygdala, with data supporting a postsynaptic site of ethanol action (Weiner et al., 1999; Carta et al., 2003; Läck et al., 2008). The kainate receptors in this brain region seem to be formed by GluRs 5 and 6 and KA2 subunits (Li and Rogawski, 1998). Ion current kinetics has not been examined in these neurons, and thus it is difficult to determine the contribution, if any, of desensitization to the ethanol inhibition.
V. Ethanol and Desensitization of Extracellular ATP-Gated Ion Channels
Extracellular ATP-gated ion channels (P2Xs) are structurally different from both cys-loop and glutamate-gated ionotropic receptors: these cationic channels are considered trimeric receptors with two putative TM segments, a large extracellular loop (where the endogenous ligand ATP is thought to bind), and two small cytosolic amino and carboxyl ends per subunit (North, 2002; Jiang et al., 2003; Vial et al., 2004). Compared with other ionotropic receptors gated by extracellular ligands, P2Xs constitute a relatively new family, yet their alcohol sensitivity (for review, see Weight et al., 1999; Dilger, 2002; Davies et al., 2006) has been explored since their initial pharmacological characterization. First reported in isolated bullfrog dorsal root ganglion neurons (Li et al., 1993), ethanol inhibition of native P2X receptors was also found in rat hippocampal CA1 neurons (Li et al., 2000) and in a slice preparation of rat ventral tegmental area that contains dopamine neurons and the GABAergic nerve endings impinging upon them, the P2X receptors being presumably located in the GABAergic presynaptic membrane (Xiao et al., 2008). Ethanol inhibition of native P2X was replicated in recombinant homomeric P2X2 and P2X4Rs expressed in X. laevis oocytes and HEK293 cells. Data from X. laevis oocytes show that P2X4R are more sensitive to ethanol inhibition than P2X2R, whereas P2X3R are activated by the drug (Davies et al., 2002, 2005), underscoring that overall ethanol action on P2XR is subunit-dependent. It is noteworthy that native P2XR in the CNS, including those in dorsal root ganglia neurons, are predominantly heterooligomers, such as P2X2+P2X3 (Robertson et al., 2001; Vial et al., 2004; Alexander et al., 2008), so the relative contribution of subunit composition versus other cell factors when studying ethanol actions on recombinants versus native P2XRs remains to be established.
Most P2XRs, whether native receptors or recombinant proteins after heterologous espression, display desensitization with maintained application of ATP or structurally related receptor agonists. However, desensitization rate in the continuous presence of agonist is also greatly dependent on subunit composition, with P2X3R-mediated current decaying in several seconds and P2X7R-mediated currents remaining relatively steady for several minutes (Conley, 1996; Vial et al., 2004). Studies on native P2X channels showed that ethanol (50–100 mM) inhibition of ATP-evoked macroscopic current was related to a significant acceleration of current deactivation in the absence of changes in activation. Ethanol action on deactivation, however, was independent of ATP concentration (Li et al., 1998). In the absence of detailed kinetic models, we can only speculate that a significant contribution of ethanol modulation of ATP-mediated desensitization to overall drug action on this receptor is unlikely. On the other hand, receptor recovery from desensitization is controlled by type of agonist used and, more importantly, by intracellular signals, including Ca2+i. The role of these factors in determining ethanol interaction with desensitized P2Rs and/or overall ethanol action on these receptors remains to be determined.
VI. Ethanol and Desensitization of Potassium Channels That Are Gated by Intracellular Ligands
In this section, we consider some ion channels that, based on structural and functional considerations, do not belong to the LGIC superfamily. Indeed, their gating processes are governed by biological signals other than ligand binding (e.g., transmembrane voltage). Binding of specific ligands, however, critically controls overall channel activity and may determine the channel response to ethanol.
A. Calcium- and Voltage-Gated Potassium Channel
Short-term exposure to ethanol concentrations that modify behavior in both invertebrates and vertebrates (including humans) modulate the steady-state activity of calcium- and voltage-gated potassium (BK) channels in a wide variety of preparations, as reported using native and recombinant channels (for review, see Brodie et al., 2007). BK channels are tetrameric proteins consisting of channel-forming α (encoded by Slo, Slo1,or KCNMA1) subunits. These subunits include a TM6 core that is highly conserved with that found in purely voltage-gated potassium channels (Latorre, 1994; Lu et al., 2006; Salkoff et al., 2006). However, BK channels also contain 1) an S0 segment resulting in an exoplasmic amino end (Meera et al., 1997), and 2) a large cytosolic carboxyl end that includes sensing regions for calcium and/or magnesium, and important consensus areas for channel protein membrane insertion and post-translational modification (Schubert and Nelson, 2001; Weiger et al., 2002; Wang et al., 2003; Lu et al., 2006; Salkoff et al., 2006). In most tissues, BK channel complexes consist of the association of α subunits with small (TM2), regulatory β subunits, the latter showing a differential distribution among tissues (Behrens et al., 2000; Brenner et al., 2000). There are four β subunit types encoded by separate genes (KCNMB1–4), which together with abundant KCNMA1 splicing (Fury et al., 2002), provide distinct phenotypes to native BK channels in different tissues (Orio et al., 2002).
As found with many other ion channels, short-term exposure of recombinant or native BK channels to ethanol may result in increase, no change, or decrease in channel steady-state activity (NPo). These variant responses have been documented in highly simplified systems, such as cloned slo1 channels expressed in cell-free membrane patches (for review, see Brodie et al., 2007), with some studies ruling out ethanol-induced changes in N as contributing factor to variability in drug action (Dopico et al., 1998; Liu et al., 2008). In addition, a time-dependent component of BK channel responsiveness to ethanol; that is, channel responses to short-term alcohol exposure change qualitatively and/or quantitatively with repeated or protracted (longer than 2–3 min) drug application was first noted with neurohypophysial terminal channels (Dopico et al., 1996) and since then has been confirmed with both native and recombinant channels (Liu et al., 2004; Pietrzykowski et al., 2004; Yuan et al., 2008). Experimental conditions in all these studies also rule out that changes in N could contribute to the reduced response in NPo that is evoked by ethanol after 2 to 3 min of drug exposure.
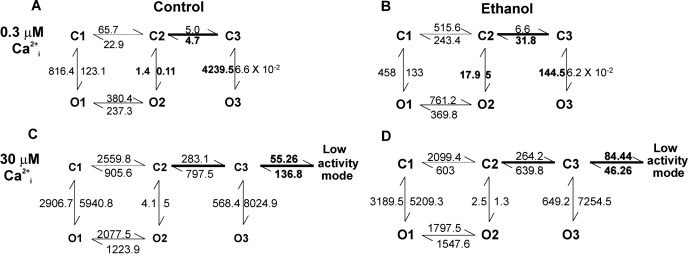
Ethanol modifies BK Po without altering ion conduction, intrinsic, voltage- or physiological magnesium-driven gating. Instead, alcohol specifically facilitates calcium-driven gating, which results in differential ethanol effects on channel activity as a function of activating ligand: potentiation at low (<10 μM) and inhibition at high (>10 μM) internal calcium concentrations (Liu et al., 2008). When internal calcium reaches 10 to 1000 μM, BK channels enter a low Po gating mode (Rothberg et al., 1996), a mode that can be conceptualized as an activating ligand-driven set of desensitized states (Liu et al., 2008; see kinetic model in Fig. 2). Remarkably, ethanol-induced reduction of BK channel activity and thus current is caused by the alcohol favoring channel dwelling into this low Po mode. Moreover, BK channel differential responses to ethanol involve specific calcium-recognition sites (the calcium-bowl and the Asp362/Asp367 calcium site) in the slo1 carboxyl end, with the Asp362/Asp367 calcium high-affinity site being necessary and sufficient to sustain ethanol-induced inhibition. Indeed, slo1 D362A/D362A mutants are not only refractory to ethanol-induced inhibition but fail to enter the calcium-driven, low-activity mode when evaluated at 100 μM internal calcium (Liu et al., 2008).
Fig. 2.
Simple, empirically derived kinetic models showing that ethanol modulation of Ca2+i-dependent channel dwelling into a low activity (Po) mode is a major determinant of the final drug action on BK channel activity. At high concentrations of activating ligand (Ca2+i), channel activity could be satisfactorily modeled only by introducing an additional component corresponding to the Ca2+i-driven low-activity mode (C versus A), which can be interpreted as a set of channel desensitized states. At low ligand concentrations, ethanol prevents channel entry into long-closed states by increasing the C3→C2 transition and decreasing O3→C3. In addition, ethanol stabilizes openings within the normal-activity mode by shifting the O2↔ C2 equilibrium toward O2 (B versus A). These kinetic changes explain ethanol-induced potentiation of BK Po at low ligand concentrations, as widely reported (Brodie et al., 2007). At high activating ligand concentrations, however, ethanol mildly diminishes the C3→C2 transition and drastically shifts the C3↔ low activity mode equilibrium toward the latter (5 times) (D versus C), favoring channel dwelling within the low-activity mode. These kinetic changes explain ethanol-induced reduction of BK Po at high activating ligand concentrations (Liu et al., 2008). Main changes introduced by ethanol and/or increase in internal calcium are bolded. [Adapted from Liu J, Vaithianathan T, Manivannan K, Parrill A, and Dopico AM (2008) Ethanol modulates BKCa channels by acting as an adjuvant of calcium. Mol Pharmacol 74:628–640. Copyright © 2008 American Society for Pharmacology and Experimental Therapeutics.
On top of the fundamental interplay within the slo1 subunit, activating Ca2+i and ethanol, BK channel responses to short-term alcohol exposure are fine-tuned by an orchestration of factors. They include post-translational modification of slo1 (Liu et al., 2006), accessory β subunit coexpression (Martin et al., 2004; Feinberg-Zadek and Treistman, 2007; Liu et al., 2008; Bukiya et al., 2009), and the lipid species around the channel protein (Crowley et al., 2003, 2005; Yuan et al., 2008). It is highly likely that some of these regulatory elements (e.g., β subunits; Feinberg-Zadek et al., 2008), alone or in concerted fashion, play a key role in modulating the temporal component of BK channel responses to ethanol.
B. G Protein-Coupled Inwardly Rectifying Potassium Channel
As found with calcium-activated channels of the BK type, short-term exposure to ethanol concentrations (and other n-alcohols) that modify behavior modulate the steady-state activity of native and recombinant G protein-coupled inwardly rectifying potassium (GIRK) channels. Remarkably, whether homomeric or heteromeric, GIRK is the only subset of inwardly rectifying potassium channels that is sensitive to clinically relevant concentrations of ethanol (Kobayashi et al., 1999; Lewohl et al., 1999; Yamakura et al., 2001; Blednov et al., 2002).
Upon Gi/Go protein-coupled receptor activation, G protein βγ subunits are released and bind to the amino and carboxyl ends of GIRK channels with consequent channel opening (Sadja et al., 2003). It is conceivable that activating ligand-induced channel modulation may condition ethanol responses. Using chimeric channels, Lewohl et al. (1999) dissected apart the carboxyl end regions that are involved in ethanol sensing and G protein activation, strongly suggesting that βγ subunit binding on GIRK channels is not sufficient for ethanol sensitivity. Likewise, experiments conducted in the presence of pertussis toxin or antisense oligonucleotides directed against Gβ1 subunits indicate that ethanol modulation of GIRK is independent of Go/Gi activation (Kobayashi et al., 1999). It should be noted, however, that desensitization of GIRK currents is evident within a few minutes, a phenomenon that is due to channel targeting by Gq downstream products (Jan and Jan, 2000). Thus, whereas GIRK1/4 channel responses to ethanol are sustained for at least 20 min (Lewohl et al., 1999), any possible interaction between GIRK channel desensitization and ethanol remains to be formally tested.
VII. Conclusions and Implications
We have briefly summarized the ample evidence documenting ethanol modulation of receptor desensitization for a wide variety of structurally unrelated ionotropic receptors. The contribution of receptor desensitization to the overall ethanol action on ion channel behavior, however, varies wildly, even among members of structurally related ion channels. For example, within the cys-loop family of ionotropic receptors, ethanol interaction with desensitized receptors is a major contributor to the final drug action on 5-HT3 and nAchRs but not on GABA-A and GlyRs. In the case of ionotropic receptors gated by glutamate, desensitization processes and their contribution to overall channel activity are complex. Thus, the degree of contribution, if any, of receptor desensitization to ethanol's final effect on receptor activity and thus ionic current remains largely unknown (see Table 1). The paucity of current data prevents us from establishing a role of receptor desensitization in ethanol actions on P2XRs. Regarding K+ channels gated by intracellular ligands, the presence of a Ca2+i-driven, low activity mode in BK channels is comparable with a set of channel desensitized states, and ethanol facilitation of channel entry into this mode explains drug inhibition of channel activity. In contrast, it is unlikely that G proteins play a significant role in ethanol overall action on GIRK, although the specific contribution of Gq signaling-mediated channel desensitization to ethanol actions remains to be tested.
TABLE 1.
Ethanol modulation of LGIC desensitization and its contribution to drug effect on channel activity
| Desensitization Impact on Overall Kinetics | Ethanol Modulation of Desensitization | Ethanol Effect on Overall Channel Activity | |
|---|---|---|---|
| Ionotropic receptors gated by extracellular ligands | |||
| The cys-loop family | |||
| 5-HT3 | Desensitization is slow (seconds) and a major determinant of overall deactivation | Variable: increase at subsaturating [agonist]; decrease at saturating [agonist] | Activates 5-HT3A, which declines as [agonist] increases; 5HT3B blunts ethanol potentiation |
| nAchR | Desensitization is complex, with fast and slow states. In general, current decay increases as [agonist] increases; desensitization is mainly dependent on channel subunit composition | In general, increase at subsaturating [agonist]. Ethanol action on desensitization might be dependent on cholesterol around the channel | Usually, activation at low [agonist]. Ethanol >250 mM may inhibit current because of stabilization of desensitized states |
| GABA-A | Fast and slow phases (milliseconds to several seconds) even in continuous presence of agonist. Several micromolar GABA render more than one desensitization component even in a single channel subtype | Undetermined for native channels and most recombinants. Increased desensitization of α6β2γ2S channels. No apparent effect in GABA-ARs with δ subunit | Variable, largely dependent on subunit composition and expression system. Consistent potentiation of recombinant channels expressed in X. laevis oocytes |
| Glycine | Slow. A fast component (τ≈5 ms) is seen with clustering of α1 or coexpression of β | Largely unexplored, but it seems not to be a major contributor of ethanol effects on gating | Potentiation of native and recombinant channels. Ethanol may decrease deactivation of current |
| Ionotropic receptors gated by glutamate | |||
| AMPA | Faster than that observed with kainate receptors | Decreased extent of steady-state desensitization. Decreased rate of exit from desensitized state induced by kainate | Inhibition, depending on agonist used and kinetic state |
| NMDA | Desensitization induced by NMDA in calcium presence. In addition, separate desensitization by either aspartate or glycine | Stabilization of desensitized state(s) | Inhibition, yet contribution of desensitization to this effect remains to be established |
| Kainate | Slower than that of AMPA | Undetermined | Inhibition |
| Ionotropic receptors gated by ATP | |||
| Desensitization in presence of continuous ATP varies with subunit composition and intracellular signaling | Formally untested, yet ethanol accelerates macroscopic current deactivation | Inhibition of native channels and most homomeric recombinants yet contribution of desensitization to drug action is unknown. | |
| K+ channels gated by intracellular ligands | |||
| BK | Channel enters a low activity mode at 10–1000 μM Ca2+ | At Ca2+ >10 μM, ethanol favors slo1 and slo1+β4 channel entry into low activity mode | Variable, depending on [agonist]. Channel entry into low-activity mode leads to inhibition |
| GIRK | Desensitization in a few minutes due to Gq protein downstream signaling | Undetermined | Potentiation. Unlikely that putative drug action on desensitization contributes to ethanol effect on current |
The interactions between ethanol and receptor desensitization are of critical importance when designing experiments to probe alcohol action on ionotropic receptors. If agonist/alcohol application is too slow, the receptors will begin to desensitize before drug concentrations have reached equilibrium. This may cause the investigator to underestimate or even miss altogether (as discussed for AMPAR) the potentiation or inhibition produced by alcohols. Ideally, drug application should be as rapid as possible, and preferably in the millisecond or even submillisecond range when dealing with LGICs that exhibit strong and rapid desensitization (e.g., AMPAR, nAchR fast desensitization, etc.). These considerations may help explain conflicting results regarding ethanol action on defined channel proteins in highly simplified in vitro systems.
The role of desensitization in alcohol action may become more prominent in special pathophysiological situations or subcellular domains. For example, during intense high-frequency neuronal firing, epileptiform activity, or stroke, extracellular glutamate concentrations become elevated over normal basal levels (Choi and Rothman, 1990; Meldrum, 1994) and thus could produce low-level AMPA receptor activation and desensitization. In these situations, ethanol may produce potent inhibition of AMPAR-mediated synaptic transmission. Likewise, whereas ethanol activates BK channels at resting, submicromolar cell calcium levels, drug action will turn into inhibition of current due to channels dwelling in a “desensitized” low activity mode when activating calcium exceeds the low micromolar range (Liu et al., 2008). These calcium levels are reached close to the BK channel in areas of neurotransmitter release (Llinás and Moreno, 1998) where these channels are clustered, and also in vascular smooth muscle during contraction (Pérez et al., 2001). Moreover, ethanol has been reported to increase cytosolic calcium in brain microsomes (Daniell and Harris, 1991), and induce apoptosis in cerebellar granule cells (Bhave and Hoffman, 1997), a process in which cystosolic calcium may reach >1 μM. Activation of BK channels has been reported to protect against apoptosis (Kim et al., 2004), and ethanol favoring BK channel dwelling in a low Po gating mode would likely impair one of the major conductances that can protect a cell from toxic calcium levels. Thus, ethanol-induced BK channel inhibition, together with internal Ca2+ release caused by ethanol itself, could synergistically favor the cell entering apoptosis.
Finally, ethanol desensitization interactions may bring critical mechanistic insights into drug abuse and coabuse. For example, the vast majority of nAchRs of the α4β2 type (prevalent in brain) will be desensitized at nicotine concentrations reached in the brain by regular smokers (Brody et al., 2006). Ethanol at toxicologically relevant concentrations interferes with this receptor desensitization, an action that could contribute to the coabuse of ethanol and nicotine, as discussed by Davis and de Fiebre (2006). In a more general way, an increased contribution of desensitized channel receptors to the overall receptor-mediated response and/or facilitation of desensitization by ethanol will reduce the receptor response, eventually contributing to acute tolerance to ethanol exposure. It is noteworthy that reduced responsiveness to ethanol is one of the characteristics thought to contribute to the risk of developing alcoholism in humans (Schuckit, 1985, 2000).
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism and by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant AA11560]. We thank Maria T. Asuncion-Chin for technical assistance.
This article is available online at http://pharmrev.aspetjournals.org.
doi:10.1124/pr.108.000430.
Footnotes
Abbreviations: LGIC, ligand-gated ion channel; CNS, central nervous system; nAchR, nicotinic acetylcholine receptor; NMDA, N-methyl-d-aspartate; Po, channel open probability; BK, large conductance, voltage- and calcium-gated potassium; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate; 5-HT, 5-hydroxytryptamine; GluR, glutamate receptor; AMPAR, AMPA receptor; NMDAR, NMDA receptor; GIRK, G protein-coupled inwardly rectifying potassium.
References
- Aguayo LG (1990) Ethanol potentiates the GABAA-activated Cl- current in mouse hippocampal and cortical neurons. Eur J Pharmacol 187 127-130. [DOI] [PubMed] [Google Scholar]
- Aguayo LG and Pancetti FC (1994) Ethanol modulation of the γ-aminobutyric acidA- and glycine-activated Cl- current in cultured mouse neurons. J Pharmacol Exp Ther 270 61-69. [PubMed] [Google Scholar]
- Aistrup GL, Marszalec W, and Narahashi T (1999) Ethanol modulation of nicotinic acetylcholine receptor currents in cultured cortical neurons. Mol Pharmacol 55 39-49. [DOI] [PubMed] [Google Scholar]
- Akinshola BE (2001) Straight-chain alcohols exhibit a cutoff in potency for the inhibition of recombinant glutamate receptor subunits. Br J Pharmacol 133 651-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Li P, Manion BD, Evers AS, and Steinbach JH (2007) Ethanol modulates the interaction of the endogenous neurosteroid allopregnanolone with the α1β2β2L GABAA receptor. Mol Pharmacol 71 461-472. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, and Peters JA (2008) Guide to receptors and channels (GRAC). Br J Pharmacol 153 S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A and Akk G (1998) Desensitization of mouse nicotinic acetylcholine receptor channels. A two-gate mechanism. J Gen Physiol 112 181-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenziger JE, Morris ML, Darsaut TE, and Ryan SE (2000) Effect of membrane lipid composition on the conformational equilibria of the nicotinic acetylcholine receptor. J Biol Chem 275 777-784. [DOI] [PubMed] [Google Scholar]
- Barrantes FJ (2003) Modulation of nicotinic acetylcholine receptor function through the outer and middle rings of transmembrane domains. Curr Opin Drug Discov Devel 6 620-632. [PubMed] [Google Scholar]
- Barrantes FJ (2004) Lipid matters: nicotinic acetylcholine receptor-lipid interactions. Mol Membr Biol 19 277-284. [DOI] [PubMed] [Google Scholar]
- Barry JA and Gawrisch K (1995) Effects of ethanol on lipid bilayers containing cholesterol, gangliosides, and sphingomyelin. Biochemistry 34 8852-8860. [DOI] [PubMed] [Google Scholar]
- Behrens R, Nolting A, Reimann F, Schwarz M, Waldschütz R, and Pongs O (2000) hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett 474 99-106. [DOI] [PubMed] [Google Scholar]
- Bhave SV and Hoffman PL (1997) Ethanol promotes apoptosis in cerebellar granule cells by inhibiting the trophic effect of NMDA. J Neurochem 68 578-586. [DOI] [PubMed] [Google Scholar]
- Bianchi MT and Macdonald RL (2001) Agonist trapping by GABAA receptor channels. J Neurosci 21 9083-9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Cooper R, Wallace D, Mane N, and Harris RA (2002) Hyperactivity and dopamine D1 receptor activation in mice lacking girk2 channels. Psychopharmacology (Berl) 159 370-378. [DOI] [PubMed] [Google Scholar]
- Bollan K, Robertson LA, Tang H, and Connolly CN (2003) Multiple assembly signals in gamma-aminobutyric acid (type A) receptor subunits combine to drive receptor construction and composition. Biochem Soc Trans 31 875-879. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Ali DN, Bleck V, and Harris RA (2002) Acetylcholine and alcohol sensitivity of neuronal nicotinic acetylcholine receptors: mutations in transmembrane domains. Alcohol Clin Exp Res 26 1764-1772. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Henderson LA, Bleck V, Trudell JR, and Harris RA (2003) Sites of excitatory and inhibitory actions of alcohols on neuronal α2β4 nicotinic acetylcholine receptors. J Pharmacol Exp Ther 307 42-52. [DOI] [PubMed] [Google Scholar]
- Borghese CM and Harris RA (2007) Studies of ethanol actions on recombinant delta-containing gamma-aminobutyric acid type A receptors yield contradictory results. Alcohol 41 155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Stórustovu S, Ebert B, Herd MB, Belelli D, Lambert JJ, Marshall G, Wafford KA, and Harris RA (2006) The δ subunit of GABAA receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther 316 1360-1368. [DOI] [PubMed] [Google Scholar]
- Botta P, Radcliffe RA, Carta M, Mameli M, Daly E, Floyd KL, Deitrich RA, and Valenzuela CF (2007) Modulation of GABAA receptors in cerebellar granule neurons by ethanol: a review of genetic and electrophysiological studies. Alcohol 41 187-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd GW, Low P, Dunlop JI, Robertson LA, Vardy A, Lambert JJ, Peters JA, and Connolly CN (2002) Assembly and cell surface expression of homomeric and heteromeric 5-HT3 receptors: the role of oligomerization and chaperone proteins. Mol Cell Neurosci 21 38-50. [DOI] [PubMed] [Google Scholar]
- Brenner R, Jegla TJ, Wickenden A, Liu Y, and Aldrich RW (2000) Cloning and characterization of novel large conductance calcium-activated potassium channel β subunits hKCNMB3 and hKCNMB4. J Biol Chem 275 6453-6461. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Scholz A, Weiger TM, and Dopico AM (2007) Ethanol interactions with calcium-dependent potassium channels. Alcohol Clin Exp Res 31 1625-1632. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, et al. (2006) Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry 63 907-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Liu J, and Dopico AM (2009) Arterial smooth muscle BK channel beta1 subunits determine ethanol-induced cerebrovascular constriction (Abstract). Biophys J 96 474a. [Google Scholar]
- Butler DH and McNamee MG (1993) FTIR analysis of nicotinic acetylcholine receptor secondary structure in reconstituted membranes. Biochim Biophys Acta 1150 17-24. [DOI] [PubMed] [Google Scholar]
- Cantor RS (2003) Receptor desensitization by neurotransmitters in membranes: are neurotransmitters the endogenous anesthetics? Biochemistry 42 11891-11897. [DOI] [PubMed] [Google Scholar]
- Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, and Harris RA (1999) Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther 289 774-780. [PubMed] [Google Scholar]
- Carta M, Ariwodola OJ, Weiner JL, and Valenzuela CF (2003) Alcohol potently inhibits the kainate receptor-dependent excitatory drive of hippocampal interneurons. Proc Natl Acad Sci U S A 100 6813-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash DJ and Subbarao K (1987) Channel opening of gamma-aminobutyric acid receptor from rat brain: molecular mechanisms of the receptor responses. Biochemistry 26 7562-7570. [DOI] [PubMed] [Google Scholar]
- Cash DJ and Subbarao K (1988) Different effects of pentobarbital on two gamma-aminobutyrate receptors from rat brain: channel opening, desensitization, and an additional conformational change. Biochemistry 27 4580-4590. [DOI] [PubMed] [Google Scholar]
- Celentano JJ, Gibbs TT, and Farb DH (1988) Ethanol potentiates GABA and glycine induced currents in chick spinal cord neurons. Brain Res 455 377-380. [DOI] [PubMed] [Google Scholar]
- Choi DW and Rothman SM (1990) The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci 13 171-182. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, and Spedding M (2009) A nomenclature for ligand-gated ion channels. Neuropharmacology 56 2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D (1998) Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol 125 924-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D and Hawkes AG (1995) A Q-matrix cookbook: how to write only one program to calculate the single-channel and macroscopic predictions for any kinetic mechanism, in Single-Channel Recording, 2nd ed. (Sakmann B and Neher E eds), pp. 589-633, Plenum Press, New York.
- Colquhoun D and Sakmann B (1985) Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol 369 501-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley EC (1996) The Ion Channel Facts Book, Vol. 1: Extracellular Ligand-Gated Channels. Academic Press, London.
- Covernton PJ and Connolly JG (1997) Differential modulation of rat neuronal nicotinic receptor subtypes by acute application of ethanol. Br J Pharmacol 122 1661-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DH, Cui J, and Aldrich RW (1997) Allosteric gating of a large conductance Ca-activated K+ channel. J Gen Physiol 110 257-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JJ, Treistman SN, and Dopico AM (2003) Cholesterol antagonizes ethanol potentiation of human brain BKCa channels reconstituted into phospholipid bilayers. Mol Pharmacol 64 365-372. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Treistman SN, and Dopico AM (2005) Distinct structural features of phospholipids differentially determine ethanol sensitivity and basal function of BK channels. Mol Pharmacol 68 4-10. [DOI] [PubMed] [Google Scholar]
- Cui C and Mayer ML (1999) Heteromeric kainate receptors formed by the coassembly of GluR5, GluR6, and GluR7. J Neurosci 19 8281-8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell LC and Harris RA (1991) Effect of chronic ethanol treatment and selective breeding for sensitivity to ethanol on calcium release induced by inositol trisphosphate or ethanol from brain and liver microsomes. Alcohol Clin Exp Res 15 224-228. [DOI] [PubMed] [Google Scholar]
- Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, and Kirkness EF (1999) The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature 397 359-363. [DOI] [PubMed] [Google Scholar]
- Davies DL, Machu TK, Guo Y, and Alkana RL (2002) Ethanol sensitivity in ATP-gated P2X receptors is subunit dependent. Alcohol Clin Exp Res 26 773-778. [PubMed] [Google Scholar]
- Davies DL, Kochegarov AA, Kuo ST, Kulkarni AA, Woodward JJ, King BF, and Alkana RL (2005) Ethanol differentially affects ATP-gated P2X(3) and P2X(4) receptor subtypes expressed in Xenopus oocytes. Neuropharmacology 49 243-253. [DOI] [PubMed] [Google Scholar]
- Davies DL, Asatryan L, Kuo ST, Woodward JJ, King BF, Alkana RL, Xiao C, Ye JH, Sun H, Zhang L, et al. (2006) Effects of ethanol on adenosine 5′-triphosphate-gated purinergic and 5-hydroxytryptamine3 receptors. Alcohol Clin Exp Res 30 349-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TJ and de Fiebre CM (2006) Alcohol's actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health 29 179-185. [PMC free article] [PubMed] [Google Scholar]
- Dilger JP and Liu Y (1992) Desensitization of acetylcholine receptors in BC3H-1 cells. Pflugers Arch 420 479-485. [DOI] [PubMed] [Google Scholar]
- Dilger JP, Vidal AM, Mody HI, and Liu Y (1994) Evidence for direct actions of general anesthetics on an ion channel protein. A new look at a unified mechanism of action. Anesthesiology 81 431-442. [DOI] [PubMed] [Google Scholar]
- Dilger JP, Liu Y, and Vidal AM (1995) Interactions of general anaesthetics with single acetylcholine receptor channels. Eur J Anaesthesiol 12 31-39. [PubMed] [Google Scholar]
- Dilger JP (2002) The effects of general anaesthetics on ligand-gated ion channels. Br J Anaesth 89 41-51. [DOI] [PubMed] [Google Scholar]
- Dopico AM, Anantharam V, and Treistman SN (1998) Ethanol increases the activity of Ca++-dependent K+ (mslo) channels: functional interaction with cytosolic Ca++. J Pharmacol Exp Ther 284 258-268. [PubMed] [Google Scholar]
- Dopico AM, Lemos JR, and Treistman SN (1996) Ethanol activates large-conductance, Ca2+-activated K+ channels in neurohypophysial terminals. Mol Pharmacol 49 40-48. [PubMed] [Google Scholar]
- Eggers ED and Berger AJ (2004) Mechanisms for the modulation of native glycine receptor channels by ethanol. J Neurophysiol 91 2685-2695. [DOI] [PubMed] [Google Scholar]
- Eggers ED, O'Brien JA, and Berger AJ (2000) Developmental changes in the modulation of synaptic glycine receptors by ethanol. J Neurophysiol 84 2409-2416. [DOI] [PubMed] [Google Scholar]
- Feinberg-Zadek PL, Martin G, and Treistman SN (2008) BK channel subunit composition modulates molecular tolerance to ethanol. Alcohol Clin Exp Res 32 1207-1216. [DOI] [PubMed] [Google Scholar]
- Feinberg-Zadek PL and Treistman SN (2007) Beta-subunits are important modulators of the acute response to alcohol in human BK channels. Alcohol Clin Exp Res 31 737-744. [DOI] [PubMed] [Google Scholar]
- Fernández Nievas GA, Barrantes FJ, and Antollini SS (2008) Modulation of nicotinic acetylcholine receptor conformational state by free fatty acids and steroids. J Biol Chem 283 21478-21486. [DOI] [PubMed] [Google Scholar]
- Fleming MF, Mihic SJ, and Harris RA (2001) Ethanol, in Goodman & Gilman's The Pharmacological Basis of Therapeutics (Gardman JG, Limbird LE, and Goodman Gilman A, eds) pp 429-446, McGraw-Hill, New York.
- Fong TM and McNamee MG (1986) Correlation between acetylcholine receptor function and structural properties of membranes. Biochemistry 25 830-840. [DOI] [PubMed] [Google Scholar]
- Forman SA, Miller KW, and Yellen G (1995) A discrete site for general anesthetics on a postsynaptic receptor. Mol Pharmacol 48 574-581. [PubMed] [Google Scholar]
- Forman SA and Zhou Q (1999) Novel modulation of a nicotinic receptor channel mutant reveals that the open state is stabilized by ethanol. Mol Pharmacol 55 102-108. [DOI] [PubMed] [Google Scholar]
- Forman SA and Zhou Q (2000) Nicotinic receptor pore mutations create a sensitive inhibitory site for ethanol. Alcohol Clin Exp Res 24 1363-1368. [PubMed] [Google Scholar]
- Forman SA, Zhou QL, and Stewart DS (2007) Photoactivated 3-azioctanol irreversibly desensitizes muscle nicotinic ACh receptors via interactions at alphaE262. Biochemistry 46 11911-11918. [DOI] [PubMed] [Google Scholar]
- Forman SA (1998) Direct interactions of anesthetics and nonanesthetics with the nicotinic acetylcholine receptor pore. Toxicol Lett 100–101: 169-178. [DOI] [PubMed] [Google Scholar]
- Fury M, Marx SO, and Marks AR (2002) Molecular BKology: the study of splicing and dicing. Sci STKE 123 PE12. [DOI] [PubMed] [Google Scholar]
- Godden EL, Harris RA, and Dunwiddie TV (2001) Correlation between molecular volume and effects of n-alcohols on human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther 296 716-722. [PubMed] [Google Scholar]
- Grosman C and Auerbach A (2000) Kinetic, mechanistic, and structural aspects of unliganded gating of acetylcholine receptor channels: a single-channel study of second transmembrane segment 12′ mutants. J Gen Physiol 115 621-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA (1999) Ethanol actions on multiple ion channels: which are important? Alcohol Clin Exp Res 23 1563-1570. [PubMed] [Google Scholar]
- Harris RA, Trudell JR, and Mihic SJ (2008) Ethanol's molecular targets. Sci Signal 1 re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayrapetyan V, Jenschke M, Dillon GH, and Machu TK (2005) Co-expression of the 5-HT(3B) subunit with the 5-HT(3A) receptor reduces alcohol sensitivity. Brain Res Mol Brain Res 142 146-150. [DOI] [PubMed] [Google Scholar]
- Hille B (2001) Ion Channels of Excitable Membranes, 3rd ed. Sinauer Assoc., Sunderland, MA.
- Hitzemann RJ, Schueler HE, Graham-Brittain C, and Kreishman GP (1986) Ethanol-induced changes in neuronal membrane order. An NMR study. Biochim Biophys Acta 859 189-197. [DOI] [PubMed] [Google Scholar]
- Homanics GE, Harrison NL, Quinlan JJ, Krasowski MD, Rick CE, de Blas AL, Mehta AK, Kist F, Mihalek RM, Aul JJ, et al. (1999) Normal electrophysiological and behavioral responses to ethanol in mice lacking the long splice variant of the gamma2 subunit of the gamma-aminobutyrate type A receptor. Neuropharmacology 38 253-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XQ, Hayrapetyan V, Gadhiya JJ, Rhubottom HE, Lovinger DM, and Machu TK (2006) Mutations of L293 in transmembrane two of the mouse 5-hydroxytryptamine3A receptor alter gating and alcohol modulatory actions. Br J Pharmacol 148 88-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir RL and Miles K (1989) Protein phosphorylation of nicotinic acetylcholine receptors. Crit Rev Biochem Mol Biol 24 183-215. [DOI] [PubMed] [Google Scholar]
- Jan LY and Jan YN (2000) Heartfelt crosstalk: desensitization of the GIRK current. Nat Cell Biol 2 E165-7. [DOI] [PubMed] [Google Scholar]
- Jenkins A, Franks NP, and Lieb WR (1996) Actions of general anaesthetics on 5-HT3 receptors in N1E-115 neuroblastoma cells. Br J Pharmacol 117 1507-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AA, Davies PA, Bräuner-Osborne H, and Krzywkowski K (2008) 3B but which 3B? And that's just one of the questions: the heterogeneity of human 5-HT(3) receptors. Trends Pharmacol Sci 29 437-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LH, Kim M, Spelta V, Bo X, Surprenant A, and North RA (2003) Subunit arrangement in P2X receptors. J Neurosci 23 8903-8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MV and Westbrook GL (1996) The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci 19 96-101. [DOI] [PubMed] [Google Scholar]
- Kalant H (1998) Research on tolerance: what can we learn from history? Alcohol Clin Exp Res 22 67-76. [DOI] [PubMed] [Google Scholar]
- Katz B and Thesleff S (1957) A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol 138 63-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin TP (2006) A Pharmacology Primer. Theory, Applications, and Methods, 2nd ed. Academic Press, Burlington, MA.
- Kim KY, Lee JH, Park JH, Yoo MA, Kwak YG, Kim SO, Yoo SE, and Hong KW (2004) Anti-apoptotic action of (2S,3S,4R)-N″-cyano-N-(6-amino-3,4-dihydro-3-hydroxy-2-methyl-2-dimethoxymethyl-2H-benzopyran-4-yl)-N′-benzylguanidine (KR-31378) by suppression of the phosphatase and tensin homolog deleted from chromosome 10 phosphorylation and increased phosphorylation of casein kinase2/Akt/cyclic AMP response element binding protein via maxi-K channel opening in neuronal cells. Eur J Pharmacol. 497 267-277. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ikeda K, Kojima H, Niki H, Yano R, Yoshioka T, and Kumanishi T (1999) Ethanol opens G-protein-activated inwardly rectifying K+ channels. Nat Neurosci 2 1091-1097. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Debus F, Linden AM, Malécot C, Leppä E, Vekovischeva O, Rabe H, Böhme I, Aller MI, Wisden W, et al. (2007) Does ethanol act preferentially via selected brain GABAA receptor subtypes? the current evidence is ambiguous. Alcohol 41 163-176. [DOI] [PubMed] [Google Scholar]
- Läck AK, Ariwodola OJ, Chappell AM, Weiner JL, and McCool BA (2008) Ethanol inhibition of kainate receptor-mediated excitatory neurotransmission in the rat basolateral nucleus of the amygdala. Neuropharmacology 55 661-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R (1994) Molecular workings of large conductance (maxi) Ca2+-activated K+ channels, in Handbook of Membrane Channels (Petracchia C ed) pp 79-102, Academic Press, San Diego, CA.
- Lewohl JM, Wilson WR, Mayfield RD, Brozowski SJ, Morrisett RA, and Harris RA (1999) G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat Neurosci 2 1084-1090. [DOI] [PubMed] [Google Scholar]
- Li H and Rogawski MA (1998) GluR5 kainate receptor mediated synaptic transmission in rat basolateral amygdala in vitro. Neuropharmacology 37 1279-1286. [DOI] [PubMed] [Google Scholar]
- Li C, Aguayo L, Peoples RW, and Weight FF (1993) Ethanol inhibits a neuronal ATP-gated ion channel. Mol Pharmacol 44 871-875. [PubMed] [Google Scholar]
- Li C, Peoples RW, and Weight FF (1998) Ethanol-induced inhibition of a neuronal P2X purinoceptor by an allosteric mechanism. Br J Pharmacol 123 1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xiong K, and Weight FF (2000) Ethanol inhibition of adenosine 5′-triphosphate-activated current in freshly isolated adult rat hippocampal CA1 neurons. Neurosci Lett 295 77-80. [DOI] [PubMed] [Google Scholar]
- Lima-Landman MT and Albuquerque EX (1989) Ethanol potentiates and blocks NMDA-activated single-channel currents in rat hippocampal pyramidal cells. FEBS Lett 247 61-67. [DOI] [PubMed] [Google Scholar]
- Lindstrom JM (2003) Nicotinic acetylcholine receptors of muscles and nerves: comparison of their structures, functional roles, and vulnerability to pathology. Ann N Y Acad Sci 998 41-52. [DOI] [PubMed] [Google Scholar]
- Lingle CJ, Maconochie D, and Steinbach JH (1992) Activation of skeletal muscle nicotinic acetylcholine receptors. J Membr Biol 126 195-217. [DOI] [PubMed] [Google Scholar]
- Little HJ (1991) Mechanisms that may underlie the behavioural effects of ethanol. Prog Neurobiol 36 171-194. [DOI] [PubMed] [Google Scholar]
- Llinás R and Moreno H (1998) Local Ca2+ signaling in neurons. Cell Calcium 24 359-366. [DOI] [PubMed] [Google Scholar]
- Liu J, Asuncion-Chin M, Liu P, and Dopico AM (2006) CaM kinase II phosphorylation of slo Thr107 regulates activity and ethanol responses of BK channels. Nat Neurosci 9 41-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Vaithianathan T, Manivannan K, Parrill A, and Dopico AM (2008) Ethanol modulates BKCa channels by acting as an adjuvant of calcium. Mol Pharmacol 74 628-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Xi Q, Ahmed A, Jaggar JH, and Dopico AM (2004) Essential role for smooth muscle BK channels in alcohol-induced cerebrovascular constriction. Proc Natl Acad Sci U S A 101 18217-18222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM (1997) Alcohols and neurotransmitter gated ion channels: past, present and future. Naunyn Schmiedebergs Arch Pharmacol 356 267-282. [DOI] [PubMed] [Google Scholar]
- Lovinger DM (1991) Ethanol potentiation of 5-HT3 receptor-mediated ion current in NCB-20 neuroblastoma cells. Neurosci Lett 122 57-60. [DOI] [PubMed] [Google Scholar]
- Lovinger DM (1993) High ethanol sensitivity of recombinant AMPA-type glutamate receptors expressed in mammalian cells. Neurosci Lett 159 83-87. [DOI] [PubMed] [Google Scholar]
- Lovinger DM and Crabbe JC (2005) Laboratory models of alcoholism: treatment target identification and insight into mechanisms. Nat Neurosci 8 1471-1480. [DOI] [PubMed] [Google Scholar]
- Lovinger DM and White G (1991) Ethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol Pharmacol 40 263-270. [PubMed] [Google Scholar]
- Lovinger DM and Zhou Q (1994) Alcohols potentiate ion current mediated by recombinant 5-HT3RA receptors expressed in a mammalian cell line. Neuropharmacology 33 1567-1572. [DOI] [PubMed] [Google Scholar]
- Lovinger DM and Zhou Q (1998) Alcohol effects on the 5-HT3 ligand-gated ion channel. Toxicol Lett 100–101: 239-246. [DOI] [PubMed] [Google Scholar]
- Lu R, Alioua A, Kumar Y, Eghbali M, Stefani E, and Toro L (2006) MaxiK channel partners: physiological impact. J Physiol 570 65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machu TK and Harris RA (1994) Alcohols and anesthetics enhance the function of 5-hydroxytryptamine3 receptors expressed in Xenopus laevis oocytes. J Pharmacol Exp Ther 271 898-905. [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, and Julius D (1991) Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science 254 432-437. [DOI] [PubMed] [Google Scholar]
- Marszalec W, Kurata Y, Hamilton BJ, Carter DB, and Narahashi T (1994) Selective effects of alcohols on gamma-aminobutyric acid A receptor subunits expressed in human embryonic kidney cells. J Pharmacol Exp Ther 269 157-163. [PubMed] [Google Scholar]
- Marszalec W, Yeh JZ, and Narahashi T (2005) Desensitization of nicotine acetylcholine receptors: modulation by kinase activation and phosphatase inhibition. Eur J Pharmacol 514 83-90. [DOI] [PubMed] [Google Scholar]
- Martin G, Puig S, Pietrzykowski A, Zadek P, Emery P, and Treistman SN (2004) Somatic localization of specific large-conductance calcium-activated potassium channel subtype controls compartimentalized ethanol sensitivity in the nucleus accumbens. J Neurosci 24 6563-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia MP, Trudell JR, and Harris RA (2000) Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci U S A 97 9305-9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Frye GD, Pulido MD, and Botting SK (2003) Effects of chronic ethanol consumption on rat GABA(A) and strychnine-sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain Res 963 165-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Wallner M, Song M, and Toro L (1997) Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0–S6), an extracellular N terminus, and an intracellular (S9–S10) C terminus. Proc Natl Acad Sci U S A 94 14066-14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum BS (1994) The role of glutamate in epilepsy and other CNS disorders. Neurology 44(11 Suppl 8): S14-S23. [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, et al. (1997) Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 389 385-389. [DOI] [PubMed] [Google Scholar]
- Millar NS and Harkness PC (2008) Assembly and trafficking of nicotinic acetylcholine receptors. Mol Membr Biol 25 279-292. [DOI] [PubMed] [Google Scholar]
- Möykkynen T, Korpi ER, and Lovinger DM (2003) Ethanol inhibits AMPA receptor function in CNS neurons by increasing desensitization. J Pharmacol Exp Ther 306 546-555. [DOI] [PubMed] [Google Scholar]
- Murrell RD and Haydon DA (1991) Actions of n-alcohols on nicotinic acetylcholine receptor ion channels in cultured rat muscle cells. Ann N Y Acad Sci 625 365-374. [DOI] [PubMed] [Google Scholar]
- Murrell RD, Braun MS, and Haydon DA (1991) Actions of n-alcohols on nicotinic acetylcholine receptor channels in cultured rat myotubes. J Physiol 437 431-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Aistrup GL, Huang CS, Marszalec W, Song JH, Yeh JZ, and Narahashi T (1996) Potent modulation of neuronal nicotinic acetylcholine receptor-channel by ethanol. Neurosci Lett 217 189-193. [PubMed] [Google Scholar]
- Narahashi T, Aistrup GL, Marszalec W, and Nagata K (1999) Neuronal nicotinic acetylcholine receptors: a new target site of ethanol. Neurochem Int 35 131-141. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Söderpalm B, Ericson M, Olausson P, Engel JA, Zhang X, Nordberg A, Marszalec W, Aistrup GL, Schmidt LG, et al. (2001) Mechanisms of alcohol-nicotine interactions: alcoholics versus smokers. Alcohol Clin Exp Res 25 152S-156S. [DOI] [PubMed] [Google Scholar]
- Nashmi R and Lester HA (2006) CNS localization of neuronal nicotinic receptors. J Mol Neurosci 30 181-184. [DOI] [PubMed] [Google Scholar]
- Nievas GA, Barrantes FJ, and Antollini SS (2007) Conformation-sensitive steroid and fatty acid sites in the transmembrane domain of the nicotinic acetylcholine receptor. Biochemistry 46 3503-3512. [DOI] [PubMed] [Google Scholar]
- North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82 1013-1067. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Hanchar HJ, Meera P, and Wallner M (2007) GABAA receptor subtypes: the ”one glass of wine“ receptors. Alcohol 41 201-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio P, Rojas P, Ferreira G, and Latorre R (2002) New disguises for an old channel: MaxiK channel beta-subunits. News Physiol Sci 17 156-161. [DOI] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, and Mayer ML (1994) Cyclothiazide differentially modulates desensitization of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor splice variants. Mol Pharmacol 46 129-138. [PubMed] [Google Scholar]
- Peoples RW, Li C, and Weight FF (1996) Lipid vs protein theories of alcohol action in the nervous system. Annu Rev Pharmacol Toxicol 36 185-201. [DOI] [PubMed] [Google Scholar]
- Peoples RW, White G, Lovinger DM, and Weight FF (1997) Ethanol inhibition of N-methyl-d-aspartate-activated current in mouse hippocampal neurones: whole-cell patch-clamp analysis. Br J Pharmacol 122 1035-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez GJ, Bonev AD, and Nelson MT (2001) Micromolar Ca2+ from sparks activates Ca2+-sensitive K+ channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol 281 C1769-C1775. [DOI] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Martin GE, Puig SI, Knott TK, Lemos JR, and Treistman SN (2004) Alcohol tolerance in large-conductance, calcium-activated potassium channels of CNS terminals is intrinsic and includes two components: decreased ethanol potentiation and decreased channel density. J Neurosci 24 8322-8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp RL, Lickteig RL, and Lovinger DM (1999) Factors that enhance ethanol inhibition of N-methyl-d-aspartate receptors in cerebellar granule cells. J Pharmacol Exp Ther 289 1564-1574. [PubMed] [Google Scholar]
- Raines DE, Rankin SE, and Miller KW (1995) General anesthetics modify the kinetics of nicotinic acetylcholine receptor desensitization at clinically relevant concentrations. Anesthesiology 82 276-287. [DOI] [PubMed] [Google Scholar]
- Rankin SE, Addona GH, Kloczewiak MA, Bugge B, and Miller KW (1997) The cholesterol dependence of activation and fast desensitization of the nicotinic acetylcholine receptor. Biophys J 73 2446-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Salous AK, Paul JM, Lamb KA, Dwyer DS, and Peoples RW (2008) Functional interactions of alcohol-sensitive sites in the N-methyl-d-aspartate receptor M3 and M4 domains. J Biol Chem 283 8250-8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Prasad A, and MacDonald JF (1992) Ethanol modulation of GABA receptor-activated Cl-currents neurons of the chick, rat and mouse central nervous system. Eur J Pharmacol 224 173-181. [DOI] [PubMed] [Google Scholar]
- Robertson SJ, Ennion SJ, Evans RJ, and Edwards FA (2001) Synaptic P2X receptors. Curr Opin Neurobiol 11 378-386. [DOI] [PubMed] [Google Scholar]
- Rothberg BS, Bello RA, Song L, and Magleby KL (1996) High Ca2+ concentrations induce a low activity mode and reveal Ca2+-independent long shut intervals in BK channels from rat muscle. J Physiol 493 673-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadja R, Alagem N, and Reuveny E (2003) Gating of GIRK channels: details of an intricate, membrane-delimited signaling complex. Neuron 39 9-12. [DOI] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, and Wei A (2006) High-conductance potassium channels of the SLO family. Nat Rev Neurosci 7 921-931. [DOI] [PubMed] [Google Scholar]
- Sapp DW and Yeh HH (1998) Ethanol-GABAA receptor interactions: a comparison between cell lines and cerebellar Purkinje cells. J Pharmacol Exp Ther 284 768-776. [PubMed] [Google Scholar]
- Schubert R and Nelson MT (2001) Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci 22 505-512. [DOI] [PubMed] [Google Scholar]
- Schuckit MA (1985) Genetics and the risk for alcoholism. JAMA 254 2614-2617. [PubMed] [Google Scholar]
- Schuckit MA (2000) Genetics of the risk for alcoholism. Am J Addict 9 103-112. [DOI] [PubMed] [Google Scholar]
- Schwappach B (2008) An overview of trafficking and assembly of neurotransmitter receptors and ion channels. Mol Membr Biol 25 270-278. [DOI] [PubMed] [Google Scholar]
- Shelley C and Magleby KL (2008) Linking exponential components to kinetic states in markov models for single-channel gating. J Gen Physiol 132 295-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E, Baur R, and Malherbe P (1993) Recombinant GABAA receptor function and ethanol. Fed Eur Biochem Soc Lett 324 140-142. [DOI] [PubMed] [Google Scholar]
- Solt K, Ruesch D, Forman SA, Davies PA, and Raines DE (2007) Differential effects of serotonin and dopamine on human 5-HT3A receptor kinetics: interpretation within an allosteric kinetic model. J Neurosci 27 13151-13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonner JM (2002) Issues in the design and interpretation of minimum alveolar anesthetic concentration (MAC) studies. Anesth Analg 95 609-614. [DOI] [PubMed] [Google Scholar]
- Sonner JM (2008) A hypothesis on the origin and evolution of the response to inhaled anesthetics. Anesth Analg 107 849-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak CE (1995) Correlations among Hill parameters reflect models of activating ligand-gated ion channels. Trends Pharmacol Sci 16 39-42. [DOI] [PubMed] [Google Scholar]
- Stevens R, Rüsch D, Solt K, Raines DE, and Davies PA (2005) Modulation of human 5-hydroxytryptamine type 3AB receptors by volatile anesthetics and n-alcohols. J Pharmacol Exp Ther 314 338-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A, Davies PA, Kirkness EF, Safa P, and Hales TG (2003) Introduction of the 5-HT3B subunit alters the functional properties of 5-HT3 receptors native to neuroblastoma cells. Neuropharmacology 44 214-223. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, and Smith SS (2002) Hormonally regulated alpha4beta2delta GABAA receptors are a target for alcohol. Nat Neurosci 5 721-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung KW, Engel SR, Allan AM, and Lovinger DM (2000) 5-HT3 Receptor function and potentiation by alcohols in frontal cortex neurons from transgenic mice overexpressing the receptor. Neuropharmacology 39 2346-2351. [DOI] [PubMed] [Google Scholar]
- Taylor P and Insel PA (1990) Molecular basis of drug action, in Principles of Drug Action: The Basis of Pharmacology (Taylor P and Insel PA eds) pp. 103-200, Churchill Livingstone, New York.
- Tierney KJ, Block DE, and Longo ML (2005) Elasticity and phase behavior of DPPC membrane modulated by cholesterol, ergosterol, and ethanol. Biophys J 89 2481-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO and Fischbach GD (1989) Glutamate receptor desensitization and its role in synaptic transmission. Neuron 3 209-218. [DOI] [PubMed] [Google Scholar]
- Tsujiyama S, Akaike A, Ujihara H, and Sasa M (1997) Potentiation by ethanol of GABA-induced current and facilitation of its desensitization in cultured rat cortical neurons. Gen Pharmacol 28 375-380. [DOI] [PubMed] [Google Scholar]
- Ueno S, Lin A, Nikolaeva N, Trudell JR, Mihic SJ, Harris RA, and Harrison NL (2000) Tryptophan scanning mutagenesis in TM2 of the GABAA receptor alpha subunit: effects on channel gating and regulation by ethanol. Br J Pharmacol 131 296-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela CF and Cardoso RA (1999) Acute effects of ethanol on kainate receptors with different subunit compositions. J Pharmacol Exp Ther 288 1199-1206. [PubMed] [Google Scholar]
- Valenzuela CF, Cardoso RA, Wick MJ, Weiner JL, Dunwiddie TV, and Harris RA (1998) Effects of ethanol on recombinant glycine receptors expressed in mammalian cell lines. Alcohol Clin Exp Res 22 1132-1136. [PubMed] [Google Scholar]
- Vial C, Roberts JA, and Evans RJ (2004) Molecular properties of ATP-gated P2X receptor ion channels. Trends Pharmacol Sci 25 487-493. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, and Olsen RW (2003) Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A 100 15218-15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SX, Ikeda M, and Guggino WB (2003) The cytoplasmic tail of large conductance, voltage- and Ca2+-activated K+ (MaxiK) channel is necessary for its cell surface expression. J Biol Chem 278 2713-2722. [DOI] [PubMed] [Google Scholar]
- Weiger TM, Hermann A, and Levitan IB (2002) Modulation of calcium-activated potassium channels. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 188 79-87. [DOI] [PubMed] [Google Scholar]
- Weight FF, Li C, and Peoples RW (1999) Alcohol action on membrane ion channels gated by extracellular ATP (P2X receptors). Neurochem Int 35 143-152. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Dunwiddie TV, and Valenzuela CF (1999) Ethanol inhibition of synaptically evoked kainate responses in rat hippocampal CA3 pyramidal neurons. Mol Pharmacol 56 85-90. [DOI] [PubMed] [Google Scholar]
- Wood SC, Tonner PH, de Armendi AJ, Bugge B, and Miller KW (1995) Channel inhibition by alkanols occurs at a binding site on the nicotinic acetylcholine receptor. Mol Pharmacol 47 121-130. [PubMed] [Google Scholar]
- Woodward JJ (2000) Ethanol and NMDA receptor signaling. Crit Rev Neurobiol 14 69-89. [DOI] [PubMed] [Google Scholar]
- Wright JM, Peoples RW, and Weight FF (1996) Single-channel and whole-cell analysis of ethanol inhibition of NMDA-activated currents in cultured mouse cortical and hippocampal neurons. Brain Res 738 249-256. [DOI] [PubMed] [Google Scholar]
- Wu G and Miller KW (1994) Ethanol enhances agonist-induced fast desensitization in nicotinic acetylcholine receptors. Biochemistry 33 9085-9091. [DOI] [PubMed] [Google Scholar]
- Wu G, Tonner PH, and Miller KW (1994) Ethanol stabilizes the open channel state of the Torpedo nicotinic acetylcholine receptor. Mol Pharmacol 45 102-108. [PubMed] [Google Scholar]
- Wyman J and Gill SJ (1990) Binding and Linkage: Functional Chemistry of Biological Molecules. University Science Books, Mill Valley, CA.
- Xiao C, Zhou C, Li K, Davies DL, and Ye JH (2008) Purinergic type 2 receptors at GABAergic synapses on ventral tegmental area dopamine neurons are targets for ethanol action. J Pharmacol Exp Ther 327 196-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura T, Lewohl JM, and Harris RA (2001) Differential effects of general anesthetics on G protein-coupled inwardly rectifying and other potassium channels. Anesthesiology 95 144-153. [DOI] [PubMed] [Google Scholar]
- Ye Q, Koltchine VV, Mihic SJ, Mascia MP, Wick MJ, Finn SE, Harrison NL, and Harris RA (1998) Enhancement of glycine receptor function by ethanol is inversely correlated with molecular volume at position alpha267. J Biol Chem 273 3314-3319. [DOI] [PubMed] [Google Scholar]
- Ye JH, Tao L, Ren J, Schaefer R, Krnjevic K, Liu PL, Schiller DA, and McArdle JJ (2001) Ethanol potentiation of glycine-induced responses in dissociated neurons of rat ventral tegmental area. J Pharmacol Exp Ther 296 77-83. [PubMed] [Google Scholar]
- Young AP and Sigman DS (1981) Allosteric effects of volatile anesthetics on the membrane-bound acetylcholine receptor protein. I. Stabilization of the high-affinity state. Mol Pharmacol 20 498-505. [PubMed] [Google Scholar]
- Yuan C, O'Connell RJ, Wilson A, Pietrzykowski AZ, and Treistman SN (2008) Acute alcohol tolerance is intrinsic to the BKCa protein, but is modulated by the lipid environment. J Biol Chem 283 5090-5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q and Lovinger DM (1996) Pharmacologic characteristics of potentiation of 5-HT3 receptors by alcohols and diethyl ether in NCB-20 neuroblastoma cells. J Pharmacol Exp Ther 278 732-740. [PubMed] [Google Scholar]
- Zhou QL, Zhou Q, and Forman SA (2000) The n-alcohol site in the nicotinic receptor pore is a hydrophobic patch. Biochemistry 39 14920-14926. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Verdoorn TA, and Lovinger DM (1998) Alcohols potentiate the function of 5-HT3 receptor-channels on NCB-20 neuroblastoma cells by favouring and stabilizing the open channel state. J Physiol 507 335-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF and Thio LL (1992) Properties of vertebrate glutamate receptors: calcium mobilization and desensitization. Prog Neurobiol 39 295-336. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Aistrup GL, Marszalec W, Gillespie A, Chavez-Noriega LE, Yeh JZ, and Narahashi T (2001) Dual action of n-alcohols on neuronal nicotinic acetylcholine receptors. Mol Pharmacol 60 700-711. [PubMed] [Google Scholar]
- Zuo Y, Kuryatov A, Lindstrom JM, Yeh JZ, and Narahashi T (2002) Alcohol modulation of neuronal nicotinic acetylcholine receptors is alpha subunit dependent. Alcohol Clin Exp Res 26 779-784. [PubMed] [Google Scholar]
- Zuo Y, Nagata K, Yeh JZ, and Narahashi T (2004) Single-channel analyses of ethanol modulation of neuronal nicotinic acetylcholine receptors. Alcohol Clin Exp Res 28 688-696. [DOI] [PubMed] [Google Scholar]