Abstract
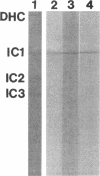
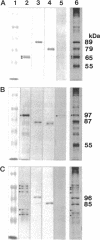
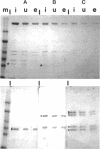
The outer arm dynein of sea urchin sperm axoneme contains three intermediate chains (IC1, IC2, and IC3; M(r) 128,000, 98,000, and 74,000, respectively). IC2 and IC3 are members of the WD family; the WD motif is responsible for a protein-protein interaction. We describe here the molecular cloning of IC1. IC1 has a unique primary structure, the N-terminal part is homologous to the sequence of thioredoxin, the middle part consists of three repetitive sequences homologous to the sequence of nucleoside diphosphate kinase, and the C-terminal part contains a high proportion of negatively charged glutamic acid residues. Thus, IC1 is a novel dynein intermediate chain distinct from IC2 and IC3 and may be a multifunctional protein. The thioredoxin-related part of IC1 is more closely related to those of two redox-active Chlamydomonas light chains than thioredoxin. Antibodies were prepared against the N-terminal and middle domains of IC1 expressed as His-tagged proteins in bacteria. These antibodies cross-reacted with some dynein polypeptides (potential homologues of IC1) from distantly related species. We propose here that the three intermediate chains are the basic core units of sperm outer arm dynein because of their ubiquitous existence. The recombinant thioredoxin-related part of IC1 and outer arm dyneins from sea urchin and distantly related species were specifically bound to and eluted from a phenylarsine oxide affinity column with 2-mercaptoethanol, indicating that they contain vicinal dithiols competent to undergo reversible oxidation/reduction.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2241–2245. doi: 10.1093/nar/19.suppl.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Weiss R. M., Terwilliger T. C. The hydrophobic moment detects periodicity in protein hydrophobicity. Proc Natl Acad Sci U S A. 1984 Jan;81(1):140–144. doi: 10.1073/pnas.81.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon C., White D., Huitorel P., Cosson J. A monoclonal antibody against the dynein IC1 peptide of sea urchin spermatozoa inhibits the motility of sea urchin, dinoflagellate, and human flagellar axonemes. Mol Biol Cell. 1994 Sep;5(9):1051–1063. doi: 10.1091/mbc.5.9.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti J. L., King S. M., Moss A. G., Witman G. B. Outer arm dynein from trout spermatozoa. Purification, polypeptide composition, and enzymatic properties. J Biol Chem. 1989 Jul 5;264(19):11450–11457. [PubMed] [Google Scholar]
- Gilles A. M., Presecan E., Vonica A., Lascu I. Nucleoside diphosphate kinase from human erythrocytes. Structural characterization of the two polypeptide chains responsible for heterogeneity of the hexameric enzyme. J Biol Chem. 1991 May 15;266(14):8784–8789. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Holzbaur E. L., Johnson K. A. Rate of ATP synthesis by dynein. Biochemistry. 1986 Jan 28;25(2):428–434. doi: 10.1021/bi00350a023. [DOI] [PubMed] [Google Scholar]
- Hoshi M., Numakunai T., Sawada H. Evidence for participation of sperm proteinases in fertilization of the solitary ascidian, Halocynthia roretzi: effects of protease inhibitors. Dev Biol. 1981 Aug;86(1):117–121. doi: 10.1016/0012-1606(81)90322-5. [DOI] [PubMed] [Google Scholar]
- Inaba K. ATP-dependent conformational changes of dynein: evidence for changes in the interaction of dynein heavy chain with the intermediate chain 1. J Biochem. 1995 Apr;117(4):903–907. doi: 10.1093/oxfordjournals.jbchem.a124794. [DOI] [PubMed] [Google Scholar]
- Inaba K., Mohri H. Dynamic conformational changes of 21 S dynein ATPase coupled with ATP hydrolysis revealed by proteolytic digestion. J Biol Chem. 1989 May 15;264(14):8384–8388. [PubMed] [Google Scholar]
- Inaba K., Morisawa M. Chymotrypsin-like protease activity associated with demembranated sperm of chum salmon. Biol Cell. 1992;76(3):329–333. doi: 10.1016/0248-4900(92)90435-4. [DOI] [PubMed] [Google Scholar]
- King S. M., Gatti J. L., Moss A. G., Witman G. B. Outer-arm dynein from trout spermatozoa: substructural organization. Cell Motil Cytoskeleton. 1990;16(4):266–278. doi: 10.1002/cm.970160406. [DOI] [PubMed] [Google Scholar]
- King S. M., Patel-King R. S., Wilkerson C. G., Witman G. B. The 78,000-M(r) intermediate chain of Chlamydomonas outer arm dynein is a microtubule-binding protein. J Cell Biol. 1995 Oct;131(2):399–409. doi: 10.1083/jcb.131.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S. M., Wilkerson C. G., Witman G. B. The Mr 78,000 intermediate chain of Chlamydomonas outer arm dynein interacts with alpha-tubulin in situ. J Biol Chem. 1991 May 5;266(13):8401–8407. [PubMed] [Google Scholar]
- King S. M., Witman G. B. Localization of an intermediate chain of outer arm dynein by immunoelectron microscopy. J Biol Chem. 1990 Nov 15;265(32):19807–19811. [PubMed] [Google Scholar]
- Kobayashi T., Kaji K., Sakai H. Nucleoside diphosphate kinase and the flagellar movement of glycerinated spermatozoa. J Biochem. 1976 Feb;79(2):413–418. doi: 10.1093/oxfordjournals.jbchem.a131084. [DOI] [PubMed] [Google Scholar]
- Kodama T., Fukui K., Kometani K. The initial phosphate burst in ATP hydrolysis by myosin and subfragment-1 as studied by a modified malachite green method for determination of inorganic phosphate. J Biochem. 1986 May;99(5):1465–1472. doi: 10.1093/oxfordjournals.jbchem.a135616. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Mierendorf R. C., Percy C., Young R. A. Gene isolation by screening lambda gt11 libraries with antibodies. Methods Enzymol. 1987;152:458–469. doi: 10.1016/0076-6879(87)52054-7. [DOI] [PubMed] [Google Scholar]
- Mitchell D. R., Kang Y. Identification of oda6 as a Chlamydomonas dynein mutant by rescue with the wild-type gene. J Cell Biol. 1991 May;113(4):835–842. doi: 10.1083/jcb.113.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. R., Kang Y. Reversion analysis of dynein intermediate chain function. J Cell Sci. 1993 Aug;105(Pt 4):1069–1078. doi: 10.1242/jcs.105.4.1069. [DOI] [PubMed] [Google Scholar]
- Moss A. G., Sale W. S., Fox L. A., Witman G. B. The alpha subunit of sea urchin sperm outer arm dynein mediates structural and rigor binding to microtubules. J Cell Biol. 1992 Sep;118(5):1189–1200. doi: 10.1083/jcb.118.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer E. J., Schmidt C. J., Nambudripad R., Smith T. F. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994 Sep 22;371(6495):297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- Ogawa K. Four ATP-binding sites in the midregion of the beta heavy chain of dynein. Nature. 1991 Aug 15;352(6336):643–645. doi: 10.1038/352643a0. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Kamiya R., Wilkerson C. G., Witman G. B. Interspecies conservation of outer arm dynein intermediate chain sequences defines two intermediate chain subclasses. Mol Biol Cell. 1995 Jun;6(6):685–696. doi: 10.1091/mbc.6.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K., Mohri H. Studies on flagellar ATPase from sea urchin spermatozoa. I. Purification and some properties of the enzyme. Biochim Biophys Acta. 1972 Jan 21;256(1):142–155. doi: 10.1016/0005-2728(72)90169-7. [DOI] [PubMed] [Google Scholar]
- Ogawa K. Primary structure and function of a dynein motor molecule. Zoolog Sci. 1992 Apr;9(2):265–274. [PubMed] [Google Scholar]
- Ogawa K., Yokota E., Shirai H. Assembly of a protein sharing epitopes with sperm dynein heavy chains into meiotic spindle in the prometaphase starfish oocyte. Biol Cell. 1988;64(1):57–66. doi: 10.1016/0248-4900(88)90093-7. [DOI] [PubMed] [Google Scholar]
- Patel-King R. S., Benashki S. E., Harrison A., King S. M. Two functional thioredoxins containg redox-senesitive vicinal dithiols from the Chlamydomonas outer dynein arm. J Biol Chem. 1996 Mar 15;271(11):6283–6291. doi: 10.1074/jbc.271.11.6283. [DOI] [PubMed] [Google Scholar]
- Porter M. E., Johnson K. A. Characterization of the ATP-sensitive binding of Tetrahymena 30 S dynein to bovine brain microtubules. J Biol Chem. 1983 May 25;258(10):6575–6581. [PubMed] [Google Scholar]
- Sale W. S., Fox L. A. Isolated beta-heavy chain subunit of dynein translocates microtubules in vitro. J Cell Biol. 1988 Nov;107(5):1793–1797. doi: 10.1083/jcb.107.5.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Kimura I. Effects of N-ethylmaleimide on dynein adenosinetriphosphatase activity and its recombining ability with outer fibers. J Biochem. 1974 Nov;76(5):1001–1008. [PubMed] [Google Scholar]
- Shimizu T. The substrate specificity of dynein from Tetrahymena cilia. J Biochem. 1987 Nov;102(5):1159–1165. doi: 10.1093/oxfordjournals.jbchem.a122154. [DOI] [PubMed] [Google Scholar]
- Stephens R. E., Prior G. Dynein from serotonin-activated cilia and flagella: extraction characteristics and distinct sites for cAMP-dependent protein phosphorylation. J Cell Sci. 1992 Dec;103(Pt 4):999–1012. doi: 10.1242/jcs.103.4.999. [DOI] [PubMed] [Google Scholar]
- Stephens R. E., Prior G. Dynein inner arm heavy chain identification in cAMP-activated flagella using class-specific polyclonal antibodies. Cell Motil Cytoskeleton. 1995;30(4):261–271. doi: 10.1002/cm.970300404. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Bell C. W., Sale W. S., Gibbons I. R. Structure of the dynein-1 outer arm in sea urchin sperm flagella. I. Analysis by separation of subunits. J Biol Chem. 1982 Jan 10;257(1):508–515. [PubMed] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994 Nov 11;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. Molecular analysis of the microtubule motor dynein. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8769–8772. doi: 10.1073/pnas.90.19.8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa T., Hasegawa S., Mohri H. The bound nucleotides of the isolated microtubules of sea-urchin sperm flagella and their possible role in flagellar movement. Exp Cell Res. 1968 Sep;52(1):86–100. doi: 10.1016/0014-4827(68)90549-1. [DOI] [PubMed] [Google Scholar]