Abstract
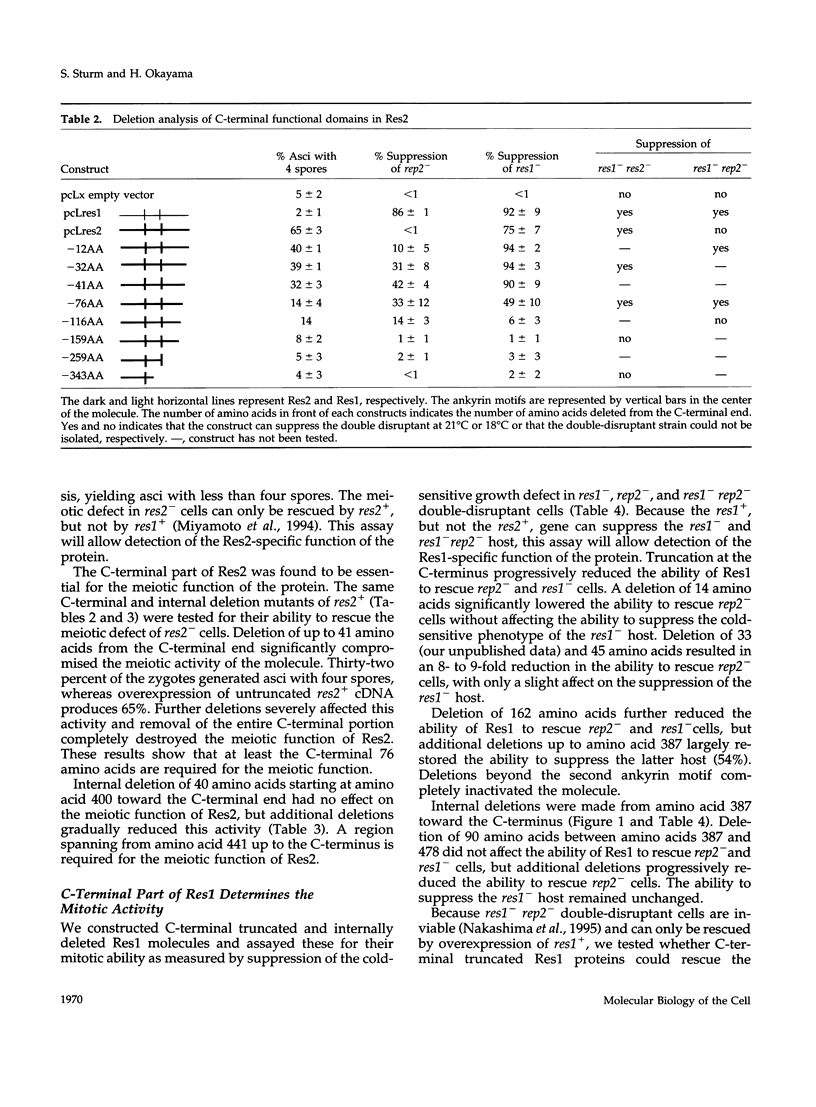
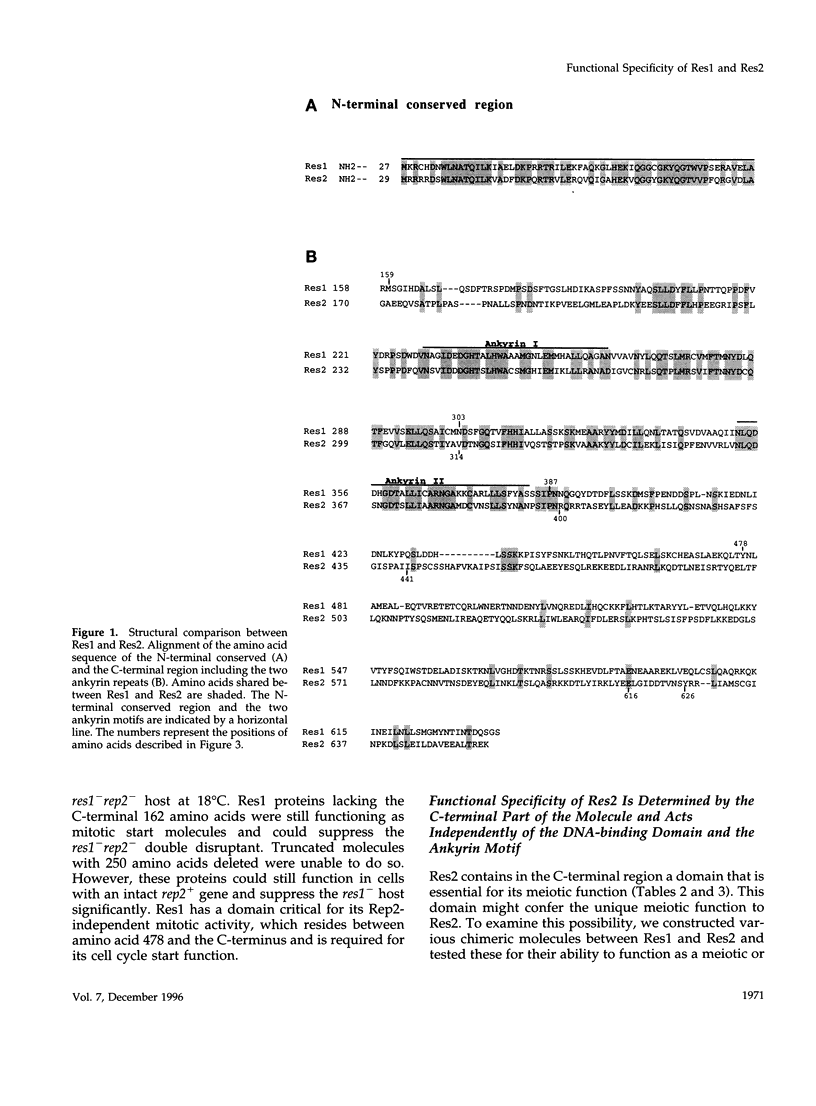
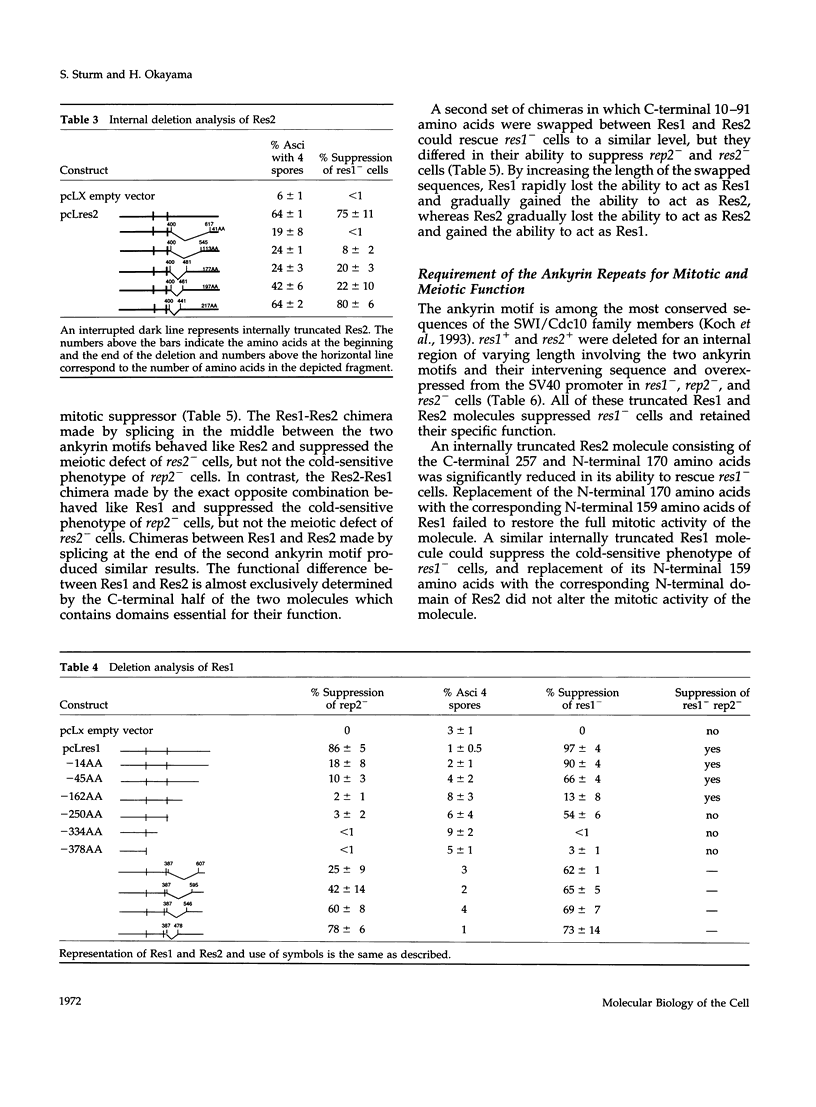
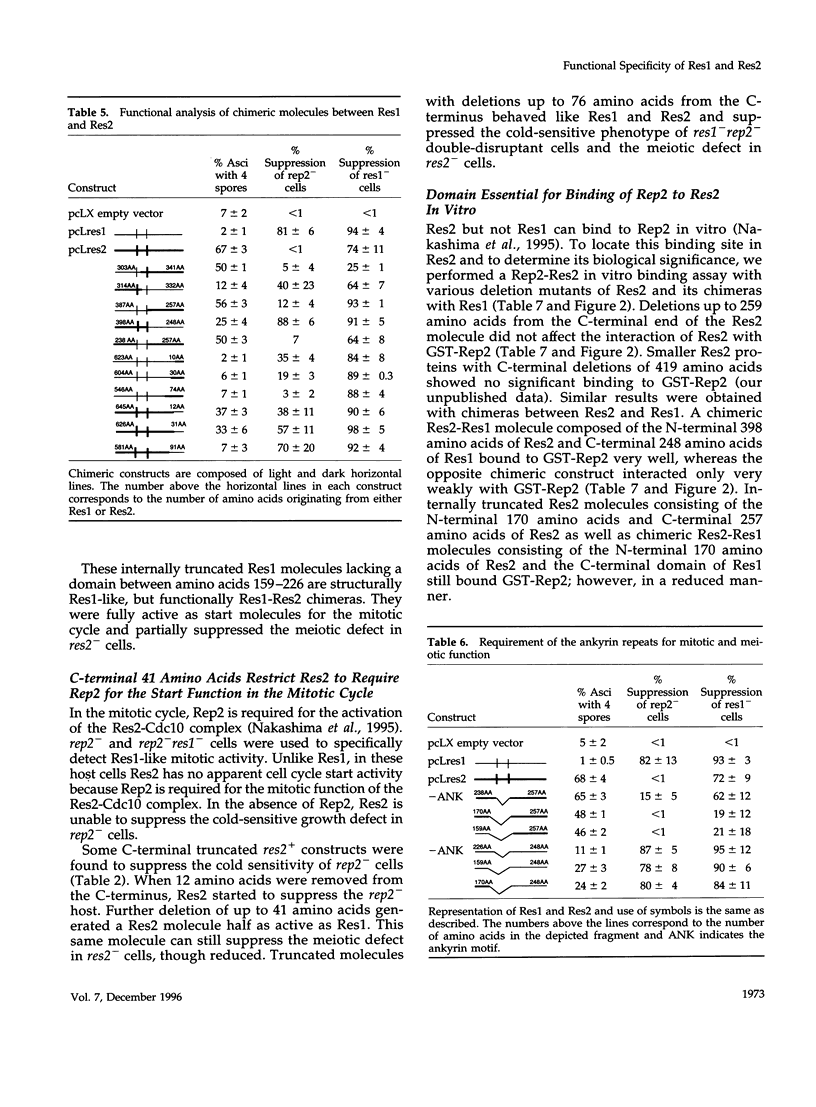
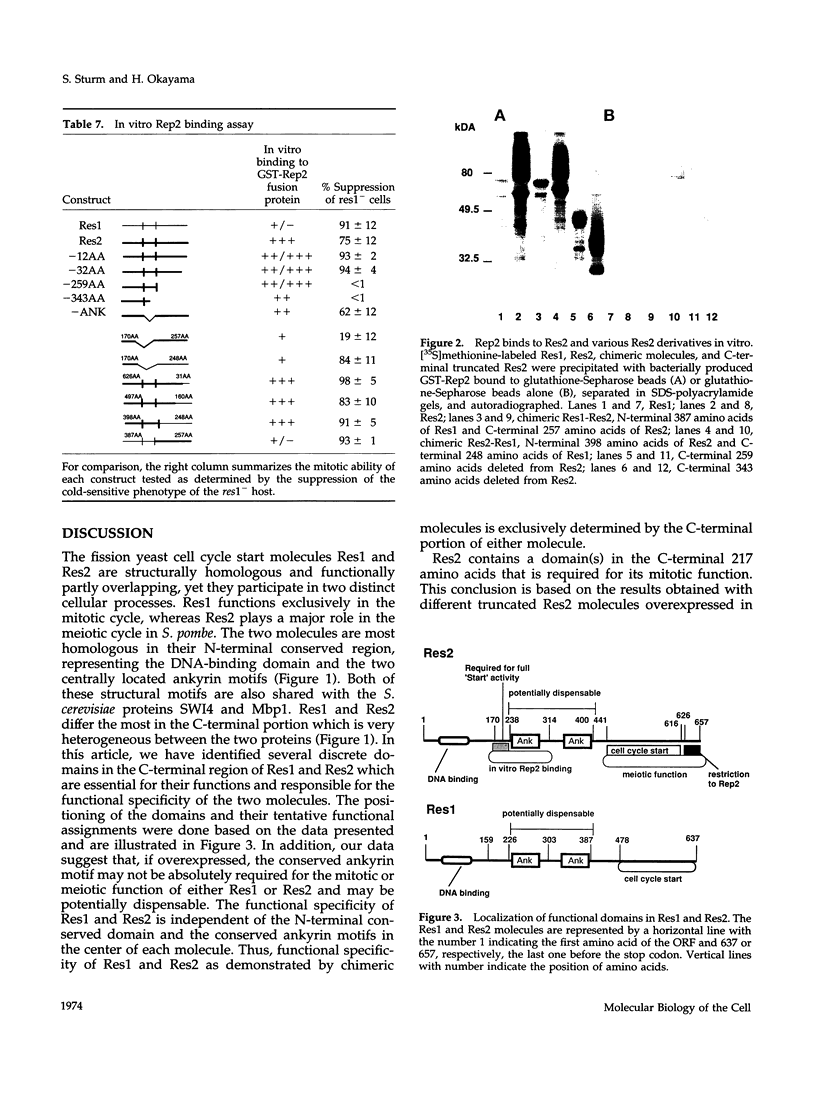
In Schizosaccharomyces pombe the "start" of the cell cycle is regulated by two parallel, functionally overlapping complexes composed of Res1-Cdc10 and Res2-Cdc10. Res1 and Res2 are structurally very homologous and are required for the start of the mitotic and meiotic cycle, respectively. We have addressed the question which parts of the proteins are essential for function and determine the functional specificity. Several discrete domains in the nonconserved C-terminal region are essential for the mitotic and meiotic start function and determine the functional specificity independently of the structurally conserved motifs at the N-terminal end and in the center. One of these domains in Res2 restricts Res2 to interact only with Rep2. Res2 without this domain behaves like a functional chimera having the properties of Res2 and Res1. Likewise, internally truncated forms of Res1 lacking the centrally located ankyrin repeats and adjacent sequences can partially suppress the meiotic defect in res2- cells. These truncated Res1 molecules behave like functional chimeras with the properties of Res1 and Res2.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. J. Gene expression. Dialogue with the cell cycle. Nature. 1992 Jan 30;355(6359):393–394. doi: 10.1038/355393a0. [DOI] [PubMed] [Google Scholar]
- Andrews B. J., Herskowitz I. The yeast SWI4 protein contains a motif present in developmental regulators and is part of a complex involved in cell-cycle-dependent transcription. Nature. 1989 Dec 14;342(6251):830–833. doi: 10.1038/342830a0. [DOI] [PubMed] [Google Scholar]
- Aves S. J., Durkacz B. W., Carr A., Nurse P. Cloning, sequencing and transcriptional control of the Schizosaccharomyces pombe cdc10 'start' gene. EMBO J. 1985 Feb;4(2):457–463. doi: 10.1002/j.1460-2075.1985.tb03651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayté J., Leis J. F., Herrera A., Tang E., Yang H., DeCaprio J. A. The Schizosaccharomyces pombe MBF complex requires heterodimerization for entry into S phase. Mol Cell Biol. 1995 May;15(5):2589–2599. doi: 10.1128/mcb.15.5.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri M., Beach D. Sct1 functions in partnership with Cdc10 in a transcription complex that activates cell cycle START and inhibits differentiation. Cell. 1993 Feb 26;72(4):607–619. doi: 10.1016/0092-8674(93)90079-6. [DOI] [PubMed] [Google Scholar]
- Dirick L., Moll T., Auer H., Nasmyth K. A central role for SWI6 in modulating cell cycle Start-specific transcription in yeast. Nature. 1992 Jun 11;357(6378):508–513. doi: 10.1038/357508a0. [DOI] [PubMed] [Google Scholar]
- Egel R., Egel-Mitani M. Premeiotic DNA synthesis in fission yeast. Exp Cell Res. 1974 Sep;88(1):127–134. doi: 10.1016/0014-4827(74)90626-0. [DOI] [PubMed] [Google Scholar]
- Gordon C. B., Fantes P. A. The cdc22 gene of Schizosaccharomyces pombe encodes a cell cycle-regulated transcript. EMBO J. 1986 Nov;5(11):2981–2985. doi: 10.1002/j.1460-2075.1986.tb04595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann J. F., Beach D. cdt1 is an essential target of the Cdc10/Sct1 transcription factor: requirement for DNA replication and inhibition of mitosis. EMBO J. 1994 Jan 15;13(2):425–434. doi: 10.1002/j.1460-2075.1994.tb06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M., Nagata A., Jinno S., Suto K., Okayama H. Wee1(+)-like gene in human cells. Nature. 1991 Sep 5;353(6339):80–83. doi: 10.1038/353080a0. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Martin G. S., Forsburg S. L., Stephen R. J., Russo A., Nurse P. The fission yeast cdc18+ gene product couples S phase to START and mitosis. Cell. 1993 Jul 30;74(2):371–382. doi: 10.1016/0092-8674(93)90427-r. [DOI] [PubMed] [Google Scholar]
- Koch C., Moll T., Neuberg M., Ahorn H., Nasmyth K. A role for the transcription factors Mbp1 and Swi4 in progression from G1 to S phase. Science. 1993 Sep 17;261(5128):1551–1557. doi: 10.1126/science.8372350. [DOI] [PubMed] [Google Scholar]
- Lowndes N. F., Johnson A. L., Breeden L., Johnston L. H. SWI6 protein is required for transcription of the periodically expressed DNA synthesis genes in budding yeast. Nature. 1992 Jun 11;357(6378):505–508. doi: 10.1038/357505a0. [DOI] [PubMed] [Google Scholar]
- Lowndes N. F., Johnson A. L., Johnston L. H. Coordination of expression of DNA synthesis genes in budding yeast by a cell-cycle regulated trans factor. Nature. 1991 Mar 21;350(6315):247–250. doi: 10.1038/350247a0. [DOI] [PubMed] [Google Scholar]
- Lowndes N. F., McInerny C. J., Johnson A. L., Fantes P. A., Johnston L. H. Control of DNA synthesis genes in fission yeast by the cell-cycle gene cdc10+. Nature. 1992 Jan 30;355(6359):449–453. doi: 10.1038/355449a0. [DOI] [PubMed] [Google Scholar]
- Marks J., Fankhauser C., Reymond A., Simanis V. Cytoskeletal and DNA structure abnormalities result from bypass of requirement for the cdc10 start gene in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1992 Mar;101(Pt 3):517–528. doi: 10.1242/jcs.101.3.517. [DOI] [PubMed] [Google Scholar]
- McIntosh E. M., Atkinson T., Storms R. K., Smith M. Characterization of a short, cis-acting DNA sequence which conveys cell cycle stage-dependent transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1991 Jan;11(1):329–337. doi: 10.1128/mcb.11.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M., Tanaka K., Okayama H. res2+, a new member of the cdc10+/SWI4 family, controls the 'start' of mitotic and meiotic cycles in fission yeast. EMBO J. 1994 Apr 15;13(8):1873–1880. doi: 10.1002/j.1460-2075.1994.tb06456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nagata A., Igarashi M., Jinno S., Suto K., Okayama H. An additional homolog of the fission yeast cdc25+ gene occurs in humans and is highly expressed in some cancer cells. New Biol. 1991 Oct;3(10):959–968. [PubMed] [Google Scholar]
- Nakashima N., Tanaka K., Sturm S., Okayama H. Fission yeast Rep2 is a putative transcriptional activator subunit for the cell cycle 'start' function of Res2-Cdc10. EMBO J. 1995 Oct 2;14(19):4794–4802. doi: 10.1002/j.1460-2075.1995.tb00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P., Bissett Y. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature. 1981 Aug 6;292(5823):558–560. doi: 10.1038/292558a0. [DOI] [PubMed] [Google Scholar]
- Nurse P., Thuriaux P., Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976 Jul 23;146(2):167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Okazaki N., Kume K., Jinno S., Tanaka K., Okayama H. High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 1990 Nov 25;18(22):6485–6489. doi: 10.1093/nar/18.22.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond A., Marks J., Simanis V. The activity of S.pombe DSC-1-like factor is cell cycle regulated and dependent on the activity of p34cdc2. EMBO J. 1993 Nov;12(11):4325–4334. doi: 10.1002/j.1460-2075.1993.tb06117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Okazaki K., Okazaki N., Ueda T., Sugiyama A., Nojima H., Okayama H. A new cdc gene required for S phase entry of Schizosaccharomyces pombe encodes a protein similar to the cdc 10+ and SWI4 gene products. EMBO J. 1992 Dec;11(13):4923–4932. doi: 10.1002/j.1460-2075.1992.tb05599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R., Smiley J., Andrews B., Campbell J. L. Regulation of the yeast DNA replication genes through the Mlu I cell cycle box is dependent on SWI6. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9479–9483. doi: 10.1073/pnas.89.20.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Takeda T., Nasmyth K., Jones N. pct1+, which encodes a new DNA-binding partner of p85cdc10, is required for meiosis in the fission yeast Schizosaccharomyces pombe. Genes Dev. 1994 Apr 15;8(8):885–898. doi: 10.1101/gad.8.8.885. [DOI] [PubMed] [Google Scholar]