Abstract
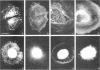
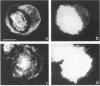
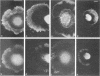
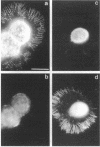
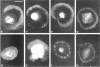
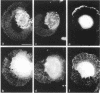
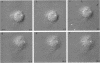
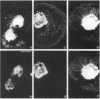
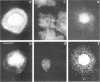
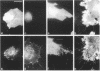
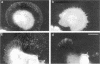
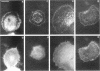
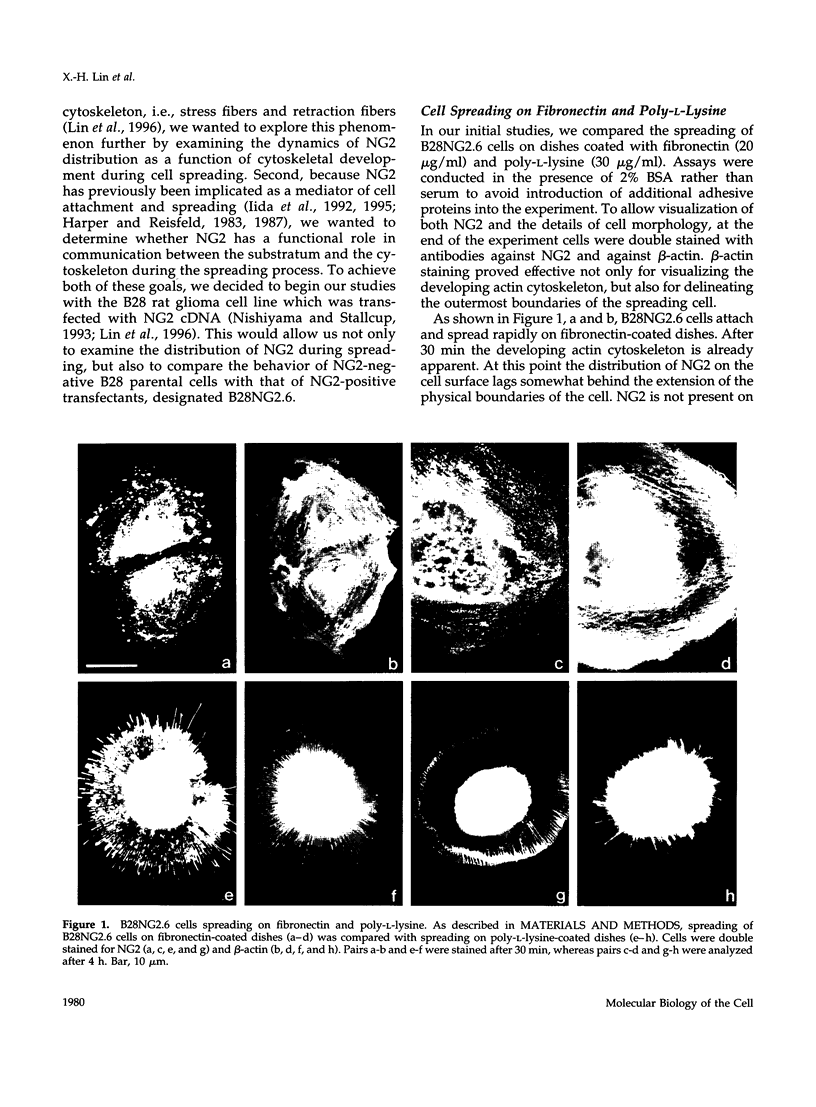
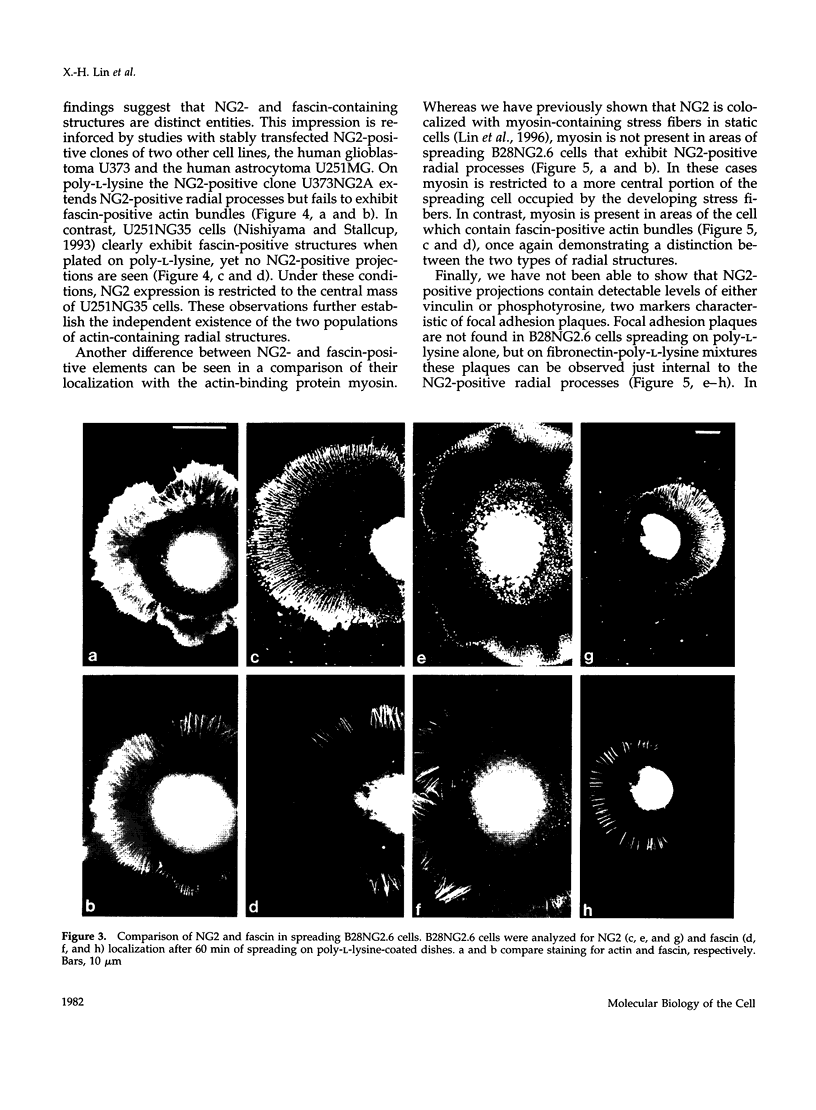
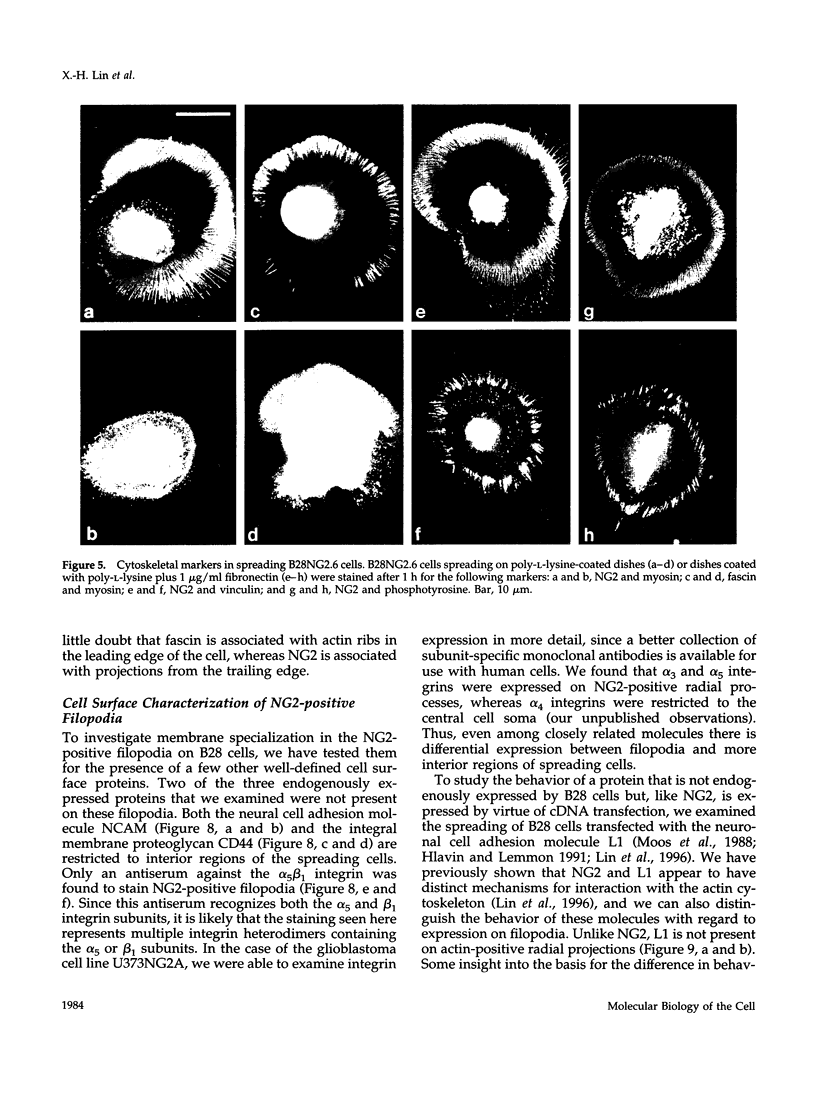
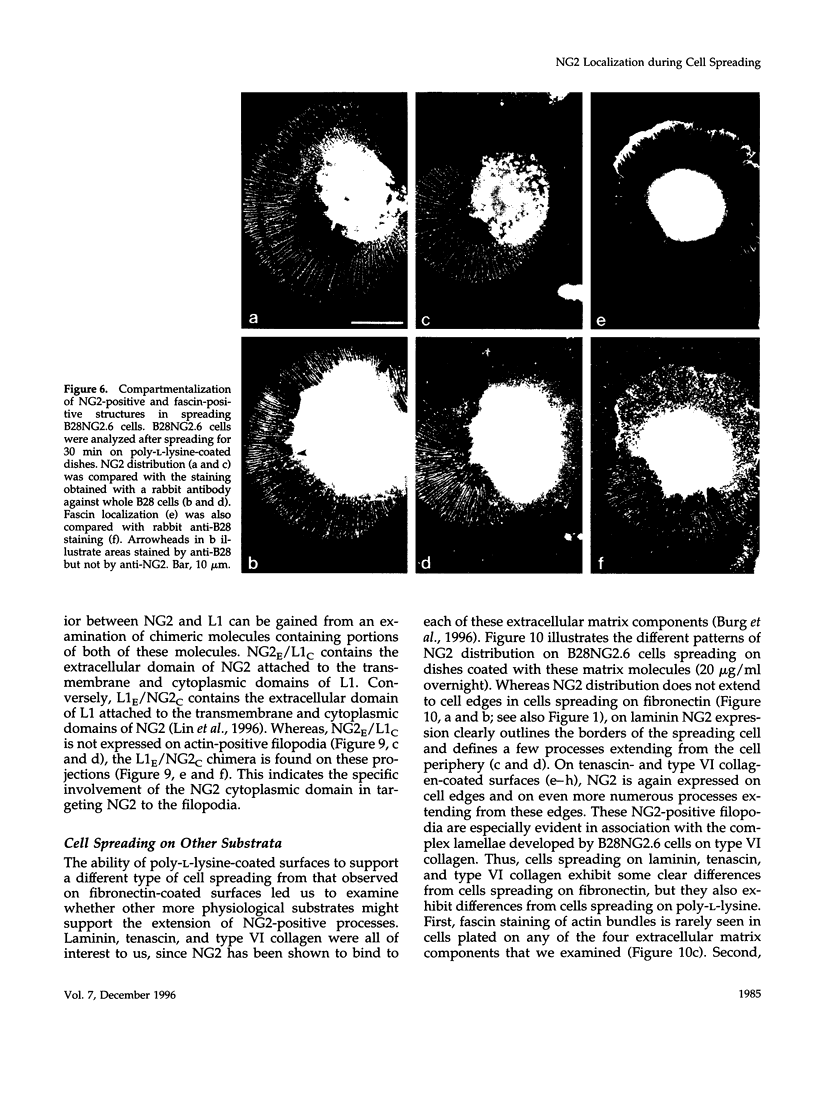
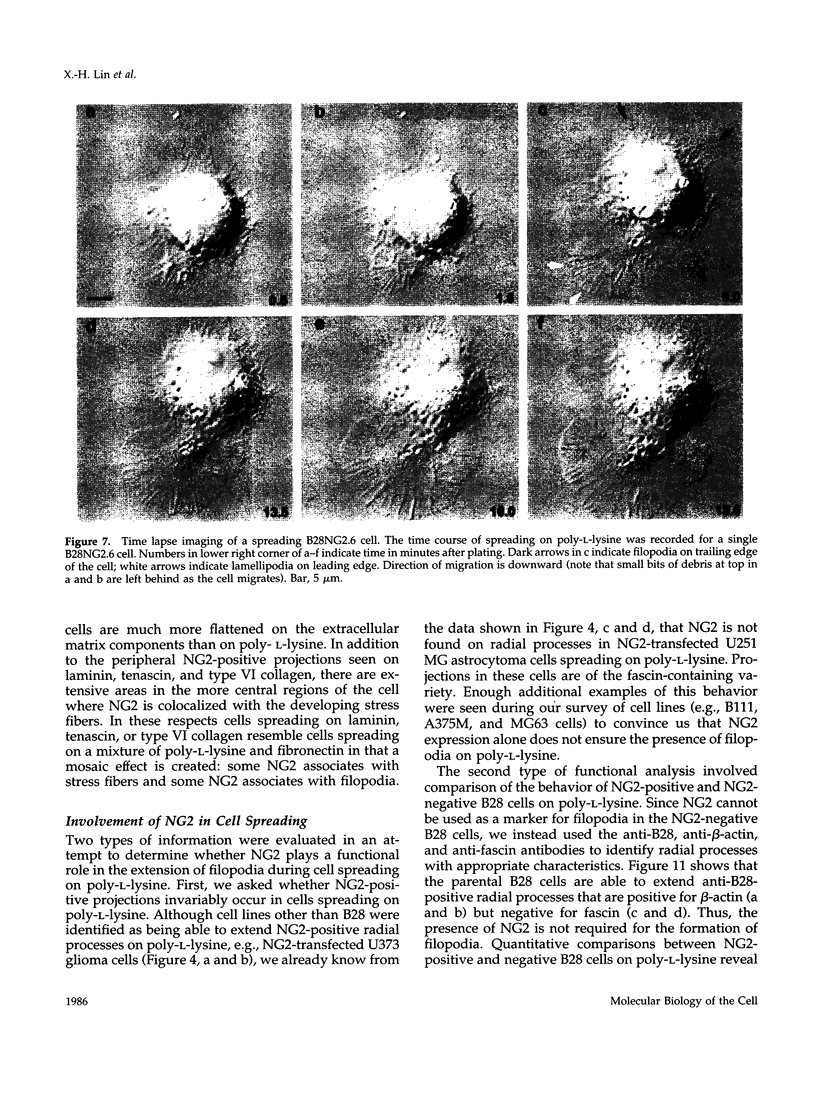
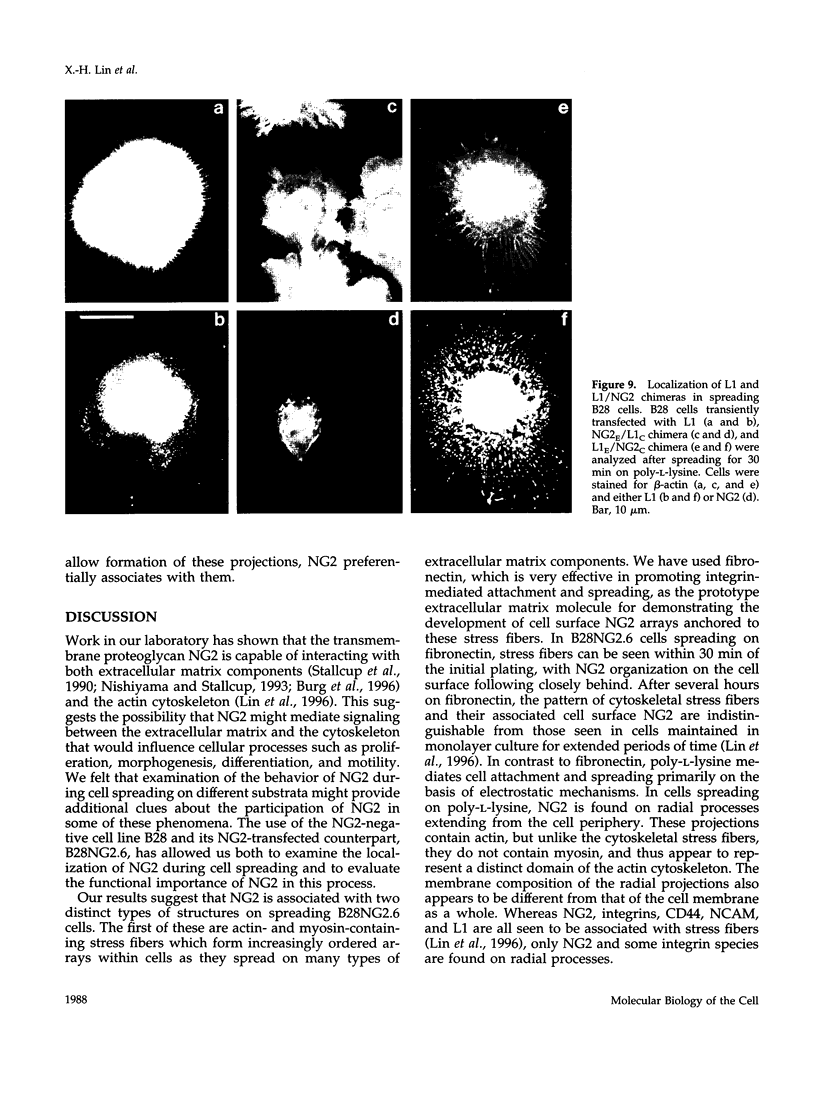
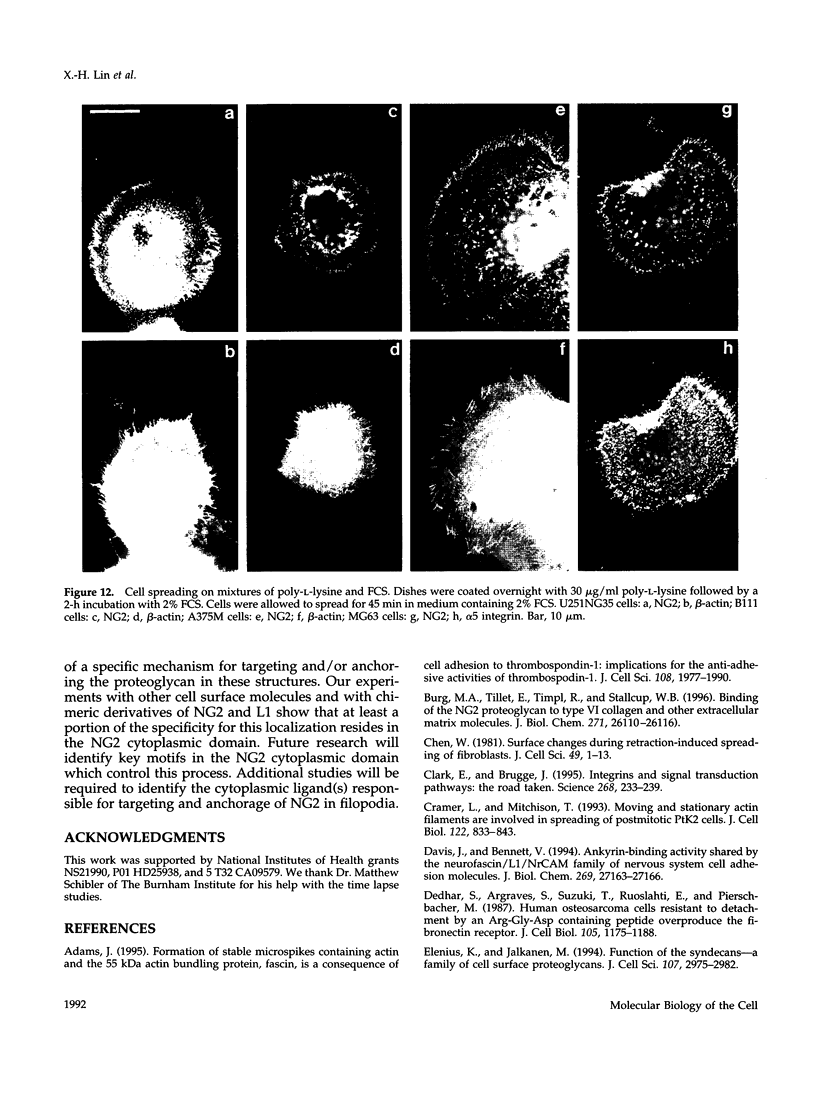
The transmembrane proteoglycan NG2 is able to interact both with components of the extracellular matrix and with the actin cytoskeleton. An examination of the distribution of NG2 during cell spreading suggests that NG2 can associate with two distinct types of actin-containing cytoskeletal structures, depending on the nature of the stimulus derived from the substratum. On fibronectin-coated dishes, cell surface NG2 associates exclusively with stress fibers developing within the cell. On poly-L-lysine-coated dishes, cell surface NG2 is associated with radial processes extending from the cell periphery. Spreading on fibronectin/poly-L-lysine mixtures, as well as on matrix components such as laminin, tenascin, and type VI collagen, produces cells with mosaic characteristics, i.e., NG2 is associated with both types of structures. NG2-positive radial processes are distinct from a second population of radial structures that contain fascin. NG2-positive extensions appear to be individual self-contained units (filopodia), whereas fascin is associated with actin ribs within sheets of membrane (lamellipodia). NG2- and fascin-positive structures are often localized to opposite poles of spreading cells, suggesting a possible role for the two classes of cellular extensions in the establishment of cell polarity during morphogenesis or migration. Time lapse imaging confirms the presence of lamellipodia on the leading edges of migrating cells, while numerous filopodia are present on trailing edges.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Formation of stable microspikes containing actin and the 55 kDa actin bundling protein, fascin, is a consequence of cell adhesion to thrombospondin-1: implications for the anti-adhesive activities of thrombospondin-1. J Cell Sci. 1995 May;108(Pt 5):1977–1990. doi: 10.1242/jcs.108.5.1977. [DOI] [PubMed] [Google Scholar]
- Burg M. A., Tillet E., Timpl R., Stallcup W. B. Binding of the NG2 proteoglycan to type VI collagen and other extracellular matrix molecules. J Biol Chem. 1996 Oct 18;271(42):26110–26116. doi: 10.1074/jbc.271.42.26110. [DOI] [PubMed] [Google Scholar]
- Chen W. T. Surface changes during retraction-induced spreading of fibroblasts. J Cell Sci. 1981 Jun;49:1–13. doi: 10.1242/jcs.49.1.1a. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Brugge J. S. Integrins and signal transduction pathways: the road taken. Science. 1995 Apr 14;268(5208):233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Cramer L., Mitchison T. J. Moving and stationary actin filaments are involved in spreading of postmitotic PtK2 cells. J Cell Biol. 1993 Aug;122(4):833–843. doi: 10.1083/jcb.122.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. Q., Bennett V. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. J Biol Chem. 1994 Nov 4;269(44):27163–27166. [PubMed] [Google Scholar]
- Dedhar S., Argraves W. S., Suzuki S., Ruoslahti E., Pierschbacher M. D. Human osteosarcoma cells resistant to detachment by an Arg-Gly-Asp-containing peptide overproduce the fibronectin receptor. J Cell Biol. 1987 Sep;105(3):1175–1182. doi: 10.1083/jcb.105.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenius K., Jalkanen M. Function of the syndecans--a family of cell surface proteoglycans. J Cell Sci. 1994 Nov;107(Pt 11):2975–2982. doi: 10.1242/jcs.107.11.2975. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Rationale and methods for the use of nude mice to study the biology and therapy of human cancer metastasis. Cancer Metastasis Rev. 1986;5(1):29–49. doi: 10.1007/BF00049529. [DOI] [PubMed] [Google Scholar]
- Grako K. A., Stallcup W. B. Participation of the NG2 proteoglycan in rat aortic smooth muscle cell responses to platelet-derived growth factor. Exp Cell Res. 1995 Nov;221(1):231–240. doi: 10.1006/excr.1995.1371. [DOI] [PubMed] [Google Scholar]
- Harper J. R., Reisfeld R. A. Inhibition of anchorage-independent growth of human melanoma cells by a monoclonal antibody to a chondroitin sulfate proteoglycan. J Natl Cancer Inst. 1983 Aug;71(2):259–263. [PubMed] [Google Scholar]
- Heath J. P., Holifield B. F. Cell locomotion: new research tests old ideas on membrane and cytoskeletal flow. Cell Motil Cytoskeleton. 1991;18(4):245–257. doi: 10.1002/cm.970180402. [DOI] [PubMed] [Google Scholar]
- Hlavin M. L., Lemmon V. Molecular structure and functional testing of human L1CAM: an interspecies comparison. Genomics. 1991 Oct;11(2):416–423. doi: 10.1016/0888-7543(91)90150-d. [DOI] [PubMed] [Google Scholar]
- Iida J., Meijne A. M., Spiro R. C., Roos E., Furcht L. T., McCarthy J. B. Spreading and focal contact formation of human melanoma cells in response to the stimulation of both melanoma-associated proteoglycan (NG2) and alpha 4 beta 1 integrin. Cancer Res. 1995 May 15;55(10):2177–2185. [PubMed] [Google Scholar]
- Iida J., Skubitz A. P., Furcht L. T., Wayner E. A., McCarthy J. B. Coordinate role for cell surface chondroitin sulfate proteoglycan and alpha 4 beta 1 integrin in mediating melanoma cell adhesion to fibronectin. J Cell Biol. 1992 Jul;118(2):431–444. doi: 10.1083/jcb.118.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalomiris E. L., Bourguignon L. Y. Mouse T lymphoma cells contain a transmembrane glycoprotein (GP85) that binds ankyrin. J Cell Biol. 1988 Feb;106(2):319–327. doi: 10.1083/jcb.106.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger D. A., Horwitz A. F. Cell migration: a physically integrated molecular process. Cell. 1996 Feb 9;84(3):359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Lebakken C. S., Rapraeger A. C. Syndecan-1 mediates cell spreading in transfected human lymphoblastoid (Raji) cells. J Cell Biol. 1996 Mar;132(6):1209–1221. doi: 10.1083/jcb.132.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T. J. Actin based motility on retraction fibers in mitotic PtK2 cells. Cell Motil Cytoskeleton. 1992;22(2):135–151. doi: 10.1002/cm.970220207. [DOI] [PubMed] [Google Scholar]
- Moos M., Tacke R., Scherer H., Teplow D., Früh K., Schachner M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988 Aug 25;334(6184):701–703. doi: 10.1038/334701a0. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Dahlin K. J., Prince J. T., Johnstone S. R., Stallcup W. B. The primary structure of NG2, a novel membrane-spanning proteoglycan. J Cell Biol. 1991 Jul;114(2):359–371. doi: 10.1083/jcb.114.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Lin X. H., Giese N., Heldin C. H., Stallcup W. B. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996 Feb 1;43(3):299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Lin X. H., Giese N., Heldin C. H., Stallcup W. B. Interaction between NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells is required for optimal response to PDGF. J Neurosci Res. 1996 Feb 1;43(3):315–330. doi: 10.1002/(SICI)1097-4547(19960201)43:3<315::AID-JNR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Stallcup W. B. Expression of NG2 proteoglycan causes retention of type VI collagen on the cell surface. Mol Biol Cell. 1993 Nov;4(11):1097–1108. doi: 10.1091/mbc.4.11.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt E., Risteli J., van Delden V., Timpl R. Structural diversity and domain composition of a unique collagenous fragment (intima collagen) obtained from human placenta. Biochem J. 1983 May 1;211(2):295–302. doi: 10.1042/bj2110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontén J., Westermark B. Properties of human malignant glioma cells in vitro. Med Biol. 1978 Aug;56(4):184–193. [PubMed] [Google Scholar]
- Schmidt C. E., Horwitz A. F., Lauffenburger D. A., Sheetz M. P. Integrin-cytoskeletal interactions in migrating fibroblasts are dynamic, asymmetric, and regulated. J Cell Biol. 1993 Nov;123(4):977–991. doi: 10.1083/jcb.123.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrappe M., Klier F. G., Spiro R. C., Waltz T. A., Reisfeld R. A., Gladson C. L. Correlation of chondroitin sulfate proteoglycan expression on proliferating brain capillary endothelial cells with the malignant phenotype of astroglial cells. Cancer Res. 1991 Sep 15;51(18):4986–4993. [PubMed] [Google Scholar]
- Schubert D., Heinemann S., Carlisle W., Tarikas H., Kimes B., Patrick J., Steinbach J. H., Culp W., Brandt B. L. Clonal cell lines from the rat central nervous system. Nature. 1974 May 17;249(454):224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P. Cell migration by graded attachment to substrates and contraction. Semin Cell Biol. 1994 Jun;5(3):149–155. doi: 10.1006/scel.1994.1019. [DOI] [PubMed] [Google Scholar]
- Stallcup W. B., Dahlin K., Healy P. Interaction of the NG2 chondroitin sulfate proteoglycan with type VI collagen. J Cell Biol. 1990 Dec;111(6 Pt 2):3177–3188. doi: 10.1083/jcb.111.6.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy R. E., Strong J. P., Newman W. P., 3rd, Malcom G. T., Oalmann M. C., Guzman M. A. Renovasculopathies of nephrosclerosis in relation to atherosclerosis at ages 25 to 54 years. Kidney Int. 1996 Feb;49(2):564–570. doi: 10.1038/ki.1996.80. [DOI] [PubMed] [Google Scholar]
- Yamashiro-Matsumura S., Matsumura F. Intracellular localization of the 55-kD actin-bundling protein in cultured cells: spatial relationships with actin, alpha-actinin, tropomyosin, and fimbrin. J Cell Biol. 1986 Aug;103(2):631–640. doi: 10.1083/jcb.103.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]