Abstract
We report development of a polymer gel with a catalytic activity that can be switched on and off when the solvent composition is changed. The gel consists of two species of monomers. The major component, N-isopropylacrylamide, makes the gel swell and shrink in response to a change in composition of ethanol/water mixtures. The minor component, vinylimidazole, which is capable of catalysis, is copolymerized into the gel network. The reaction rate for catalytic hydrolysis of p-nitrophenyl caprylate was small when the gel was swollen. In contrast, when the gel was shrunken, the reaction rate increased 5 times. The activity changes discontinuously as a function of solvent composition, thus the catalysis can be switched on and off by an infinitesimal change in solvent composition. The kinetics of catalysis by the gel in the shrunken state is well described by the Michaelis–Menten formula, indicating that the absorption of the substrate by the hydrophobic environment created by the N-isopropylacrylamide polymer in the shrunken gel is responsible for enhancement of catalytic activity. In the swollen state, the rate vs. active site concentration is linear, indicating that the substrate absorption is not a primary factor determining the kinetics. Catalytic activity of the gel is studied for substrates with various alkyl chain lengths; of those studied the switching effect is most pronounced for p-nitrophenyl caprylate.
Natural enzymes catalyze chemical reactions, and equally importantly, regulate them by reversibly and repeatedly switching on and off the catalytic activities. To mimic this special feature of enzymes, we designed a polymer gel consisting of two species of monomers, each having a specific role. The major component allows the gel to reversibly swell and shrink in response to changes in environmental parameters such as temperature and solvent (1–9). On swelling and shrinking, the local density of the hydrophobic moiety changes and the affinity to substrate molecules is altered accordingly. The minor component capable of catalysis of substrate decomposition is copolymerized with the responsive monomers into the gel network. The catalytic activity of the gel is expected to be switched on and off as the substrate is reversibly bound and released during the cycle of gel swelling and shrinking.
Materials and Methods
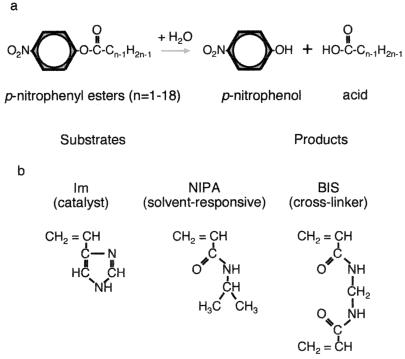
To demonstrate the feasibility of the idea, we chose hydrolysis of p-nitrophenyl esters with different alkyl chain lengths as the chemical reaction to examine (Fig. 1a). This reaction is known to be catalyzed by 4(5)-vinylimidazole (10). We, therefore, used it as the minor catalytic monomer component of the gel (Fig. 1b). As the major component, we chose the widely used hydrophobic monomer N-isopropylacrylamide (NIPA). The substrate and gel monomer components were selected because (i) the substrate and one of the hydrolysis products, p-nitrophenol, were easily detected by using UV spectroscopy; (ii) the NIPA gel underwent a discontinuous swelling/shrinking transition in response to changes in environmental parameters such as temperature and solvent; and (iii) monomers make clear homogeneous gels.
Figure 1.
(a) The reaction scheme of hydrolysis of p-nitrophenyl esters with different alkyl chain lengths. (b) The chemical structures of the monomers. The reaction presented is catalyzed by the gels made of N-isopropylacrylamide (NIPA), 4(5)-vinylimidazole (Im), and N,N′-methylenebisacrylamide (BIS).
In preparing the gel, NIPA (2.04 g, 18 mmol); 4(5)-vinylimidazole (28.2 mg, 0.3 mmol); N,N′-methylenebisacrylamide as the cross-linker (18.5 mg, 0.12 mmol); and azobis(isobutylonitrile) as the initiator (4.93 mg, 0.03 mmol) were dissolved in 0.5 ml of dimethyl sulfoxide. The solution was degassed and polymerized in glass pipettes with 500-μm i.d. at 60°C for 24 h. The cylindrical gels were taken out of the pipettes and washed thoroughly with water before use.
Results and Discussion
Volume Transition in Ethanol/Water Mixtures.
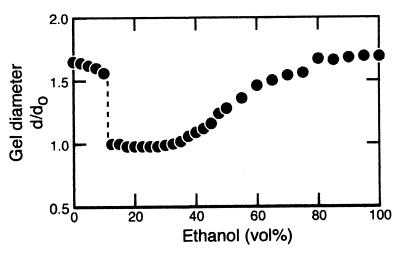
To control the degree of gel swelling and shrinking, samples were placed in mixtures of ethanol and buffer (0.05 M Tris⋅HCl, pH 8.0) with different ethanol compositions at 23°C. The equilibrium gel diameter, d, normalized by that on polymerization, do, is plotted as a function of ethanol concentration in Fig. 2 for the gel containing 4(5)-vinylimidazole. The gel was swollen in water. With the increase in ethanol, the gel shrank, undergoing a discontinuous volume phase transition at 12.5 vol %. The gel remained in the shrunken state until 35 vol %. Further increase in ethanol concentration led to continuous swelling of the gel. The gel was fully swollen at 100 vol % ethanol.
Figure 2.
Gel diameter, d, as a function of ethanol concentration in ethanol/buffer (pH 8.0) mixtures. It is normalized by the gel diameter on synthesis, do. There is a discontinuous volume transition at approximately 12.5 vol % ethanol. The gel shrinks once and swells again as ethanol concentration increases.
Initial Rate in Hydrolytic Reaction.
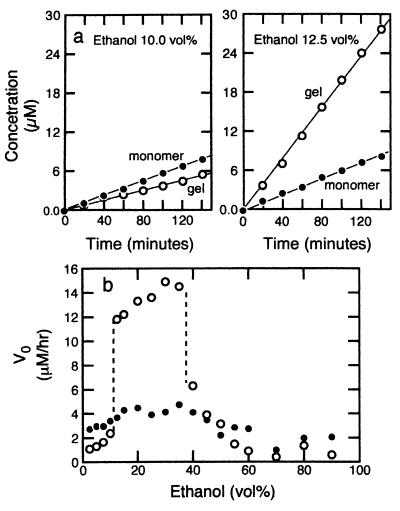
In the catalytic hydrolysis experiment, the gels were first immersed in mixed solvents of ethanol and buffer with various ethanol compositions. At each ethanol composition, 5 μl of p-nitrophenyl caprylate (25 mM in dioxane) was added to 3 ml of the solution containing the gels by using a microsyringe. The gel started to catalyze the esterolic hydrolysis and the reaction was monitored by measuring the concentration of released product, p-nitrophenol, by using the UV spectrometer at 400 nm (11). As Fig. 3a shows, the hydrolysis proceeded with a constant speed, with a linear increase in accumulated product with time. The linearity was observed up until 20% conversion of the substrate. As a control, similar hydrolysis experiments were performed without the gel, when imidazole molecules were in the solutions in the same total amounts as in the gel.
Figure 3.
(a) The concentration of the catalytic product, p-nitrophenol, as a function of time. ○, Catalysis by the gel; and ●, catalysis by an imidazole monomer solution. There is a marked increase in the catalysis rate by the gel in the shrunken state at 12.5 vol % ethanol. In the swollen state at 10 vol % ethanol, the reaction rate is small. (b) The initial reaction rate, V0, as a function of ethanol concentration for catalysis by the gel (○) and imidazole solution (●). In parallel to the swelling and shrinking of the gel, the catalytic activity undergoes discontinuous but reversible change.
When gel was in the swollen state (Fig. 3a Left), the reaction rate was small, and even smaller than that of the imidazole monomer solution in all of the ethanol concentrations. In contrast, when the gel was shrunken (Fig. 3a Right), the reaction rate increased dramatically to 5 times higher than that catalyzed by the monomer solution.
To quantitatively analyze the above result, we measured the initial reaction rates as the slopes of the graphs similar to Fig. 3a (it is molar consumption of substrate per unit time) and plotted those rates as a function of ethanol concentration in Fig. 3b. Note that the rate of catalysis by the imidazole solution depended only slightly and smoothly on ethanol concentration. In contrast, the catalysis by the gel showed discontinuous transitions twice as the ethanol concentration was varied. Namely, at low ethanol concentrations, the catalytic rate was very low. As the ethanol concentration was increased, the catalytic rate discontinuously increased at the condition where the gel discontinuously shrank. As the ethanol concentration was further increased, there was another decreasing, discontinuous transition of catalytic rate, although in this case the swelling volume transition was smooth and continuous.
The gel-catalyzed reaction was slower than that catalyzed by the imidazole solution whenever the gel was swollen at either low or high ethanol concentrations. Quite the opposite, the shrunken gel increased the reaction rate much higher than the control. We stress that both transitions were discontinuous in terms of catalysis, namely, an infinitesimal change in solvent composition increased the catalysis rate severalfold. Interestingly, catalysis changed discontinuously even when gel diameter changed continuously in the swelling transition as shown in Fig. 3b at higher ethanol concentration near 35 vol %. The latter mechanism is not fully understood at this moment.
Reaction Kinetics.
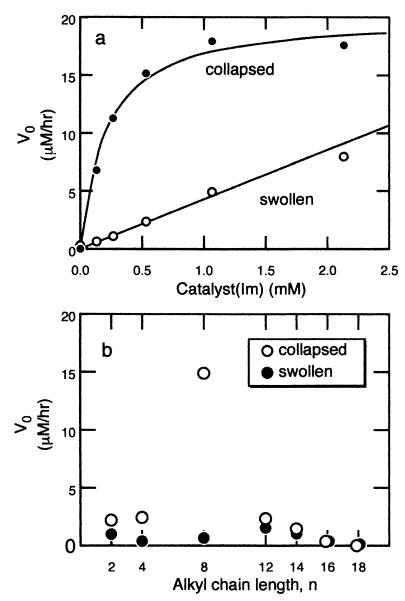
To understand the mechanism of the switching on and off of gel catalysis, we examined the dependence of the initial rate on the catalyst concentration. It is known that a hydrolytic reaction catalyzed by an enzyme follows the Michaelis–Menten mechanism that involves the formation of a substrate–catalyst complex, ES, illustrated in Eq. 1 (12)
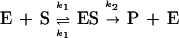 |
1 |
where E denotes a catalyst (imidazole in our case), S, a substrate, and P, a product. Based on this mechanism, the initial rate for the condition [E] ≫ [S] can be derived in the steady-state approximation:
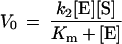 |
2 |
where V0 is an initial rate and Km (which = [k−1 + k2]/k1) is the Michaelis constant. Under our experimental conditions, the catalytic site (imidazole) concentration was indeed in excess of the substrate, [E] ≫ [S], which reflected the low solubility of the substrate, p-nitrophenyl caprylate, in the solvent. We applied these theoretical predictions to analyze the gel-catalyzed hydrolysis. As Fig. 4a shows, the curve, V0 vs. [E], for the shrunken gel (15% ethanol in mixed solvent) was well fit by Eq. 2. The values of Km and k2 were found to be 0.23 mM and 0.49 h−1, respectively. This kinetic behavior indicated that the gel-catalyzed reaction involved formation of the catalyst/substrate, ES. On the other hand, the swollen gels did not exhibit such a kinetic pattern; instead the relation was linear in this concentration regime of catalytic sites. Thus, the hydrolysis catalyzed by the swollen gel involved the bimolecular reaction given by Eq. 3 with a rate constant, k.
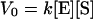 |
3 |
These observations were explained in terms of substrate binding by the major component of the gel, NIPA. The NIPA polymer components created a strong hydrophobic environment on shrinking that increased the hydrophobic affinity between the polymer and the substrate, in particular with its alkyl chain. Because of the affinity, the effective substrate concentration within the gel was increased, which enhanced the overall catalytic rate. This effect disappeared when the gel was swollen, and the substrate absorption is not a primary factor determining the kinetics. These results suggested that the substrate absorption was responsible for the discontinuous transition in the catalysis in parallel to the diameter change of the gels shown in Fig. 3b.
Figure 4.
(a) The initial reaction rate, V0, is plotted as a function of catalyst concentration at two ethanol concentrations. The catalyst concentration was increased by adding more gel samples to a constant volume of solution. The reaction rate catalyzed by the collapsed gel in 15 vol % ethanol obeys the Michaelis–Menten kinetics, but the swollen gel in 10 vol % ethanol does not in this catalyst-concentration region. (b) Catalysis rate as a function of alkyl chain length of substrate. ○, Reaction rate for the collapsed gel in 30% ethanol mixture, where the p-nitrophenyl caprylate (n = 8) has the largest catalysis rate. ●, The swollen gel in 5% ethanol mixture.
The reversible affinity of hydrophobic molecules by NIPA polymers was indeed reported by Kanazawa et al. (13) and was used for reversible affinity chromatography.
Alkyl Chain-Length Dependence.
We further examined hydrolysis of substrates with alkyl groups of various lengths (n = 2 to 18) catalyzed by the gels in both the swollen and shrunken states. The results are presented in Fig. 4b. The shrunken gel (30% ethanol in mixed solvent) exhibited slightly higher catalytic activity than the swollen one (5% ethanol in mixed solvent) for all substrates except for n = 8 (p-nitrophenyl caprylate). The hydrolysis of p-nitrophenyl caprylate was catalyzed 5 times faster in the shrunken state than in the swollen state. The discontinuous switching effect was observed on this substrate, but not on the other substrates.
The dependence on chain length may be understood in terms of strength of hydrophobic interactions. The shorter alkyl chain (n = 2 or 4) may not produce strong enough hydrophobic attraction between the gel and the substrate. Thus, not much effective absorption and the higher catalytic rate are expected. The longer alkyl chain is more hydrophobic, but may have steric hindrance that prevents substrate absorption onto the catalytic sites within the gel and/or the chemical reaction. Another possibility is that for the longer alkyl chains, the hydrophobic affinity was so strong that diffusion of the substrates to reach the reaction sites (imidazole groups) may be prohibited.
Induced Fit.
It was interesting to observe that an addition of substrate helped shrinkage of gel near the transition threshold during chemical reaction (the data are not shown); in this way, substrate itself can accelerate the catalysis. This may be compared with the “induced fit” between some enzymes and substrates. Such conjecture was supported by observation that if imidazole was acylated, the polymer became more hydrophobic and the gel shrinkage was indeed induced.
Discontinuous Transition of Catalytic Rate.
It is interesting to observe that catalytic activity changed discontinuously around 38% ethanol solvent, whereas the diameter of the gel changed only continuously. The decrease in catalytic rate at high ethanol concentration may result from a decrease in the hydrophobic affinity in the swollen gel for the substrate. The enhancement of catalytic rate is caused by the substrate binding presented as ES complex formation in Eq. 1. There has to be some cooperativity in catalysis and absorption processes in determination of the overall reaction rate, but further investigation is necessary for a better understanding of this intriguing observation.
Conclusions
We have developed gels that not only catalyze a chemical reaction but also can switch their catalysis on and off in response to an infinitesimal change in solvent composition. Triggers other than solvent composition, such as temperature, light (14), and magnetic field (15), can, in principle, be used by properly designing the gel composition.
Kokufuta (16) showed switching on/off of the enzyme reaction in the gel by thermal swelling–shrinking change. The enzyme was immobilized in thermoresponsive poly(vinyl methyl ether) gel. The enzymatic reaction proceeded in the swollen gel below critical temperature, Tc, but it was entirely depressed in the collapsed gel above Tc. This is explained by the difference in substrate diffusion within the swollen and shrunken gel. The shrunken gel decreases permeance of the substrate to the enzyme within the gel. In the present work, we incorporated small catalytic groups, imidazole, instead of a natural enzyme. The NIPA network increases the affinity for the substrate by hydrophobic interaction on shrinking, which leads to enhancement of catalytic activity.
Although the catalysis rate of the current gel is much slower than that of enzymes, there are several ways to improve the rate. In improving the gel catalysts toward artificial enzymes, it is necessary to enhance both the specificity and the strength of absorption of substrates and the chemical reactivity of the catalytic center. In our experiment, the absorption by polymers was performed by nonspecific hydrophobic interaction. The molecular recognition of substrate may be enhanced by using a variety of groups capable of hydrogen bonding and electrostatic interaction (17) in addition to the hydrophobic interaction we used here. The imprinting of the substrate/monomer interaction onto the gels may further increase the specificity and affinity for the substrate (18).
The catalytic activity in reactive sites may be increased by incorporating multiple groups that can cooperatively act in catalyzing reactions of substrate molecules. For example, in chymotrypsin, molecular groups such as COOH and OH work as a group for proton donor/acceptor relay along with the imidazole group. In our case, only imidazole participated in catalysis. These are exciting scientific and technological challenges.
The design idea shown in this paper can be applied to development of gels that can be used for many other purposes where enzymes have been used. The gel system, once its catalytic rate is well improved, will have advantages over enzymes in some important aspects—i.e., it can be applied where enzymatic action is needed but enzymes cannot be used. First, temperature dependence of enzymatic catalysis is gradual, whereas gel catalysts can perform an infinitely sharp, discontinuous transition. Although there are quite a few studies successfully mimicking various aspects of enzymes by using synthetic polymers, only gradual change in catalytic activity in response to changing environment has been reported (19–22). Second, the environmental condition for enzymatic activity is extremely limited and narrow. In particular, the threshold temperature of the enzyme should be around body temperature and the salt and pH and aqueous conditions should be physiological. In our case, any temperature for switching on and off can be chosen by using a proper chemical composition of polymers. Third, enzymes are expensive, whereas gels are orders of magnitudes cheaper. Finally, enzymes are fragile and can be easily denatured and inactivated. The gels are much more robust in a sense that they are denatured on swelling but are reversibly activated, or renatured on shrinking.
Acknowledgments
This work was supported by Department of Energy Contract DE-FG02–96ER45606.
Abbreviation
- NIPA
N-isopropylacrylamide
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.180192597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.180192597
References
- 1.Tanaka T. Phys Rev Lett. 1978;40:820–823. [Google Scholar]
- 2.Gutowska A, Bark J S, Kwon I C, Bae Y H, Cha Y, Kim S W. J Contr Rel. 1997;48:141–148. [Google Scholar]
- 3.Osada Y, Okuzaki H, Hori H. Nature (London) 1992;355:242–244. [Google Scholar]
- 4.Yoshida R, Uchida K, Kaneko Y, Sakai K, Kikuchi A, Sakurai Y, Okano T. Nature (London) 1995;374:240–242. [Google Scholar]
- 5.Hirotsu S, Hirokawa Y, Tanaka T. J Chem Phys. 1987;87:1392–1395. [Google Scholar]
- 6.Hoffman A S. Artificial Organs. 1992;16:43–49. doi: 10.1111/j.1525-1594.1992.tb00266.x. [DOI] [PubMed] [Google Scholar]
- 7.Siegel R A. Adv Polym Sci. 1993;109:233–267. [Google Scholar]
- 8.Ilmain F, Tanaka T, Kokufuta E. Nature (London) 1991;349:400–401. [Google Scholar]
- 9.Amiya T, Hirokawa Y, Hirose Y, Li Y, Tanaka T. J Chem Phys. 1987;86:2375–2379. [Google Scholar]
- 10.Overberger C G, Salamone J C. Acc Chem Res. 1969;2:217–224. [Google Scholar]
- 11.Sellergren B, Shea K J. Tetrahedron Asymmetry. 1994;5:1403–1406. [Google Scholar]
- 12.Jencks W P. Catalysis in Chemistry and Enzymology. New York: McGraw–Hill; 1969. pp. 555–614. [Google Scholar]
- 13.Kanazawa H, Yamamoto K, Matsushima Y, Takai N, Kikuchi A, Sakurai Y, Okano T. Anal Chem. 1996;68:100–105. doi: 10.1021/ac950359j. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki A, Tanaka T. Nature (London) 1990;346:345–347. [Google Scholar]
- 15.Mitwalli A H, Denison T A, Jackson D K, Leeb S B, Tanaka T. J Intell Mat Syst Struct. 1997;8:596–604. [Google Scholar]
- 16.Kokufuta E. In: The Polymeric Materials Encyclopedia. Salamone J C, editor. Vol. 4. Boca Raton, FL: CRC; 1996. pp. 2614–2621. [Google Scholar]
- 17.Kunitake Y. In: Polymer-Supported Reactions in Organic Synthesis. Hodge P, Sherrington D C, editors. New York: Wiley; 1980. pp. 195–247. [Google Scholar]
- 18.Pande V S, Grosberg A Yu, Tanaka T. Proc Natl Acad Sci USA. 1994;91:12976–12979. doi: 10.1073/pnas.91.26.12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang G J, Fife W K. J Am Chem Soc. 1998;120:883–887. [Google Scholar]
- 20.Robinson D K, Mosbach K. J Chem Soc Chem Commun. 1989;14:969–970. [Google Scholar]
- 21.Leonhardt A, Mosbach K. React Polym. 1987;6:285–290. [Google Scholar]
- 22.Overberger C G, Morimoto M. J Am Chem Soc. 1971;93:3222–3227. [Google Scholar]