Abstract
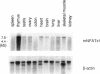
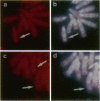
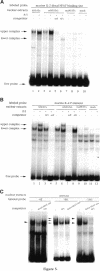
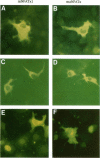
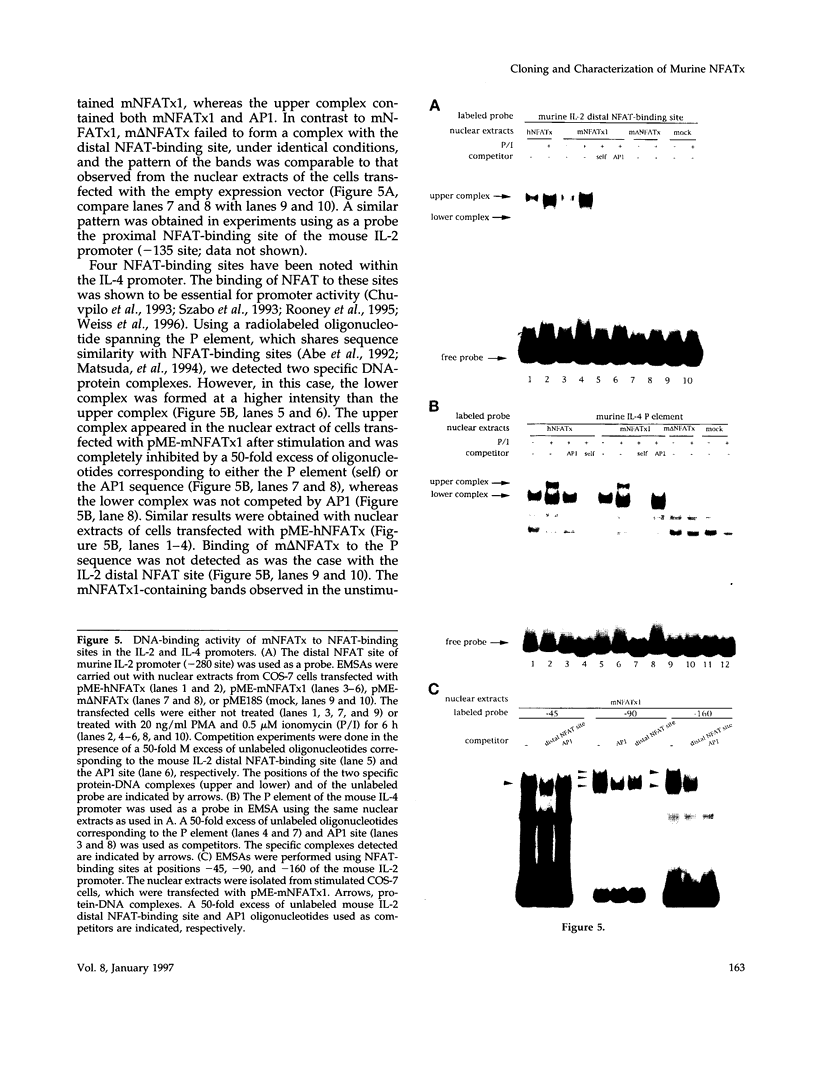
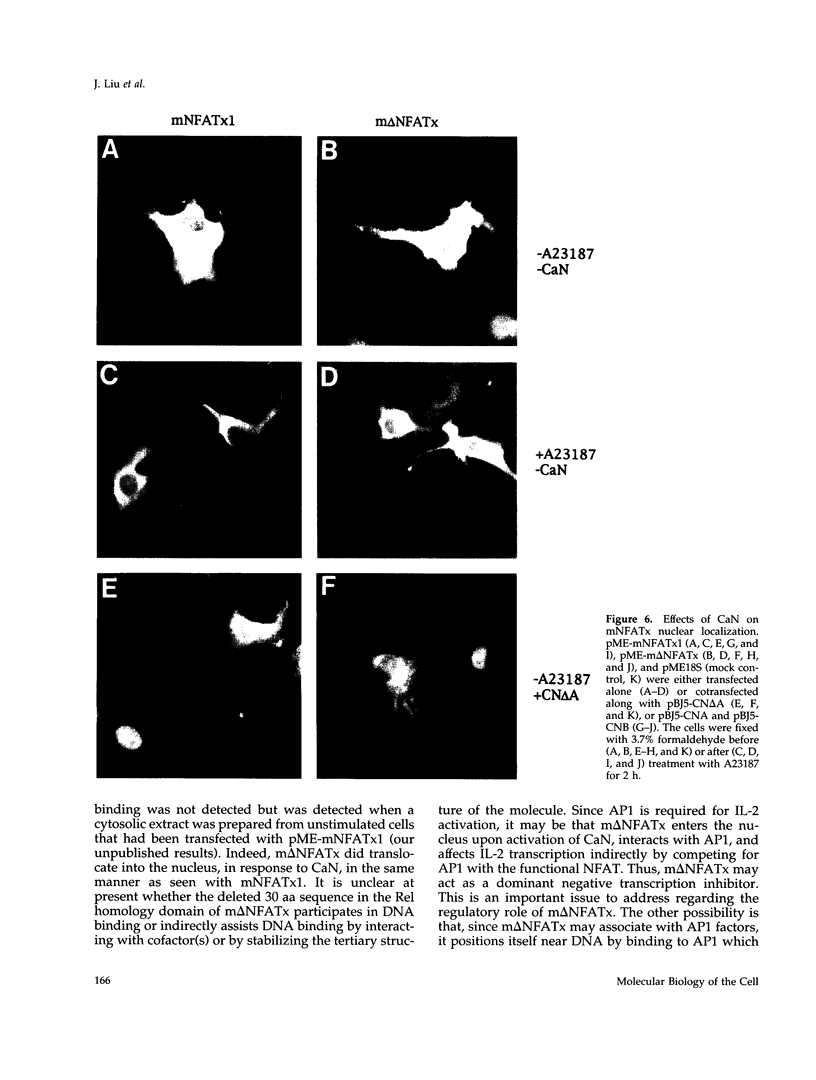
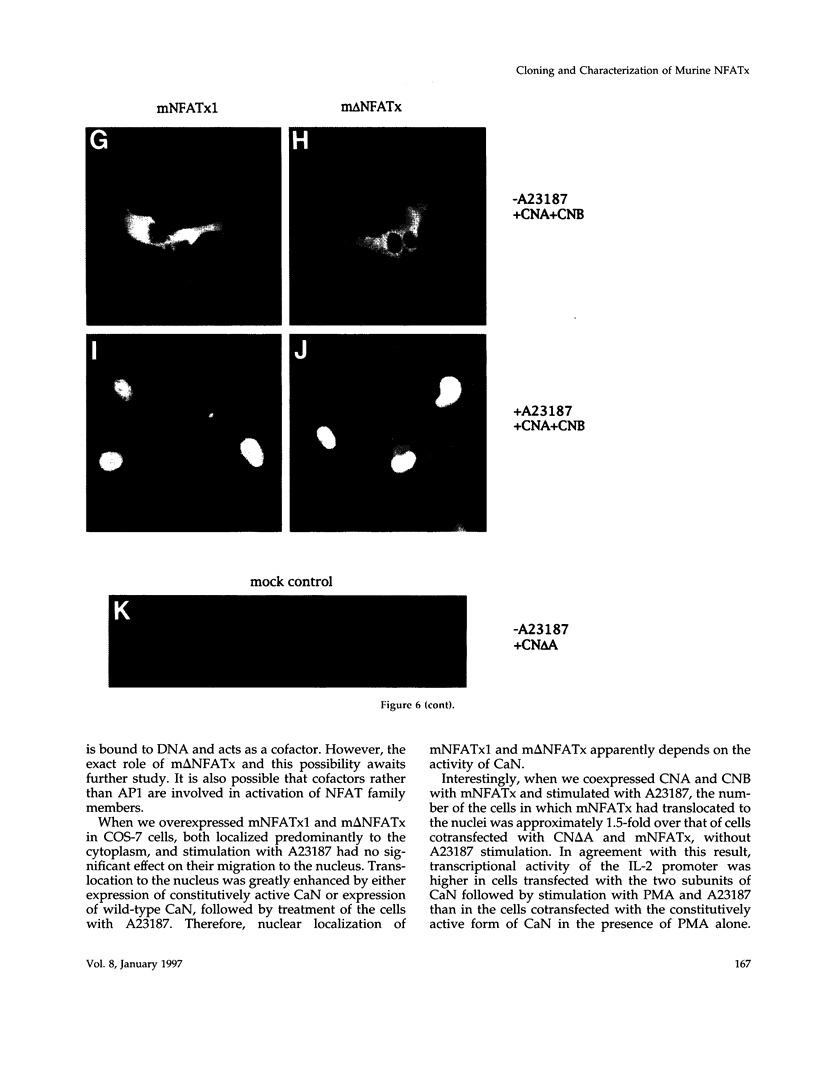
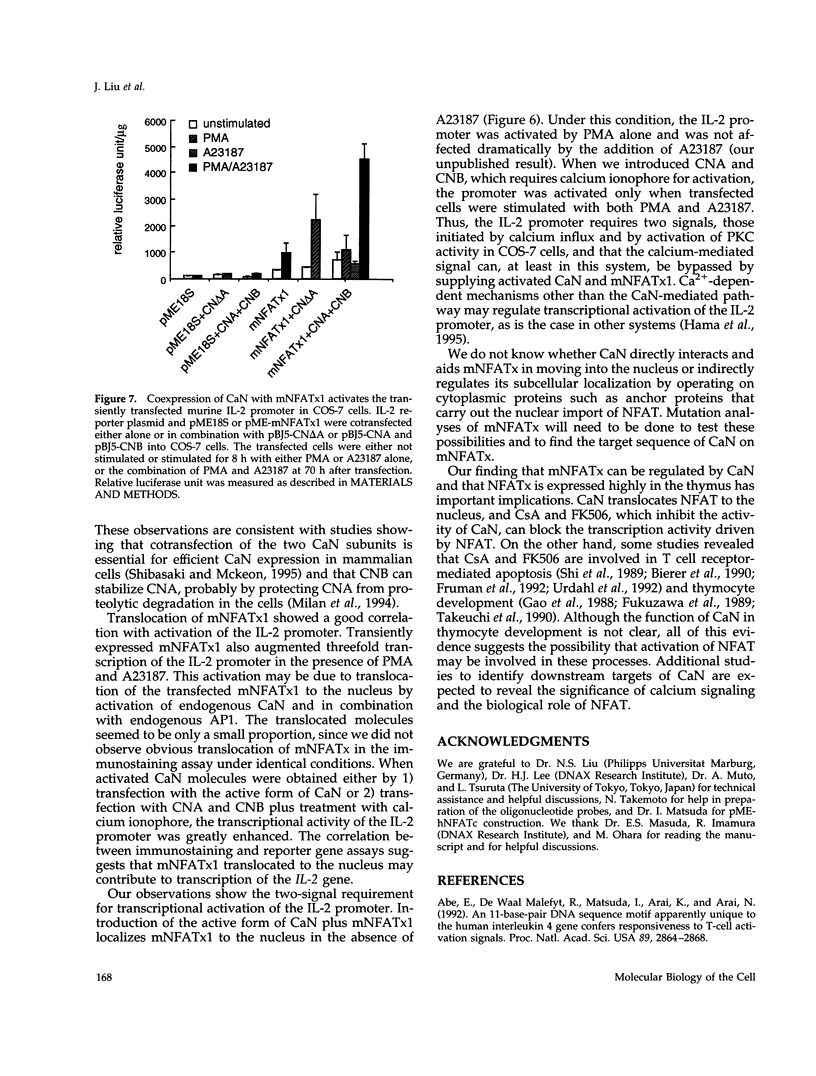
Members of the nuclear factor of activated T cells (NFAT) are involved in the induction of a number of cytokine genes. We report here cDNA cloning and chromosomal localization of a murine homologue of human NFATx, designated as mNFATx1, and its splicing variants mNFATx2 and m delta NFATx. Northern blot analysis showed mNFATx1 to be predominantly expressed in the thymus. mNFATx1, but not m delta NFATx, produced in COS-7 cells, bound to all NFAT-binding sites of the interleukin (IL)-2 and IL-4 promoters tested. Immunofluorescence assay showed that both mNFATx1 and m delta NFATx introduced into COS-7 cells localized predominantly to the cytoplasm, but did translocate to the nucleus, either by cotransfection with an active form of calcineurin or wild-type calcineurin followed by stimulation with calcium ionophore. Translocation of mNFATx1 correlated well with activation of the murine IL-2 promoter; mNFATx1 translocated under conditions described above, in combination with phorbol 12-myristate 13-acetate, activated the transiently transfected murine IL-2 promoter. Thus, nuclear-translocated mNFATx1 is involved in activation of the IL-2 promoter. These results provide the first evidence for the requirement of calcineurin in the control of mNFATx imported from the cytoplasm to the nucleus and implies that mNFATx may possibly be a substrate of calcineurin in vivo.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe E., De Waal Malefyt R., Matsuda I., Arai K., Arai N. An 11-base-pair DNA sequence motif apparently unique to the human interleukin 4 gene confers responsiveness to T-cell activation signals. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2864–2868. doi: 10.1073/pnas.89.7.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer B. E. Cyclosporin A, FK506, and rapamycin: binding to immunophilins and biological action. Chem Immunol. 1994;59:128–155. [PubMed] [Google Scholar]
- Bierer B. E., Somers P. K., Wandless T. J., Burakoff S. J., Schreiber S. L. Probing immunosuppressant action with a nonnatural immunophilin ligand. Science. 1990 Oct 26;250(4980):556–559. doi: 10.1126/science.1700475. [DOI] [PubMed] [Google Scholar]
- Chuvpilo S., Schomberg C., Gerwig R., Heinfling A., Reeves R., Grummt F., Serfling E. Multiple closely-linked NFAT/octamer and HMG I(Y) binding sites are part of the interleukin-4 promoter. Nucleic Acids Res. 1993 Dec 11;21(24):5694–5704. doi: 10.1093/nar/21.24.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clipstone N. A., Crabtree G. R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992 Jun 25;357(6380):695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Cockerill P. N., Bert A. G., Jenkins F., Ryan G. R., Shannon M. F., Vadas M. A. Human granulocyte-macrophage colony-stimulating factor enhancer function is associated with cooperative interactions between AP-1 and NFATp/c. Mol Cell Biol. 1995 Apr;15(4):2071–2079. doi: 10.1128/mcb.15.4.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Fruman D. A., Mather P. E., Burakoff S. J., Bierer B. E. Correlation of calcineurin phosphatase activity and programmed cell death in murine T cell hybridomas. Eur J Immunol. 1992 Oct;22(10):2513–2517. doi: 10.1002/eji.1830221008. [DOI] [PubMed] [Google Scholar]
- Fukuzawa M., Sharrow S. O., Shearer G. M. Effect of cyclosporin A on T cell immunity. II. Defective thymic education of CD4 T helper cell function in cyclosporin A-treated mice. Eur J Immunol. 1989 Jun;19(6):1147–1152. doi: 10.1002/eji.1830190627. [DOI] [PubMed] [Google Scholar]
- Fuleihan R., Ramesh N., Horner A., Ahern D., Belshaw P. J., Alberg D. G., Stamenkovic I., Harmon W., Geha R. S. Cyclosporin A inhibits CD40 ligand expression in T lymphocytes. J Clin Invest. 1994 Mar;93(3):1315–1320. doi: 10.1172/JCI117089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao E. K., Lo D., Cheney R., Kanagawa O., Sprent J. Abnormal differentiation of thymocytes in mice treated with cyclosporin A. Nature. 1988 Nov 10;336(6195):176–179. doi: 10.1038/336176a0. [DOI] [PubMed] [Google Scholar]
- Goldfeld A. E., McCaffrey P. G., Strominger J. L., Rao A. Identification of a novel cyclosporin-sensitive element in the human tumor necrosis factor alpha gene promoter. J Exp Med. 1993 Oct 1;178(4):1365–1379. doi: 10.1084/jem.178.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad P., Wargnier A., Bourge J. F., Sasportes M., Paul P. A promoter element of the human serine esterase granzyme B gene controls specific transcription in activated T cells. Eur J Immunol. 1993 Mar;23(3):625–629. doi: 10.1002/eji.1830230307. [DOI] [PubMed] [Google Scholar]
- Hama N., Paliogianni F., Fessler B. J., Boumpas D. T. Calcium/calmodulin-dependent protein kinase II downregulates both calcineurin and protein kinase C-mediated pathways for cytokine gene transcription in human T cells. J Exp Med. 1995 Mar 1;181(3):1217–1222. doi: 10.1084/jem.181.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Thomas D. J., Timmerman L. A., Li X., Francke U., Crabtree G. R. NFATc3, a lymphoid-specific NFATc family member that is calcium-regulated and exhibits distinct DNA binding specificity. J Biol Chem. 1995 Aug 25;270(34):19898–19907. doi: 10.1074/jbc.270.34.19898. [DOI] [PubMed] [Google Scholar]
- Hoey T., Sun Y. L., Williamson K., Xu X. Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity. 1995 May;2(5):461–472. doi: 10.1016/1074-7613(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Hori T., Takahashi E., Ayusawa D., Takeishi K., Kaneda S., Seno T. Regional assignment of the human thymidylate synthase (TS) gene to chromosome band 18p11.32 by nonisotopic in situ hybridization. Hum Genet. 1990 Oct;85(6):576–580. doi: 10.1007/BF00193577. [DOI] [PubMed] [Google Scholar]
- Jain J., Burgeon E., Badalian T. M., Hogan P. G., Rao A. A similar DNA-binding motif in NFAT family proteins and the Rel homology region. J Biol Chem. 1995 Feb 24;270(8):4138–4145. [PubMed] [Google Scholar]
- Jain J., McCaffrey P. G., Valge-Archer V. E., Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature. 1992 Apr 30;356(6372):801–804. doi: 10.1038/356801a0. [DOI] [PubMed] [Google Scholar]
- Jain J., Miner Z., Rao A. Analysis of the preexisting and nuclear forms of nuclear factor of activated T cells. J Immunol. 1993 Jul 15;151(2):837–848. [PubMed] [Google Scholar]
- Jenkins F., Cockerill P. N., Bohmann D., Shannon M. F. Multiple signals are required for function of the human granulocyte-macrophage colony-stimulating factor gene promoter in T cells. J Immunol. 1995 Aug 1;155(3):1240–1251. [PubMed] [Google Scholar]
- Kitamura T., Hayashida K., Sakamaki K., Yokota T., Arai K., Miyajima A. Reconstitution of functional receptors for human granulocyte/macrophage colony-stimulating factor (GM-CSF): evidence that the protein encoded by the AIC2B cDNA is a subunit of the murine GM-CSF receptor. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5082–5086. doi: 10.1073/pnas.88.12.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Masuda E. S., Arai N., Arai K., Yokota T. Definition of cis-regulatory elements of the mouse interleukin-5 gene promoter. Involvement of nuclear factor of activated T cell-related factors in interleukin-5 expression. J Biol Chem. 1995 Jul 21;270(29):17541–17550. doi: 10.1074/jbc.270.29.17541. [DOI] [PubMed] [Google Scholar]
- Liou H. C., Baltimore D. Regulation of the NF-kappa B/rel transcription factor and I kappa B inhibitor system. Curr Opin Cell Biol. 1993 Jun;5(3):477–487. doi: 10.1016/0955-0674(93)90014-h. [DOI] [PubMed] [Google Scholar]
- Masuda E. S., Naito Y., Tokumitsu H., Campbell D., Saito F., Hannum C., Arai K., Arai N. NFATx, a novel member of the nuclear factor of activated T cells family that is expressed predominantly in the thymus. Mol Cell Biol. 1995 May;15(5):2697–2706. doi: 10.1128/mcb.15.5.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda E. S., Tokumitsu H., Tsuboi A., Shlomai J., Hung P., Arai K., Arai N. The granulocyte-macrophage colony-stimulating factor promoter cis-acting element CLE0 mediates induction signals in T cells and is recognized by factors related to AP1 and NFAT. Mol Cell Biol. 1993 Dec;13(12):7399–7407. doi: 10.1128/mcb.13.12.7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda I., Masuda E. S., Tsuboi A., Behnam S., Arai N., Arai K. Characterization of NF(P), the nuclear factor that interacts with the regulatory P sequence (5'-CGAAAATTTCC-3') of the human interleukin-4 gene: relationship to NF-kappa B and NF-AT. Biochem Biophys Res Commun. 1994 Mar 15;199(2):439–446. doi: 10.1006/bbrc.1994.1248. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Harada Y. N., Natsuume-Sakai S., Lee K., Shiomi T., Chapman V. M. Location of the mouse complement factor H gene (cfh) by FISH analysis and replication R-banding. Cytogenet Cell Genet. 1992;61(4):282–285. doi: 10.1159/000133423. [DOI] [PubMed] [Google Scholar]
- McCaffrey P. G., Goldfeld A. E., Rao A. The role of NFATp in cyclosporin A-sensitive tumor necrosis factor-alpha gene transcription. J Biol Chem. 1994 Dec 2;269(48):30445–30450. [PubMed] [Google Scholar]
- McCaffrey P. G., Luo C., Kerppola T. K., Jain J., Badalian T. M., Ho A. M., Burgeon E., Lane W. S., Lambert J. N., Curran T. Isolation of the cyclosporin-sensitive T cell transcription factor NFATp. Science. 1993 Oct 29;262(5134):750–754. doi: 10.1126/science.8235597. [DOI] [PubMed] [Google Scholar]
- Milan D., Griffith J., Su M., Price E. R., McKeon F. The latch region of calcineurin B is involved in both immunosuppressant-immunophilin complex docking and phosphatase activation. Cell. 1994 Nov 4;79(3):437–447. doi: 10.1016/0092-8674(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Nolan G. P. NF-AT-AP-1 and Rel-bZIP: hybrid vigor and binding under the influence. Cell. 1994 Jun 17;77(6):795–798. doi: 10.1016/0092-8674(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Northrop J. P., Ho S. N., Chen L., Thomas D. J., Timmerman L. A., Nolan G. P., Admon A., Crabtree G. R. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature. 1994 Jun 9;369(6480):497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- Park J., Yaseen N. R., Hogan P. G., Rao A., Sharma S. Phosphorylation of the transcription factor NFATp inhibits its DNA binding activity in cyclosporin A-treated human B and T cells. J Biol Chem. 1995 Sep 1;270(35):20653–20659. doi: 10.1074/jbc.270.35.20653. [DOI] [PubMed] [Google Scholar]
- Rooney J. W., Hoey T., Glimcher L. H. Coordinate and cooperative roles for NF-AT and AP-1 in the regulation of the murine IL-4 gene. Immunity. 1995 May;2(5):473–483. doi: 10.1016/1074-7613(95)90028-4. [DOI] [PubMed] [Google Scholar]
- Rooney J. W., Sun Y. L., Glimcher L. H., Hoey T. Novel NFAT sites that mediate activation of the interleukin-2 promoter in response to T-cell receptor stimulation. Mol Cell Biol. 1995 Nov;15(11):6299–6310. doi: 10.1128/mcb.15.11.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito F., Sasaki S., Chepelinsky A. B., Fushimi K., Marumo F., Ikeuchi T. Human AQP2 and MIP genes, two members of the MIP family, map within chromosome band 12q13 on the basis of two-color FISH. Cytogenet Cell Genet. 1995;68(1-2):45–48. doi: 10.1159/000133885. [DOI] [PubMed] [Google Scholar]
- Shaw J. P., Utz P. J., Durand D. B., Toole J. J., Emmel E. A., Crabtree G. R. Identification of a putative regulator of early T cell activation genes. Science. 1988 Jul 8;241(4862):202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- Shaw K. T., Ho A. M., Raghavan A., Kim J., Jain J., Park J., Sharma S., Rao A., Hogan P. G. Immunosuppressive drugs prevent a rapid dephosphorylation of transcription factor NFAT1 in stimulated immune cells. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):11205–11209. doi: 10.1073/pnas.92.24.11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. F., Sahai B. M., Green D. R. Cyclosporin A inhibits activation-induced cell death in T-cell hybridomas and thymocytes. Nature. 1989 Jun 22;339(6226):625–626. doi: 10.1038/339625a0. [DOI] [PubMed] [Google Scholar]
- Shibasaki F., McKeon F. Calcineurin functions in Ca(2+)-activated cell death in mammalian cells. J Cell Biol. 1995 Nov;131(3):735–743. doi: 10.1083/jcb.131.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo S. J., Gold J. S., Murphy T. L., Murphy K. M. Identification of cis-acting regulatory elements controlling interleukin-4 gene expression in T cells: roles for NF-Y and NF-ATc. Mol Cell Biol. 1993 Aug;13(8):4793–4805. doi: 10.1128/mcb.13.8.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Horiuchi T., Sugimoto T., Matsuda H., Yagita H., Okumura K. Effects of cyclosporin A on T-cell development in organ-cultured foetal thymus. Immunology. 1990 Oct;71(2):158–165. [PMC free article] [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi A., Masuda E. S., Naito Y., Tokumitsu H., Arai K., Arai N. Calcineurin potentiates activation of the granulocyte-macrophage colony-stimulating factor gene in T cells: involvement of the conserved lymphokine element 0. Mol Biol Cell. 1994 Jan;5(1):119–128. doi: 10.1091/mbc.5.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta L., Lee H. J., Masuda E. S., Koyano-Nakagawa N., Arai N., Arai K., Yokota T. Cyclic AMP inhibits expression of the IL-2 gene through the nuclear factor of activated T cells (NF-AT) site, and transfection of NF-AT cDNAs abrogates the sensitivity of EL-4 cells to cyclic AMP. J Immunol. 1995 May 15;154(10):5255–5264. [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Verweij C. L., Crabtree G. R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- Urdahl K. B., Pardoll D. M., Jenkins M. K. Self-reactive T cells are present in the peripheral lymphoid tissues of cyclosporin A-treated mice. Int Immunol. 1992 Dec;4(12):1341–1349. doi: 10.1093/intimm/4.12.1341. [DOI] [PubMed] [Google Scholar]
- Wang D. Z., McCaffrey P. G., Rao A. The cyclosporin-sensitive transcription factor NFATp is expressed in several classes of cells in the immune system. Ann N Y Acad Sci. 1995 Sep 7;766:182–194. doi: 10.1111/j.1749-6632.1995.tb26661.x. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Muramatsu M., Hirai H., Suzuki T., Fujisawa J., Yoshida M., Arai K., Arai N. HTLV-I encoded Tax in association with NF-kappa B precursor p105 enhances nuclear localization of NF-kappa B p50 and p65 in transfected cells. Oncogene. 1993 Nov;8(11):2949–2958. [PubMed] [Google Scholar]
- Weiss D. L., Hural J., Tara D., Timmerman L. A., Henkel G., Brown M. A. Nuclear factor of activated T cells is associated with a mast cell interleukin 4 transcription complex. Mol Cell Biol. 1996 Jan;16(1):228–235. doi: 10.1128/mcb.16.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodrow M., Clipstone N. A., Cantrell D. p21ras and calcineurin synergize to regulate the nuclear factor of activated T cells. J Exp Med. 1993 Nov 1;178(5):1517–1522. doi: 10.1084/jem.178.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]