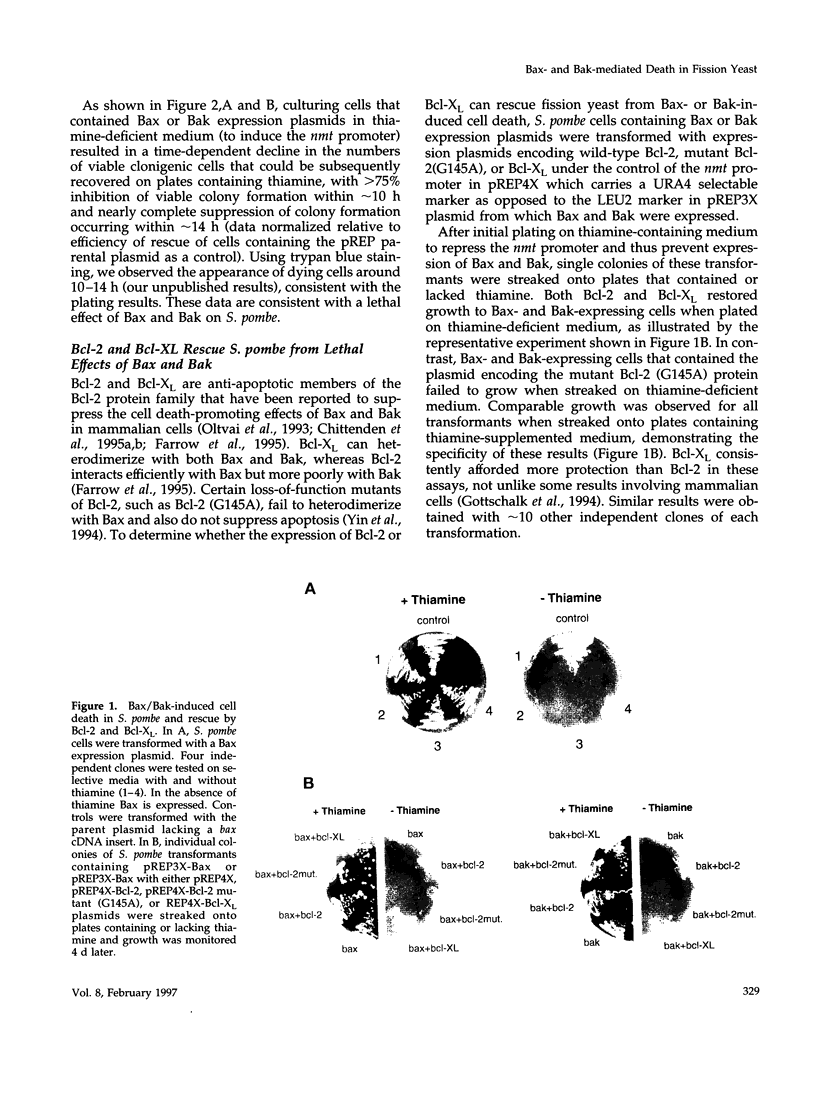
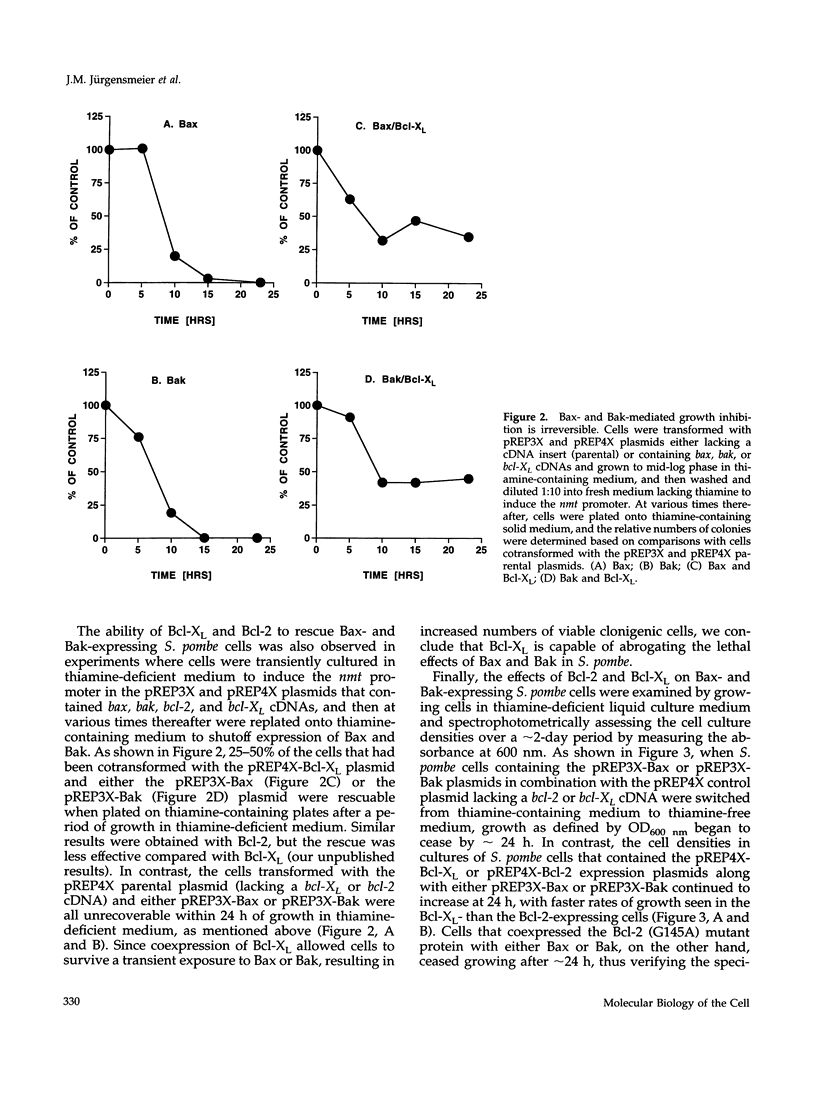
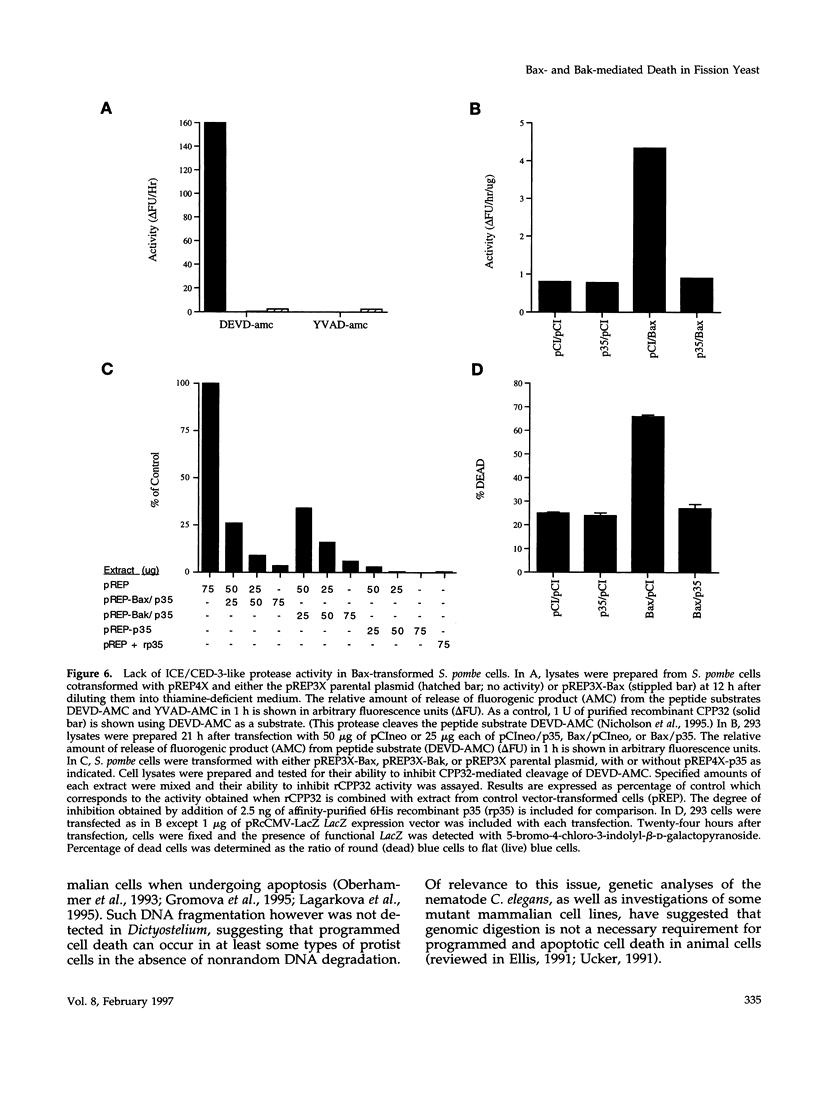
Abstract
The effects of the expression of the human Bcl-2 family proteins Bax, Bak, Bcl-2, and Bcl-XL were examined in the fission yeast Schizosaccharomyces pombe and compared with Bax-induced cell death in mammalian cells. Expression of the proapoptotic proteins Bax and Bak conferred a lethal phenotype in this yeast, which was strongly suppressed by coexpression of the anti-apoptotic protein Bcl-XL. Bcl-2 also partially abrogated Bax-mediated cytotoxicity in S. pombe, whereas a mutant of Bcl-2 (Gly145Ala) that fails to heterodimerize with Bax or block apoptosis in mammalian cells was inactive. However, other features distinguished Bax- and Bak-induced death in S. pombe from animal cell apoptosis. Electron microscopic analysis of S. pombe cells dying in response to Bax or Bak expression demonstrated massive cytosolic vacuolization and multifocal nuclear chromatin condensation, thus distinguishing this form of cell death from the classical morphological features of apoptosis seen in animal cells. Unlike Bax-induced apoptosis in 293 cells that led to the induction of interleukin-1 beta-converting enzyme (ICE)/CED-3-like protease activity, Bax- and Bak-induced cell death in S. pombe was accompanied neither by internucleosomal DNA fragmentation nor by activation of proteases with specificities similar to the ICE/CED-3 family. In addition, the baculovirus protease inhibitor p35, which is a potent inhibitor of ICE/CED-3 family proteases and a blocker of apoptosis in animal cells, failed to prevent cell death induction by Bax or Bak in fission yeast, whereas p35 inhibited Bax-induced cell death in mammalian cells. Taken together, these findings suggest that Bcl-2 family proteins may retain an evolutionarily conserved ability to regulate cell survival and death but also indicate differences in the downstream events that are activated by overexpression of Bax or Bak in divergent cell types.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnemri E. S., Robertson N. M., Fernandes T. F., Croce C. M., Litwack G. Overexpressed full-length human BCL2 extends the survival of baculovirus-infected Sf9 insect cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7295–7299. doi: 10.1073/pnas.89.16.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameisen J. C., Estaquier J., Idziorek T., De Bels F. Programmed cell death and AIDS: significance, perspectives and unanswered questions. Cell Death Differ. 1995 Jan;2(1):9–22. [PubMed] [Google Scholar]
- Ameisen J. C. The origin of programmed cell death. Science. 1996 May 31;272(5266):1278–1279. doi: 10.1126/science.272.5266.1278. [DOI] [PubMed] [Google Scholar]
- Apte S. S., Mattei M. G., Seldin M. F., Olsen B. R. The highly conserved defender against the death 1 (DAD1) gene maps to human chromosome 14q11-q12 and mouse chromosome 14 and has plant and nematode homologs. FEBS Lett. 1995 Apr 24;363(3):304–306. doi: 10.1016/0014-5793(95)00321-y. [DOI] [PubMed] [Google Scholar]
- Armstrong R. C., Aja T., Xiang J., Gaur S., Krebs J. F., Hoang K., Bai X., Korsmeyer S. J., Karanewsky D. S., Fritz L. C. Fas-induced activation of the cell death-related protease CPP32 Is inhibited by Bcl-2 and by ICE family protease inhibitors. J Biol Chem. 1996 Jul 12;271(28):16850–16855. doi: 10.1074/jbc.271.28.16850. [DOI] [PubMed] [Google Scholar]
- Bertin J., Mendrysa S. M., LaCount D. J., Gaur S., Krebs J. F., Armstrong R. C., Tomaselli K. J., Friesen P. D. Apoptotic suppression by baculovirus P35 involves cleavage by and inhibition of a virus-induced CED-3/ICE-like protease. J Virol. 1996 Sep;70(9):6251–6259. doi: 10.1128/jvi.70.9.6251-6259.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodrug S. E., Aimé-Sempé C., Sato T., Krajewski S., Hanada M., Reed J. C. Biochemical and functional comparisons of Mcl-1 and Bcl-2 proteins: evidence for a novel mechanism of regulating Bcl-2 family protein function. Cell Death Differ. 1995 Jul;2(3):173–182. [PubMed] [Google Scholar]
- Brancolini C., Benedetti M., Schneider C. Microfilament reorganization during apoptosis: the role of Gas2, a possible substrate for ICE-like proteases. EMBO J. 1995 Nov 1;14(21):5179–5190. doi: 10.1002/j.1460-2075.1995.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bump N. J., Hackett M., Hugunin M., Seshagiri S., Brady K., Chen P., Ferenz C., Franklin S., Ghayur T., Li P. Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science. 1995 Sep 29;269(5232):1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan A. M., Orth K., O'Rourke K., Duan H., Poirier G. G., Dixit V. M. Molecular ordering of the cell death pathway. Bcl-2 and Bcl-xL function upstream of the CED-3-like apoptotic proteases. J Biol Chem. 1996 Mar 1;271(9):4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- Chittenden T., Flemington C., Houghton A. B., Ebb R. G., Gallo G. J., Elangovan B., Chinnadurai G., Lutz R. J. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 1995 Nov 15;14(22):5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittenden T., Harrington E. A., O'Connor R., Flemington C., Lutz R. J., Evan G. I., Guild B. C. Induction of apoptosis by the Bcl-2 homologue Bak. Nature. 1995 Apr 20;374(6524):733–736. doi: 10.1038/374733a0. [DOI] [PubMed] [Google Scholar]
- Clem R. J., Miller L. K. Control of programmed cell death by the baculovirus genes p35 and iap. Mol Cell Biol. 1994 Aug;14(8):5212–5222. doi: 10.1128/mcb.14.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornillon S., Foa C., Davoust J., Buonavista N., Gross J. D., Golstein P. Programmed cell death in Dictyostelium. J Cell Sci. 1994 Oct;107(Pt 10):2691–2704. doi: 10.1242/jcs.107.10.2691. [DOI] [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Farrow S. N., White J. H., Martinou I., Raven T., Pun K. T., Grinham C. J., Martinou J. C., Brown R. Cloning of a bcl-2 homologue by interaction with adenovirus E1B 19K. Nature. 1995 Apr 20;374(6524):731–733. doi: 10.1038/374731a0. [DOI] [PubMed] [Google Scholar]
- Forsburg S. L. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 1993 Jun 25;21(12):2955–2956. doi: 10.1093/nar/21.12.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk A. R., Boise L. H., Thompson C. B., Quintáns J. Identification of immunosuppressant-induced apoptosis in a murine B-cell line and its prevention by bcl-x but not bcl-2. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7350–7354. doi: 10.1073/pnas.91.15.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalf W., Stephan C., Chaudhuri B. Role of mitochondria and C-terminal membrane anchor of Bcl-2 in Bax induced growth arrest and mortality in Saccharomyces cerevisiae. FEBS Lett. 1996 Feb 12;380(1-2):169–175. doi: 10.1016/0014-5793(96)00044-0. [DOI] [PubMed] [Google Scholar]
- Gromova I. I., Nielsen O. F., Razin S. V. Long-range fragmentation of the eukaryotic genome by exogenous and endogenous nucleases proceeds in a specific fashion via preferential DNA cleavage at matrix attachment sites. J Biol Chem. 1995 Aug 4;270(31):18685–18690. doi: 10.1074/jbc.270.31.18685. [DOI] [PubMed] [Google Scholar]
- Hanada M., Aimé-Sempé C., Sato T., Reed J. C. Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J Biol Chem. 1995 May 19;270(20):11962–11969. doi: 10.1074/jbc.270.20.11962. [DOI] [PubMed] [Google Scholar]
- Hay B. A., Wolff T., Rubin G. M. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994 Aug;120(8):2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- Henderson S., Huen D., Rowe M., Dawson C., Johnson G., Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner M. O., Ellis R. E., Horvitz H. R. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature. 1992 Apr 9;356(6369):494–499. doi: 10.1038/356494a0. [DOI] [PubMed] [Google Scholar]
- Hengartner M. O., Horvitz H. R. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell. 1994 Feb 25;76(4):665–676. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Kane D. J., Ord T., Anton R., Bredesen D. E. Expression of bcl-2 inhibits necrotic neural cell death. J Neurosci Res. 1995 Feb 1;40(2):269–275. doi: 10.1002/jnr.490400216. [DOI] [PubMed] [Google Scholar]
- Kane D. J., Sarafian T. A., Anton R., Hahn H., Gralla E. B., Valentine J. S., Ord T., Bredesen D. E. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993 Nov 19;262(5137):1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- Kayalar C., Ord T., Testa M. P., Zhong L. T., Bredesen D. E. Cleavage of actin by interleukin 1 beta-converting enzyme to reverse DNase I inhibition. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):2234–2238. doi: 10.1073/pnas.93.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M. C., Brauer M. J., Powers V. C., Wu J. J., Umansky S. R., Tomei L. D., Barr P. J. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature. 1995 Apr 20;374(6524):736–739. doi: 10.1038/374736a0. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992 Aug 15;80(4):879–886. [PubMed] [Google Scholar]
- Krajewski S., Blomqvist C., Franssila K., Krajewska M., Wasenius V. M., Niskanen E., Nordling S., Reed J. C. Reduced expression of proapoptotic gene BAX is associated with poor response rates to combination chemotherapy and shorter survival in women with metastatic breast adenocarcinoma. Cancer Res. 1995 Oct 1;55(19):4471–4478. [PubMed] [Google Scholar]
- Krajewski S., Krajewska M., Shabaik A., Wang H. G., Irie S., Fong L., Reed J. C. Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Res. 1994 Nov 1;54(21):5501–5507. [PubMed] [Google Scholar]
- Krajewski S., Tanaka S., Takayama S., Schibler M. J., Fenton W., Reed J. C. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993 Oct 1;53(19):4701–4714. [PubMed] [Google Scholar]
- Krajewski S., Zapata J. M., Reed J. C. Detection of multiple antigens on western blots. Anal Biochem. 1996 May 1;236(2):221–228. doi: 10.1006/abio.1996.0160. [DOI] [PubMed] [Google Scholar]
- Lagarkova M. A., Iarovaia O. V., Razin S. V. Large-scale fragmentation of mammalian DNA in the course of apoptosis proceeds via excision of chromosomal DNA loops and their oligomers. J Biol Chem. 1995 Sep 1;270(35):20239–20241. doi: 10.1074/jbc.270.35.20239. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Green D. R. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995 Aug 11;82(3):349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- Martin S. J., O'Brien G. A., Nishioka W. K., McGahon A. J., Mahboubi A., Saido T. C., Green D. R. Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J Biol Chem. 1995 Mar 24;270(12):6425–6428. doi: 10.1074/jbc.270.12.6425. [DOI] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J Biol Chem. 1990 Jul 5;265(19):10857–10864. [PubMed] [Google Scholar]
- Messmer U. K., Reimer D. M., Reed J. C., Brüne B. Nitric oxide induced poly(ADP-ribose) polymerase cleavage in RAW 264.7 macrophage apoptosis is blocked by Bcl-2. FEBS Lett. 1996 Apr 15;384(2):162–166. doi: 10.1016/0014-5793(96)00311-0. [DOI] [PubMed] [Google Scholar]
- Miloshev G., Venkov P., van Holde K., Zlatanova J. Low levels of exogenous histone H1 in yeast cause cell death. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11567–11570. doi: 10.1073/pnas.91.24.11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Muchmore S. W., Sattler M., Liang H., Meadows R. P., Harlan J. E., Yoon H. S., Nettesheim D., Chang B. S., Thompson C. B., Wong S. L. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996 May 23;381(6580):335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- Nicholson D. W., Ali A., Thornberry N. A., Vaillancourt J. P., Ding C. K., Gallant M., Gareau Y., Griffin P. R., Labelle M., Lazebnik Y. A. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995 Jul 6;376(6535):37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Nuñez G., Clarke M. F. The Bcl-2 family of proteins: regulators of cell death and survival. Trends Cell Biol. 1994 Nov;4(11):399–403. doi: 10.1016/0962-8924(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Oberhammer F., Wilson J. W., Dive C., Morris I. D., Hickman J. A., Wakeling A. E., Walker P. R., Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993 Sep;12(9):3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltvai Z. N., Korsmeyer S. J. Checkpoints of dueling dimers foil death wishes. Cell. 1994 Oct 21;79(2):189–192. doi: 10.1016/0092-8674(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Ottilie S., Chernoff J., Hannig G., Hoffman C. S., Erikson R. L. The fission yeast genes pyp1+ and pyp2+ encode protein tyrosine phosphatases that negatively regulate mitosis. Mol Cell Biol. 1992 Dec;12(12):5571–5580. doi: 10.1128/mcb.12.12.5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk G. J., Ramer K., Amiri P., Williams L. T. Requirement of an ICE-like protease for induction of apoptosis and ceramide generation by REAPER. Science. 1996 Feb 9;271(5250):808–810. doi: 10.1126/science.271.5250.808. [DOI] [PubMed] [Google Scholar]
- Quan L. T., Caputo A., Bleackley R. C., Pickup D. J., Salvesen G. S. Granzyme B is inhibited by the cowpox virus serpin cytokine response modifier A. J Biol Chem. 1995 May 5;270(18):10377–10379. doi: 10.1074/jbc.270.18.10377. [DOI] [PubMed] [Google Scholar]
- Rabizadeh S., LaCount D. J., Friesen P. D., Bredesen D. E. Expression of the baculovirus p35 gene inhibits mammalian neural cell death. J Neurochem. 1993 Dec;61(6):2318–2321. doi: 10.1111/j.1471-4159.1993.tb07477.x. [DOI] [PubMed] [Google Scholar]
- Rao L., Debbas M., Sabbatini P., Hockenbery D., Korsmeyer S., White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Tanaka S., Cuddy M., Cho D., Smith J., Kallen R., Saragovi H. U., Torigoe T. A strategy for generating monoclonal antibodies against recombinant baculovirus-produced proteins: application to the Bcl-2 oncoprotein. Anal Biochem. 1992 Aug 15;205(1):70–76. doi: 10.1016/0003-2697(92)90580-z. [DOI] [PubMed] [Google Scholar]
- Sato T., Hanada M., Bodrug S., Irie S., Iwama N., Boise L. H., Thompson C. B., Golemis E., Fong L., Wang H. G. Interactions among members of the Bcl-2 protein family analyzed with a yeast two-hybrid system. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9238–9242. doi: 10.1073/pnas.91.20.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak T. W., Oltvai Z. N., Yang E., Wang K., Boise L. H., Thompson C. B., Korsmeyer S. J. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7834–7838. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Eguchi Y., Kamiike W., Itoh Y., Hasegawa J., Yamabe K., Otsuki Y., Matsuda H., Tsujimoto Y. Induction of apoptosis as well as necrosis by hypoxia and predominant prevention of apoptosis by Bcl-2 and Bcl-XL. Cancer Res. 1996 May 1;56(9):2161–2166. [PubMed] [Google Scholar]
- Shub D. A. Bacterial viruses. Bacterial altruism? Curr Biol. 1994 Jun 1;4(6):555–556. doi: 10.1016/s0960-9822(00)00124-x. [DOI] [PubMed] [Google Scholar]
- Subramanian T., Tarodi B., Chinnadurai G. p53-independent apoptotic and necrotic cell deaths induced by adenovirus infection: suppression by E1B 19K and Bcl-2 proteins. Cell Growth Differ. 1995 Feb;6(2):131–137. [PubMed] [Google Scholar]
- Sugimoto A., Friesen P. D., Rothman J. H. Baculovirus p35 prevents developmentally programmed cell death and rescues a ced-9 mutant in the nematode Caenorhabditis elegans. EMBO J. 1994 May 1;13(9):2023–2028. doi: 10.1002/j.1460-2075.1994.tb06475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig R., Gilman A. G. Mammalian membrane-bound adenylyl cyclases. J Biol Chem. 1995 Jan 6;270(1):1–4. doi: 10.1074/jbc.270.1.1. [DOI] [PubMed] [Google Scholar]
- Tewari M., Quan L. T., O'Rourke K., Desnoyers S., Zeng Z., Beidler D. R., Poirier G. G., Salvesen G. S., Dixit V. M. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995 Jun 2;81(5):801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- Ucker D. S. Death by suicide: one way to go in mammalian cellular development? New Biol. 1991 Feb;3(2):103–109. [PubMed] [Google Scholar]
- Ucker D. S., Wilson J. D., Hebshi L. D. Target cell death triggered by cytotoxic T lymphocytes: a target cell mutant distinguishes passive pore formation and active cell suicide mechanisms. Mol Cell Biol. 1994 Jan;14(1):427–436. doi: 10.1128/mcb.14.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D. L., Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci U S A. 1996 Mar 19;93(6):2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D. L. Toward an understanding of the molecular mechanisms of physiological cell death. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):786–789. doi: 10.1073/pnas.90.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zelenski N. G., Yang J., Sakai J., Brown M. S., Goldstein J. L. Cleavage of sterol regulatory element binding proteins (SREBPs) by CPP32 during apoptosis. EMBO J. 1996 Mar 1;15(5):1012–1020. [PMC free article] [PubMed] [Google Scholar]
- Weiss L. M., Warnke R. A., Sklar J., Cleary M. L. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med. 1987 Nov 5;317(19):1185–1189. doi: 10.1056/NEJM198711053171904. [DOI] [PubMed] [Google Scholar]
- Welburn S. C., Dale C., Ellis D., Beecroft R., Pearson T. W. Apoptosis in procyclic Trypanosoma brucei rhodesiense in vitro. Cell Death Differ. 1996 Apr;3(2):229–236. [PubMed] [Google Scholar]
- White K., Tahaoglu E., Steller H. Cell killing by the Drosophila gene reaper. Science. 1996 Feb 9;271(5250):805–807. doi: 10.1126/science.271.5250.805. [DOI] [PubMed] [Google Scholar]
- Williams G. T. Programmed cell death: apoptosis and oncogenesis. Cell. 1991 Jun 28;65(7):1097–1098. doi: 10.1016/0092-8674(91)90002-g. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- Xue D., Horvitz H. R. Inhibition of the Caenorhabditis elegans cell-death protease CED-3 by a CED-3 cleavage site in baculovirus p35 protein. Nature. 1995 Sep 21;377(6546):248–251. doi: 10.1038/377248a0. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky M. B. Programmed cell death in bacterial populations. Science. 1995 Feb 10;267(5199):836–837. doi: 10.1126/science.7846528. [DOI] [PubMed] [Google Scholar]
- Yin X. M., Oltvai Z. N., Korsmeyer S. J. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994 May 26;369(6478):321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- Yuan J., Shaham S., Ledoux S., Ellis H. M., Horvitz H. R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993 Nov 19;75(4):641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Zha H., Aimé-Sempé C., Sato T., Reed J. C. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem. 1996 Mar 29;271(13):7440–7444. doi: 10.1074/jbc.271.13.7440. [DOI] [PubMed] [Google Scholar]
- Zha H., Fisk H. A., Yaffe M. P., Mahajan N., Herman B., Reed J. C. Structure-function comparisons of the proapoptotic protein Bax in yeast and mammalian cells. Mol Cell Biol. 1996 Nov;16(11):6494–6508. doi: 10.1128/mcb.16.11.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]