Abstract
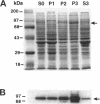
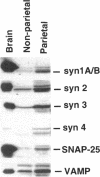
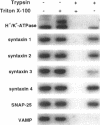
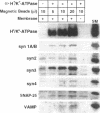
H+/K(+)-ATPase is the proton pump in the gastric parietal cell that is responsible for gastric acid secretion. Stimulation of acid secretion is associated with a reorganization of the parietal cells resulting in the incorporation of H+/K(+)-ATPase from a cytoplasmic membrane pool, the tubulovesicle compartment, into the apical canalicular membrane. To better characterize the role of membrane trafficking events in the morphological and physiological changes associated with acid secretion from parietal cells, we have characterized the expression and localization of soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) in these cells. Each of the six different SNARE proteins examined [syntaxins 1 through 4 of 25-kDa synaptosome-associated protein, and vesicle-associated membrane protein] were found to be expressed in parietal cells. Furthermore, two of these SNAREs, vesicle-associated membrane protein and syntaxin 3, were associated with H+/K(+)-ATPase-containing tubulovesicles while the remainder were excluded from this compartment. The expression of syntaxin 1 and synaptosome-associated protein of 25 kDa in parietal cells, two SNAREs previously thought to be restricted to neuroendocrine tissues, suggests that parietal cells may utilize membrane trafficking machinery that is similar to that utilized for regulated exocytosis in neurons. Furthermore, the localization of syntaxin 3, a putative target membrane SNARE, to the tubulovesicle compartment indicates that syntaxin 3 may have an alternative function. These observations support a role for intracellular membrane trafficking events in the regulated recruitment of H+/K(+)-ATPase to the plasma membrane after parietal cell stimulation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bark I. C., Hahn K. M., Ryabinin A. E., Wilson M. C. Differential expression of SNAP-25 protein isoforms during divergent vesicle fusion events of neural development. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1510–1514. doi: 10.1073/pnas.92.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable C. J., Hofstein R., Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985 Jun;352(2):286–290. doi: 10.1016/0165-3806(85)90116-6. [DOI] [PubMed] [Google Scholar]
- Baumert M., Maycox P. R., Navone F., De Camilli P., Jahn R. Synaptobrevin: an integral membrane protein of 18,000 daltons present in small synaptic vesicles of rat brain. EMBO J. 1989 Feb;8(2):379–384. doi: 10.1002/j.1460-2075.1989.tb03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. K., Calakos N., Scheller R. H. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992 Jul 10;257(5067):255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., García-Arrarás J. E., Elferink L. A., Peterson K., Fleming A. M., Hazuka C. D., Scheller R. H. The syntaxin family of vesicular transport receptors. Cell. 1993 Sep 10;74(5):863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Scheller R. H. A molecular description of synaptic vesicle membrane trafficking. Annu Rev Biochem. 1994;63:63–100. doi: 10.1146/annurev.bi.63.070194.000431. [DOI] [PubMed] [Google Scholar]
- Braun J. E., Fritz B. A., Wong S. M., Lowe A. W. Identification of a vesicle-associated membrane protein (VAMP)-like membrane protein in zymogen granules of the rat exocrine pancreas. J Biol Chem. 1994 Feb 18;269(7):5328–5335. [PubMed] [Google Scholar]
- Cain C. C., Trimble W. S., Lienhard G. E. Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J Biol Chem. 1992 Jun 15;267(17):11681–11684. [PubMed] [Google Scholar]
- Chew C. S., Ljungström M., Smolka A., Brown M. R. Primary culture of secretagogue-responsive parietal cells from rabbit gastric mucosa. Am J Physiol. 1989 Jan;256(1 Pt 1):G254–G263. doi: 10.1152/ajpgi.1989.256.1.G254. [DOI] [PubMed] [Google Scholar]
- Chilcote T. J., Galli T., Mundigl O., Edelmann L., McPherson P. S., Takei K., De Camilli P. Cellubrevin and synaptobrevins: similar subcellular localization and biochemical properties in PC12 cells. J Cell Biol. 1995 Apr;129(1):219–231. doi: 10.1083/jcb.129.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow D. C., Forte J. G. Characterization of the beta-subunit of the H(+)-K(+)-ATPase using an inhibitory monoclonal antibody. Am J Physiol. 1993 Dec;265(6 Pt 1):C1562–C1570. doi: 10.1152/ajpcell.1993.265.6.C1562. [DOI] [PubMed] [Google Scholar]
- Chow D. C., Forte J. G. Functional significance of the beta-subunit for heterodimeric P-type ATPases. J Exp Biol. 1995 Jan;198(Pt 1):1–17. doi: 10.1242/jeb.198.1.1. [DOI] [PubMed] [Google Scholar]
- Dotti C. G., Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990 Jul 13;62(1):63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- Ferro-Novick S., Jahn R. Vesicle fusion from yeast to man. Nature. 1994 Jul 21;370(6486):191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Forte J. G., Black J. A., Forte T. M., Machen T. E., Wolosin J. M. Ultrastructural changes related to functional activity in gastric oxyntic cells. Am J Physiol. 1981 Nov;241(5):G349–G358. doi: 10.1152/ajpgi.1981.241.5.G349. [DOI] [PubMed] [Google Scholar]
- Forte J. G., Yao X. The membrane-recruitment-and-recycling hypothesis of gastric HCl secretion. Trends Cell Biol. 1996 Feb;6(2):45–48. doi: 10.1016/0962-8924(96)81009-9. [DOI] [PubMed] [Google Scholar]
- Forte T. M., Machen T. E., Forte J. G. Ultrastructural changes in oxyntic cells associated with secretory function: a membrane-recycling hypothesis. Gastroenterology. 1977 Oct;73(4 Pt 2):941–955. [PubMed] [Google Scholar]
- Gaisano H. Y., Ghai M., Malkus P. N., Sheu L., Bouquillon A., Bennett M. K., Trimble W. S. Distinct cellular locations of the syntaxin family of proteins in rat pancreatic acinar cells. Mol Biol Cell. 1996 Dec;7(12):2019–2027. doi: 10.1091/mbc.7.12.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisano H. Y., Sheu L., Foskett J. K., Trimble W. S. Tetanus toxin light chain cleaves a vesicle-associated membrane protein (VAMP) isoform 2 in rat pancreatic zymogen granules and inhibits enzyme secretion. J Biol Chem. 1994 Jun 24;269(25):17062–17066. [PubMed] [Google Scholar]
- Garcia E. P., McPherson P. S., Chilcote T. J., Takei K., De Camilli P. rbSec1A and B colocalize with syntaxin 1 and SNAP-25 throughout the axon, but are not in a stable complex with syntaxin. J Cell Biol. 1995 Apr;129(1):105–120. doi: 10.1083/jcb.129.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenring J. R., Soroka C. J., Shen K. R., Tang L. H., Rodriguez W., Vaughan H. D., Stoch S. A., Modlin I. M. Enrichment of rab11, a small GTP-binding protein, in gastric parietal cells. Am J Physiol. 1994 Aug;267(2 Pt 1):G187–G194. doi: 10.1152/ajpgi.1994.267.2.G187. [DOI] [PubMed] [Google Scholar]
- Helander H. F., Hirschowitz B. I. Quantitative ultrastructural studies on gastric parietal cells. Gastroenterology. 1972 Dec;63(6):951–961. [PubMed] [Google Scholar]
- Hirst B. H., Forte J. G. Redistribution and characterization of (H+ + K+)-ATPase membranes from resting and stimulated gastric parietal cells. Biochem J. 1985 Nov 1;231(3):641–649. doi: 10.1042/bj2310641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhne-Zell B., Ecker A., Weller U., Gratzl M. Synaptobrevin cleavage by the tetanus toxin light chain is linked to the inhibition of exocytosis in chromaffin cells. FEBS Lett. 1994 Nov 28;355(2):131–134. doi: 10.1016/0014-5793(94)01192-3. [DOI] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Ouimet C., Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low S. H., Chapin S. J., Weimbs T., Kömüves L. G., Bennett M. K., Mostov K. E. Differential localization of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1996 Dec;7(12):2007–2018. doi: 10.1091/mbc.7.12.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon H. T., Ushkaryov Y. A., Edelmann L., Link E., Binz T., Niemann H., Jahn R., Südhof T. C. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 1993 Jul 22;364(6435):346–349. doi: 10.1038/364346a0. [DOI] [PubMed] [Google Scholar]
- Oyler G. A., Higgins G. A., Hart R. A., Battenberg E., Billingsley M., Bloom F. E., Wilson M. C. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989 Dec;109(6 Pt 1):3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevsner J., Hsu S. C., Braun J. E., Calakos N., Ting A. E., Bennett M. K., Scheller R. H. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994 Aug;13(2):353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Pevsner J. The role of Sec1p-related proteins in vesicle trafficking in the nerve terminal. J Neurosci Res. 1996 Jul 15;45(2):89–95. doi: 10.1002/(SICI)1097-4547(19960715)45:2<89::AID-JNR1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R. Rab GTPases: master regulators of membrane trafficking. Curr Opin Cell Biol. 1994 Aug;6(4):522–526. doi: 10.1016/0955-0674(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Ravichandran V., Chawla A., Roche P. A. Identification of a novel syntaxin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues. J Biol Chem. 1996 Jun 7;271(23):13300–13303. doi: 10.1074/jbc.271.23.13300. [DOI] [PubMed] [Google Scholar]
- Reenstra W. W., Forte J. G. Isolation of H+,K(+)-ATPase-containing membranes from the gastric oxyntic cell. Methods Enzymol. 1990;192:151–165. doi: 10.1016/0076-6879(90)92068-o. [DOI] [PubMed] [Google Scholar]
- Regazzi R., Wollheim C. B., Lang J., Theler J. M., Rossetto O., Montecucco C., Sadoul K., Weller U., Palmer M., Thorens B. VAMP-2 and cellubrevin are expressed in pancreatic beta-cells and are essential for Ca(2+)-but not for GTP gamma S-induced insulin secretion. EMBO J. 1995 Jun 15;14(12):2723–2730. doi: 10.1002/j.1460-2075.1995.tb07273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994 Mar 1;4(3):220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Roush D. L., Gottardi C. J., Caplan M. J. Sorting of the gastric H,K-ATPase in endocrine and epithelial cells. Ann N Y Acad Sci. 1994 Sep 15;733:212–222. doi: 10.1111/j.1749-6632.1994.tb17271.x. [DOI] [PubMed] [Google Scholar]
- Smolka A., Helander H. F., Sachs G. Monoclonal antibodies against gastric H+ + K+ ATPase. Am J Physiol. 1983 Oct;245(4):G589–G596. doi: 10.1152/ajpgi.1983.245.4.G589. [DOI] [PubMed] [Google Scholar]
- Söllner T., Bennett M. K., Whiteheart S. W., Scheller R. H., Rothman J. E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993 Nov 5;75(3):409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Südhof T. C. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995 Jun 22;375(6533):645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Tang L. H., Stoch S. A., Modlin I. M., Goldenring J. R. Identification of rab2 as a tubulovesicle-membrane-associated protein in rabbit gastric parietal cells. Biochem J. 1992 Aug 1;285(Pt 3):715–719. doi: 10.1042/bj2850715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble W. S., Cowan D. M., Scheller R. H. VAMP-1: a synaptic vesicle-associated integral membrane protein. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4538–4542. doi: 10.1073/pnas.85.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volchuk A., Sargeant R., Sumitani S., Liu Z., He L., Klip A. Cellubrevin is a resident protein of insulin-sensitive GLUT4 glucose transporter vesicles in 3T3-L1 adipocytes. J Biol Chem. 1995 Apr 7;270(14):8233–8240. doi: 10.1074/jbc.270.14.8233. [DOI] [PubMed] [Google Scholar]
- Wolosin J. M., Forte J. G. Stimulation of oxyntic cell triggers K+ and Cl- conductances in apical H+-K+-ATPase membrane. Am J Physiol. 1984 May;246(5 Pt 1):C537–C545. doi: 10.1152/ajpcell.1984.246.5.C537. [DOI] [PubMed] [Google Scholar]