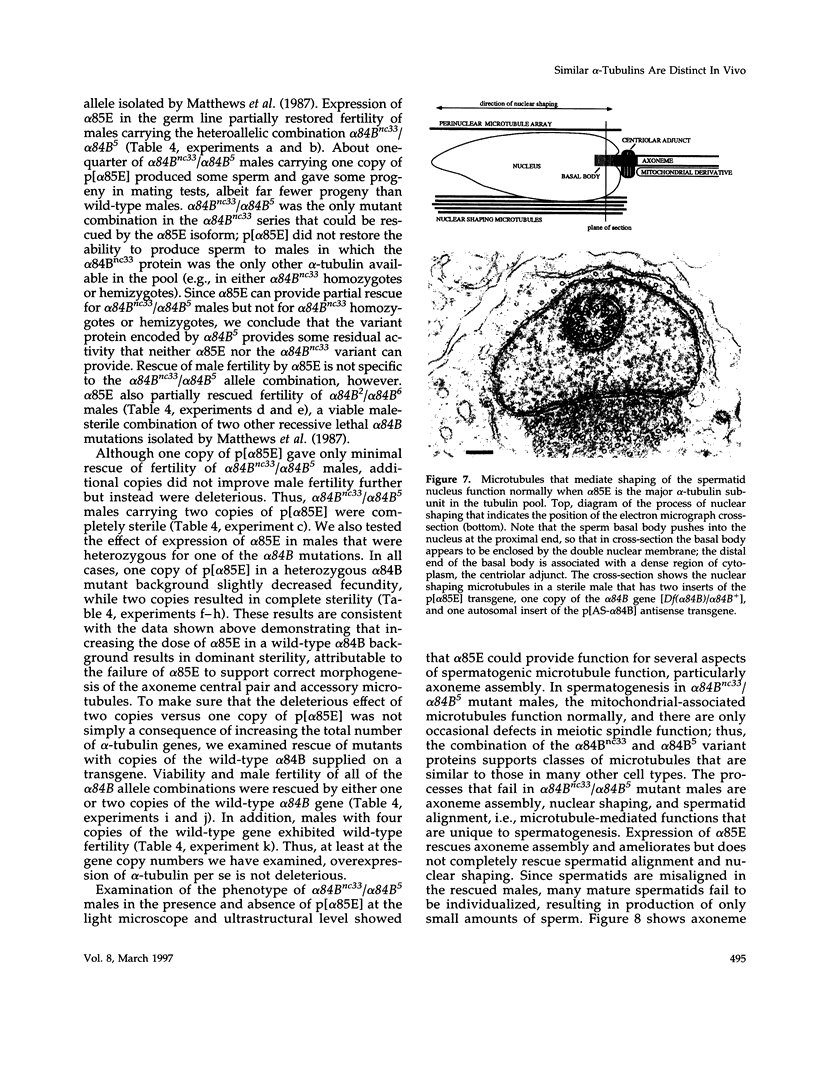
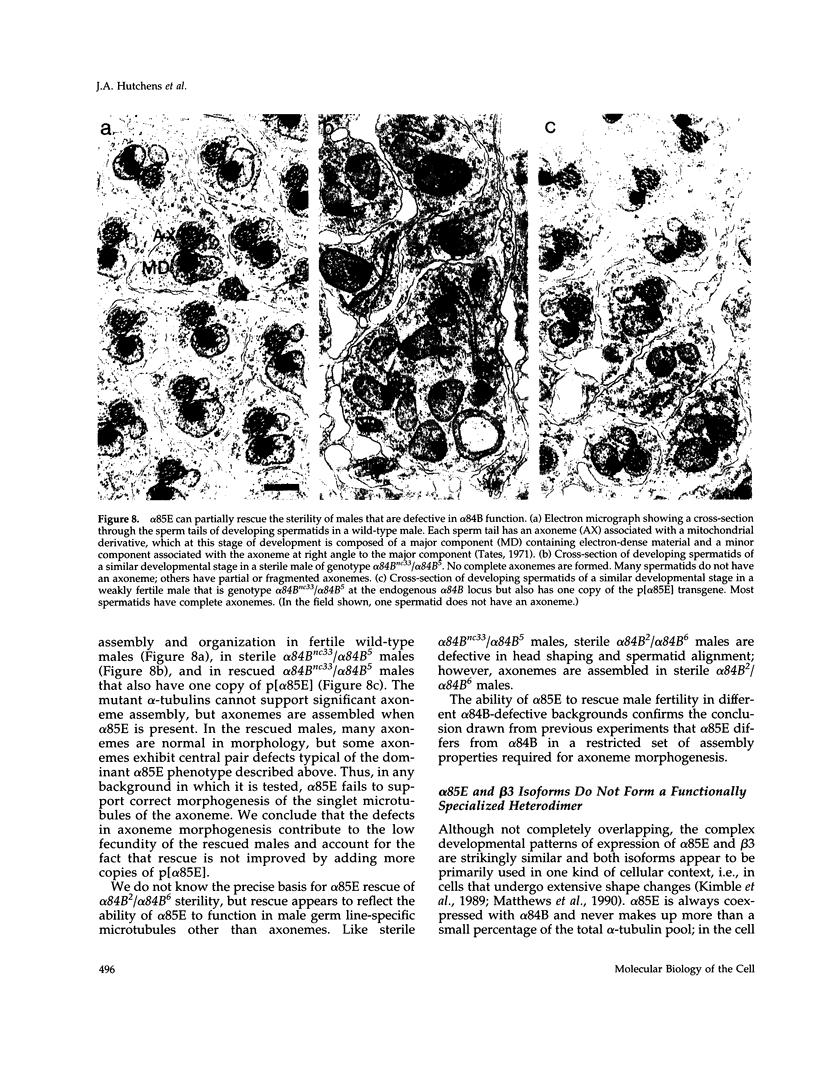
Abstract
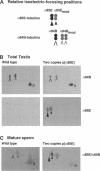
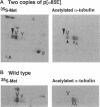
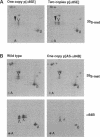
We used transgenic analysis in Drosophila to compare the ability of two structurally similar alpha-tubulin isoforms to support microtubule assembly in vivo. Our data revealed that even closely related alpha-tubulin isoforms have different functional capacities. Thus, in multicellular organisms, even small changes in tubulin structure may have important consequences for regulation of the microtubule cytoskeleton. In spermatogenesis, all microtubule functions in the postmitotic male germ cells are carried out by a single tubulin heterodimer composed of the major Drosophila alpha-84B tubulin isoform and the testis-specific beta 2-tubulin isoform. We tested the ability of the developmentally regulated alpha 85E-tubulin isoform to replace alpha 84B in spermatogenesis. Even though it is 98% similar in sequence, alpha 85E is not functionally equivalent to alpha 84B. alpha 85E can support some functional microtubules in the male germ cells, but alpha 85E causes dominant male sterility if it makes up more than one-half of the total alpha-tubulin pool in the spermatids. alpha 85E does not disrupt meiotic spindle or cytoplasmic microtubules but causes defects in morphogenesis of the two classes of singlet microtubules in the sperm tail axoneme, the central pair and the accessory microtubules. Axonemal defects caused by alpha 85E are precisely reciprocal to dominant defects in doublet microtubules we observed in a previous study of ectopic germ-line expression of the developmentally regulated beta 3-tubulin isoform. These data demonstrate that the doublet and singlet axoneme microtubules have different requirements for alpha- and beta-tubulin structure. In their normal sites of expression, alpha 85E and beta 3 are coexpressed during differentiation of several somatic cell types, suggesting that alpha 85E and beta 3 might form a specialized heterodimer. Our tests of different alpha-beta pairs in spermatogenesis did not support this model. We conclude that if alpha 85E and beta 3 have specialized properties required for their normal functions, they act independently to modulate the properties of microtubules into which they are incorporated.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audebert S., Desbruyères E., Gruszczynski C., Koulakoff A., Gros F., Denoulet P., Eddé B. Reversible polyglutamylation of alpha- and beta-tubulin and microtubule dynamics in mouse brain neurons. Mol Biol Cell. 1993 Jun;4(6):615–626. doi: 10.1091/mbc.4.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bré M. H., de Néchaud B., Wolff A., Fleury A. Glutamylated tubulin probed in ciliates with the monoclonal antibody GT335. Cell Motil Cytoskeleton. 1994;27(4):337–349. doi: 10.1002/cm.970270406. [DOI] [PubMed] [Google Scholar]
- Dettman R. W., Turner F. R., Raff E. C. Genetic analysis of the Drosophila beta3-tubulin gene demonstrates that the microtubule cytoskeleton in the cells of the visceral mesoderm is required for morphogenesis of the midgut endoderm. Dev Biol. 1996 Jul 10;177(1):117–135. doi: 10.1006/dbio.1996.0150. [DOI] [PubMed] [Google Scholar]
- Eddé B., Rossier J., Le Caer J. P., Berwald-Netter Y., Koulakoff A., Gros F., Denoulet P. A combination of posttranslational modifications is responsible for the production of neuronal alpha-tubulin heterogeneity. J Cell Biochem. 1991 Jun;46(2):134–142. doi: 10.1002/jcb.240460207. [DOI] [PubMed] [Google Scholar]
- Eddé B., Rossier J., Le Caer J. P., Desbruyères E., Gros F., Denoulet P. Posttranslational glutamylation of alpha-tubulin. Science. 1990 Jan 5;247(4938):83–85. doi: 10.1126/science.1967194. [DOI] [PubMed] [Google Scholar]
- Fackenthal J. D., Hutchens J. A., Turner F. R., Raff E. C. Structural analysis of mutations in the Drosophila beta 2-tubulin isoform reveals regions in the beta-tubulin molecular required for general and for tissue-specific microtubule functions. Genetics. 1995 Jan;139(1):267–286. doi: 10.1093/genetics/139.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackenthal J. D., Turner F. R., Raff E. C. Tissue-specific microtubule functions in Drosophila spermatogenesis require the beta 2-tubulin isotype-specific carboxy terminus. Dev Biol. 1993 Jul;158(1):213–227. doi: 10.1006/dbio.1993.1180. [DOI] [PubMed] [Google Scholar]
- Fouquet J. P., Edde B., Kann M. L., Wolff A., Desbruyeres E., Denoulet P. Differential distribution of glutamylated tubulin during spermatogenesis in mammalian testis. Cell Motil Cytoskeleton. 1994;27(1):49–58. doi: 10.1002/cm.970270106. [DOI] [PubMed] [Google Scholar]
- Fuller M. T., Caulton J. H., Hutchens J. A., Kaufman T. C., Raff E. C. Genetic analysis of microtubule structure: a beta-tubulin mutation causes the formation of aberrant microtubules in vivo and in vitro. J Cell Biol. 1987 Mar;104(3):385–394. doi: 10.1083/jcb.104.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller M. T., Caulton J. H., Hutchens J. A., Kaufman T. C., Raff E. C. Mutations that encode partially functional beta 2 tubulin subunits have different effects on structurally different microtubule arrays. J Cell Biol. 1988 Jul;107(1):141–152. doi: 10.1083/jcb.107.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays T. S., Deuring R., Robertson B., Prout M., Fuller M. T. Interacting proteins identified by genetic interactions: a missense mutation in alpha-tubulin fails to complement alleles of the testis-specific beta-tubulin gene of Drosophila melanogaster. Mol Cell Biol. 1989 Mar;9(3):875–884. doi: 10.1128/mcb.9.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelrigg T., Kaufman T. C. Revertants of Dominant Mutations Associated with the Antennapedia Gene Complex of DROSOPHILA MELANOGASTER: Cytology and Genetics. Genetics. 1983 Nov;105(3):581–600. doi: 10.1093/genetics/105.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle H. D., Hutchens J. A., Turner F. R., Raff E. C. Regulation of beta-tubulin function and expression in Drosophila spermatogenesis. Dev Genet. 1995;16(2):148–170. doi: 10.1002/dvg.1020160208. [DOI] [PubMed] [Google Scholar]
- Hoyle H. D., Raff E. C. Two Drosophila beta tubulin isoforms are not functionally equivalent. J Cell Biol. 1990 Sep;111(3):1009–1026. doi: 10.1083/jcb.111.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues K. J., Kaufman T. C., Raff R. A., Raff E. C. The testis-specific beta-tubulin subunit in Drosophila melanogaster has multiple functions in spermatogenesis. Cell. 1982 Dec;31(3 Pt 2):655–670. doi: 10.1016/0092-8674(82)90321-x. [DOI] [PubMed] [Google Scholar]
- Kimble M., Dettman R. W., Raff E. C. The beta 3-tubulin gene of Drosophila melanogaster is essential for viability and fertility. Genetics. 1990 Dec;126(4):991–1005. doi: 10.1093/genetics/126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble M., Incardona J. P., Raff E. C. A variant beta-tubulin isoform of Drosophila melanogaster (beta 3) is expressed primarily in tissues of mesodermal origin in embryos and pupae, and is utilized in populations of transient microtubules. Dev Biol. 1989 Feb;131(2):415–429. doi: 10.1016/s0012-1606(89)80014-4. [DOI] [PubMed] [Google Scholar]
- Kirk K. E., Morris N. R. Either alpha-tubulin isogene product is sufficient for microtubule function during all stages of growth and differentiation in Aspergillus nidulans. Mol Cell Biol. 1993 Aug;13(8):4465–4476. doi: 10.1128/mcb.13.8.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Hernault S. W., Rosenbaum J. L. Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry. 1985 Jan 15;24(2):473–478. doi: 10.1021/bi00323a034. [DOI] [PubMed] [Google Scholar]
- LeDizet M., Piperno G. Identification of an acetylation site of Chlamydomonas alpha-tubulin. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5720–5724. doi: 10.1073/pnas.84.16.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K. A., Kaufman T. C. Developmental consequences of mutations in the 84B alpha-tubulin gene of Drosophila melanogaster. Dev Biol. 1987 Jan;119(1):100–114. doi: 10.1016/0012-1606(87)90211-9. [DOI] [PubMed] [Google Scholar]
- Matthews K. A., Miller D. F., Kaufman T. C. Developmental distribution of RNA and protein products of the Drosophila alpha-tubulin gene family. Dev Biol. 1989 Mar;132(1):45–61. doi: 10.1016/0012-1606(89)90203-0. [DOI] [PubMed] [Google Scholar]
- Matthews K. A., Miller D. F., Kaufman T. C. Functional implications of the unusual spatial distribution of a minor alpha-tubulin isotype in Drosophila: a common thread among chordotonal ligaments, developing muscle, and testis cyst cells. Dev Biol. 1990 Jan;137(1):171–183. doi: 10.1016/0012-1606(90)90018-e. [DOI] [PubMed] [Google Scholar]
- Matthews K. A., Rees D., Kaufman T. C. A functionally specialized alpha-tubulin is required for oocyte meiosis and cleavage mitoses in Drosophila. Development. 1993 Mar;117(3):977–991. doi: 10.1242/dev.117.3.977. [DOI] [PubMed] [Google Scholar]
- May G. S. The highly divergent beta-tubulins of Aspergillus nidulans are functionally interchangeable. J Cell Biol. 1989 Nov;109(5):2267–2274. doi: 10.1083/jcb.109.5.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels F., Gasch A., Kaltschmidt B., Renkawitz-Pohl R. A 14 bp promoter element directs the testis specificity of the Drosophila beta 2 tubulin gene. EMBO J. 1989 May;8(5):1559–1565. doi: 10.1002/j.1460-2075.1989.tb03540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels F., Wolk A., Renkawitz-Pohl R. Further sequence requirements for male germ cell-specific expression under the control of the 14 bp promoter element (beta 2UE1) of the Drosophila beta 2 tubulin gene. Nucleic Acids Res. 1991 Aug 25;19(16):4515–4521. doi: 10.1093/nar/19.16.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., Fuller M. T. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol. 1985 Dec;101(6):2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff E. C., Fackenthal J. D., Hutchens J. A., Hoyle H. D., Turner F. R. Microtubule architecture specified by a beta-tubulin isoform. Science. 1997 Jan 3;275(5296):70–73. doi: 10.1126/science.275.5296.70. [DOI] [PubMed] [Google Scholar]
- Raff E. C., Fuller M. T., Kaufman T. C., Kemphues K. J., Rudolph J. E., Raff R. A. Regulation of tubulin gene expression during embryogenesis in Drosophila melanogaster. Cell. 1982 Jan;28(1):33–40. doi: 10.1016/0092-8674(82)90372-5. [DOI] [PubMed] [Google Scholar]
- Robertson H. M., Preston C. R., Phillis R. W., Johnson-Schlitz D. M., Benz W. K., Engels W. R. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics. 1988 Mar;118(3):461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph J. E., Kimble M., Hoyle H. D., Subler M. A., Raff E. C. Three Drosophila beta-tubulin sequences: a developmentally regulated isoform (beta 3), the testis-specific isoform (beta 2), and an assembly-defective mutation of the testis-specific isoform (B2t8) reveal both an ancient divergence in metazoan isotypes and structural constraints for beta-tubulin function. Mol Cell Biol. 1987 Jun;7(6):2231–2242. doi: 10.1128/mcb.7.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüdiger M., Plessman U., Klöppel K. D., Wehland J., Weber K. Class II tubulin, the major brain beta tubulin isotype is polyglutamylated on glutamic acid residue 435. FEBS Lett. 1992 Aug 10;308(1):101–105. doi: 10.1016/0014-5793(92)81061-p. [DOI] [PubMed] [Google Scholar]
- Rüdiger M., Plessmann U., Rüdiger A. H., Weber K. Beta tubulin of bull sperm is polyglycylated. FEBS Lett. 1995 May 8;364(2):147–151. doi: 10.1016/0014-5793(95)00373-h. [DOI] [PubMed] [Google Scholar]
- Schatz P. J., Solomon F., Botstein D. Genetically essential and nonessential alpha-tubulin genes specify functionally interchangeable proteins. Mol Cell Biol. 1986 Nov;6(11):3722–3733. doi: 10.1128/mcb.6.11.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez F., Natzle J. E., Cleveland D. W., Kirschner M. W., McCarthy B. J. A dispersed multigene family encoding tubulin in Drosophila melanogaster. Cell. 1980 Dec;22(3):845–854. doi: 10.1016/0092-8674(80)90561-9. [DOI] [PubMed] [Google Scholar]
- Theurkauf W. E., Baum H., Bo J., Wensink P. C. Tissue-specific and constitutive alpha-tubulin genes of Drosophila melanogaster code for structurally distinct proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8477–8481. doi: 10.1073/pnas.83.22.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf W. E. Behavior of structurally divergent alpha-tubulin isotypes during Drosophila embryogenesis: evidence for post-translational regulation of isotype abundance. Dev Biol. 1992 Nov;154(1):205–217. doi: 10.1016/0012-1606(92)90060-t. [DOI] [PubMed] [Google Scholar]