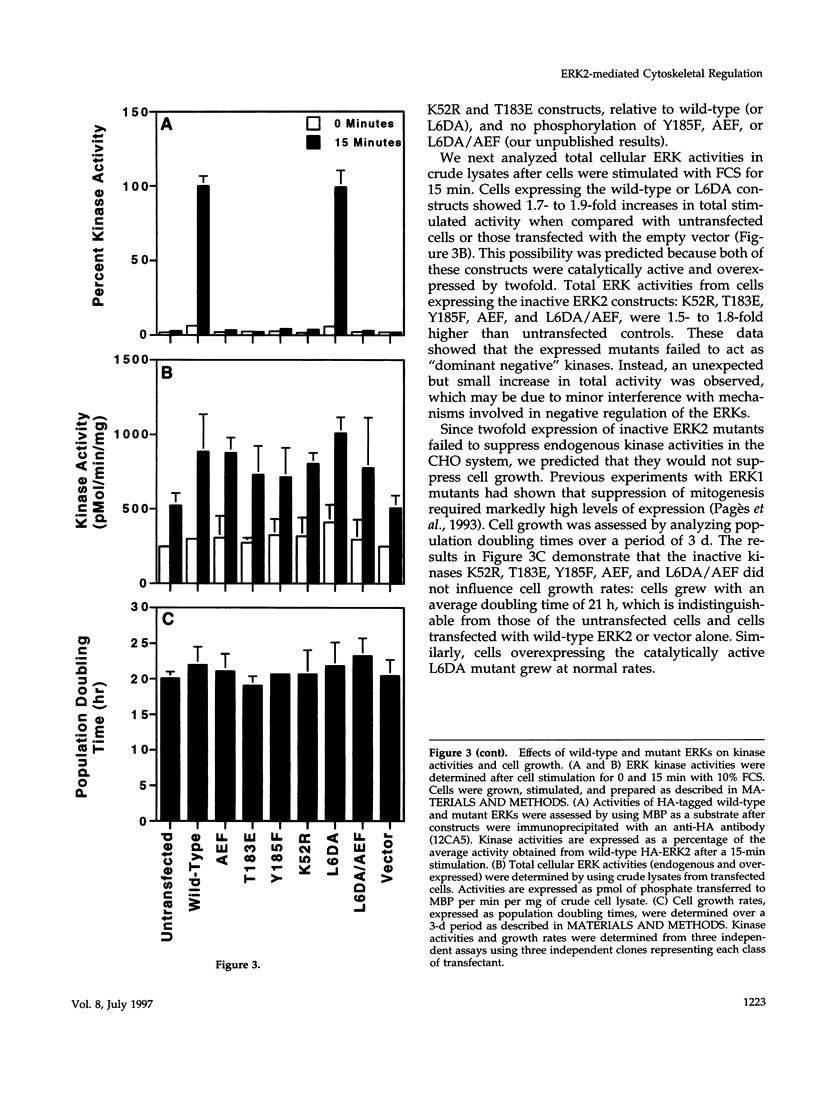
Abstract
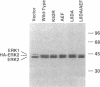
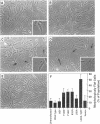
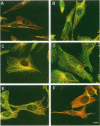
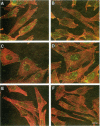
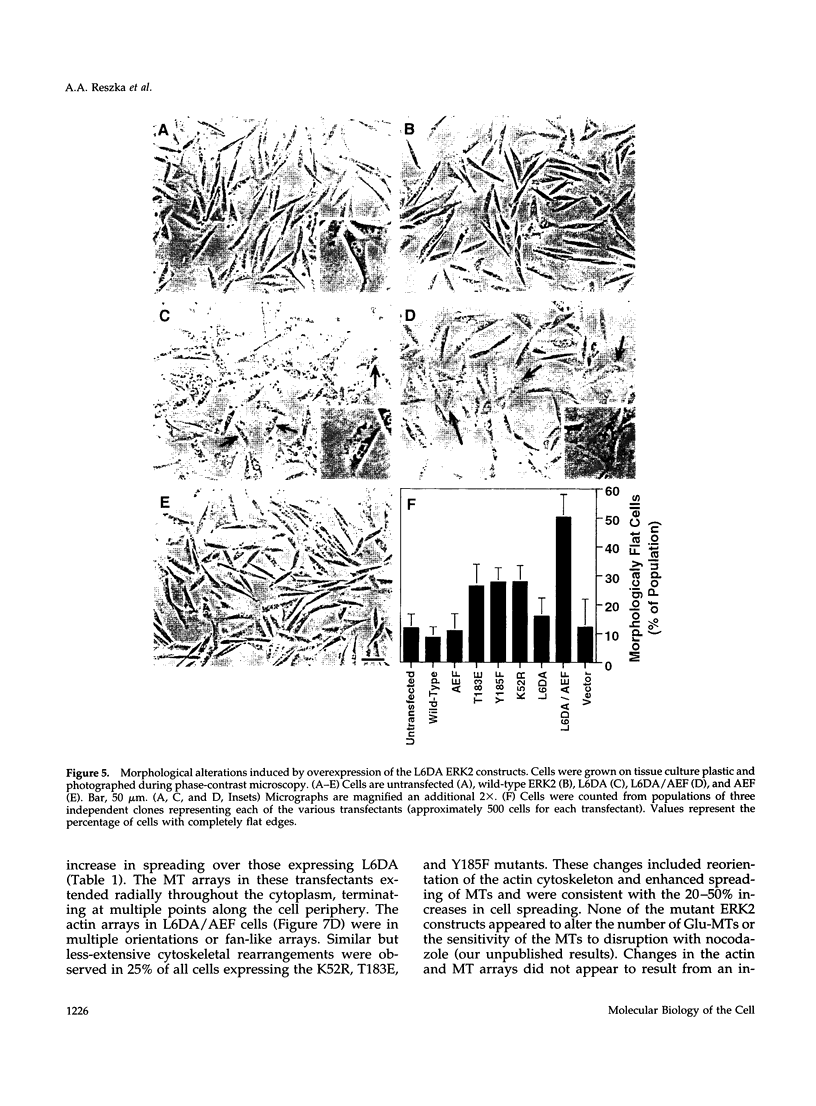
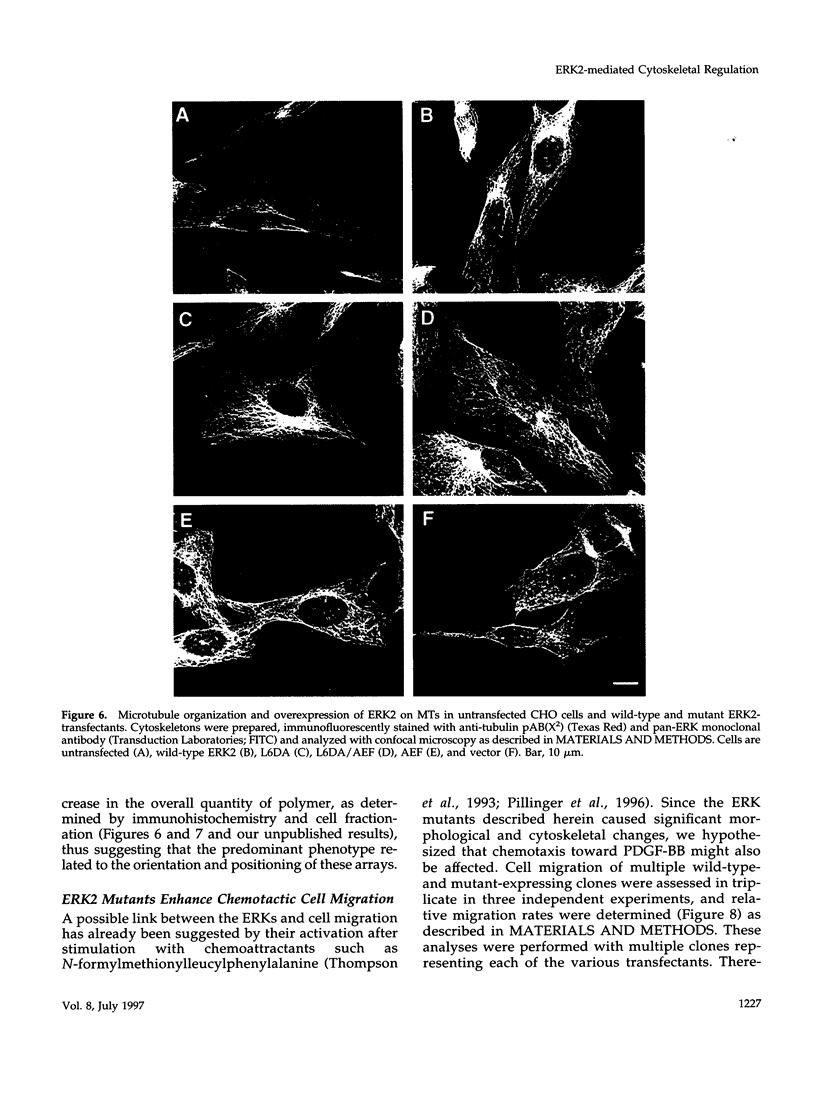
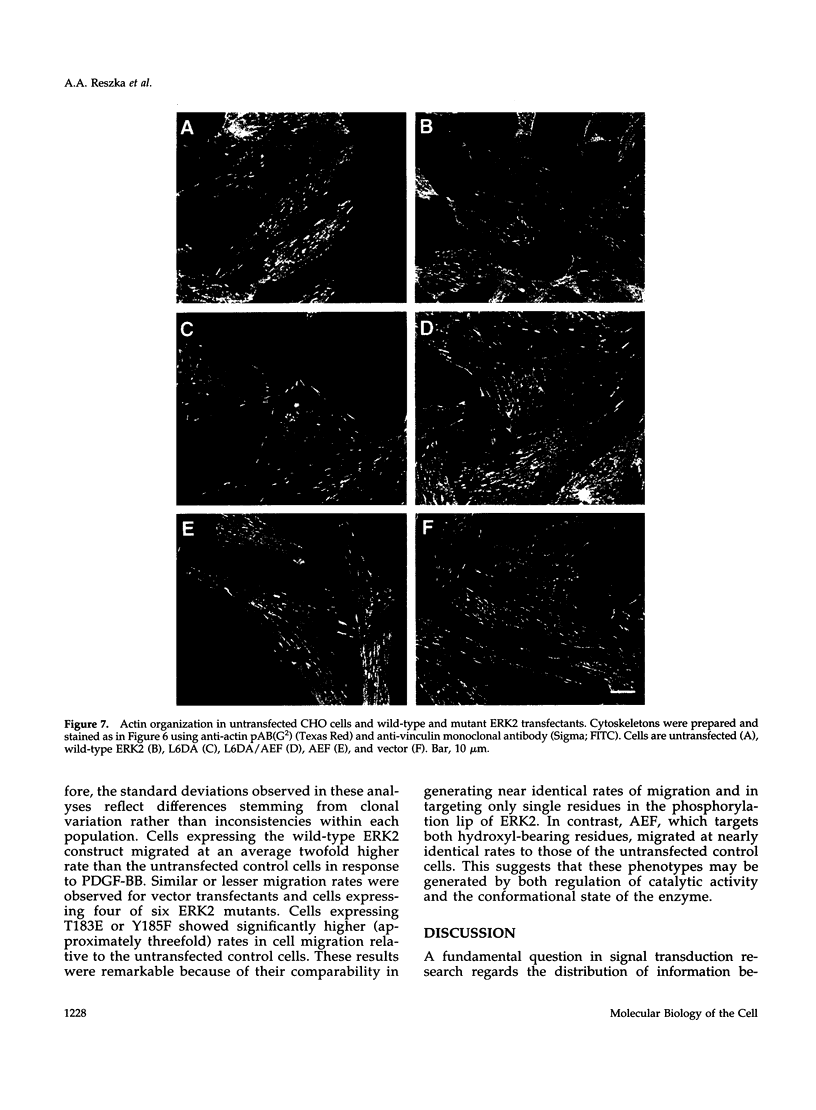
The extracellular signal-regulated kinases (ERKs) 1 and 2 are mitogen-activated protein kinases that act as key components in a signaling cascade linking growth factor receptors to the cytoskeleton and the nucleus. ERK2 mutants have been used to alter cytoskeletal regulation in Chinese hamster ovary cells without affecting cell growth or feedback signaling. Mutation of the unique loop L6 (residues 91-95), which is in a portion of the molecule that is cryptic upon the binding of ERK2 to the microtubules (MTs), generated significant morphological alterations. Most notable phenotypes were observed after expression of a combined mutant incorporating changes to both L6 and the TEY phosphorylation lip, including a 70% increase in cell spreading. Actin stress fibers in these cells, which normally formed a single broad parallel array, were arranged in three or more orientations or in fan-like arrays. MTs, which ordinarily extend longitudinally from the centrosome, spread radially, covering a larger surface area. Single, but not the double, mutations of the Thr and Tyr residues of the TEY phosphorylation lip caused a ca. 25% increase in cell spreading, accompanied by a threefold increase in chemotactic cell migration. Mutation of Lys-52 triggered a 48% increase in cell spreading but no alteration to chemotaxis. These findings suggest that wild-type ERK2 inhibits the organization of the cytoskeleton, the spreading of the cell, and chemotactic migration. This involves control of the orientation of actin and MTs and the positioning of focal adhesions via regulatory interactions that may occur on the MTs.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagrodia S., Dérijard B., Davis R. J., Cerione R. A. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995 Nov 24;270(47):27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- Bornfeldt K. E., Raines E. W., Nakano T., Graves L. M., Krebs E. G., Ross R. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J Clin Invest. 1994 Mar;93(3):1266–1274. doi: 10.1172/JCI117081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski J. C., Borisy G. G. Microtubule-associated proteins from cultured HeLa cells. Analysis of molecular properties and effects on microtubule polymerization. J Biol Chem. 1980 Dec 10;255(23):11570–11576. [PubMed] [Google Scholar]
- Chen Q., Kinch M. S., Lin T. H., Burridge K., Juliano R. L. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994 Oct 28;269(43):26602–26605. [PubMed] [Google Scholar]
- Cherniack A. D., Klarlund J. K., Czech M. P. Phosphorylation of the Ras nucleotide exchange factor son of sevenless by mitogen-activated protein kinase. J Biol Chem. 1994 Feb 18;269(7):4717–4720. [PubMed] [Google Scholar]
- Childs T. J., Watson M. H., Sanghera J. S., Campbell D. L., Pelech S. L., Mak A. S. Phosphorylation of smooth muscle caldesmon by mitogen-activated protein (MAP) kinase and expression of MAP kinase in differentiated smooth muscle cells. J Biol Chem. 1992 Nov 15;267(32):22853–22859. [PubMed] [Google Scholar]
- Clark E. A., Hynes R. O. Ras activation is necessary for integrin-mediated activation of extracellular signal-regulated kinase 2 and cytosolic phospholipase A2 but not for cytoskeletal organization. J Biol Chem. 1996 Jun 21;271(25):14814–14818. doi: 10.1074/jbc.271.25.14814. [DOI] [PubMed] [Google Scholar]
- Corbalan-Garcia S., Yang S. S., Degenhardt K. R., Bar-Sagi D. Identification of the mitogen-activated protein kinase phosphorylation sites on human Sos1 that regulate interaction with Grb2. Mol Cell Biol. 1996 Oct;16(10):5674–5682. doi: 10.1128/mcb.16.10.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coso O. A., Chiariello M., Yu J. C., Teramoto H., Crespo P., Xu N., Miki T., Gutkind J. S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995 Jun 30;81(7):1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- Cowley S., Paterson H., Kemp P., Marshall C. J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994 Jun 17;77(6):841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Drechsel D. N., Hyman A. A., Cobb M. H., Kirschner M. W. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992 Oct;3(10):1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore R. S., Bayer V. E., Pelech S. L., Posada J., Cooper J. A., Baraban J. M. p42 mitogen-activated protein kinase in brain: prominent localization in neuronal cell bodies and dendrites. Neuroscience. 1993 Jul;55(2):463–472. doi: 10.1016/0306-4522(93)90516-i. [DOI] [PubMed] [Google Scholar]
- Frost J. A., Geppert T. D., Cobb M. H., Feramisco J. R. A requirement for extracellular signal-regulated kinase (ERK) function in the activation of AP-1 by Ha-Ras, phorbol 12-myristate 13-acetate, and serum. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3844–3848. doi: 10.1073/pnas.91.9.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Matsuda S., Shiina N., Kosako H., Shiokawa K., Akiyama T., Ohta K., Sakai H. In vitro effects on microtubule dynamics of purified Xenopus M phase-activated MAP kinase. Nature. 1991 Jan 17;349(6306):251–254. doi: 10.1038/349251a0. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Feld M. K. Fibronectin adsorption on hydrophilic and hydrophobic surfaces detected by antibody binding and analyzed during cell adhesion in serum-containing medium. J Biol Chem. 1982 May 10;257(9):4888–4893. [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Chemoattractant-induced tyrosine phosphorylation and activation of microtubule-associated protein kinase in human neutrophils. J Biol Chem. 1992 Sep 5;267(25):18122–18125. [PubMed] [Google Scholar]
- Hanks S. K., Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995 May;9(8):576–596. [PubMed] [Google Scholar]
- Herzog W., Weber K. Fractionation of brain microtubule-associated proteins. Isolation of two different proteins which stimulate tubulin polymerization in vitro. Eur J Biochem. 1978 Dec 1;92(1):1–8. doi: 10.1111/j.1432-1033.1978.tb12716.x. [DOI] [PubMed] [Google Scholar]
- Holt K. H., Kasson B. G., Pessin J. E. Insulin stimulation of a MEK-dependent but ERK-independent SOS protein kinase. Mol Cell Biol. 1996 Feb;16(2):577–583. doi: 10.1128/mcb.16.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi M., Ohta K., Gotoh Y., Mori A., Murofushi H., Sakai H., Nishida E. Mitogen-activated-protein-kinase-catalyzed phosphorylation of microtubule-associated proteins, microtubule-associated protein 2 and microtubule-associated protein 4, induces an alteration in their function. Eur J Biochem. 1992 Jan 15;203(1-2):43–52. doi: 10.1111/j.1432-1033.1992.tb19825.x. [DOI] [PubMed] [Google Scholar]
- Joneson T., White M. A., Wigler M. H., Bar-Sagi D. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of RAS. Science. 1996 Feb 9;271(5250):810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995 Jul 14;270(28):16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Khalil R. A., Menice C. B., Wang C. L., Morgan K. G. Phosphotyrosine-dependent targeting of mitogen-activated protein kinase in differentiated contractile vascular cells. Circ Res. 1995 Jun;76(6):1101–1108. doi: 10.1161/01.res.76.6.1101. [DOI] [PubMed] [Google Scholar]
- Lu Q., Soria J. P., Wood J. G. p44mpk MAP kinase induces Alzheimer type alterations in tau function and in primary hippocampal neurons. J Neurosci Res. 1993 Jul 1;35(4):439–444. doi: 10.1002/jnr.490350411. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. M., Drewes G., Biernat J., Gustke N., Van Lint J., Vandenheede J. R., Mandelkow E. Glycogen synthase kinase-3 and the Alzheimer-like state of microtubule-associated protein tau. FEBS Lett. 1992 Dec 21;314(3):315–321. doi: 10.1016/0014-5793(92)81496-9. [DOI] [PubMed] [Google Scholar]
- Mansour S. J., Matten W. T., Hermann A. S., Candia J. M., Rong S., Fukasawa K., Vande Woude G. F., Ahn N. G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994 Aug 12;265(5174):966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- Minden A., Lin A., Claret F. X., Abo A., Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995 Jun 30;81(7):1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M., Kosik K. S. The pool of map kinase associated with microtubules is small but constitutively active. Mol Biol Cell. 1996 Jun;7(6):893–905. doi: 10.1091/mbc.7.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes C. D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995 Apr 7;81(1):53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Metz R., Chen L., Mattéi M. G., Carrasco D., Bravo R. Structure, mapping, and expression of erp, a growth factor-inducible gene encoding a nontransmembrane protein tyrosine phosphatase, and effect of ERP on cell growth. Mol Cell Biol. 1993 Sep;13(9):5195–5205. doi: 10.1128/mcb.13.9.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagès G., Lenormand P., L'Allemain G., Chambard J. C., Meloche S., Pouysségur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillinger M. H., Feoktistov A. S., Capodici C., Solitar B., Levy J., Oei T. T., Philips M. R. Mitogen-activated protein kinase in neutrophils and enucleate neutrophil cytoplasts: evidence for regulation of cell-cell adhesion. J Biol Chem. 1996 May 17;271(20):12049–12056. doi: 10.1074/jbc.271.20.12049. [DOI] [PubMed] [Google Scholar]
- Redwood C. S., Marston S. B., Gusev N. B. The functional effects of mutations Thr673-->Asp and Ser702-->Asp at the Pro-directed kinase phosphorylation sites in the C-terminus of chicken gizzard caldesmon. FEBS Lett. 1993 Jul 19;327(1):85–89. doi: 10.1016/0014-5793(93)81045-2. [DOI] [PubMed] [Google Scholar]
- Reszka A. A., Seger R., Diltz C. D., Krebs E. G., Fischer E. H. Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8881–8885. doi: 10.1073/pnas.92.19.8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992 Aug 7;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Robbins D. J., Zhen E., Owaki H., Vanderbilt C. A., Ebert D., Geppert T. D., Cobb M. H. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J Biol Chem. 1993 Mar 5;268(7):5097–5106. [PubMed] [Google Scholar]
- Robinson M. J., Harkins P. C., Zhang J., Baer R., Haycock J. W., Cobb M. H., Goldsmith E. J. Mutation of position 52 in ERK2 creates a nonproductive binding mode for adenosine 5'-triphosphate. Biochemistry. 1996 May 7;35(18):5641–5646. doi: 10.1021/bi952723e. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., van der Geer P., Mbamalu G., Pawson T. MAP kinase phosphorylation of mSos1 promotes dissociation of mSos1-Shc and mSos1-EGF receptor complexes. Oncogene. 1995 Oct 5;11(7):1417–1426. [PubMed] [Google Scholar]
- Seger R., Seger D., Reszka A. A., Munar E. S., Eldar-Finkelman H., Dobrowolska G., Jensen A. M., Campbell J. S., Fischer E. H., Krebs E. G. Overexpression of mitogen-activated protein kinase kinase (MAPKK) and its mutants in NIH 3T3 cells. Evidence that MAPKK involvement in cellular proliferation is regulated by phosphorylation of serine residues in its kinase subdomains VII and VIII. J Biol Chem. 1994 Oct 14;269(41):25699–25709. [PubMed] [Google Scholar]
- Shiina N., Moriguchi T., Ohta K., Gotoh Y., Nishida E. Regulation of a major microtubule-associated protein by MPF and MAP kinase. EMBO J. 1992 Nov;11(11):3977–3984. doi: 10.1002/j.1460-2075.1992.tb05491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. On the crawling of animal cells. Science. 1993 May 21;260(5111):1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Thompson H. L., Shiroo M., Saklatvala J. The chemotactic factor N-formylmethionyl-leucyl-phenylalanine activates microtubule-associated protein 2 (MAP) kinase and a MAP kinase kinase in polymorphonuclear leucocytes. Biochem J. 1993 Mar 1;290(Pt 2):483–488. doi: 10.1042/bj2900483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlhac M. H., Kubiak J. Z., Clarke H. J., Maro B. Microtubule and chromatin behavior follow MAP kinase activity but not MPF activity during meiosis in mouse oocytes. Development. 1994 Apr;120(4):1017–1025. doi: 10.1242/dev.120.4.1017. [DOI] [PubMed] [Google Scholar]
- Verlhac M. H., Kubiak J. Z., Weber M., Géraud G., Colledge W. H., Evans M. J., Maro B. Mos is required for MAP kinase activation and is involved in microtubule organization during meiotic maturation in the mouse. Development. 1996 Mar;122(3):815–822. doi: 10.1242/dev.122.3.815. [DOI] [PubMed] [Google Scholar]
- Verlhac M. H., de Pennart H., Maro B., Cobb M. H., Clarke H. J. MAP kinase becomes stably activated at metaphase and is associated with microtubule-organizing centers during meiotic maturation of mouse oocytes. Dev Biol. 1993 Aug;158(2):330–340. doi: 10.1006/dbio.1993.1192. [DOI] [PubMed] [Google Scholar]
- Wang X. M., Peloquin J. G., Zhai Y., Bulinski J. C., Borisy G. G. Removal of MAP4 from microtubules in vivo produces no observable phenotype at the cellular level. J Cell Biol. 1996 Feb;132(3):345–357. doi: 10.1083/jcb.132.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wary K. K., Mainiero F., Isakoff S. J., Marcantonio E. E., Giancotti F. G. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996 Nov 15;87(4):733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Waters S. B., Holt K. H., Ross S. E., Syu L. J., Guan K. L., Saltiel A. R., Koretzky G. A., Pessin J. E. Desensitization of Ras activation by a feedback disassociation of the SOS-Grb2 complex. J Biol Chem. 1995 Sep 8;270(36):20883–20886. doi: 10.1074/jbc.270.36.20883. [DOI] [PubMed] [Google Scholar]
- Worthen G. S., Avdi N., Buhl A. M., Suzuki N., Johnson G. L. FMLP activates Ras and Raf in human neutrophils. Potential role in activation of MAP kinase. J Clin Invest. 1994 Aug;94(2):815–823. doi: 10.1172/JCI117401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Strand A., Robbins D., Cobb M. H., Goldsmith E. J. Atomic structure of the MAP kinase ERK2 at 2.3 A resolution. Nature. 1994 Feb 24;367(6465):704–711. doi: 10.1038/367704a0. [DOI] [PubMed] [Google Scholar]
- Zhang J., Zhang F., Ebert D., Cobb M. H., Goldsmith E. J. Activity of the MAP kinase ERK2 is controlled by a flexible surface loop. Structure. 1995 Mar 15;3(3):299–307. doi: 10.1016/s0969-2126(01)00160-5. [DOI] [PubMed] [Google Scholar]
- Zhang S., Han J., Sells M. A., Chernoff J., Knaus U. G., Ulevitch R. J., Bokoch G. M. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J Biol Chem. 1995 Oct 13;270(41):23934–23936. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]