Abstract
The p38 mitogen-activated protein (MAP) kinases (p38) are important signaling molecules that regulate various cellular processes. Four isoforms of p38 family, p38α, p38β, p38γ, and p38δ, have been identified in mammalian cells. Previous studies have shown that p38α knockout is embryonic lethal in mice. At the cellular level, p38α is abundantly expressed in mouse embryonic stem cells (ESCs), but p38α knockout (p38α−/−) ESCs can differentiate to endothelial cells (ECs), smooth muscle cells (SMCs), and neurons. We speculate that the lost function of p38α in p38α−/−ESCs may be compensated for by the redundant function of other isoforms. To test this hypothesis, we used siRNA approach to knock down the expression of p38δ, the second abundant isoform in ESCs. ESCs stably expressing p38δ siRNA were established from p38α−/−ESCs, resulting in 80% reduction of p38δ mRNA expression. However, these ESCs, deficient of both p38α and p38δ, could still differentiate into ECs and SMCs. We extended our investigation to test if these cells can differentiate into epithelial cells in which p38δ has been shown to regulate epidermis differentiation. Our results demonstrate again that ESC differentiation to epithelial cells is independent of p38α and p38δ. We conclude that p38α and p38δ are not essential for ESC differentiating into ECs, SMCs, or epithelial cells although numerous studies have shown that the two kinases regulate various cellular activities in aforementioned cells. Our results highlight the possibility that p38 MAP kinases may play less significant roles in ESC differentiation than in the regulation of cellular activities of fully differentiated somatic cells.
Keywords: p38 MAP kinases, Differentiation, Embryonic stem cells, Endothelial cells, Smooth muscle cells, Epithelial cells
1. Introduction
Mitogen-activated protein (MAP) kinases are widely expressed kinases that regulate a wide variety of cellular activities. Three distinct subtypes of MAP kinases have been characterized. Extracellular signal-regulated kinases (ERK) are strongly activated by growth factors, thus they are critical for cell proliferation (Cobb et al., 1991). Two other subtypes, known as stress-activated protein kinases, are c-Jun N-terminal protein kinases (JNK) and p38 MAP kinases. They are strongly activated by cytokines and cellular stresses, and generally promote inhibition of cell growth and induce apoptosis (Kyriakis and Avruch, 1996). However, it is now recognized that MAP kinases represent a family of signaling molecules that have many diverse functions (Nebreda and Porras, 2000). Four p38 isoforms have been identified in mammalian cells. They are named p38α, p38β, p38γ, and p38δ (Ono and Han, 2000). Depending on cell types and the nature of stimuli, different isoforms may have redundant, specific, or even opposite functions (Nebreda and Porras, 2000; Ono and Han, 2000). Most of the functions described in the literature are those of p38α or p38β, or both due to the availability of pyridinylimidazole derivatives (such as SB203580) (Jackson et al., 1998) as selective inhibitors of p38α and p38β without effect on p38γ or p38δ. In comparison with p38α and p38β, we know much less about p38γ and p38δ.
Most of our knowledge of p38 MAP kinases is derived from cultured somatic cells. Little is known about their roles in developmental biology. The generation of p38 knockout mice has provided some important insight. A pivotal role of p38α in the regulation of growth and development is suggested in animal models by the finding that p38α knockout is embryonic lethal (Adams et al., 2000). However, further studies revealed that the lethality of p38α knockout is mainly due to the defective placental organogenesis since the p38α−/− mouse embryos could be rescued by tetraploid complementation and they could develop to term with normal appearance (Adams et al., 2000). This suggests that p38α may not be essential for mouse development once the embryos are independent of the placenta for nutrient supply. This is surprising since it is difficult to envision that such a widely expressed kinase in various cells is dispensable for embryo development except for placental formation. In contrast to p38α knockout, p38β−/−mice are viable and exhibit no apparent health problems (Beardmore et al., 2005). p38β−/− mice showed normal T-cell development and responses to TNF and LPS in the immune system. It is concluded that p38β is not critical for overall growth and development (Beardmore et al., 2005). Similarly, p38γ knockout, p38δ knockout, and p38γ/δ double knockout mice are viable without apparent phenotypic abnormality suggesting that neither p38γ, p38δ, nor p38γ/p38δ, is essential for embryogenesis (Sabio et al., 2005).
Gene knockout technique represents one of the most significant advances in the study of gene functions in animal models. This approach has been recently extended to embryonic stem cells (ESCs), which can differentiate to various cell types, mimicking in vivo differentiation process. Gene knockout in ESCs have proven to be a useful in vitro model to analyze developmental roles of specific genes at the cellular level and the results can provide important complimentary information to in vivo knockout animal studies. For instance, using a VEGF receptor (FLK) knockout (flk-1−/−) ESC line, Schuh et al. (Schuh et al., 1999) have found the specific defective steps in endothelial differentiation and vessel assembly caused by flk deletion. These results not only demonstrate the importance of VEGF signaling in endothelial differentiation and vascular development at cellular level, but also support and complement the findings from in vivo studies (Shalaby et al., 1995). The knowledge of p38 isoforms from knockout mice has provided valuable information of their relative importance during embryogenesis, but we have little knowledge about their developmental roles at the cellular level. The embryonic lethality limits further in-depth analysis of the developmental role of p38α at the cellular level in animal models, but the generation of p38α−/− ESCs (Roach et al., 1995; Kim et al., 2005) provides a valuable alternative system. Taking the advantage of the availability of p38α−/−ESCs, we have attempted to elucidate its developmental roles. We have shown that p38α−/− ESCs display altered cell adhesion to different extracellular matrix proteins (Guo and Yang, 2006), but they can differentiate into endothelial cells (ECs), smooth muscle cells (SMCs), and neurons (Guo et al., 2007). Furthermore, p38α−/− ESC-differentiated cells can express cell adhesion molecules in response to TNF-α stimulus, similar to cells derived from wild type ESCs (Rajan et al., 2008). The current study extends our investigation to p38δ in ESC differentiation. We have found that, like p38α, p38δ is dispensable for ESC differentiation. We are somewhat surprised by this finding since numerous studies have shown the importance of p38α and p38δ in the regulation of various cellular activities of the aforementioned cells in culture. Nevertheless, the data reported in the current and our previous studies (Guo et al., 2007; Rajan et al., 2008) suggest the possibility that p38 MAP kinases may not be critical for ESC differentiation process per se although they may play prominent roles in the regulation of various cellular activities in differentiated somatic cells (Kyriakis and Avruch, 2001). This conclusion is in fact consistent with the results obtained from knockout animals (Adams et al., 2000; Beardmore et al., 2005; Sabio et al., 2005)and provides support at the cellular level for the hypothesis that the embryonic lethality of p38α knockout is a result of insufficient nutrient/oxygen supply due to the defective placental organogenesis, but not a direct effect of p38α knockout on the overall embryo developmental process (Adams et al., 2000). Our results highlight the importance of rational interpretation of the results obtained from in vivo gene knockout studies (especially when a gene knockout is embryonic lethal) and those from in vitro cellular and biochemical studies.
2. Materials and methods
2.1. ESC culture
Generation of p38α+/+ and p38α−/− ESCs has been previously described (Roach et al., 1995; Allen et al., 2000). They were maintained in DMEM containing 15% fetal bovine serum (FBS) and 1000 U/ml leukemia inhibitory factor (LIF) (Guo et al., 2007). ESCs were routinely maintained in cell culture dishes coated with 0.1% gelatin at 37 °C in a humidified atmosphere at 5% CO2. Cell culture medium was refreshed every other day.
2.2. Construction of p38δ shRNA plasmid, cell transfection, and selection of cells stably expressing shRNA
Twoo ligos, 5′GCAGTGGATCCGGCCAAATCCTATATTCAGTTCAAGAGACTGAATATAGGATTT-GGCCTTTTTGGAAAAGCTTGCTGC-3′ and 5′GCAGCAAGCTTTTCCAAAAAGGCCAAATCCTATATTCAGTCTCTTGAACTGAA-TATAGGATTTGGCCGGATCCACTGC-3′, were used for shRNA plasmid construction. They were synthesized by Integrated DNA Technologies and purified by 8% polyacrylamide gel electrophoresis. The two fragments at 100 μM were denatured at 95 °C for 1 min and allowed to cool down for annealing for 10 min. The double stranded (ds) DNA fragment was than purified using agarose gel. The purified dsDNA was first digested with BamHI in a buffer containing 50mM Tris-HCl (pH7.9), 100mM NaCl, 10mM MgCl2, and 1mM dithiothreitol at 37 °C for 4–5 h, followed by precipitation with 7.5 M ammonium acetate and ethanol. After the precipitation, the dsDNA was subjected to the second digestion with HindIII in a buffer containing 10mM Tris-HCl (pH7.9), 50mM NaCl, 10mM MgCl2, 1mM dithiothreitol at 37 °C for 4–5 h, followed by ammonium acetate and ethanol precipitation. The resulting dsDNA fragment was suspended in 15 μl of sterile water. The pSilencer 2.1 U6 hygro vector was also digested sequentially with BamHI and HindIII in the similar fashion. After the digestion and ethanol precipitation, the concentration of both the digested vector and the digested DNA fragment were determined using a spectrophotometer. The digested vector and the digested DNA fragment were taken in the exact ratio of 1:5 and ligated using T4 DNA ligase overnight at 16 °C. The ligation mixture was than transformed into E. coli DH5α strain for amplification of the recombinant plasmid. The recombinant pSilencer 2.1 U6 hygro p38δ was verified by sequencing (SEQWRIGHT Inc).
p38α−/−ESCs (2.4×105) were transfected with siRNAp38δ plasmid DNA (8 μg) in a 25 cm2 flask using Lipofectamine according to the protocols recommended by the manufacturer (Invitrogen). After transfection for 48 h, cells were selected with ESC complete medium containing 200 μg/ml hygromycin B for two weeks. The selected (surviving) cells can stably express siRNAp38δ and were designated as p38α−/−siδ ESCs. Control cells were generated in parallel by transfecting p38α−/−ESCs with a plasmid encoding a universal negative control sequence (Ambion) and were designated as p38α−/−siCON ESCs.
2.3. ESC differentiation
ESC differentiation was performed as previously described (Guo et al., 2007). Briefly, ESCs went through two consecutive steps of differentiation. ESCs (1×105 cells/ml) were first allowed to differentiate in embryoid bodies (EBs). ESCs were suspended in a bacterial culture dish in which ESCs clumped and formed EBs. After incubation for 24 h, the medium was changed to LIF-free medium containing 15% FBS to initiate cell differentiation. After incubation for 5 days, EBs were allowed to further differentiate in suspension for additional 5 days to generate 10 day EBs; alternatively, 5-day old EBs were transferred to gelatin-coated cell culture dishes or coverglasses. In the latter case, EBs adhered to the culture dishes where the cells within the EBs grew out to form monolayer-like structures, which we defined as EB-derived monolayers. To test the effect of SB203580 on p38 activation and ESC differentiation, 5 μM SB203580 was added to the cell culture medium on the second day of EB formation. The medium was refreshed every other day. EBs and EB-derived monolayers were used as the sources to identify the differentiated cells and for further analyses as specified in individual experiments.
2.4. Immunocytochemistry and microscopic analysis
EB-derived monolayers were fixed with 4% paraformaldehyde and washed with PBS. Immunocytochemical analysis was performed according to our published protocols (Guo et al., 2007). Briefly, fixed EB-derived monolayers were permeabilized with PBS containing 0.25% Triton X-100 for 30 min. After being blocked in 2% bovine serum albumin and 5% preimmune serum, the cells were incubated with primary antibodies overnight at 4 °C. The positive cells were detected either by fluorescein isothiocyanate (FITC)- or rhodamine-conjugated secondary antibodies. In some experiments, the nuclei were stained with 10 μM Hoescht 33258. The cells were examined with a LSM 510 laser-scanning confocal microscope (Zeiss). Image analysis was performed using LSM Image Examiner software (Zeiss). The details for each experiment are described in the figure legend.
2.5. RNA extraction, reverse transcription, and polymerase chain reaction (RT-PCR)
Total RNA was extracted from ESCs or EBs using Tri-reagent (Sigma). cDNA was prepared by M-MLV reverse transcriptase. The specificity of the PCR was determined by the dissociation curve and confirmed by agarose gel electrophoresis. Quantitative real time PCR (RT-qPCR) was performed using SYBR green jumpstart Taq ready mix (Sigma) on a MX3000PTM Real-time PCR system (Stratagene) as previously reported (Guo et al., 2007). Sequences of the primer sets are as follows (F, forward; R, reverse)
| B-actin, F: 5′-CATGTACGTAGCCATCCAGGC-3′ | R: 5′-CTCTTTGATGTCACGCACGAT-3′ |
| p38δ, F: 5′-ACATGCACCATGAGAACGTCA-3′ | R: 5′-CTGGACCTTATCCTCGCTGAA-3′ |
| p38β, F: 5′-TGGCTGTAAAGAAGCTGTCTCG-3′ | R: 5′-GTCCTATGACGTTCTCGTGCT-3′ |
| p38γ, F: 5′-TACACGCAGACAGTGGACATT-3′ | R: 5′-GCGTTGGTCAGGACAGAGG-3′ |
| VE-cadherin, F: 5′-CACTGCTTTGGGAGCCTTC -3′ | R: 5′-GGGGCAGCGATTCATTTTTCT-3′ |
| SMA, F: 5′-GGACGTACAACTGGTATTGTGC-3′ | R: 5′-CGGCAGTAGTCACGAAGGAAT-3′ |
| Keratin 8, F: 5′-CCCCGGGCCTTCAGC-3′ | R: 5′-GTTGGGGTCCACCTCCAG-3′ |
| Keratin 14, F: 5′-AGCGGCAAGAGTGAGATTTCT-3′ | R: 5′-CCTCCAGGTTATTCTCCAGGG-3′ |
| Keratin 17, F: 5′-CAATGACCGCCTGGCCTCCTA-3′ | R: 5′-GCCATTGATGTCGGCCTCCAC-3′ |
3. Results
3.1. Relative expression levels of p38 isoforms in ESCs
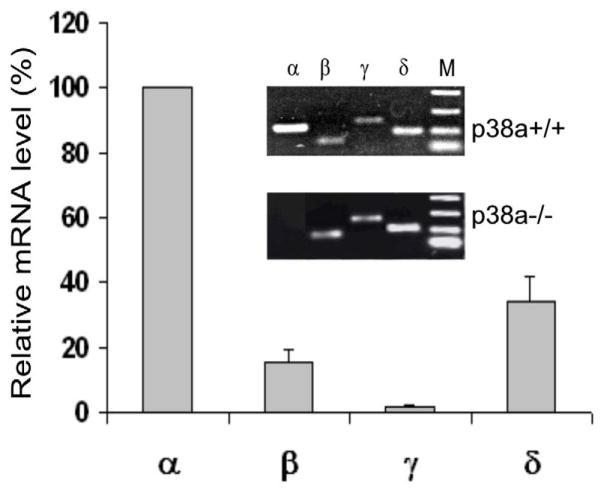
We have previously shown that p38α is abundantly expressed in mouse ESCs (Guo and Yang, 2006). In order to determine whether the dispensability of p38α for ESC differentiation to ECs and SMCs (Guo et al., 2007) was due to the compensatory effect by the other isoforms, we first determined the relative expression levels of the four p38 isoforms by real-time quantitative PCR (RT-qPCR). As shown in Fig. 1, the relative mRNA levels are p38α>p38δ>p38β>p38γ in wild-type ESCs. Expression of p38α was completely eliminated inp38 α−/−ESCs. Deletion of p38α gene did not alter the expression of other three isoforms since the mRNA levels and the relative ratios of p38δ, p38γ and p38β in p38α −/−ESCs were similar to their counterparts in p38α+/+ESCs (Fig. 1 insets).
Fig. 1. Relative mRNA levels of p38 isoformsin p38 α+/+ and p38α−/− ESCs.
The graph represents the relative mRNA levels determined by RT-qPCR in p38α+/+ ESC. The mRNA levels of the four isoforms were first normalized to β-actin mRNA. The expression level of p38α was then set as 100 % for comparison. Results are means ± SEM of three independent experiments. The insets show the PCR products of the isoforms derived from p38α +/+ and p38α−/− ESCs analyzed by agarose gel electrophoresis. p38α is the most abundant isoform expressed in p38α+/+ ESCs, but it is completely eliminated in p38α−/− ESCs.
3.2. Construction of the small hairpin RNA (shRNA) vector and generation of ESCs stably expressing siRNA targeting p38δ
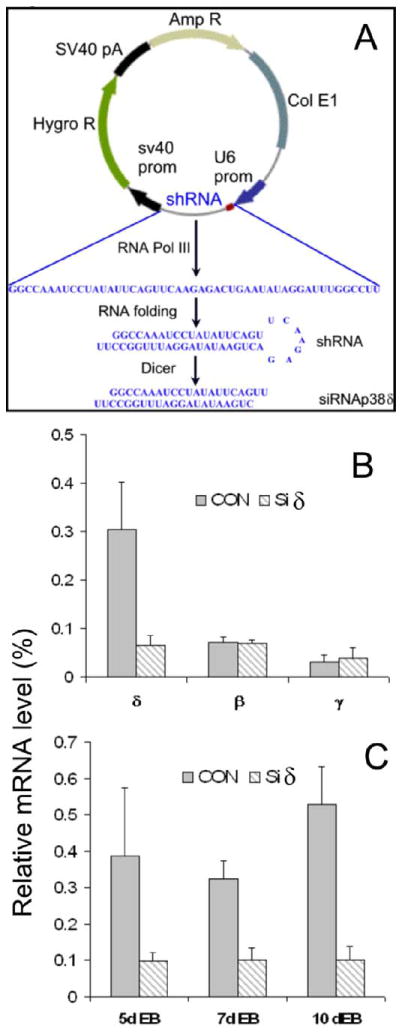
If the dispensability of p38α for ESC differentiation is due to the functional redundancy of other p38 isoforms, we would expect that p38β, which shares the highest homology with p38 α (Ono and Han, 2000), is the best candidate. However, our previous results showed that SB, which inhibits both p38α and p38β, did not affect ESC differentiation to SMCs, ECs and neurons, which argues against a role of p38β for ESC differentiation to these cells (Guo et al., 2007). Therefore, we have chosen to target p38δ by siRNA technique because of its relative higher level of expression (Fig. 1). Although the conventional gene knockdown by synthetic siRNA has proven to be an effective method, the transient nature of this method is not suitable for the long-term differentiation experiments. To circumvent this problem, we have constructed a plasmid vector that expresses a shRNA against p38δ. The sequence of the shRNA and the mechanism of siRNA production are illustrated in Fig. 2A. In this method, a DNA sequence encoding a shRNA targeted to p38δ sequence was inserted to the pSilencer 2.1-U6 hygro expression vector. Once expressed in the cell, the RNA product forms a double-stranded small hairpin RNA (shRNA), which is processed to siRNA targeting p38δ (SiRNAp38δ) through the endogenous Dicer. After transfection of p38α−/−ESCs with the siRNAp38δ plasmid, cells were selected with hygromycin, which killed all other cells except for the cells that were stably transfected with the plasmid that expressed the hygromycin resistance gene. The selected cells were designated as p38α−/−siδ ESCs. The control cells were established in parallel and designated as p38α-/siCON. To analyze the specificity and efficacy of expressed siRNA, p38δ mRNA was analyzed in ESCs and EBs, as shown in Fig. 2B & C. The p38δ mRNA level of siRNA-expressing cells was reduced to less than 20% of the control cells, while the mRNA of p38β and p38γ was not affected. These results demonstrated the efficiency and specificity of this method to knockdown the expression of p38δ.
Fig. 2. Construction of p38δ siRNA expression plasmid vector and the effects of p38δ knockdwon.
A, Schematic illustration of shRNA expressing vector, the sequence of shRNA, and the production of siRNA targeting p38δ (siRNAp38δ). B, Specific knockdown of p38δ in p38α−/− ESCs stably expressing p38δ siRNA vectors (siδ). CON, control p38α−/−siCON ESCs. C, Effective knockdown of p38δ in EBs formed from p38α−/− ESCs stably expressing p38δ siRNA vectors (p38α−/−siδ ESCs, siδ). CON, EBs formed from control cells. The mRNA levels (p38δ, p38β and p38γ in B and p38δ in C) were determined by RT-qPCR and normalized to β-actin mRNA. The expression level of β-actin was set as 100 %. Results are means ± SEM of three independent experiments.
3.3. p38δ knockdown does not have apparent effects on the morphology of ESCs and EB formation
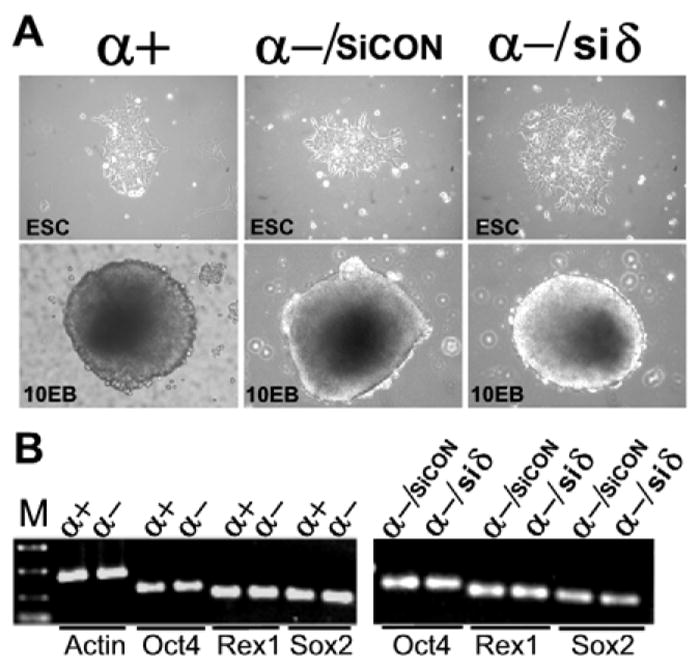
ESCs grow in colonies in cell culture dishes and they are kept in an undifferentiated state when cultured in the presence of leukemia inhibitory factor (LIF). When deprived of cell adhesion and cultured in suspension, ESCs grow in aggregates and form structures called embryoid bodies (EBs). Fig. 3A shows the morphology of ECS colonies grown on cell culture dishes and EBs in suspension generated from wild type ESCs (α+), p38α−/−siCON ESCs (α-/siCON) and ESC p38α−/−siδ ESCs(α-/siδ). Neither the morphology of ESCs nor the EB formation was affected by p38δ knockdown (α-/sicon vs α-/siδ). The colonies and EBs formed from all three types of cells are comparable in number, size, and morphology. We further analyzed the expression of ESC marker genes, Oct4, Sox2, and Rex1, three major genes that are responsible for the maintenance of ESC pluripotency and self-renewal (Niwa, 2007). They were similar among p38α+/+, p38α−/−, and p38α−/−/siRNAp38δ ESCs (Fig. 3B).
Fig. 3. p38δ knockdown does not affect the morphology of ESCs, EB formation, and the expression of ESC pluripotency makers.
A, ESC and EB morphology of p38α+/+ESCs (α+), p38α−/−/siCON ESCs (α-/siCON), and p38α−/− siδ ESCs (α-/siδ). A representative of ESC colony (200x magnification) or a 10 day EB (100x magnification) formed from each genotype of ESCs were photographed under a phase contrast microscope. B, expression levels of ESC pluripotency markers, Oct4, Rex1 and Sox2, was determined by RT-PCR and analyzed by agarose gel electrophoresis. Actin was analyzed in parallel and was used as an internal control. The similar expression levels of ESC pluripotency markers among different ESCs were conformed by RT-qPCR (data not shown).
3.4. ESCs deficient of both p38α and p38δ can differentiate to ECs and SMCs
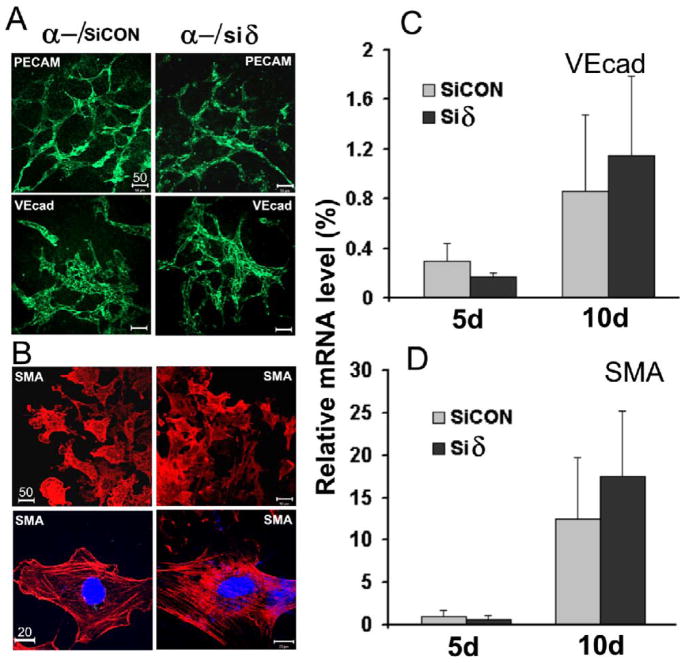
To test the effect of p38δ knockdown on the potential of ESC differentiation, we used our well-established ESC differentiation model (Guo et al., 2007). This method, defined as EB-monolayer model, consists of two steps: ESCs were first allowed to differentiate in three-dimensional EB structure cultured in suspension, followed by transferring EBs to a culture dish where cells within the EBs grow out to form monolayer-like structures. The EB structure resembles an early embryo in which ESCs can differentiate into all cell types derived from the three germ layers (Hwang et al., 2008). The monolayers allow for convenient immunostaining and image analysis. As shown in Fig. 4A, p38α−/−Siδ ESCs and control cells could differentiate into ECs and assembled vascular networks with very similar density and morphology, as detected by immunostaining of two EC markers, PECAM-1 and VE-cadherin. Similar to ECs, SMCs are among the early-differentiated cell types during embryogenesis (Wobus and Boheler, 2005). We further analyzed the expression of smooth muscle α-actin (SMA), a marker that is widely used to identity SMCs. The positive cells were scattered sparsely in many locations of EB-monolayers, but a large population was detected around the edges of the EB outgrowths. Fig. 4B shows a group of these cells differentiated from control cells and from p38α−/−Siδ ESCs. They have similar morphology and fluorescent staining pattern (Fig. 4B, upper panels). At high magnification, the distinctive SMA filament networks were clearly identifiable in single cells and they were arranged in similar patterns in control cells and in p38α−/−Siδ ESCs (Fig. 4B, lower panels). The patterns of vascular structures assembled from ECs and SMCs shown in Fig. 4 were similar to those differentiated from wild type ESCs as we previously reported (Guo et al., 2007). Quantitative RT-PCR analysis confirmed that the expression levels of cell specific markers VE-cadherin and SMA were also comparable in control cells and in p38δ kncokdown cells (Fig. 4C and D). Together, these data suggest that neither p38α nor the combination of p38α and p38δ is critical for EC and SMC differentiation.
Fig. 4. Differentiation of ESCs to ECs and SMCs.
EB-monolayers derived from p38α−/−/siCON ESCs (α-/siCON), and p38α−/−siδ ESCs (α-/siδ) were immunostained with; A, antibodies against EC markers PECAM-1 (PECAM) or VE-cadherin (VEcad) followed by FITC-conjugated secondary antibodies (green); B, antibodies against SMC marker SMA followed by rhodamine-conjugated secondary antibodies (red). The cells were examined and photographed with a confocal microscope (scale bar unit =μm). C & D, quantitative determination of VEcad and SMA expression by RT-qPCR. The mRNA of each gene was determined from 5 and 10 day EBs and normalized to β-actin mRNA. The expression level of β-actin was set as 100 %. Results are means ± SEM of three independent experiments.
3.5. ESCs deficient of both p38α and p38δ can differentiate to epithelial cells
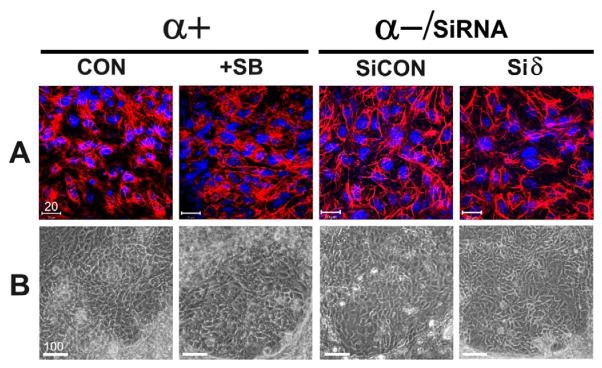
We extended our study to investigate if p38α and p38δ play any roles in epithelial cell lineage differentiation since there were a number of reports suggesting that they regulate keratinocyte differentiation during epidermis maturation (Sayama et al., 2001; Efimova et al., 2003). We performed ESC differentiation using different ESCs of genotypes under the following condition; p38α+/+ ESCs in the absence or presence of SB (Fig. 5, α+, CON and +SB, respectively), p38α−/−ESC control cells and p38α−/−Siδ ESCs (Fig. 5, α-, CON and Siδ, respectively). To identify epithelial cell lineages, we immunostained the EB-monolayers with a monoclonal antibody C-2562 (Sigma), which recognizes multiple forms of cytokeratins (Hasan et al., 1998). The cells that were recognized by the antibodies showed similar staining patterns in differentiated cells from the four types of ESCs (Fig. 5A). Although positive cells can been detected in multiple locations of EB-monolayers, the cells within the “patch –like” areas (Fig. 5B) showed intensive staining of typical intermediate filaments of cytokeratins (Fig. 5A). The morphology and the cytokeratin staining patterns of these cells have been positively identified as keratinocytes by others investigators (Troy and Turksen, 2009; Haase et al., 2007). We did not detect apparent differences, either in morphology or in cytokeratin immunostaining, among the cells differentiated from control cells (wild types ESCs, α+, CON), ESCs in which both p38α and p38β were inhibited (α+, +SB), p38α−/−ESCs (α−, CON), and ESCs deficiency of both p38α and p38δ (α-, Siδ).
Fig. 5. Differentiation of ESCs to keratinocytes.
EB-derived monolayers were differentiated from ESCs of the following genotypes: p38α+/+ESCs without treatment (p38α+, CON); p38α+/+ ESCs in the presence of 5 μM SB (p38α+, SB); p38α−/−/siCON ESCs (α-/SiCON), and p38α−/−siδ ESCs (α-/siδ). A, keratinocytes identified by immunocytochemistry. Differentiated cells were immunostained with an antibody against cytokeratins (C-2562, Sigma) followed by rhodamine-conjugated secondary antibodies and photographed under a confocal microscope. B, the cytokeratin positive cells identified in A were examined and photographed under a phase contrast microscope. These cells were detected in “patch-like” areas and have typical morphology of keratinocytes (scale bar unit = μm).
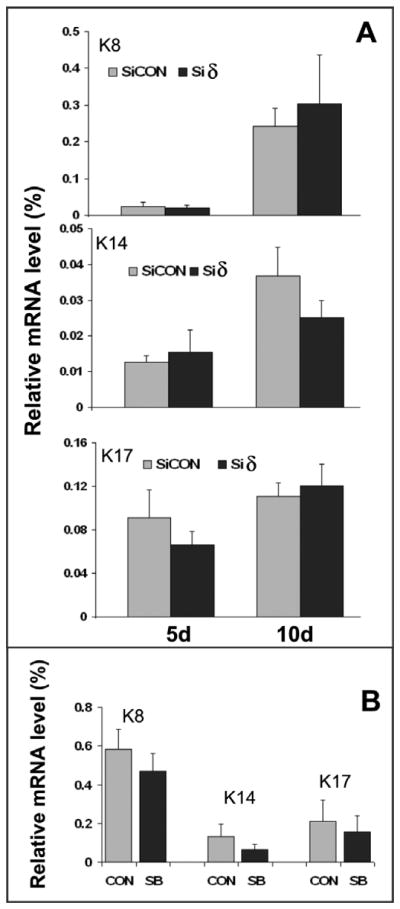
We further quantitatively measured expression levels of cytokeratins 8, 14, and 17, which have been used as markers for keratinocyte differentiation (Troy and Turksen, 2009; Haase et al., 2007). As shown in Fig. 6, the mRNA levels of cytokeratins determined by RT-qPCR are comparable in all differentiated cells tested at two differentiation periods, consistent with the results derived from morphological and immunocytochemical analysis (Fig, 5). These results indicate that differentiation of ESCs to keratinocytes does not depend on either p38α or the combination of p38α and p38δ.
Fig. 6. Effect of p38α knockout, p38α/β inhibition, and p38δ knockdown on the expression of cytokeratins.
A, 5 day and 10 day EBs differentiated from p38α−/−/siCON ESCs (SiCON) and p38α−/−siδ ESCs (siδ) were analyzed for the expression of keratin 8 (K8), keratin14 (K14), and keratin17 (K17) by qRT-PCR. The mRNA of each gene was determined and normalized to β-actin mRNA. The expression level of β-actin was set as 100 %. Results are means ± SEM of three independent experiments. B, expression of cytokeratins in 10 day EBs differentiated from p38α+/+ ESCs in the absence of 5 μM SB (CON) or in the presence of 5 μM SB (SB). The mRNA level of each gene was determined by the same method as described in A.
4. DISCUSSION
Numerous studies have demonstrated the importance of p38 MAP kinases in the regulation of a wide range of cellular activities, such as cell proliferation, apoptosis, and inflammation (Ono and Han, 2000; Kyriakis and Avruch, 2001). Most studies described in the literature are derived from experiments using SB inhibitors or by overexpressing their wild types or dominant negative mutants of p38 isoforms. These studies have generated a large body of information and have indeed advanced our understanding of the functions of p38 isoforms, but the concerns associated with the aforementioned methods are nonspecific effects of chemical inhibitors and the insufficient efficacy of dominant negative mutants. The shortfalls of these approaches may contribute to many conflicting results and confusions in the field. Therefore, there is a need to verify previous results with genetically defined cells. The p38α−/−ESC and their differentiated cells may represent the best tools available for this purpose and for investigating the developmental roles of p38 family members, of which we have little knowledge.
Previous studies using primary endothelial cells (ECs) in three-dimensional cell culture models (Matsumoto et al., 2002; Sweeney et al., 2003), including our own work (Yang et al., 2004), have demonstrated that inhibition of p38 with SB affects cell proliferation, viability and EC morphogenesis during in vitro vessel assembly. Coincidentally, one of the major phenotypic features of p38α−/− embryo is the overall underdeveloped vascular structure (Mudgett et al., 2000). Therefore, one would assume that p38α may play an important role in endothelial differentiation and vascular assembly. However, our recent study showed that p38α−/−ESCs are able to differentiate into ECs and assemble into complex vessel structures like wild type ESCs (Guo et al., 2007). Additional data revealed that differentiation of ESCs to SMCs or neurons was also not affected by the deletion of p38α, although the roles of p38α in differentiated SMCs (Gerthoffer, 2005) and neurons (Takeda and Ichijo, 2002) have been well recognized. A similar conclusion has been reported for lymphocyte (Kim et al., 2005) and myeloid differentiation (Allen et al., 2000) in which p38α is apparently dispensable. A rational explanation for these observations is that p38α function may be compensated for by other p38 isoforms, namely p38β, p38γ orp38 δ in knockout animals or ESCs. In this aspect, one would speculate that p38β, which shares the highest homology with p38 α (Ono and Han, 2000), is the best candidate. However, the fact that the SB inhibitor, which inhibits both p38α and p38β, did not affect ESC differentiation to SMCs, ECs and neurons, argues against the requirement of p38β (Guo et al., 2007). Therefore, the current study focused on p38δ, the second most abundantly expressed isoform after p38α. We showed that the constitutive expression of siRNA against p38δ in p38α−/−ESCs significantly reduced p38δ mRNA, but neither ESC morphology, EB formation, nor the expression of pluripotency markers was affected. Importantly, the lack of impact of p38δ knockdown on ESC differentiation to ECs and SMCs once again demonstrated a non-essential role of p38δ in the process. Several studies have suggested that p38α andp38 δ regulate epithelial cell maturation in skin epidermis, which is a self-renewing tissue maintained by keratinocyte (the major constituent of the epidermis) proliferation and differentiation (Fuchs, 1990). Differentiation is the most important way by which keratinocytes form a multilayered epidermis, during which cells undergo a series of morphological and biochemical changes and form the protective covering of the skin. Using SB203580 inhibitor, Sayama reported that ASK1-p38 MAP kinase cascade regulates keratinocyte terminal differentiation (Sayama et al., 2001). p38δ has been suggested to regulate human keratinocyte differentiation through its interaction with ERK and to mediate the effect of protein kinase C (Balasubramanian and Eckert, 2007). Therefore, we extended our investigation to test the possibility that p38α and p38δ may regulate ESC differentiation into keratinocyes. Our results clearly demonstrated that ESCs lacking p38α or bothp38 α and p38δ do not show apparent defects, despite the demonstrated roles of the two kinases in aforementioned keratinocyte differentiation and in many other functions, such as stress response of keratinocytes (Cao et al., 2008).
Taken together, the results from our previous studies (Guo et al., 2007), the current report, and these reported by others (Allen et al., 2000; Kim et al., 2005) are somewhat surprising considering the well documented functions of p38α andp38 δ in somatic cells, but they are not completely unexpected considering their non-essential roles in embryogenesis (except for placental development in the case of p38α) as demonstrated in knockout animal models (Adams et al., 2000; Sabio et al., 2005). It is reported that the differentiation potential and commitment of p38α−/−ESCs to cardiomyocytes seemed to be compromised (Aouadi et al., 2006), however, an opposite conclusion has been reported in a recent study using SB inhibitors (Graichen et al., 2008). Therefore, whether or not p38α plays a role in cardiomyocyte differentiation is inconclusive. It is not clear at this point if the functions of differentiated p38α−/−ECs, SMCs, and epithelial cells are compromised, but apparently p38α andp38 δ are non-essential for ESC differentiation to the aforementioned cell types. What remains to be answered is how their functions on ESC differentiation during embryogenesis, if any, are compensated for. In this aspect, p38γ could be a logical candidate, but its very low expression level in ESCs (Fig. 1) and the lack of phenotype in knockout mice (Sabio et al., 2005) dampen the enthusiasm for the investigation in the current study. It is possible that p38 MAP kinases may intrinsically play less significant roles in ESC differentiation than in the regulation of cellular activities of fully differentiated somatic cells. Alternatively, one can speculate that other closely related kinases, such as JNK, may have functional redundancy to substitute the roles of the p38 family members since these enzymes share some functional similarities (Kyriakis and Avruch, 2001). This hypothesis may be tested by knockdown of JNK expression using the same strategy described in this study, which we would like to pursue in a further investigation. While these questions remain to be answered, we can conclude that mouse ESCs lacking p38α and p38δ can differentiate to endothelial cells, smooth muscle cells, and epithelial cells.
Acknowledgments
We thank Dr. Christopher Gabel for providing mouse ESCs and Mississippi Functional Genomics Network for the use of the facility. This work was supported by a NIH grant HL082731 (Y.-L.G.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RH, Porras A, Alonso G, Jones M, Vintersten K, Panelli S, Valladares A, Perez L, Klein R, Nebreda AR. Essential role of MAP kinase α in placental but not embryonic cardiovascular development. Mol Cell. 2000;6:109–116. [PubMed] [Google Scholar]
- Allen M, Svensson L, Roach M, Hambor J, McNeish J, Gabel CA. Deficiency of the stress kinase p38α results in embryonic lethality: characterization of the kinase dependence of stress responses of enzyme-deficient embryonic stem cells. J Exp Med. 2000;191:859–870. doi: 10.1084/jem.191.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouadi M, Bost F, Caron L, Laurent K, Marchand Brustel Y, Binetruy B. P38MAPK activity commits embryonic stem cells to either neurogenesis or cardiomyogenesis. Stem Cells. 2006;24:1399–1406. doi: 10.1634/stemcells.2005-0398. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Eckert RL. Keratinocyte proliferation, differentiation, and apoptosis--Differential mechanisms of regulation by curcumin, EGCG and apigenin. Toxicology and Applied Pharmacology. 2007;224:214–219. doi: 10.1016/j.taap.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardmore VA, Hinton HJ, Eftychi C, Apostolaki M, Armaka M, Darragh J, McIlrath J, Carr JM, Armit LJ, Clacher C, Malone L, Kollias G, Arthur JS. Generation and characterization of p38β (MAPK11) gene-targeted mice. Mol Cell Biol. 2005;25:10454–10464. doi: 10.1128/MCB.25.23.10454-10464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Lu S, Kivlin R, Wallin B, Card E, Bagdasarian A, Tamakloe T, Chu Wm, Guan Kl, Wan Y. AMP-activated Protein kinase contributes to UV- and H2O2-induced apoptosis in human skin keratinocytes. J Biol Chem. 2008;283:28897–28908. doi: 10.1074/jbc.M804144200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cobb MH, Boulton TG, Robbins DJ. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991;2:965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova T, Broome AM, Eckert RL. A Regulatory Role for p38δ MAPK in keratinocyte differentiation: evidence for p38δ-ERK1/2 complex formation. J Biol Chem. 2003;278:34277–34285. doi: 10.1074/jbc.M302759200. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Epidermal differentiation: the bare essentials. J Cell Biol. 1990;111:2807–2814. doi: 10.1083/jcb.111.6.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerthoffer WT. Signal-transduction pathways that regulate visceral smooth muscle function III. coupling of muscarinic receptors to signaling kinases and effector proteins in gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2005;288:G849–G853. doi: 10.1152/ajpgi.00530.2004. [DOI] [PubMed] [Google Scholar]
- Graichen R, Xu X, Braam SR, Balakrishnan T, Rahmat SN, Sieh S, Tham SC, Freund C, Moore J, Mummery C, Colman A, Zweigerdt R, Davidson BP. Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation. 2008;76:357–370. doi: 10.1111/j.1432-0436.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- Guo YL, Yang B. Altered cell adhesion and cell viability in a p38α mitogen-activated protein kinase-deficient mouse embryonic stem cell line. Stem Cells Dev. 2006;15:655–664. doi: 10.1089/scd.2006.15.655. [DOI] [PubMed] [Google Scholar]
- Guo YL, Ye J, Huang F. p38α MAP kinase-deficient mouse embryonic stem cells can differentiate to endothelial cells, smooth muscle cells, and neurons. Dev Dyn. 2007;236:3383–3392. doi: 10.1002/dvdy.21374. [DOI] [PubMed] [Google Scholar]
- Haase I, Knaup R, Wartenberg M, Sauer H, Hescheler Jn, Mahrle G. In vitro differentiation of murine embryonic stem cells into keratinocyte-like cells. Eur J Cell Biol. 2007;86:801–805. doi: 10.1016/j.ejcb.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Hasan AAK, Zisman T, Schmaier AH. Identification of cytokeratin 1 as a binding protein and presentation receptor for kininogens on endothelial cells. Proc Natl Acad Sci USA. 1998;95:3615–3620. doi: 10.1073/pnas.95.7.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deli Rev. 2008;60:199–214. doi: 10.1016/j.addr.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JR, Bolognese B, Hillegass L, Kassis S, Adams J, Griswold DE, Winkler JD. Pharmacological effects of SB 220025, a selective inhibitor of p38 mitogen-activated protein kinase, in angiogenesis and chronic inflammatory disease models. J Pharmacol Exp Ther. 1998;284:687–692. [PubMed] [Google Scholar]
- Kim JM, White JM, Shaw AS, Sleckman BP. MAPK p38α is dispensable for lymphocyte development and proliferation. J Immunol. 2005;174:1239–1244. doi: 10.4049/jimmunol.174.3.1239. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Turesson I, Book M, Gerwins P, Claesson-Welsh L. p38 MAP kinase negatively regulates endothelial cell survival, proliferation, and differentiation in FGF-2-stimulated angiogenesis. J Cell Biol. 2002;156:149–160. doi: 10.1083/jcb.200103096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgett JS, Ding J, Guh-Siesel L, Chartrain NA, Yang L, Gopal S, Shen MM. Essential role for p38α mitogen-activated protein kinase in placental angiogenesis. Proc Natl Acad Sci USA. 2000;97:10454–10459. doi: 10.1073/pnas.180316397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda AR, Porras A. p38 MAP kinases: beyond the stress response. Trend Biochem Sci. 2000;25:257–260. doi: 10.1016/s0968-0004(00)01595-4. [DOI] [PubMed] [Google Scholar]
- Niwa H. How is pluripotency determined and maintained? Development. 2007;134:635–646. doi: 10.1242/dev.02787. [DOI] [PubMed] [Google Scholar]
- Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Rajan R, Ye J, Bai S, Huang F, Guo YL. NF-κB, but not p38 MAP Kinase, is required for TNF-α-induced expression of cell adhesion molecules in endothelial cells. J Cell Biochem. 2008;105:477–486. doi: 10.1002/jcb.21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach ML, Stock JL, Byrum R, Koller BH, McNeish JD. A new embryonic stem cell line from DBA/1lacJ mice allows genetic modification in a murine model of human inflammation. Exp Cell Res. 1995;221:520–525. doi: 10.1006/excr.1995.1403. [DOI] [PubMed] [Google Scholar]
- Sabio G, Arthur J, Kumar Y, Peggie M, Carr J, Murray-Tait V, Centeno F, Goedert M, Morrice N, Cuenda A. p38 regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 2005;24:1134–1145. doi: 10.1038/sj.emboj.7600578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayama K, Hanakawa Y, Shirakata Y, Yamasaki K, Sawada Y, Sun L, Yamanishi K, Ichijo H, Hashimoto K. Apoptosis signal-regulating kinase 1 (ask1) is an intracellular inducer of keratinocyte differentiation. J Biol Chem. 2001;276:999–1004. doi: 10.1074/jbc.M003425200. [DOI] [PubMed] [Google Scholar]
- Schuh AC, Faloon P, Hu QL, Bhimani M, Choi K. In vitro hematopoietic and endothelial potential of flk-1(−/−) embryonic stem cells and embryos. Proc Natl Acad Sci USA. 1999;96:2159–2164. doi: 10.1073/pnas.96.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Sweeney SM, DiLullo G, Slater SJ, Martinez J, Iozzo RV, Lauer-Fields JL, Fields GB, Antonio JDS. Angiogenesis in collagen I requires α2β1 ligation of a GFP*GER sequence and possibly p38 MAPK activation and focal adhesion disassembly. J Biol Chem. 2003;278:30516–30524. doi: 10.1074/jbc.M304237200. [DOI] [PubMed] [Google Scholar]
- Takeda K, Ichijo H. Neuronal p38 MAPK signalling: an emerging regulator of cell fate and function in the nervous system. Gene Cell. 2002;7:1099–1111. doi: 10.1046/j.1365-2443.2002.00591.x. [DOI] [PubMed] [Google Scholar]
- Troy T, Turksen K. Commitment of embryonic stem cells to an epidermal cell fate and differentiation in vitro. Dev Dyn. 2009;132:293–300. doi: 10.1002/dvdy.20223. [DOI] [PubMed] [Google Scholar]
- Wobus AM, Boheler KR. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev. 2005;85:635–678. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- Yang BH, Cao DJ, Colman RW, Y-L Guo. Different roles of ERK and p38 MAP kinases during tube formation from endothelial cells cultured in 3-dimensional collagen matrices. J Cell Physiol. 2004;200:360–369. doi: 10.1002/jcp.20025. [DOI] [PubMed] [Google Scholar]