Abstract
Cervical cancer originates with human papillomavirus (HPV) infection and progresses via histologically-defined premalignant stages. Here we compare normal cervical epithelium and patient-matched high grade squamous intraepithelial lesions (HSIL) with cervical carcinoma tissue from the same patient population (n=10 per group). Specimens were analyzed by combined laser capture microdissection and 2D-DIGE. Significant expression changes were seen with 53 spots resulting in identification of 23 unique proteins at the molecular level. These include eight that uniquely distinguish normal epithelium and HSIL and four that uniquely distinguish HSIL and carcinoma. In addition, one protein, cornulin, distinguishes all three states. Other identified proteins included differentiation markers, oncogene DJ-1, serpins, stress and interferon-responsive proteins, detoxifying enzymes, and serum transporters. A literature review, performed for all identified proteins, allowed most changes to be assigned to one of three causes: direct or indirect HPV oncoprotein interactions, growth selection during latency, or interactions in the lesion microenvironment. Selected findings were confirmed by immunohistochemistry using either frozen sections from the same cohort or formalin fixed paraffin embedded samples from a tissue microarray. Novel markers described here have potential applications for increasing the predictive value of current screening methods.
Keywords: 2D-DIGE, cervical cancer, human papillomavirus, laser capture microdissection, HSIL
1 INTRODUCTION
Cancer of the uterine cervix is a significant cause of mortality, responsible for about 200,000 deaths per year among women worldwide [1]. Screening for early detection, using the Papanicolaou (Pap) test, has reduced mortality about four-fold in developed countries [2, 3]. Exfoliated cervical cells are evaluated based on alterations in nuclear and cellular morphology using the Bethesda classification system [4].
Despite its success in reducing mortality, the Pap test has shortcomings. Abnormal or ambiguous findings, which occur in about 3 million of the 55 million Pap smears performed annually in the US, necessitate costly and sometimes invasive follow-up. The accuracy of the Pap test has been studied extensively, and meta-analysis indicates that high specificity and sensitivity cannot be achieved concurrently [5]. Classification of both Pap smears and follow-up biopsies is subject to high inter-observer variability, with agreement on grading of biopsy specimens only 40% to 80% more than expected by chance alone [6]. In addition, the natural history of cervical premalignant lesions shows great individual variability. Some 40–70% of low grade lesions will regress without treatment, whereas smaller percentages will progress to a higher-grade lesion or to invasive cancer [7]. The decision to surgically ablate low-grade lesions is particularly problematic, as only one to two women per 1000 progress to invasive carcinoma within 24 months, and the procedure itself carries risk [8, 9]. Molecular markers to distinguish individual patients with a high risk of progression would clearly be valuable. Such markers might also be therapeutic targets, expanding the options for non-surgical treatment.
One approach that has been explored for improving the accuracy of cervical cancer screening is to test for the presence of high-risk type human papillomavirus (HPV) DNA following an ambiguous Pap test result. The rationale is that high-risk type HPV is the initiating agent in virtually all cervical carcinomas (reviewed in refs. [10, 11]). In patients with ambiguous Pap test results, HPV DNA assays have been shown to be preferable to repeat cytology [12]. Surrogate protein markers for HPV infection have also been used, including high-level expression of the cyclin-dependent kinase inhibitor, p16(Ink4a), and the expression of a marker of cell proliferation, Ki-67, in normally non-dividing cells of the upper layers of the epithelium [13–15]. A limitation in using HPV or surrogate markers for diagnosis is that infection with high-risk type HPV is relatively common (point prevalence = 3.4% [16]) and many infections clear spontaneously. It would be useful to have a test to detect the transition from infected cells, which proliferate simply in response to viral oncoprotein expression, and virally transformed cells, which have accumulated additional genetic and epigenetic changes during a latency period. There are currently no clinically useful molecular markers for detecting this transition. Such markers might be combined with existing screening technologies to achieve a higher positive predictive value.
Proteomic methodologies provide a general route to biomarker discovery. They have been used previously to compare cervical squamous cell carcinoma with normal cervical tissue or cervical cell lines [17, 18]. There has also been one proteomic study of the premalignant lesions that are the target of population screening. This study reported a large number of differences in exfoliated cervical cells from normal and abnormal liquid cytology-based Pap smears [19].
In the present work, we compared normal tissue, patient-matched high-grade squamous intraepithelial lesions (HSIL), and invasive carcinoma. HSIL is of particular interest as it is a high-risk premalignant condition where the probabilities of progression versus spontaneous regression are almost evenly balanced [7, 20]. Laser capture microdissection (LCM) and 2D-difference gel electrophoresis were used to obtain protein profiles from as little as 1 μg of total protein, a procedure validated previously where sample abundance was limiting [21–26]. Following quantification and statistical analysis, 53 protein spots were identified as discriminatory between sample groups, with 23 unique proteins identified to date at the molecular level.
2 MATERIALS AND METHODS
2.1 Experimental Design
There were three experimental groups: normal, patient-matched HSIL, and cancer (Fig. 1). Specimens were obtained from the Instituto Nacional de Enfermedades Neoplásicas (INEN, Lima, Peru). Patients who had positive Pap smears and were scheduled to undergo gynecologic surgery were eligible. Following institutional review board guidelines, subjects were asked to provide informed consent for use of their tissue in research. Patients with a finding of HSIL contributed both lesional tissue and normal tissue from elsewhere in the cervix. Patients with a finding of invasive cancer contributed lesional tissue only (typically, no normal anatomy remained). Three comparisons were made: (1) cancer vs. normal (unpaired), (2) HSIL vs. normal (paired), and (3) cancer vs. HSIL (unpaired). Tissues were snap frozen, and epithelial or lesional tissue was later collected by LCM as described [23]. An invariant internal standard was prepared as a mixture of normal cervical tissue from a patient who underwent transabdominal hysterectomy for symptomatic leiomyomas and cervical squamous cell carcinoma from a different patient who underwent radical hysterectomy. Samples and an internal standard were labeled with different dyes, so the abundance of each spot could be quantified relative to the corresponding spot in the internal standard [27]. Candidate biomarkers were ranked using the Significance Analysis of Microarrays (SAM, version 3.0) add-in for Microsoft Excel (available at http://www-stat.stanford.edu/~tibs/SAM/). An FDR of 10% was used as a threshold cutoff for each spot. Details of the analytical methodology are given in Supplementary Methods.
FIG. 1. Experimental design and analytical workflow.
A. Experimental design. Specimens were obtained as described in Materials and Methods. Three experimental groups were analyzed. Normal and HSIL samples were patient-matched. Carcinoma samples came from different patients from whom normal samples were unavailable. The design permits paired comparison of specimens in normal and HSIL groups as indicated, and unpaired comparison of specimens in the cancer group with specimens in the other two groups. The order of analysis was randomized to preclude systematic bias. B. Analytical workflow. Following LCM and 2D-DIGE, data analysis was performed and potential biomarkers were ranked in order of priority as described in the text. A separate preparative gel was run containing Cy3 labeled standard proteins alone (500 μg), matched to the analytical gel, and candidate biomarkers were excised, digested with trypsin, and analyzed by MALDI TOF/TOF mass spectrometry for protein identification.
2.2 Preparative Gel and Mass Spectroscopy
Spots of interest were matched to a preparative gel, and proteins were identified by mass spectrometry [23] (see also Supplementary Methods). Following trypsin digestion, extracted peptides were spotted onto a 192-well MALDI-TOF target plate for the Applied Biosystems Incorporated (ABI) 4700 Proteomics Analyzer. Automated MALDI-TOF mass spectrometry provided a peptide mass fingerprint. In addition, for each analysis the 20 most prominent peptides (excluding trypsin peaks) were subjected to collision-induced dissociation to obtain sequence information. Spectra were searched using the GPS Explorer (ABI) search tool and Mascot algorithm (Matrix Biosciences) against the NCBInr protein database.
2.3 Immunohistochemistry
Immunohistochemistry was performed using 6 μM replicate frozen sections from normal, patient-matched HSIL, and carcinoma samples (n=3 per group). Slides were air dried, fixed in 10% neutral buffered formalin for 5 min, and rinsed with distilled water. Endogenous peroxidase was quenched by incubating twice in 0.3% H2O2 for 5 min., then washing twice in PBS for 5 min. Slides were blocked with normal donkey serum for 20 min, then incubated with the following primary antibodies for 30 min: 1:100 anti-cornulin (Alexis, San Diego, CA), 1:1000 anti-Hsp27 (HSPB1) (Assay Designs, Ann Arbor, MI), 1:1000 anti-Manganese Superoxide Dismutase 2 (Abcam, Cambridge, UK), or 1:100 anti-PA28β (Abnova, Taipei, Taiwan). Slides were washed twice with PBS, then with HRP-conjugated goat anti-rabbit immunoglobulin (cornulin and superoxide dismutase), or goat anti-mouse immunoglobulin (PA28β and Hsp27) (Envision+ HRP kit, Dako Corp. Carpinteria, CA.). Slides were rinsed twice with PBS, and bound antibody was detected using diaminobenzidine. Slides were counterstained with hematoxylin. Scoring was determined by a board-certified pathologist.
Commercial tissue microarrays containing histologically confirmed cervical tissue from a variety of disease states were purchased from Biomax, Inc. (Rockville, MD). Each microarray contained 30 carcinoma specimens, 10 CIN specimens, 10 inflamed cervical tissue specimens, and 10 normal specimens. Slides were deparaffinized and run through graded alcohols to distilled water. Slides were pretreated with Target Retrieval Solution PH 6.0, (Dako Corp, Carpinteria, CA.) using a steamer (Black and Decker rice steamer) and rinsed in distilled water. Antibody staining and development were the same as for the frozen sections.
3 RESULTS
3.1 Collection and analysis of proteomic data
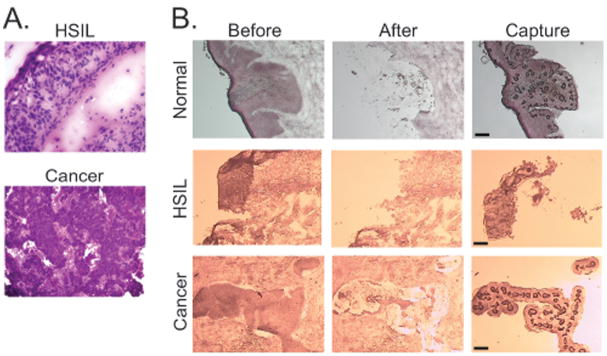
Pilot sections for each specimen were stained with hematoxylin and eosin to reveal morphological detail. Examples are shown in Fig. 2A. HSIL samples demonstrated >90% involvement of the epithelium with high-grade dysplastic cells that had not invaded through the basement membrane. Cervical cancer samples demonstrated moderately differentiated, non-keratinizing squamous cell carcinomas. All preparative sections were stained with Nuclear Fast Red for LCM. Fig. 2B shows representative sections before and after LCM, as well as the captured tissue. The more intensely stained epithelial or lesional tissue was collected, leaving behind the lighter-stained stroma.
FIG. 2. Representative histological sections.
A. Histopathology of HSIL and cancer specimens. Prior to performing LCM, pilot sections were stained with hematoxylin and eosin to screen for lesional tissue (HSIL, 200X; cancer, 100X). B. Typical LCM results. Frozen sections of normal cervical epithelium, high-grade squamous intra-epithelial lesion (HSIL) and cervical squamous carcinoma (cancer) were stained with Nuclear Fast Red. Microscopic images before LCM and after LCM are shown along with the annealed tissue captured on the cap, Dark circles in the captured tissue represent sites of direct laser energy deposition, where the cap polymer annealed to the underlying tissue. Scale bar, 30 μm.
We performed a pilot study to evaluate technical reproducibility of the combined LCM and 2D-DIGE procedure and enable a power analysis. We carried out independent LCM sampling of normal cervical tissue and cervical cancer, analyzed protein abundance by 2D-DIGE using an invariant internal standard, and evaluated reproducibility based on coefficients of variation (Supplementary Figure 1). The median coefficient of variation was 23% for both normal cervical tissue and cervical cancer. Because the analytical methodology is the same, we expect that the distribution of coefficients for the HSIL group is the same, although it was not possible to perform the same replicate sampling because of the small size and scarcity of the lesions.
To estimate statistical power for biomarker discovery, we considered a hypothetical marker with a between-group difference of 2-fold and a CV of 30% for technical variation, both of which were within the observed range. We assumed that within-group biological variation would be on the same order as technical variation and that tests would be conducted on the log-scale so that the CV roughly corresponds to the standard deviation of the log-transformed data. A study would require 10 subjects per group to obtain 80% power to identify such features using a two-sided alpha of 0.05.
For the main analysis, proteins from the 30 samples (n=10 per group) were extracted, labeled, and analyzed by 2D-DIGE. An average of 2257 spots was identified in each gel, of which an average of 1489 spots was matched to the master map. Of these, 135 spots were selected for further analysis, based on manual inspection showing unequivocal alignment across spot maps generated from all 30 samples. To prioritize spots for analysis, protein abundance values were calculated as described in Materials and Methods and used as input data for Significance Analysis of Microarray (SAM). For each of the three comparisons (cancer versus normal, HSIL versus normal, and cancer versus HSIL), SAM calculated a relative difference score, d(i), and a false discovery rate based on analysis of permuted data sets. We applied a threshold value for d score based on a false discovery rate (FDR) of 10% or less and an additional filter to exclude spots with an absolute change in expression level of <2.0-fold. We reasoned that tissue biomarkers with changes of <2.0-fold might be difficult to measure reliably in a clinical laboratory (e.g. by immunohistochemistry) and thus would be unlikely to be widely adopted. Application of a filter based on fold change has been shown to further reduce FDR [28]. Based on these criteria, we identified 53 features (spots) as candidate biomarkers.
3.2 Proteomic patterns in normal, HSIL, and cancer
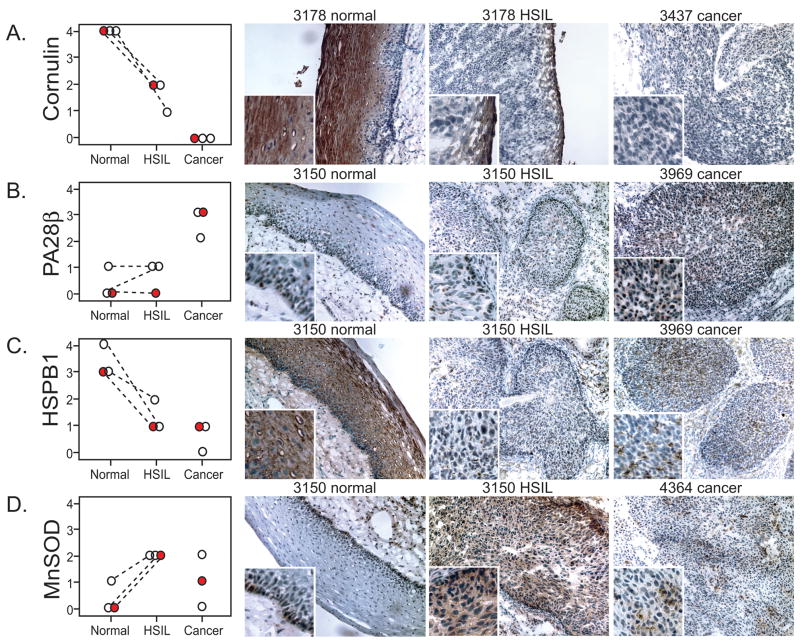
Based on the SAM analysis, there were 42 spots that distinguished cancer from normal, 23 that distinguished HSIL from normal, and 9 that distinguished cancer from HSIL. Some spots were significant in two or more of these pairwise comparisons (20/53) and one distinguished all three sample groups. Individual data values for four representative markers are presented in Fig 3A–D. The vertical axis represents the “internal ratio” (IR) of expression for each spot relative to the internal standard in the same gel. Data are plotted as log2 IR, such that each unit on the vertical axis corresponds to a 2-fold change. Dashed lines, which connect paired normal and HSIL specimens from the same patient, illustrate how the availability of paired samples reveal consistent expression trends that might not otherwise have been apparent. Similar graphs for the other candidate biomarkers are shown in Supplementary Figure 2. Viewing group means, in addition to the individual values, provides additional insight. Fig. 3A shows an overview of results as a heat map, with red indicating more expression and green indicating less. HSIL has its own, distinctive, pattern of expression, with some markers more cancer-like, and others more normal-like (see Discussion).
FIG. 3. Differential expression analysis.
A–D. Graphical representations of four candidate biomarkers. Graphs display the relative abundance of 4 proteins differentially expressed between normal, HSIL, and cancer samples. Relative abundance values (Y axis) is expressed on a logarithmic scale, with each unit increment representing a 2-fold change. Each circle indicates an individual tissue sample. Patient-matched samples are connected by the dashed lines. E. Heat map. The mean of each of the experimental groups was calculated and expressed as a heat map. Green indicates lesser relative abundance, red indicates greater (see scale). Arrows denote the candidate biomarkers detailed in preceding panels.
3.3 Match to preparative gel and mass spectrometry analysis
To identify spots at the molecular level, we ran a separate preparative gel with 500 μg of Cy3-labeled mixed internal standard, matched the spot map to the master map from the analytical gels, picked spots of interest, and obtained mass spectrometry identifications as described in Materials and Methods. We picked 31 spots, including only those that could be unambiguously matched between the preparative gel and the master map and that were well resolved from abundant neighboring spots, and obtained definite identifications for 29. Among these, there were five instances where nearby, co-regulated spots proved to be the same protein, leaving the 23 unique proteins listed in Table 1. Many of the proteins are known by more than one name; when possible we have used systematic nomenclature that reflects identities of proteins as members of gene families, with synonyms listed only when they are widely used in the literature. Mascot scores from peptide mass fingerprinting and collisionally-induced dissociation were greater than 80 (60 is the threshold for significance), and all protein identifications achieved a 100% protein score confidence interval. Supplementary Fig. 3 shows the mass spectrum for a candidate biomarker. MS coverage of 30% and sequence information from 15 peptides unequivocally identified this protein as the differentiation marker, cornulin. Supplementary Fig. 4 shows the 28 identified spots projected onto an image of a representative 2D gel. With one exception noted in the Figure Legend, calculated mass and pI values were consistent with migration. The identified proteins have a diverse set of mass and pI values, indicating that the selection criteria for potential biomarkers did not introduce any obvious bias with respect to protein size or charge.
Table 1.
Identified proteins ranked by d score
| Spot numbera | Accession number | Protein name | Mass (kDa) | pI | Peptide (coverage)b | Mascot score c | Comparison (CN)d | Abs(d score)e | FDR (%)f | Comparison 2 (HN) | Abs(d score) | FDR (%) | Comparison 3 (CH) | Abs(d score) | FDR (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 667 | Q9UBG3 | cornulin | 53 | 5.73 | 15 (33%) | 143 | (7.8) | 4.0 | - | (3.9) | 4.1 | - | (2.0) | 1.6 | 4.7 |
| 1608 | Q9UL46 | PA28 β | 27 | 5.44 | 8 (40%) | 382 | 3.6 | 3.3 | - | 1.8 | 2.0 | 3.0 | 2.0 | 1.9 | 7.5 |
| 1926 | Q99497 | DJ-1 protein | 20 | 6.33 | 5 (30%) | 83 | 2.1 | 2.7 | - | 1.7 | 2.7 | - | 1.3 | 2.0 | 31 |
| 1094 | P63261 | actin | 40 | 5.55 | 12 (37%) | 317 | 2.5 | 2.8 | - | 1.4 | 1.8 | 3.0 | 1.8 | 1.9 | 7.5 |
| 2234 | P02766 | transthyretin | 13 | 5.57 | 9 (80%) | 361 | (3.0) | 2.8 | - | (2.0) | 2.1 | - | (1.5) | 1.1 | 7.5 |
| 1809 | P04792 | HSPB1 | 22 | 7.83 | 9 (46%) | 289 | (2.8) | 2.7 | - | (1.3) | 1.2 | 12 | (2.2) | 2.0 | 0 |
| 1586 | Q5SRT3 | Cl− intracellular channel 1 | 26 | 4.95 | 7 (29%) | 179 | 2.3 | 2.6 | - | 1.6 | 2.4 | - | 1.4 | 1.1 | 20 |
| 830 | P05787 | Cytokeratin 8 | 53 | 5.52 | 14 (32%) | 141 | 3.6 | 2.4 | - | 3.2 | 2.52 | - | 1.1 | 0.22 | 49 |
| 455 | P02787 | transferrin | 55 | 6.00 | 7 (21%) | 106 | (2.8) | 2.3 | - | (1.3) | 0.77 | 16 | (2.1) | 2.2 | - |
| 2093 | O14558 | Hspβ6 (HSP20) | 16 | 5.95 | 5 (38%) | 162 | (2.8) | 2.3 | - | (1.6) | 1.1 | 9.0 | (1.8) | 1.4 | 5.9 |
| 1306 | O43488 | aflatoxin reductase | 40 | 6.70 | 8 (30%) | 227 | (2.2) | 2.1 | - | (1.6) | 2.02 | - | (1.3) | .76 | 19 |
| 237 | P08123 | α2 type I Collagen | 12 9 |
9.08 | 11 (10%) | 175 | (2.6) | 2.0 | - | (1.0 | 0.080 | 39 | (2.6) | 2.0 | - |
| 1069 | P12277 | Creatine kinase B | 42 | 5.34 | 15 (30%) | 609 | 2.1 | 2.0 | 1.0 | 1.2 | 0.71 | 32 | 1.8 | 1.4 | 16 |
| 870 | P13646 | Cytokeratin 13 | 49 | 4.87 | 19 (34%) | 514 | (4.1) | 1.9 | - | (2.6) | 2.6 | - | (1.6) | 0.61 | 23 |
| 1912 | P09211 | GST π | 23 | 5.43 | 10 (62%) | 125 | (2.1) | 1.6 | - | (1.2) | 0.56 | 20 | (1.7) | 1.4 | 5.9 |
| 1609 | Q06323 | PA28 α | 28 | 5.78 | 14 (54%) | 99 | 2.3 | 1.6 | 2.8 | (1.3) | 0.62 | 32 | 1.8 | 1.0 | 23 |
| 2006 | P04179 | Manganese SOD | 22 | 6.86 | 7 (37%) | 89 | 1.3 | 0.40 | 16 | 2.3 | 3.3 | - | (1.8) | 1.5 | 4.7 |
| 612 | Q5TCJ3 | Lamin A/C | 65 | 6.40 | 19 (30%) | 80 | (1.6) | 1.0 | 5.3 | (2.6) | 2.6 | - | 1.6 | 1.2 | 19 |
| 1141 | P30740 | Serpin B1 (elastase inhibitor) | 42 | 5.90 | 18 (44%) | 402 | (1.2) | 1.3 | 1.9 | (2.1) | 2.4 | - | 1.9 | 0.47 | 45 |
| 1103 | Q8IXI3 | Serpin B2 (SCCA1) | 44 | 6.35 | 16 (52%) | 546 | (1.7) | 0.29 | 16 | (2.0) | 2.2 | - | 1.2 | 1.5 | 16 |
| 1464 | P13645 | Cytokeratin 10 | 59 | 5.09 | 14 (22%) | 122 | (2.1) | 1.3 | 1.9 | (2.1) | 2.1 | - | (1.0) | 0.044 | 49 |
| 753 | P02538 | Cytokeratin 6A | 60 | 7.59 | 18 (31%) | 274 | (1.8) | 0.88 | 6.7 | (2.5) | 1.8 | 1.6 | 1.4 | 0.56 | 43 |
| 801 | P23381 | trp-tRNA synthetase | 53 | 6.03 | 6 (35%) | 235 | 1.7 | 0.97 | 13 | (1.4) | 0.98 | 13 | 2.4 | 2.3 | 7.1 |
Spots were ranked in order of decreasing absolute value of d score as determined by SAM algorithm. Dark grey shading denotes up-regulation in the indicated comparison, light grey shading denotes down-regulation, lack of shading denotes not significant. Spot numbers are as they appear on the master analytical gel. Protein accession numbers are from the SwissProt database. Predicted protein masses and isoelectric points are based on conceptual translation. CN: comparison of cancer and normal. HN: comparison of HSIL and normal. CH: comparison of cancer and HSIL.
Number of peptides that matched the identified protein sequence, followed by percent sequence coverage.
Mascot score based on combined peptide mass fingerprinting and masses of collisionally-induced dissociation peptides
Fold change in expression. Values in parentheses are decreases, other values are increases.
Absolute value of d(i) from SAM calculation.
False discovery rate from SAM calculation.
3.4 Literature Review
As a first step in understanding the significance of the findings, we performed a literature review to identify relevant genetic, structural, and biological data for each protein. Relevant citations are summarized in Supplementary Table 1. Several of the cytokeratins, two of the detoxifying enzymes, HSPB1, and Serpin 3 (SCCA1), have all been previously characterized in the context of cervical cancer development (see references in Supplementary Table 1). About half of the candidate markers, however, had not been previously associated with cervical cancer or HSIL. In many cases, biomarker increase and decrease can be rationalized in terms of the known effects of HPV E6 and E7 oncoproteins, selection for growth advantage during latency, or host-lesion interactions. We consider these general patterns in the Discussion.
3.5 Validation by immunohistochemical staining
To increase confidence in the 2-DIGE and mass spectrometry findings, we randomly selected three specimens from each group in the original cohort and performed immunohistochemistry. We investigated four markers, chosen because of the commercial availability of antibodies suitable for immunochemistry and because the markers were (a) novel in the context of cervical cancer or (b) there was a discrepancy between our data and previous reports. We stained serial sections with antibodies to cornulin, PA28β, HSPB1 and MnSOD and scored the slides on a standard scale of 0 to 3 based on intensity of staining (Fig. 4). Results showed generally good agreement with the 2D-DIGE quantification (compare Fig. 4 with Fig. 3). In panel A, prominent cornulin staining is evident in the maturing squamous cells of the normal epithelium, in only a thin rim of non-dysplastic cells representing the outermost layer of epithelium in HSIL, and not at all in an invasive cancer sample. In Panel B, PA28β staining was evident only in cancer, and not in normal or HSIL. In panel C, the pattern of HSPB1 staining was similar to cornulin, with intense staining in the normal epithelium, consistent with reports that this small heat shock protein is a cornification chaperone [29, 30]. HSPB1 staining was also present, but at a lower level in HSIL and cancer (panel C), consistent with a prior immunohistochemical study of HSPB1 expression in cervical pre-cancerous lesions and cancer [31]. There was HSPB1 staining in areas of necrosis in cancer samples (not shown) but necrotic areas were excluded in the LCM procedure and thus not represented in the 2D-DIGE sampling. Immunohistochemical staining of MnSOD showed expression in a thin layer of cells along the basal layer of the normal squamous epithelium, an increase in expression in HSIL, and somewhat of a decline in cancer, again consistent with 2D-DIGE.
FIG. 4. Immunohistochemistry of selected samples within the original cohort.
Immunostaining was performed as described in Materials and Methods using representative frozen sections from patients in the indicated experimental groups. A., anti-cornulin, B., anti- PA28β, C., anti-Hsp27 (HSPB1), D., anti-manganese superoxide dismutase. Graphs at left in each panel represent results of scoring on a standard 0–3 scale. Red symbols correspond to the images shown. Images at right are labeled according to sample code number. Brown chromogen represents positive staining.
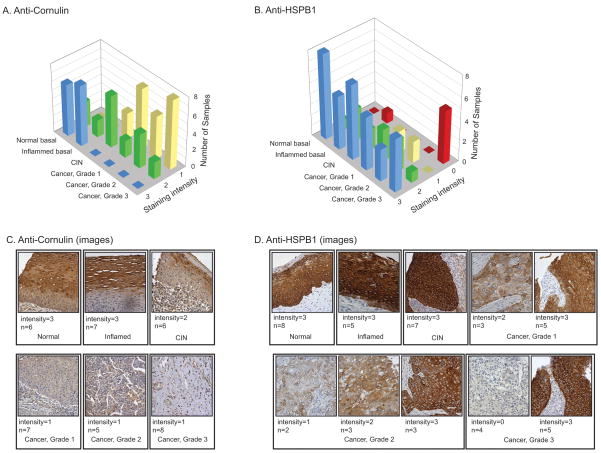
To increase statistical power and extend the findings to a different cohort of patients, we performed additional immunohistochemistry experiments using formalin fixed paraffin embedded tissue microarrrays (Fig. 5). The microarrays include more patients (n=60) and additional experimental groups (e.g., benign inflammation and lower grades of cervical intraepithelial neoplasia (CIN)). Tissue microarrays were stained with anti-cornulin or anti- Hsp27 (HSPB1), Staining intensity was scored on the same 0 to 3 scale. Statistical analysis was performed by one-way ANOVA. Differences contributing to group variance were calculated in pair-wise comparisons using the Tukey’s Honestly Significant Difference Test.
FIG. 5. Immunohistochemistry with tissue microarray.
Commercial microarray with samples drawn from an independent patient cohort. Scoring was as in Fig. 4. A, B, Histograms of staining intensity. C. Representative sections stained with anti-cornulin demonstrating intense staining in normal and inflamed tissue, moderate staining in CIN, and reduced staining in cancer. D. Representative sections stained with anti-HSPB1 demonstrating intense staining in normal, inflamed, and CIN, and large variance in cancer.
Anti-cornulin staining (Fig. 5A,C) showed no apparent difference between normal and inflamed tissue, but a highly significant difference between these two groups and cancer (p<0.001). The CIN samples had a wide distribution of values centered in between normal and cancer. We attribute the variance to the presence of multiple grades of CIN in this cohort. Because of the within-group variance, comparisons of CIN to the other groups did not reveal a statistically significant difference.
Anti-HSPB1 staining confirmed that expression of this molecular chaperone is high in normal epithelium, inflamed tissue, and HSIL, consistent with results obtained with 2D-DIGE. Surprisingly, expression in cancer was far more variable than in the original cohort. This was particularly true of grade 3 cancers, where HSPB1 was present either in high amounts or not at all.
4 DISCUSSION
The purpose of this study was to identify biomarkers that correlate with progression to neoplasia in cervical cancer. Possibly, such markers could be used to increase the positive predictive value of current screening modalities. In addition, they may provide insights into the biology of cervical cancer and thus provide leads for the development of nonsurgical therapies. To identify biomarkers, we analyzed proteomic patterns from samples representing normal, premalignant, and cancer tissue. We used a dedicated patient sample collection system, LCM to separate lesional tissue from surrounding normal tissue, and a sensitive analytical methodology to allow profiling with only a few micrograms of protein. To our knowledge it is the first study to simultaneously compare normal cervical tissue, cervical intraepithelial neoplasia, and invasive cervical cancer tissue using the same proteomic methodology.
There were significant changes in expression of many proteins, of which 23 have so far been identified at the molecular level (Table 1). Twelve have been seen before in HSIL or cancer, and results are generally concordant with the prior literature. Eleven proteins are novel. Initial (technical) validation was performed by randomly sampling a small number of specimens from the original cohort. Results agreed with the 2D-DIGE analysis, lending confidence in the technical quality of the 2D-DIGE and MS data. We also carried out a more extensive immunohistochemistry study for two proteins of particular interest, cornulin and HSPB1, which drew on a different patient cohort with larger numbers of specimens and additional disease states.
The results emphasize the power of using matched patient samples. In the 2D-DIGE experiments we identified several proteins where there was a significant change in expression between individual normal-HSIL pairs, even though the range of expression values for the normal and HSIL groups as a whole overlapped. These pairings were preserved in the technical validation study using the frozen sections. In the tissue microarrays however, samples were not patient-matched. Although this makes the tissue microarray somewhat less powerful, results (discussed in more detail in the following sections) extended the initial 2D-DIGE findings.
We focus here on overall patterns apparent in the data. In principle, there are at least three processes that have the potential to change the proteomic profile during cervical cancer progression: (1) effects resulting from direct interaction of HPV oncoproteins with cellular proteins, (2) stochastic effects resulting from the combination of cell proliferation, genomic instability, and selective pressure during the latency period that is required for development of HSIL and cancer, and (3) emergent properties resulting from interactions of lesional tissue with the tissue microenvironment. Patterns ascribable to all three processes appear to be present in the proteomic data.
4.1 Markers that potentially arise from direct interactions of HPV oncoproteins with cellular proteins
HPV E6 and E7 bind directly to p53, Rb, and a number of other cellular proteins (reviewed in references [10, 11]). Effects potentially attributable to direct interactions of HPV oncoproteins with these and other cellular proteins account for at least a quarter of the changes in the study. Serpin B1, a member of a large family of serine protease inhibitors, binds directly to E7 in a pull-down assay [32]. It is down-regulated in vitro in E7-transfected cells [33], consistent with the down-regulation observed here in HSIL. Glutathione-S-transferase similarly decreases in E7-transfected cells, although it is unknown if this reflects a direct protein-protein interaction [37]. Three other proteins identified in this study are known products of p53 target genes. Creatine kinase B and tryptophanyl tRNA synthetase are p53-repressible enzymes [34, 35] that increased significantly in cancer. Although expression of these proteins may be influenced by factors in addition to p53, the direction of the changes in expression, in both cases, is consistent with HPV E6-mediated loss of p53 function.
Other identified proteins may be regulated indirectly as a result of compromised Rb function in E7-expressing cells, which fosters continued proliferation of cells in the upper layers of squamous epithelium, reducing or blocking terminal differentiation and cornification. The differentiation marker, cornulin, declines in HSIL and further declines in cancer. Cornulin is a member of the “fused gene” family, binds calcium, and is up-regulated in response to deoxycholate-induced stress [36, 37]. It is normally expressed late during epidermal differentiation, but its function is otherwise unknown, and it has not previously been described as a cervical cancer marker. Cornulin was the only protein that showed statistically significant differences in all three pairwise 2D-DIGE comparisons. This, taken with the result of the immunohistochemistry experiments, suggests that cornulin might be uniquely useful as a diagnostic marker of disease state.
Changes in cytokeratin expression can also be ascribed to loss of the differentiated state. Expression of three cytokeratins (6A, 10, and 13) decreased in HSIL and cancer relative to normal tissue. These three proteins are known markers of keratinocyte differentiation, and the decline is consistent both with loss of the differentiated state and with previous studies of cervical cancer [15]. Cytokeratin 8 was increased in cancer relative to normal tissue, again consistent with previous work [38].
HSPB1 apparently falls into the same category of differentiation markers. The observed decline in cancer specimens was paradoxical, in that expression of this and other HSPs have been widely observed to increase in proteomic studies of cancer cells. Although there are conflicting prior reports about expression in HPV-induced lesions [14, 31, 39], it is believed that HSPB1 may have a specialized function as a cornification chaperone, and it is expressed at high levels in the upper levels of normal stratified epithelium and in in vitro differentiated keratinocytes (Fig. 4 and references [29, 39]). We saw relatively high levels in normal cervix, a slight decline in HSIL, and a marked decline in cancer, especially in some specimens. A decline in expression in less-differentiated lesions plausibly reflects their inability to undergo terminal differentiation in the presence of HPV oncoproteins. Consistent with this, in the tissue microarray, the highest frequency of HSPB1-negative specimens was in the least-differentiated (grade 3) tumors. It will be of interest to investigate the mechanism of heterogeneity in high-grade cancers and to determine whether HSPB1 status has independent prognostic or predictive value. This will require a separate study, as clinical outcome data are not available for the subjects used here.
Expression of another small heat shock protein, Hspβ6 (Hsp20) also declined in HSIL and cancer. We did not observe any examples of HSPs that increased significantly in HSIL or cancer.
4.2 Markers that are potentially selected during the latency period
Like other human cancers, cervical cancer typically develops only after a long latency period. Effects attributable to variation and selection for growth advantage are expected to occur stochastically during latency; that is, both the timing and whether a given change occurs at all will vary between patients. The oncoprotein, DJ-1, may fall into this category. DJ-1 significantly increased in cancer versus normal tissue, whereas expression values in HSIL showed considerable dispersion. DJ-1 transforms mouse NIH3T3 cells in vitro and is overexpressed in many cancers including: breast, lung, pancreatic, ovarian, and prostate [40–44]. Mechanistic studies show that DJ-1 is a negative regulator of the tumor suppressor, PTEN [45]. Interestingly, although down-regulation of PTEN expression is a negative prognostic indicator in cervical cancer [46, 47], direct mutation or loss of heterozygosity at the PTEN locus is rare [46]. Overexpression of DJ-1 could provide a mechanism for down-regulation in the absence of direct mutation or loss of the PTEN gene. It is well established that deficiency of DJ-1 (also known as PARK7) sensitizes dopaminergic neurons to stress-mediated apoptosis in hereditary Parkinson’s disease(reviewed in reference [48]). Regulation of apoptosis appears to be the common link explaining the role of DJ-1 in these disparate diseases. Interestingly, expression of Serpin B1, another biomarker discovered in this study, has previously been shown to be PTEN dependent [49]. Thus down-regulation of PTEN in HSIL could provide another explanation for the observed down regulation of Serpin B1 (in addition to direct interaction of HPV E7 with Serpin B1).
Several other proteins may fall into the category of proteins that are selected during the latency period. Manganese superoxide dismutase, which increased in HSIL, protects against free radical toxicity. High expression has previously been correlated with poor outcomes in cervical cancer [50]. Serpin B3 (SCCA1) declined in HSIL, and Chloride intracellular channel 1 protein increased in cancer; both results are novel in the context of cervical disease.
4.3 Markers that are potentially influenced by interaction of lesions with the microenvironment
Three IFN-γ inducible proteins were identified as up-regulated in cancer. Unlike IFN-α and IFN-β, which are expressed by many cell types, IFN-γ is expressed only by T cells and NK cells. Thus, expression of IFN-γ-inducible genes in cancers cells is expected to occur only as a consequence of cell-cell interactions within the tissue microenvironment. Two of the IFN-γ-inducible proteins, PA28 α and PA28 β, activate the 20S proteasome complex, which presents antigens via the MHC I pathway. Although the up-regulation of these proteins is novel in cervical cancer, up-regulation of PA 28 α has been described previously in infiltrating ductal breast carcinoma [51]. Another IFN-γ-inducible tryptophanyl protein, tRNA synthetase, has been hypothesized to protect cells from tryptophan starvation following IFN-γ-mediated induction of the catabolic enzyme, indoleamine 2,3 dioxygenase [52].
Other proteins that may fall into the category of changes attributable to host-lesion interactions include the serum transporter, transthyretin, which was decreased in cancer and HSIL. Transthyretin is a negative acute-phase serum protein that decreases in inflammatory conditions including many cancers [53]. Transferrin, another serum transporter that decreased in cancer, has been reported to decrease in ovarian cancer [54]. The decrease in extracellular matrix protein, α2-type 1 collagen that occurred in HSIL may be an indirect effect of IFN-γ, mediated via stimulation of the IRF-1 transcription factor [55].
4.4 Potential for clinical translation
Cervical cancer differs from many other common epithelial cancers in that a successful population screening method (the Pap test) has been widely adopted. Given this success, we assume that molecular biomarkers will be used to augment, rather than replace, the current screening regime. Molecular markers that objectively distinguish low and high-grade lesions have the potential to improve upon classification based on cell morphology alone. Reducing subjectivity should increase both positive and negative predictive value. Additionally, biomarkers may reflect molecular progression not observable cytologically. Markers that better represent disease progression have the potential to decrease the resources devoted to clinical follow-up and reduce the risk of overtreatment.
Although proof of clinical utility in these settings is well beyond the scope of the present study, several of the proteins identified here appear promising. The 2D-DIGE data showed that cornulin expression differs very significantly in normal and cancer, with intermediate expression in cervical intraepithelial neoplasia. A similar pattern of results was seen by immunohistochemistry, both in the initial cohort and in a larger validation cohort. DJ-1 provides another example of a protein that differs significantly in normal and cancer, with intermediate (and variable) levels in preneoplasia. Although immunohistochemistry results for DJ-1 are not available (staining was inconclusive, possibly because of poor quality of the commercial antibody), the biology of the protein suggests a possible mechanistic link with carcinogenesis. Our results lead to a testable hypothesis that quantitation of cornulin or DJ-1 levels might be used to predict risk of progression of low-grade lesions. HSPB1 provides an example of a different expression pattern, where levels are uniformly high in normal tissue and HSIL, but vary widely in cancer. The results support a hypothesis that HSPB1-negative cancers may have a different prognosis or response to treatment. Testing these hypotheses will require larger patient cohorts, outcomes data, and further development of methods for protein quantification in very small samples available from clinical screening.
Supplementary Material
Acknowledgments
We thank Drs. Carlos Santos, Carlos Velarde, and Oscar Galdos at the Departamento de Ginecología, Instituto de Enfermedades Neoplásicas, Lima, Peru, and Dr. Eileen Dickman, Medical College of Georgia, for invaluable assistance in sample acquisition and selection; Dr. Olga Roberts for contributions to the design of the experiments to measure technical variation (Supplementary Figure 1); and Eric Miller of the Medical College of Georgia proteomics core for data collection.
Abbreviations
- HPV
Human Papillomavirus
- HSIL
High-grade Squamous Intraepithelial Lesion
- LCM
Laser Capture Microdissection
- SAM
Significance Analysis of Microarray
5 LITERATURE CITED
- 1.Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006;20:207–225. doi: 10.1016/j.bpobgyn.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Loos AH, McCarron P, Weiderpass E, et al. Trends in cervical squamous cell carcinoma incidence in 13 European countries: changing risk and the effects of screening. Cancer Epidemiol Biomarkers Prev. 2005;14:677–686. doi: 10.1158/1055-9965.EPI-04-0569. [DOI] [PubMed] [Google Scholar]
- 3.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics. CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 4.Solomon D, Davey D, Kurman R, Moriarty A, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. Jama. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 5.Nanda K, McCrory DC, Myers ER, Bastian LA, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132:810–819. doi: 10.7326/0003-4819-132-10-200005160-00009. [DOI] [PubMed] [Google Scholar]
- 6.Malpica A, Matisic JP, Niekirk DV, Crum CP, et al. Kappa statistics to measure interrater and intrarater agreement for 1790 cervical biopsy specimens among twelve pathologists: qualitative histopathologic analysis and methodologic issues. Gynecol Oncol. 2005;99:S38–52. doi: 10.1016/j.ygyno.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 7.Cantor SB, Atkinson EN, Cardenas-Turanzas M, Benedet JL, et al. Natural history of cervical intraepithelial neoplasia: a meta-analysis. Acta Cytol. 2005;49:405–415. doi: 10.1159/000326174. [DOI] [PubMed] [Google Scholar]
- 8.Sadler L, Saftlas A, Wang W, Exeter M, et al. Treatment for cervical intraepithelial neoplasia and risk of preterm delivery. JAMA. 2004;291:2100–2106. doi: 10.1001/jama.291.17.2100. [DOI] [PubMed] [Google Scholar]
- 9.Samson SL, Bentley JR, Fahey TJ, McKay DJ, Gill GH. The effect of loop electrosurgical excision procedure on future pregnancy outcome. Obstet Gynecol. 2005;105:325–332. doi: 10.1097/01.AOG.0000151991.09124.bb. [DOI] [PubMed] [Google Scholar]
- 10.Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006;110:525–541. doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- 11.Munger K, Basile JR, Duensing S, Eichten A, et al. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene. 2001;20:7888–7898. doi: 10.1038/sj.onc.1204860. [DOI] [PubMed] [Google Scholar]
- 12.Arbyn M, Buntinx F, Van Ranst M, Paraskevaidis E, et al. Virologic versus cytologic triage of women with equivocal Pap smears: a meta-analysis of the accuracy to detect high-grade intraepithelial neoplasia. J Natl Cancer Inst. 2004;96:280–293. doi: 10.1093/jnci/djh037. [DOI] [PubMed] [Google Scholar]
- 13.Kong CS, Balzer BL, Troxell ML, Patterson BK, Longacre TA. p16INK4A immunohistochemistry is superior to HPV in situ hybridization for the detection of high-risk HPV in atypical squamous metaplasia. Am J Surg Pathol. 2007;31:33–43. doi: 10.1097/01.pas.0000213347.65014.ee. [DOI] [PubMed] [Google Scholar]
- 14.El-Ghobashy AA, Shaaban AM, Innes J, Prime W, Herrington CS. Differential expression of cyclin-dependent kinase inhibitors and apoptosis-related proteins in endocervical lesions. Eur J Cancer. 2007;43:2011–2018. doi: 10.1016/j.ejca.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Baak JP, Kruse AJ, Robboy SJ, Janssen EA, et al. Dynamic behavioural interpretation of cervical intraepithelial neoplasia with molecular biomarkers. J Clin Pathol. 2006;59:1017–1028. doi: 10.1136/jcp.2005.027839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunne EF, Unger ER, Sternberg M, McQuillan G, et al. Prevalence of HPV infection among females in the United States. Jama. 2007;297:813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 17.Bae SM, Lee CH, Cho YL, Nam KH, et al. Two-dimensional gel analysis of protein expression profile in squamous cervical cancer patients. Gynecol Oncol. 2005;99:26–35. doi: 10.1016/j.ygyno.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 18.Choi YP, Kang S, Hong S, Xie X, Cho NH. Proteomic analysis of progressive factors in uterine cervical cancer. Proteomics. 2005;5:1481–1493. doi: 10.1002/pmic.200401021. [DOI] [PubMed] [Google Scholar]
- 19.Gu Y, Wu SL, Meyer JL, Hancock WS, et al. Proteomic Analysis of High-Grade Dysplastic Cervical Cells Obtained from ThinPrep Slides Using Laser Capture Microdissection and Mass Spectrometry. Journal of Proteome Research. 2007 doi: 10.1021/pr070319j. [DOI] [PubMed] [Google Scholar]
- 20.Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92:727–735. doi: 10.1016/s0029-7844(98)00245-2. [DOI] [PubMed] [Google Scholar]
- 21.Shaw J, Rowlinson R, Nickson J, Stone T, et al. Evaluation of saturation labelling two-dimensional difference gel electrophoresis fluorescent dyes. Proteomics. 2003;3:1181–1195. doi: 10.1002/pmic.200300439. [DOI] [PubMed] [Google Scholar]
- 22.Sitek B, Potthoff S, Schulenborg T, Stegbauer J, et al. Novel approaches to analyse glomerular proteins from smallest scale murine and human samples using DIGE saturation labelling. Proteomics. 2006;6:4337–4345. doi: 10.1002/pmic.200500739. [DOI] [PubMed] [Google Scholar]
- 23.Greengauz-Roberts O, Stoppler H, Nomura S, Yamaguchi H, et al. Saturation labeling with cysteine-reactive cyanine fluorescent dyes provides increased sensitivity for protein expression profiling of laser-microdissected clinical specimens. Proteomics. 2005;5:1746–1757. doi: 10.1002/pmic.200401068. [DOI] [PubMed] [Google Scholar]
- 24.Wilson KE, Marouga R, Prime JE, Pashby DP, et al. Comparative proteomic analysis using samples obtained with laser microdissection and saturation dye labelling. Proteomics. 2005;5:3851–3858. doi: 10.1002/pmic.200401255. [DOI] [PubMed] [Google Scholar]
- 25.Shekouh AR, Thompson CC, Prime W, Campbell F, et al. Application of laser capture microdissection combined with two-dimensional electrophoresis for the discovery of differentially regulated proteins in pancreatic ductal adenocarcinoma. Proteomics. 2003;3:1988–2001. doi: 10.1002/pmic.200300466. [DOI] [PubMed] [Google Scholar]
- 26.Seike M, Kondo T, Fujii K, Yamada T, et al. Proteomic signature of human cancer cells. Proteomics. 2004;4:2776–2788. doi: 10.1002/pmic.200300795. [DOI] [PubMed] [Google Scholar]
- 27.Alban A, David SO, Bjorkesten L, Andersson C, et al. A novel experimental design for comparative two-dimensional gel analysis: two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics. 2003;3:36–44. doi: 10.1002/pmic.200390006. [DOI] [PubMed] [Google Scholar]
- 28.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonak C, Klosner G, Kokesch C, D FO, et al. Subcorneal colocalization of the small heat shock protein, hsp27, with keratins and proteins of the cornified cell envelope. Br J Dermatol. 2002;147:13–19. doi: 10.1046/j.1365-2133.2002.04667.x. [DOI] [PubMed] [Google Scholar]
- 30.O’Shaughnessy RF, Welti JC, Cooke JC, Avilion AA, et al. AKT-dependent HspB1 (Hsp27) activity in epidermal differentiation. J Biol Chem. 2007;282:17297–17305. doi: 10.1074/jbc.M610386200. [DOI] [PubMed] [Google Scholar]
- 31.Ciocca DR, Lo Castro G, Alonio LV, Cobo MF, et al. Effect of human papillomavirus infection on estrogen receptor and heat shock protein hsp27 phenotype in human cervix and vagina. Int J Gynecol Pathol. 1992;11:113–121. doi: 10.1097/00004347-199204000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Lee KA, Kang JW, Shim JH, Kho CW, et al. Protein profiling and identification of modulators regulated by human papillomavirus 16 E7 oncogene in HaCaT keratinocytes by proteomics. Gynecol Oncol. 2005;99:142–152. doi: 10.1016/j.ygyno.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 33.Lee KA, Shim JH, Kho CW, Park SG, et al. Protein profiling and identification of modulators regulated by the E7 oncogene in the C33A cell line by proteomics and genomics. Proteomics. 2004;4:839–848. doi: 10.1002/pmic.200300626. [DOI] [PubMed] [Google Scholar]
- 34.Rahman-Roblick R, Roblick UJ, Hellman U, Conrotto P, et al. p53 targets identified by protein expression profiling. Proc Natl Acad Sci U S A. 2007;104:5401–5406. doi: 10.1073/pnas.0700794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao J, Schmieg FI, Simmons DT, Molloy GR. Mouse p53 represses the rat brain creatine kinase gene but activates the rat muscle creatine kinase gene. Mol Cell Biol. 1994;14:8483–8492. doi: 10.1128/mcb.14.12.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Contzler R, Favre B, Huber M, Hohl D. Cornulin, a new member of the “fused gene” family, is expressed during epidermal differentiation. J Invest Dermatol. 2005;124:990–997. doi: 10.1111/j.0022-202X.2005.23694.x. [DOI] [PubMed] [Google Scholar]
- 37.Darragh J, Hunter M, Pohler E, Nelson L, et al. The calcium-binding domain of the stress protein SEP53 is required for survival in response to deoxycholic acid-mediated injury. Febs J. 2006;273:1930–1947. doi: 10.1111/j.1742-4658.2006.05206.x. [DOI] [PubMed] [Google Scholar]
- 38.Manavi M, Hudelist G, Fink-Retter A, Gschwandtler-Kaulich D, et al. Gene profiling in Pap-cell smears of high-risk human papillomavirus-positive squamous cervical carcinoma. Gynecol Oncol. 2007;105:418–426. doi: 10.1016/j.ygyno.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 39.Trautinger F, Kindas-Mugge I, Dekrout B, Knobler RM, Metze D. Expression of the 27-kDa heat shock protein in human epidermis and in epidermal neoplasms: an immunohistological study. Br J Dermatol. 1995;133:194–202. doi: 10.1111/j.1365-2133.1995.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 40.Nagakubo D, Taira T, Kitaura H, Ikeda M, et al. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- 41.Le Naour F, Misek DE, Krause MC, Deneux L, et al. Proteomics-based identification of RS/DJ-1 as a novel circulating tumor antigen in breast cancer. Clin Cancer Res. 2001;7:3328–3335. [PubMed] [Google Scholar]
- 42.MacKeigan JP, Clements CM, Lich JD, Pope RM, et al. Proteomic profiling drug-induced apoptosis in non-small cell lung carcinoma: identification of RS/DJ-1 and RhoGDIalpha. Cancer Res. 2003;63:6928–6934. [PubMed] [Google Scholar]
- 43.Hod Y. Differential control of apoptosis by DJ-1 in prostate benign and cancer cells. J Cell Biochem. 2004;92:1221–1233. doi: 10.1002/jcb.20159. [DOI] [PubMed] [Google Scholar]
- 44.Davidson B, Hadar R, Schlossberg A, Sternlicht T, et al. Expression and clinical role of DJ-1, a negative regulator of PTEN, in ovarian carcinoma. Hum Pathol. 2008;39:87–95. doi: 10.1016/j.humpath.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Kim RH, Peters M, Jang Y, Shi W, et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7:263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Cheung TH, Lo KW, Yim SF, Chan LK, et al. Epigenetic and genetic alternation of PTEN in cervical neoplasm. Gynecol Oncol. 2004;93:621–627. doi: 10.1016/j.ygyno.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 47.Lee JS, Choi YD, Lee JH, Nam JH, et al. Expression of PTEN in the progression of cervical neoplasia and its relation to tumor behavior and angiogenesis in invasive squamous cell carcinoma. J Surg Oncol. 2006;93:233–240. doi: 10.1002/jso.20493. [DOI] [PubMed] [Google Scholar]
- 48.Morris HR. Genetics of Parkinson’s disease. Annals of medicine. 2005;37:86–96. doi: 10.1080/07853890510007269. [DOI] [PubMed] [Google Scholar]
- 49.Hong TM, Yang PC, Peck K, Chen JJ, et al. Profiling the downstream genes of tumor suppressor PTEN in lung cancer cells by complementary DNA microarray. Am J Respir Cell Mol Biol. 2000;23:355–363. doi: 10.1165/ajrcmb.23.3.4002. [DOI] [PubMed] [Google Scholar]
- 50.Nakano T, Oka K, Taniguchi N. Manganese superoxide dismutase expression correlates with p53 status and local recurrence of cervical carcinoma treated with radiation therapy. Cancer Res. 1996;56:2771–2775. [PubMed] [Google Scholar]
- 51.Somiari RI, Sullivan A, Russell S, Somiari S, et al. High-throughput proteomic analysis of human infiltrating ductal carcinoma of the breast. Proteomics. 2003;3:1863–1873. doi: 10.1002/pmic.200300560. [DOI] [PubMed] [Google Scholar]
- 52.Boasso A, Herbeuval JP, Hardy AW, Winkler C, Shearer GM. Regulation of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA-synthetase by CTLA-4-Fc in human CD4+ T cells. Blood. 2005;105:1574–1581. doi: 10.1182/blood-2004-06-2089. [DOI] [PubMed] [Google Scholar]
- 53.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 54.Kozak KR, Su F, Whitelegge JP, Faull K, et al. Characterization of serum biomarkers for detection of early stage ovarian cancer. Proteomics. 2005;5:4589–4596. doi: 10.1002/pmic.200500093. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka S, Ramirez F. The first intron of the human alpha2(I) collagen gene (COL1A2) contains a novel interferon-gamma responsive element. Matrix Biol. 2007;26:185–189. doi: 10.1016/j.matbio.2006.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.