Abstract
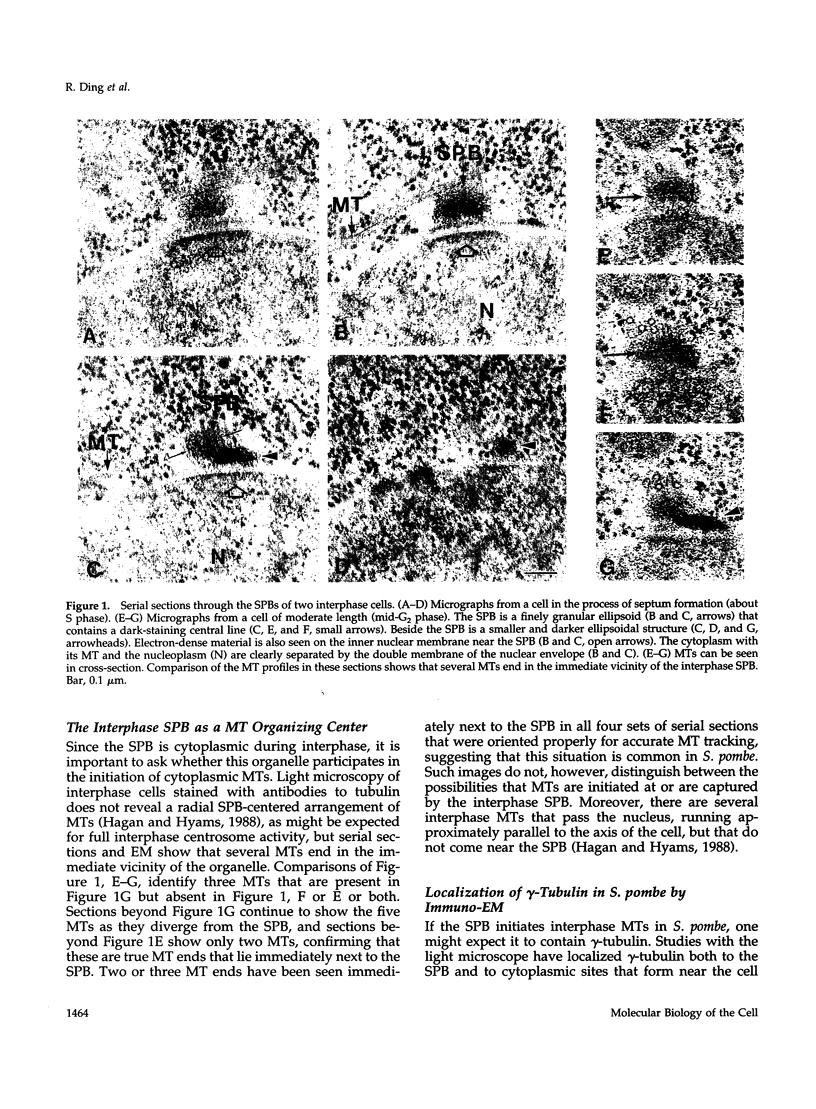
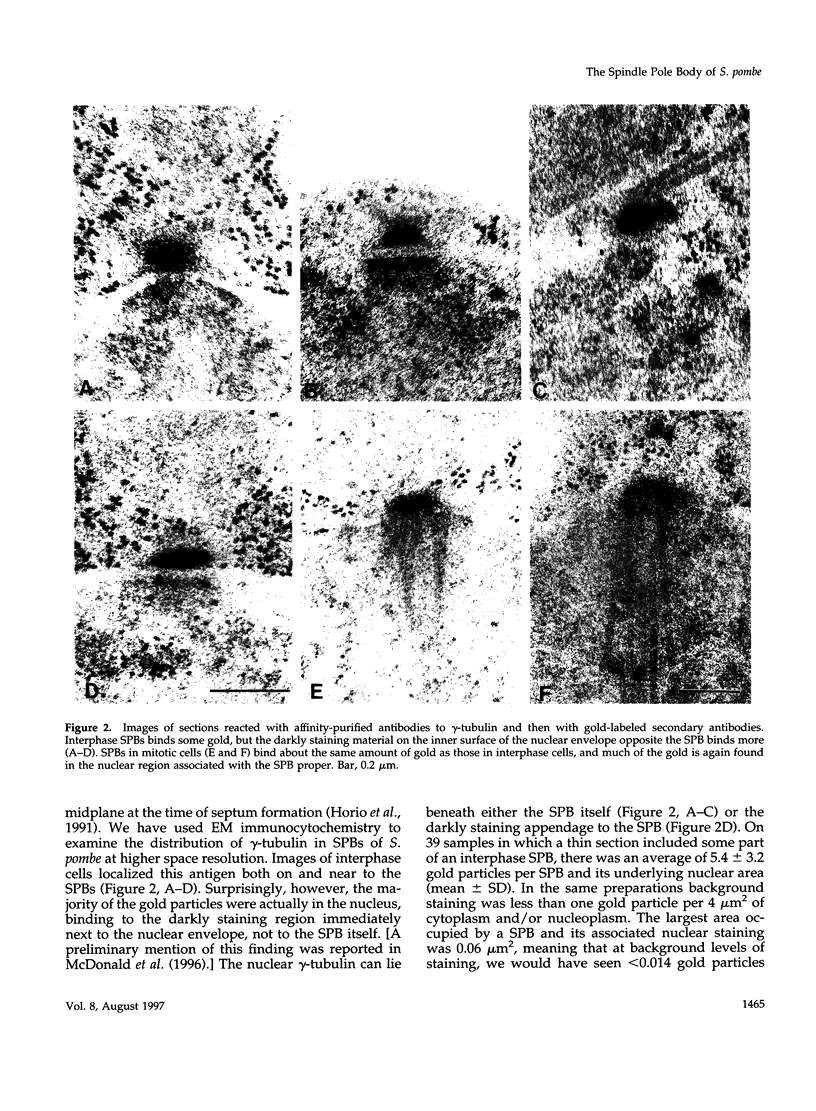
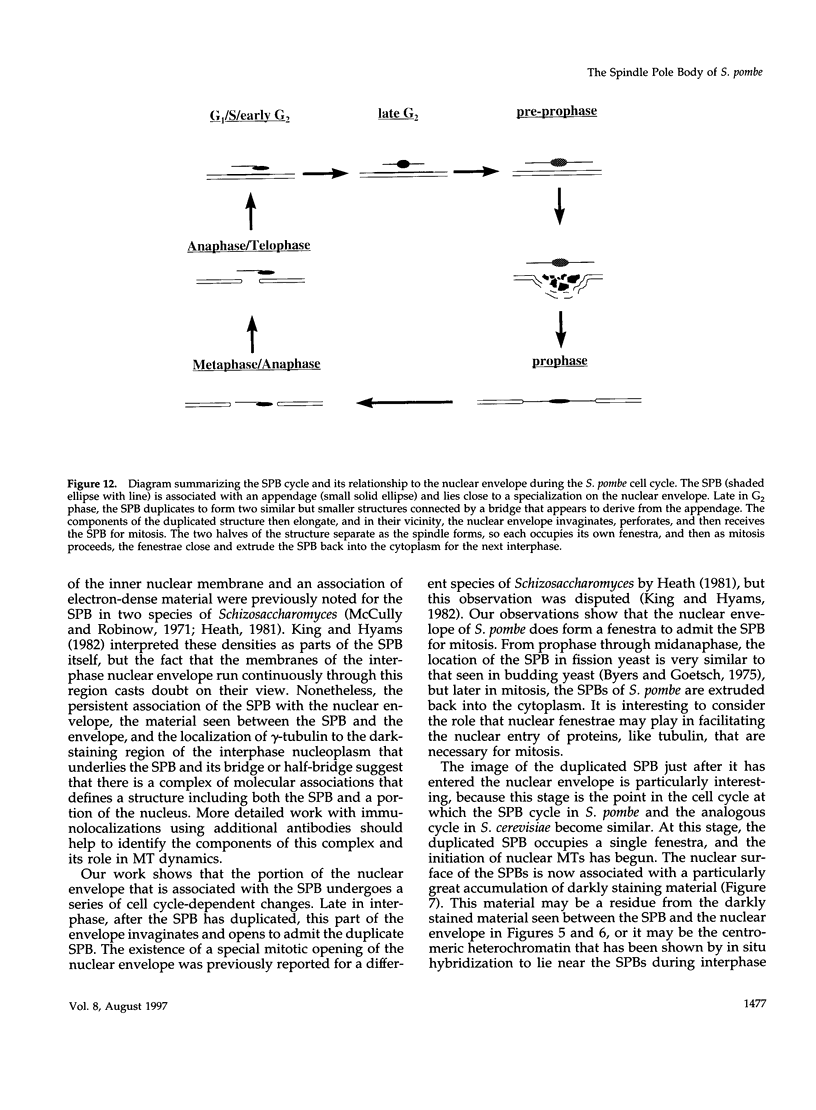
The cycle of spindle pole body (SPB) duplication, differentiation, and segregation in Schizosaccharomyces pombe is different from that in some other yeasts. Like the centrosome of vertebrate cells, the SPB of S. pombe spends most of interphase in the cytoplasm, immediately next to the nuclear envelope. Some gamma-tubulin is localized on the SPB, suggesting that it plays a role in the organization of interphase microtubules (MTs), and serial sections demonstrate that some interphase MTs end on or very near to the SPB. gamma-Tubulin is also found on osmiophilic material that lies near the inner surface of the nuclear envelope, immediately adjacent to the SPB, even though there are no MTs in the interphase nucleus. Apparently, the MT initiation activities of gamma-tubulin in S. pombe are regulated. The SPB duplicates in the cytoplasm during late G2 phase, and the two resulting structures are connected by a darkly staining bridge until the mitotic spindle forms. As the cell enters mitosis, the nuclear envelope invaginates beside the SPB, forming a pocket of cytoplasm that accumulates dark amorphous material. The nuclear envelope then opens to form a fenestra, and the duplicated SPB settles into it. Each part of the SPB initiates intranuclear MTs, and then the two structures separate to lie in distinct fenestrae as a bipolar spindle forms. Through metaphase, the SPBs remain in their fenestrae, bound to the polar ends of spindle MTs; at about this time, a small bundle of cytoplasmic MTs forms in association with each SPB. These MTs are situated with one end near to, but not on, the SPBs, and they project into the cytoplasm at an orientation that is oblique to the simple axis. As anaphase proceeds, the nuclear fenestrae close, and the SPBs are extruded back into the cytoplasm. These observations define new fields of enquiry about the control of SPB duplication and the dynamics of the nuclear envelope.
Full text
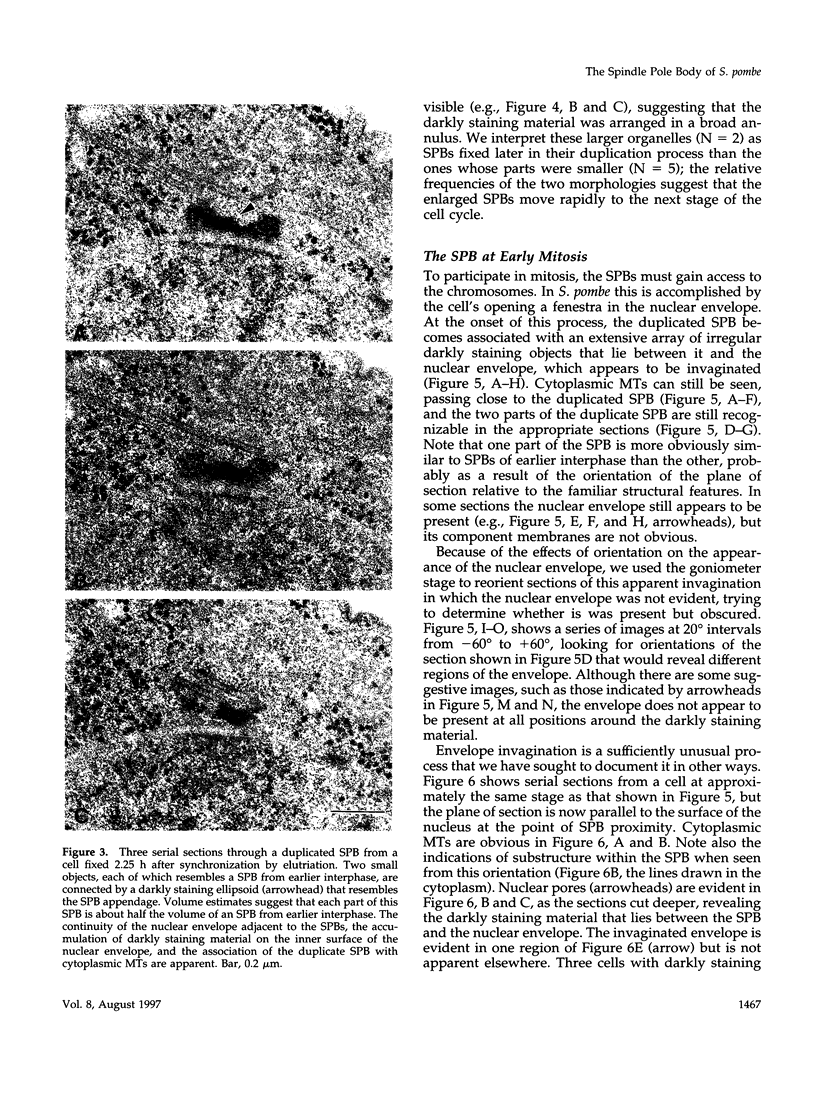
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergen L. G., Kuriyama R., Borisy G. G. Polarity of microtubules nucleated by centrosomes and chromosomes of Chinese hamster ovary cells in vitro. J Cell Biol. 1980 Jan;84(1):151–159. doi: 10.1083/jcb.84.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B., Goetsch L. Behavior of spindles and spindle plaques in the cell cycle and conjugation of Saccharomyces cerevisiae. J Bacteriol. 1975 Oct;124(1):511–523. doi: 10.1128/jb.124.1.511-523.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
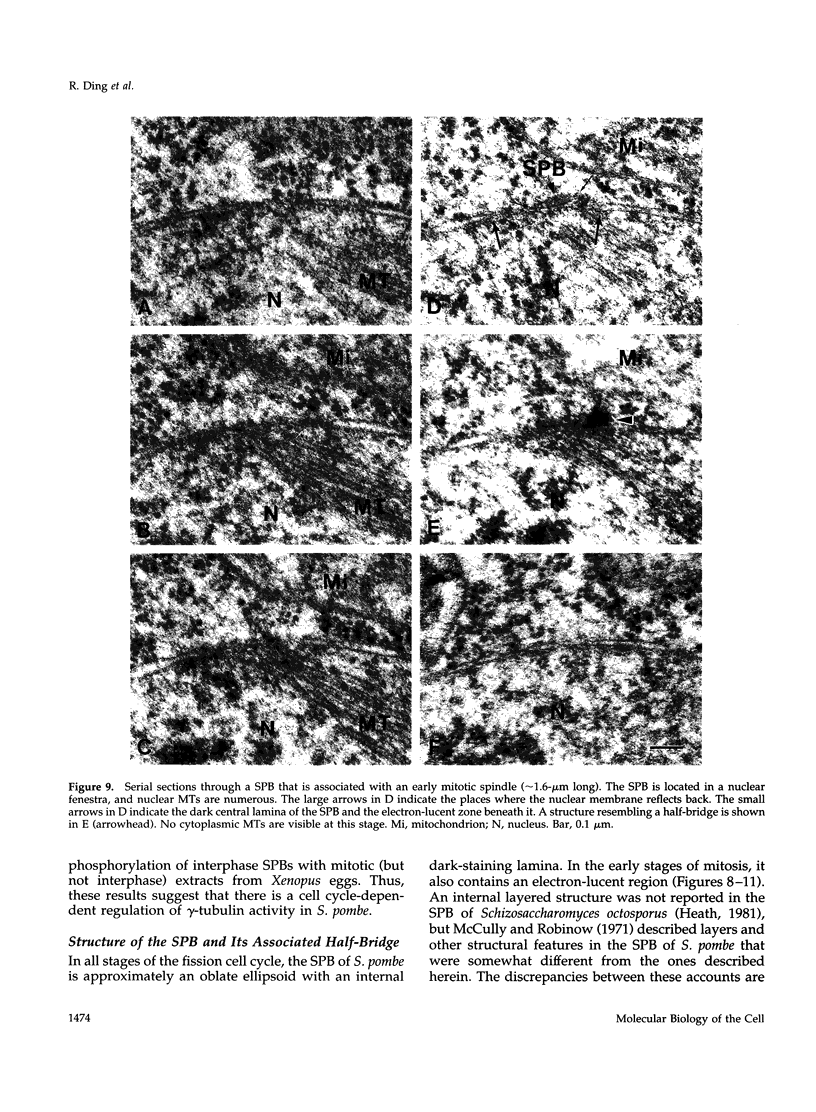
- Ding R., McDonald K. L., McIntosh J. R. Three-dimensional reconstruction and analysis of mitotic spindles from the yeast, Schizosaccharomyces pombe. J Cell Biol. 1993 Jan;120(1):141–151. doi: 10.1083/jcb.120.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson A. D., Kilmartin J. V. Spc42p: a phosphorylated component of the S. cerevisiae spindle pole body (SPD) with an essential function during SPB duplication. J Cell Biol. 1996 Mar;132(5):887–901. doi: 10.1083/jcb.132.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L., Mitchison T., Kirschner M. Influence of the centrosome on the structure of nucleated microtubules. J Cell Biol. 1985 Apr;100(4):1185–1191. doi: 10.1083/jcb.100.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. B., Sundberg H. A., Huang E. Y., Davis T. N. The 110-kD spindle pole body component of Saccharomyces cerevisiae is a phosphoprotein that is modified in a cell cycle-dependent manner. J Cell Biol. 1996 Mar;132(5):903–914. doi: 10.1083/jcb.132.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki H., Hagan I., Uzawa S., Yanagida M. Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J Cell Biol. 1993 Jun;121(5):961–976. doi: 10.1083/jcb.121.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan I. M., Hyams J. S. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1988 Mar;89(Pt 3):343–357. doi: 10.1242/jcs.89.3.343. [DOI] [PubMed] [Google Scholar]
- Heath I. B., Rethoret K. Mitosis in the fungus Zygorhynchus moelleri: evidence for stage specific enhancement of microtubule preservation by freeze substitution. Eur J Cell Biol. 1982 Oct;28(2):180–189. [PubMed] [Google Scholar]
- Heidemann S. R., McIntosh J. R. Visualization of the structural polarity of microtubules. Nature. 1980 Jul 31;286(5772):517–519. doi: 10.1038/286517a0. [DOI] [PubMed] [Google Scholar]
- Horio T., Uzawa S., Jung M. K., Oakley B. R., Tanaka K., Yanagida M. The fission yeast gamma-tubulin is essential for mitosis and is localized at microtubule organizing centers. J Cell Sci. 1991 Aug;99(Pt 4):693–700. doi: 10.1242/jcs.99.4.693. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Aist J. R. Hyphal tip cell ultrastructure of the fungus Fusarium: improved preservation by freeze-substitution. J Ultrastruct Res. 1979 Mar;66(3):224–234. doi: 10.1016/s0022-5320(79)90120-5. [DOI] [PubMed] [Google Scholar]
- Kanbe T., Hiraoka Y., Tanaka K., Yanagida M. The transition of cells of the fission yeast beta-tubulin mutant nda3-311 as seen by freeze-substitution electron microscopy. Requirement of functional tubulin for spindle pole body duplication. J Cell Sci. 1990 Jun;96(Pt 2):275–282. doi: 10.1242/jcs.96.2.275. [DOI] [PubMed] [Google Scholar]
- Keating T. J., Peloquin J. G., Rodionov V. I., Momcilovic D., Borisy G. G. Microtubule release from the centrosome. Proc Natl Acad Sci U S A. 1997 May 13;94(10):5078–5083. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J. V. Genetic and biochemical approaches to spindle function and chromosome segregation in eukaryotic microorganisms. Curr Opin Cell Biol. 1994 Feb;6(1):50–54. doi: 10.1016/0955-0674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Kremer J. R., Mastronarde D. N., McIntosh J. R. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996 Jan-Feb;116(1):71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Kubai D. F. The evolution of the mitotic spindle. Int Rev Cytol. 1975;43:167–227. doi: 10.1016/s0074-7696(08)60069-8. [DOI] [PubMed] [Google Scholar]
- Kuriyama R., Borisy G. G. Microtubule-nucleating activity of centrosomes in Chinese hamster ovary cells is independent of the centriole cycle but coupled to the mitotic cycle. J Cell Biol. 1981 Dec;91(3 Pt 1):822–826. doi: 10.1083/jcb.91.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Sevik M., Cande W. Z. In vitro microtubule-nucleating activity of spindle pole bodies in fission yeast Schizosaccharomyces pombe: cell cycle-dependent activation in xenopus cell-free extracts. J Cell Biol. 1992 Jun;117(5):1055–1066. doi: 10.1083/jcb.117.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D. Centrosomes and mitotic poles. Exp Cell Res. 1984 Jul;153(1):1–15. doi: 10.1016/0014-4827(84)90442-7. [DOI] [PubMed] [Google Scholar]
- McCully E. K., Robinow C. F. Mitosis in the fission yeast Schizosaccharomyces pombe: a comparative study with light and electron microscopy. J Cell Sci. 1971 Sep;9(2):475–507. doi: 10.1242/jcs.9.2.475. [DOI] [PubMed] [Google Scholar]
- McDonald K., O'Toole E. T., Mastronarde D. N., Winey M., Richard McIntosh J. Mapping the three-dimensional organization of microtubules in mitotic spindles of yeast. Trends Cell Biol. 1996 Jun;6(6):235–239. doi: 10.1016/0962-8924(96)40003-4. [DOI] [PubMed] [Google Scholar]
- Mitchison J. M., Nurse P. Growth in cell length in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1985 Apr;75:357–376. doi: 10.1242/jcs.75.1.357. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nicolas G. Advantages of fast-freeze fixation followed by freeze-substitution for the preservation of cell integrity. J Electron Microsc Tech. 1991 Aug;18(4):395–405. doi: 10.1002/jemt.1060180408. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Oakley C. E., Yoon Y., Jung M. K. Gamma-tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergillus nidulans. Cell. 1990 Jun 29;61(7):1289–1301. doi: 10.1016/0092-8674(90)90693-9. [DOI] [PubMed] [Google Scholar]
- Oakley C. E., Oakley B. R. Identification of gamma-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature. 1989 Apr 20;338(6217):662–664. doi: 10.1038/338662a0. [DOI] [PubMed] [Google Scholar]
- Raff J. W. Centrosomes and microtubules: wedded with a ring. Trends Cell Biol. 1996 Jul;6(7):248–251. doi: 10.1016/0962-8924(96)20020-0. [DOI] [PubMed] [Google Scholar]
- Rattner J. B., Phillips S. G. Independence of centriole formation and DNA synthesis. J Cell Biol. 1973 May;57(2):359–372. doi: 10.1083/jcb.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G. Centrosomes and the cell cycle. J Cell Sci Suppl. 1989;12:253–275. doi: 10.1242/jcs.1989.supplement_12.21. [DOI] [PubMed] [Google Scholar]
- Snyder J. A., McIntosh J. R. Initiation and growth of microtubules from mitotic centers in lysed mammalian cells. J Cell Biol. 1975 Dec;67(3):744–760. doi: 10.1083/jcb.67.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. The spindle pole body of yeast. Chromosoma. 1994 Oct;103(6):369–380. doi: 10.1007/BF00362281. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Kanbe T. Mitosis in the fission yeast Schizosaccharomyces pombe as revealed by freeze-substitution electron microscopy. J Cell Sci. 1986 Feb;80:253–268. doi: 10.1242/jcs.80.1.253. [DOI] [PubMed] [Google Scholar]
- Uzawa S., Yanagida M. Visualization of centromeric and nucleolar DNA in fission yeast by fluorescence in situ hybridization. J Cell Sci. 1992 Feb;101(Pt 2):267–275. doi: 10.1242/jcs.101.2.267. [DOI] [PubMed] [Google Scholar]
- Vorobjev I. A., Nadezhdina E. S. The centrosome and its role in the organization of microtubules. Int Rev Cytol. 1987;106:227–293. doi: 10.1016/s0074-7696(08)61714-3. [DOI] [PubMed] [Google Scholar]
- Wente S. R., Blobel G. A temperature-sensitive NUP116 null mutant forms a nuclear envelope seal over the yeast nuclear pore complex thereby blocking nucleocytoplasmic traffic. J Cell Biol. 1993 Oct;123(2):275–284. doi: 10.1083/jcb.123.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M., Byers B. Assembly and functions of the spindle pole body in budding yeast. Trends Genet. 1993 Sep;9(9):300–304. doi: 10.1016/0168-9525(93)90247-f. [DOI] [PubMed] [Google Scholar]
- Winey M., Hoyt M. A., Chan C., Goetsch L., Botstein D., Byers B. NDC1: a nuclear periphery component required for yeast spindle pole body duplication. J Cell Biol. 1993 Aug;122(4):743–751. doi: 10.1083/jcb.122.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]