Abstract
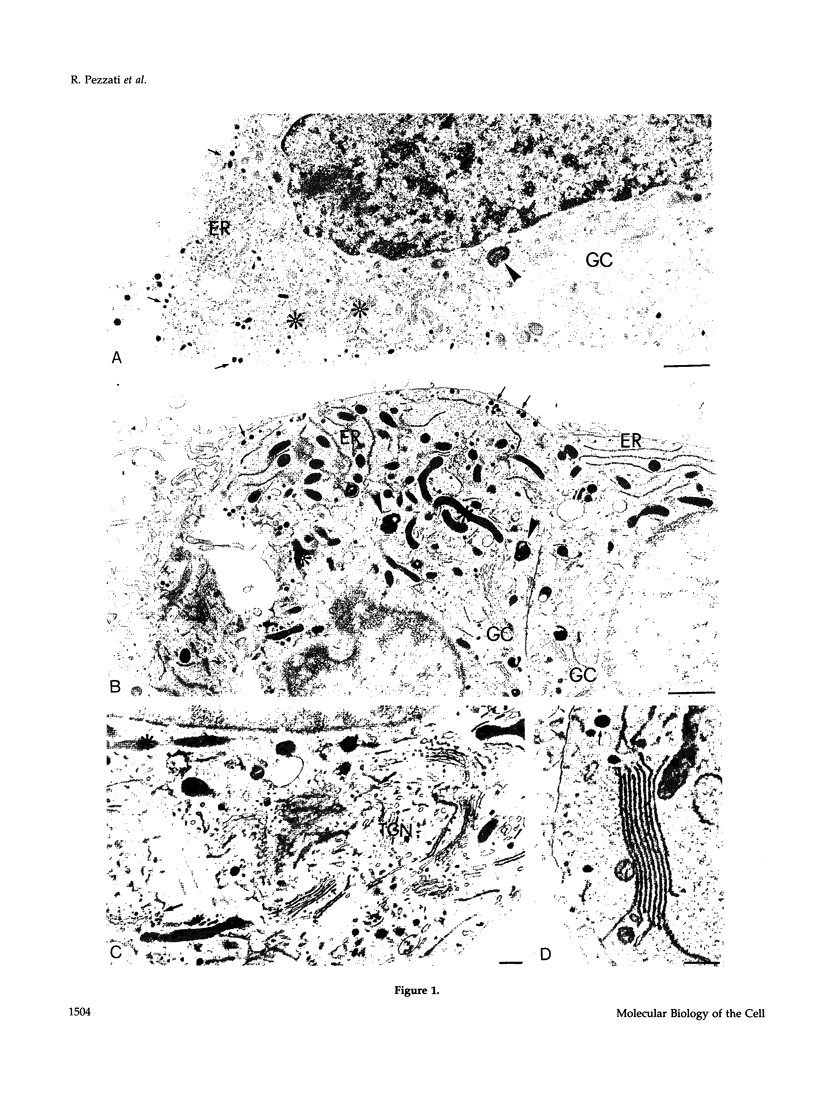
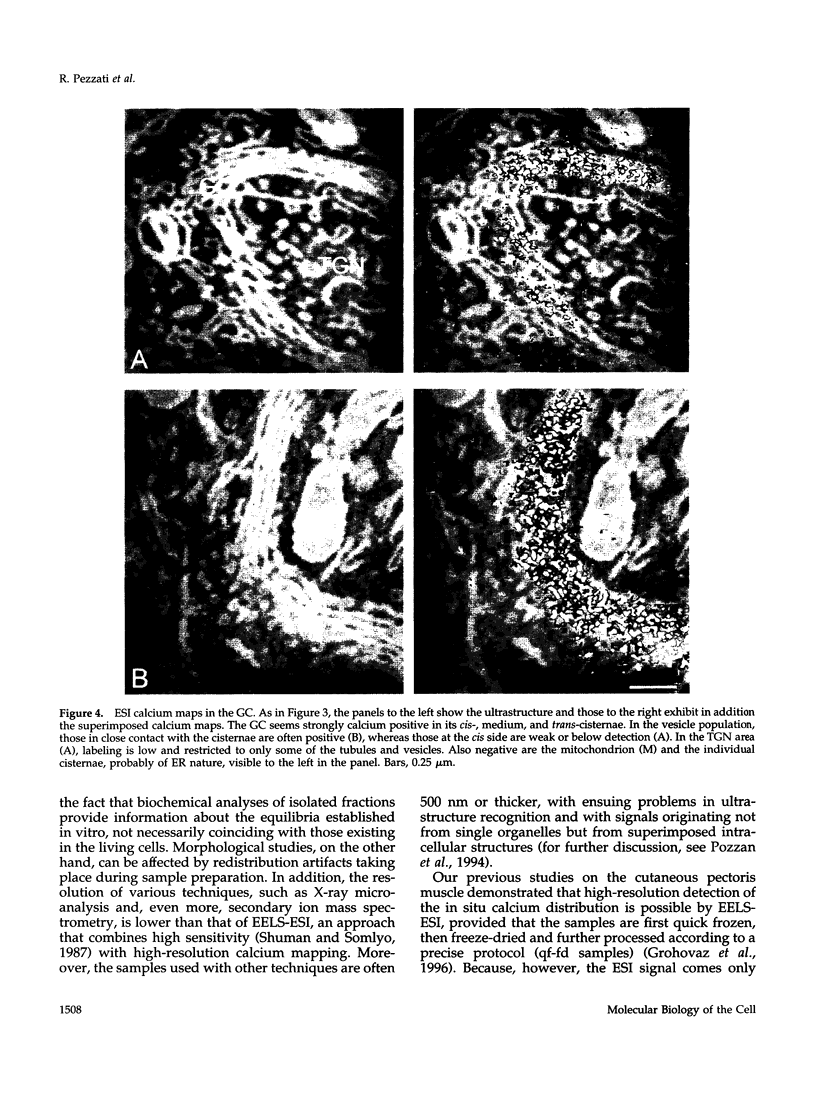
The calcium pools segregated within the endoplasmic reticulum, Golgi complex, exocytic, and other organelles are believed to participate in the regulation of a variety of cell functions. Until now, however, the precise intracellular distribution of the element had not been established. Here, we report about the first high-resolution calcium mapping obtained in neurosecretory PC12 cells by the imaging mode of the electron energy loss spectroscopy technique. The preparation procedure used included quick freezing of cell monolayers, followed by freeze-drying, fixation with OSO4 vapors, resin embedding, and cutting of very thin sections. Conventional electron microscopy and high-resolution immunocytochemistry revealed a high degree of structural preservation, a condition in which inorganic elements are expected to maintain their native distribution. Within these cells, calcium signals of nucleus, cytosol, and most mitochondria remained below detection, whereas in other organelles specific patterns were identified. In the endoplasmic reticulum, the distribution was heterogeneous with strongly positive cisternae (more often the nuclear envelope and stacks of parallel elements that are frequent in quick frozen preparations) lying in the proximity of or even in direct continuity with other, apparently negative cisternae. The Golgi complexes were labeled strongly and uniformly in all cisternae and part of their vesicles, with no appreciable differences along the cis-trans axis. Weaker or negative signals were recorded from the trans-Golgi network elements and from scattered vesicles, whereas in contrast secretion granules were strongly positive for calcium. These results are discussed in relation to the existing knowledge about the mechanisms of calcium transport in the variations organelles, and about the processes and functions regulated by organelle lumenal calcium in eukaryotic cells.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews S. B., Reese T. S. Intracellular structure and elemental analysis in rapid-frozen neurons. Ann N Y Acad Sci. 1986;483:284–294. doi: 10.1111/j.1749-6632.1986.tb34534.x. [DOI] [PubMed] [Google Scholar]
- Badminton M. N., Campbell A. K., Rembold C. M. Differential regulation of nuclear and cytosolic Ca2+ in HeLa cells. J Biol Chem. 1996 Dec 6;271(49):31210–31214. doi: 10.1074/jbc.271.49.31210. [DOI] [PubMed] [Google Scholar]
- Bastianutto C., Clementi E., Codazzi F., Podini P., De Giorgi F., Rizzuto R., Meldolesi J., Pozzan T. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their lumenal microenvironment and function. J Cell Biol. 1995 Aug;130(4):847–855. doi: 10.1083/jcb.130.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer P. A., Lynch K. Stimulation-associated changes in frog neuromuscular junctions. A quantitative ultrastructural comparison of rapid-frozen and chemically fixed nerve terminals. Neuroscience. 1986 Mar;17(3):881–895. doi: 10.1016/0306-4522(86)90052-7. [DOI] [PubMed] [Google Scholar]
- Buchanan R. A., Leapman R. D., O'Connell M. F., Reese T. S., Andrews S. B. Quantitative scanning transmission electron microscopy of ultrathin cryosections: subcellular organelles in rapidly frozen liver and cerebellar cortex. J Struct Biol. 1993 May-Jun;110(3):244–255. doi: 10.1006/jsbi.1993.1027. [DOI] [PubMed] [Google Scholar]
- Canaff L., Brechler V., Reudelhuber T. L., Thibault G. Secretory granule targeting of atrial natriuretic peptide correlates with its calcium-mediated aggregation. Proc Natl Acad Sci U S A. 1996 Sep 3;93(18):9483–9487. doi: 10.1073/pnas.93.18.9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnell L., Moore H. P. Transport via the regulated secretory pathway in semi-intact PC12 cells: role of intra-cisternal calcium and pH in the transport and sorting of secretogranin II. J Cell Biol. 1994 Nov;127(3):693–705. doi: 10.1083/jcb.127.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersen C., Treiman M. Thapsigargin discriminates strongly between Ca(2+)-ATPase phosphorylated intermediates with different subcellular distributions in bovine adrenal chromaffin cells. FEBS Lett. 1995 Dec 11;377(1):31–36. doi: 10.1016/0014-5793(95)01304-0. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B., Grohovaz F., Hurlbut W. P. Freeze-fracture studies of frog neuromuscular junctions during intense release of neurotransmitter. II. Effects of electrical stimulation and high potassium. J Cell Biol. 1979 Apr;81(1):178–192. doi: 10.1083/jcb.81.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanat E., Huttner W. B. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol. 1991 Dec;115(6):1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanat E., Weiss U., Huttner W. B. The disulfide bond in chromogranin B, which is essential for its sorting to secretory granules, is not required for its aggregation in the trans-Golgi network. FEBS Lett. 1994 Sep 5;351(2):225–230. doi: 10.1016/0014-5793(94)00865-5. [DOI] [PubMed] [Google Scholar]
- Chandra S., Fewtrell C., Millard P. J., Sandison D. R., Webb W. W., Morrison G. H. Imaging of total intracellular calcium and calcium influx and efflux in individual resting and stimulated tumor mast cells using ion microscopy. J Biol Chem. 1994 May 27;269(21):15186–15194. [PubMed] [Google Scholar]
- Colliex C. Electron energy-loss spectroscopy analysis and imaging of biological specimens. Ann N Y Acad Sci. 1986;483:311–325. doi: 10.1111/j.1749-6632.1986.tb34538.x. [DOI] [PubMed] [Google Scholar]
- ERANKO O. Quenching of tissues for freeze-drying. Acta Anat (Basel) 1954;22(4):331–336. [PubMed] [Google Scholar]
- Fiori C. E., Leapman R. D., Swyt C. R., Andrews S. B. Quantitative X-ray mapping of biological cryosections. Ultramicroscopy. 1988;24(2-3):237–249. doi: 10.1016/0304-3991(88)90313-0. [DOI] [PubMed] [Google Scholar]
- Greber U. F., Gerace L. Depletion of calcium from the lumen of endoplasmic reticulum reversibly inhibits passive diffusion and signal-mediated transport into the nucleus. J Cell Biol. 1995 Jan;128(1-2):5–14. doi: 10.1083/jcb.128.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohovaz F., Bossi M., Pezzati R., Meldolesi J., Tarelli F. T. High resolution ultrastructural mapping of total calcium: electron spectroscopic imaging/electron energy loss spectroscopy analysis of a physically/chemically processed nerve-muscle preparation. Proc Natl Acad Sci U S A. 1996 May 14;93(10):4799–4803. doi: 10.1073/pnas.93.10.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunteski-Hamblin A. M., Clarke D. M., Shull G. E. Molecular cloning and tissue distribution of alternatively spliced mRNAs encoding possible mammalian homologues of the yeast secretory pathway calcium pump. Biochemistry. 1992 Aug 25;31(33):7600–7608. doi: 10.1021/bi00148a023. [DOI] [PubMed] [Google Scholar]
- Hall T. A. Capabilities and limitations of probe methods for the microanalysis of chemical elements in biology: a brief introduction. Ultramicroscopy. 1988;24(2-3):181–184. doi: 10.1016/0304-3991(88)90310-5. [DOI] [PubMed] [Google Scholar]
- Hebert D. N., Foellmer B., Helenius A. Calnexin and calreticulin promote folding, delay oligomerization and suppress degradation of influenza hemagglutinin in microsomes. EMBO J. 1996 Jun 17;15(12):2961–2968. [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Landis D. M. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J Neurocytol. 1974 Mar;3(1):109–131. doi: 10.1007/BF01111936. [DOI] [PubMed] [Google Scholar]
- Jorgensen A. O., McGuffee L. J. Immunoelectron microscopic localization of sarcoplasmic reticulum proteins in cryofixed, freeze-dried, and low temperature-embedded tissue. J Histochem Cytochem. 1987 Jul;35(7):723–732. doi: 10.1177/35.7.2953782. [DOI] [PubMed] [Google Scholar]
- Kasai H., Takagi H., Ninomiya Y., Kishimoto T., Ito K., Yoshida A., Yoshioka T., Miyashita Y. Two components of exocytosis and endocytosis in phaeochromocytoma cells studied using caged Ca2+ compounds. J Physiol. 1996 Jul 1;494(Pt 1):53–65. doi: 10.1113/jphysiol.1996.sp021475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger E., Johansen R., Maeder M., Bohrmann B., Stauffer E., Villiger W. Artefacts and morphological changes during chemical fixation. J Microsc. 1992 Nov;168(Pt 2):181–201. doi: 10.1111/j.1365-2818.1992.tb03260.x. [DOI] [PubMed] [Google Scholar]
- Kuznetsov G., Brostrom M. A., Brostrom C. O. Demonstration of a calcium requirement for secretory protein processing and export. Differential effects of calcium and dithiothreitol. J Biol Chem. 1992 Feb 25;267(6):3932–3939. [PubMed] [Google Scholar]
- Kuznetsov G., Brostrom M. A., Brostrom C. O. Role of endoplasmic reticular calcium in oligosaccharide processing of alpha 1-antitrypsin. J Biol Chem. 1993 Jan 25;268(3):2001–2008. [PubMed] [Google Scholar]
- LeFurgey A., Bond M., Ingram P. Frontiers in electron probe microanalysis: application to cell physiology. Ultramicroscopy. 1988;24(2-3):185–219. doi: 10.1016/0304-3991(88)90311-7. [DOI] [PubMed] [Google Scholar]
- Leapman R. D., Ornberg R. L. Quantitative electron energy loss spectroscopy in biology. Ultramicroscopy. 1988;24(2-3):251–268. doi: 10.1016/0304-3991(88)90314-2. [DOI] [PubMed] [Google Scholar]
- Linton R. W., Goldsmith J. G. The role of secondary ion mass spectrometry (SIMS) in biological microanalysis: technique comparisons and prospects. Biol Cell. 1992;74(1):147–160. doi: 10.1016/0248-4900(92)90021-r. [DOI] [PubMed] [Google Scholar]
- Lièvremont J. P., Hill A. M., Hilly M., Mauger J. P. The inositol 1,4,5-trisphosphate receptor is localized on specialized sub-regions of the endoplasmic reticulum in rat liver. Biochem J. 1994 Jun 1;300(Pt 2):419–427. doi: 10.1042/bj3000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Kong N. Perturbation of cellular calcium blocks exit of secretory proteins from the rough endoplasmic reticulum. J Biol Chem. 1990 Jul 5;265(19):10893–10899. [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Wikström L. Calcium is required for folding of newly made subunits of the asialoglycoprotein receptor within the endoplasmic reticulum. J Biol Chem. 1992 Jun 25;267(18):12753–12760. [PubMed] [Google Scholar]
- Mayor S., Rothberg K. G., Maxfield F. R. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science. 1994 Jun 24;264(5167):1948–1951. doi: 10.1126/science.7516582. [DOI] [PubMed] [Google Scholar]
- Nash P. D., Opas M., Michalak M. Calreticulin: not just another calcium-binding protein. Mol Cell Biochem. 1994 Jun 15;135(1):71–78. doi: 10.1007/BF00925962. [DOI] [PubMed] [Google Scholar]
- Nigam S. K., Goldberg A. L., Ho S., Rohde M. F., Bush K. T., Sherman MYu A set of endoplasmic reticulum proteins possessing properties of molecular chaperones includes Ca(2+)-binding proteins and members of the thioredoxin superfamily. J Biol Chem. 1994 Jan 21;269(3):1744–1749. [PubMed] [Google Scholar]
- Oliver J. D., Hresko R. C., Mueckler M., High S. The glut 1 glucose transporter interacts with calnexin and calreticulin. J Biol Chem. 1996 Jun 7;271(23):13691–13696. doi: 10.1074/jbc.271.23.13691. [DOI] [PubMed] [Google Scholar]
- Ottensmeyer F. P., Andrew J. W. High-resolution microanalysis of biological specimens by electron energy loss spectroscopy and by electron spectroscopic imaging. J Ultrastruct Res. 1980 Sep;72(3):336–348. doi: 10.1016/s0022-5320(80)90069-6. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Rizzuto R., Volpe P., Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994 Jul;74(3):595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Brini M., Pizzo P., Murgia M., Pozzan T. Chimeric green fluorescent protein as a tool for visualizing subcellular organelles in living cells. Curr Biol. 1995 Jun 1;5(6):635–642. doi: 10.1016/s0960-9822(95)00128-x. [DOI] [PubMed] [Google Scholar]
- Rooney E., Meldolesi J. The endoplasmic reticulum in PC12 cells. Evidence for a mosaic of domains differently specialized in Ca2+ handling. J Biol Chem. 1996 Nov 15;271(46):29304–29311. doi: 10.1074/jbc.271.46.29304. [DOI] [PubMed] [Google Scholar]
- Rudolph H. K., Antebi A., Fink G. R., Buckley C. M., Dorman T. E., LeVitre J., Davidow L. S., Mao J. I., Moir D. T. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell. 1989 Jul 14;58(1):133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- Satoh T., Ross C. A., Villa A., Supattapone S., Pozzan T., Snyder S. H., Meldolesi J. The inositol 1,4,5,-trisphosphate receptor in cerebellar Purkinje cells: quantitative immunogold labeling reveals concentration in an ER subcompartment. J Cell Biol. 1990 Aug;111(2):615–624. doi: 10.1083/jcb.111.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer P. E., Lederkremer G. Z., Williams S., Fogliano M., Baldini G., Lodish H. F. Cab45, a novel (Ca2+)-binding protein localized to the Golgi lumen. J Cell Biol. 1996 Apr;133(2):257–268. doi: 10.1083/jcb.133.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp A. H., Snyder S. H., Nigam S. K. Inositol 1,4,5-trisphosphate receptors. Localization in epithelial tissue. J Biol Chem. 1992 Apr 15;267(11):7444–7449. [PubMed] [Google Scholar]
- Shuman H., Somlyo A. P. Electron energy loss analysis of near-trace-element concentrations of calcium. Ultramicroscopy. 1987;21(1):23–32. doi: 10.1016/0304-3991(87)90004-0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Bond M., Somlyo A. V. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985 Apr 18;314(6012):622–625. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L., Perez-Terzic C., Clapham D. E. Diffusion across the nuclear envelope inhibited by depletion of the nuclear Ca2+ store. Science. 1995 Dec 15;270(5243):1835–1838. doi: 10.1126/science.270.5243.1835. [DOI] [PubMed] [Google Scholar]
- Takei K., Mignery G. A., Mugnaini E., Südhof T. C., De Camilli P. Inositol 1,4,5-trisphosphate receptor causes formation of ER cisternal stacks in transfected fibroblasts and in cerebellar Purkinje cells. Neuron. 1994 Feb;12(2):327–342. doi: 10.1016/0896-6273(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Torri-Tarelli F., Grohovaz F., Fesce R., Ceccarelli B. Temporal coincidence between synaptic vesicle fusion and quantal secretion of acetylcholine. J Cell Biol. 1985 Oct;101(4):1386–1399. doi: 10.1083/jcb.101.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves S., De Mattei M., Landfredi M., Villa A., Green N. M., MacLennan D. H., Meldolesi J., Pozzan T. Calreticulin is a candidate for a calsequestrin-like function in Ca2(+)-storage compartments (calciosomes) of liver and brain. Biochem J. 1990 Oct 15;271(2):473–480. doi: 10.1042/bj2710473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van P. N., Rupp K., Lampen A., Söling H. D. CaBP2 is a rat homolog of ERp72 with proteindisulfide isomerase activity. Eur J Biochem. 1993 Apr 15;213(2):789–795. doi: 10.1111/j.1432-1033.1993.tb17821.x. [DOI] [PubMed] [Google Scholar]
- Velasco A., Hendricks L., Moremen K. W., Tulsiani D. R., Touster O., Farquhar M. G. Cell type-dependent variations in the subcellular distribution of alpha-mannosidase I and II. J Cell Biol. 1993 Jul;122(1):39–51. doi: 10.1083/jcb.122.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A., Podini P., Clegg D. O., Pozzan T., Meldolesi J. Intracellular Ca2+ stores in chicken Purkinje neurons: differential distribution of the low affinity-high capacity Ca2+ binding protein, calsequestrin, of Ca2+ ATPase and of the ER lumenal protein, Bip. J Cell Biol. 1991 May;113(4):779–791. doi: 10.1083/jcb.113.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A., Podini P., Panzeri M. C., Söling H. D., Volpe P., Meldolesi J. The endoplasmic-sarcoplasmic reticulum of smooth muscle: immunocytochemistry of vas deferens fibers reveals specialized subcompartments differently equipped for the control of Ca2+ homeostasis. J Cell Biol. 1993 Jun;121(5):1041–1051. doi: 10.1083/jcb.121.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virk S. S., Kirk C. J., Shears S. B. Ca2+ transport and Ca2+-dependent ATP hydrolysis by Golgi vesicles from lactating rat mammary glands. Biochem J. 1985 Mar 15;226(3):741–748. doi: 10.1042/bj2260741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe P., Villa A., Damiani E., Sharp A. H., Podini P., Snyder S. H., Meldolesi J. Heterogeneity of microsomal Ca2+ stores in chicken Purkinje neurons. EMBO J. 1991 Nov;10(11):3183–3189. doi: 10.1002/j.1460-2075.1991.tb04880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe O., Torda M., Meldolesi J. The effect of alpha-latrotoxin on the neurosecretory PC12 cell line: electron microscopy and cytotoxicity studies. Neuroscience. 1983 Nov;10(3):1011–1024. doi: 10.1016/0306-4522(83)90239-7. [DOI] [PubMed] [Google Scholar]
- Winkler H., Westhead E. The molecular organization of adrenal chromaffin granules. Neuroscience. 1980;5(11):1803–1823. doi: 10.1016/0306-4522(80)90031-7. [DOI] [PubMed] [Google Scholar]