Abstract
The consequences of exposure to developmental neurotoxicants are influenced by environmental factors. In the present study, we examined the role of dietary fat intake. We administered parathion to neonatal rats and then evaluated whether a high-fat diet begun in adulthood could modulate the persistent effects on 5HT and DA systems. Neonatal rats received parathion on postnatal days 1-4 at 0.1 or 0.2 mg/kg/day, straddling the cholinesterase inhibition threshold. In adulthood, half the animals in each exposure group were given a high-fat diet for 8 weeks. We assessed 5HT and DA concentrations and turnover in brain regions containing their respective cell bodies and projections. In addition, we monitored 5HT1A and 5HT2 receptor binding and the concentration of 5HT presynaptic transporters. Neonatal parathion exposure evoked widespread increases in neurotransmitter turnover, indicative of presynaptic hyperactivity, further augmented by 5HT receptor upregulation. In control rats, consumption of a high-fat diet recapitulated many of the changes seen with neonatal parathion exposure; the effects represented convergent mechanisms, since the high-fat diet often obtunded further increases caused by parathion. Neonatal parathion exposure causes lasting hyperactivity of 5HT and DA systems accompanied by 5HT receptor upregulation, consistent with “miswiring” of neuronal projections. A high-fat diet obtunds the effect of parathion, in part by eliciting similar changes itself. Thus, dietary factors may produce similar synaptic changes as do developmental neurotoxicants, potentially contributing to the increasing incidence in neurodevelopmental disorders.
Keywords: Brain development, Dopamine, High-fat diet, Organophosphate insecticides, Parathion, Serotonin
INTRODUCTION
Increased exposure to environmental toxicants is thought to underlie the rising incidence of neurodevelopmental disorders [21]. For widely-used agents, such as organophosphate pesticides, human exposures are virtually ubiquitous [7,10], and animal models clearly show that organophosphates disrupt brain development, leading to lasting behavioral deficits [36]. Nevertheless, it is obvious that the adverse effects are not distributed uniformly throughout the population but rather, there are vulnerable subgroups. Considerable attention has focused on the social and genetic factors that contribute to increased exposure and sensitivity, such as socioeconomic status and geographical location, e.g. inner-city, immigrant and agricultural populations [9,15,34,54], and gene-environment interactions involving polymorphisms that affect organophosphate degradation [14,18,19]. In contrast, relatively little work has been done on factors later in life that could influence the long-term consequences of fetal or neonatal toxicant exposure, either increasing or decreasing the vulnerability of specific individuals or populations in terms of functional outcome rather than initial exposure or injury. We recently explored how consumption of a diet high in saturated fat influenced the outcome of neonatal parathion exposure, basing our rationale on the successful use of “ketogenic” diets in treating refractory epilepsies [8,13,23,24] and on pilot studies showing salutary effects on attention deficit hyperactivity disorder, autism and animal models of neurobehavioral disorders [13,16,33,50]. In our studies, we found that introduction of a high-fat diet worsened the metabolic consequences of neonatal parathion exposure but ameliorated many of the synaptic deficits in cholinergic pathways that control learning, memory and other essential functions [26,37]. These results provided some of the first evidence that the consequences of early-life organophosphate exposure could be modulated by nongenetic factors that are readily manipulated, testable in animal models, and potentially, easily translated to human studies.
The effects of a high-fat diet on synaptic responses are likely due to global changes in the composition of synaptic membrane lipids [22,32], and therefore can span multiple brain regions and neurotransmitter systems [11,20,25]. In the present work, we explored the lasting effects of neonatal parathion exposure on serotonin (5-hydroxytryptamine, 5HT) pathways in rat brain regions and the effects of dietary manipulation in adulthood on the outcome. There are a number of reasons for a focus on 5HT. Organophosphates appear to target the development of 5HT systems as much as, or even more than cholinergic pathways [4,5,39,40,43-45,48], leading to corresponding behavioral deficits [1,35,51,52]. In keeping with the known role of 5HT abnormalities in affective disorders [30,31], rats exposed to low doses of organophosphates as neonates show depression-like behavioral patterns in adulthood [1]; further, a clear connection appears to be emerging between human organophosphate exposure and depression and suicide [27,28]. Here, we focused on parathion treatment regimens that span the threshold for barely-detectable cholinesterase inhibition [48]. Then, in adulthood, we switched some of the animals to a high-fat, “ketogenic” diet that more than doubles serumβ-hydroxybutyrate concentrations [26]. We then performed evaluations of multiple indices of 5HT synaptic function in all the brain regions comprising the major 5HT projections (frontal/parietal cortex, temporal/occipital cortex, hippocampus, striatum) as well as those containing 5HT cell bodies (midbrain, brainstem). We assessed the concentration of 5HT as well as 5HT turnover as an index of presynaptic neuronal activity. In addition, we measured three 5HT synaptic proteins known to be highly affected by developmental exposure to organophosphates [3-5,39,40,45,48], the 5HT1A and 5HT2 receptors (5HT1AR, 5HT2R), and the presynaptic 5HT transporter (5HTT). The two receptors play major roles in 5HT-related mental disorders, particularly depression [6,17,56,57], and the transporter, which regulates the synaptic concentration of 5HT, is the primary target for antidepressant drugs [29-31]. Finally, we compared effects on 5HT to those on dopamine (DA), a monoamine that has similar properties in terms of transmitter biosynthesis, storage and release, but that subserves different functions and whose cell bodies and projections have different regional distributions from those of 5HT.
MATERIALS AND METHODS
Animal treatments and diet
All experiments were carried out humanely and with regard for alleviation of suffering, with protocols approved by the Duke University Institutional Animal Care and Use Committee and in accordance with all federal and state guidelines. Timed-pregnant Sprague-Dawley rats (Charles River, Raleigh, NC) were housed in breeding cages, with a 12 h light-dark cycle and free access to water and food (LabDiet 5001, PMI Nutrition, St. Louis, MO). On the day after birth, all pups were randomized and redistributed to the dams with a litter size of 10 (5 males, 5 females) to maintain a standard nutritional status. Parathion (99.2% purity; Chem Service, West Chester, PA) was dissolved in dimethylsulfoxide to provide consistent absorption [38,48,53] and was injected subcutaneously in a volume of 1 ml/kg once daily on postnatal days 1-4; control animals received equivalent injections of the dimethylsulfoxide vehicle. Doses of 0.1 and 0.2 mg/kg/day were chosen because they straddle the threshold for barely-detectable cholinesterase inhibition and the first signs of reduced weight gain or impaired viability [38,48]. Brain cholinesterase inhibition 24 hr after the last dose of 0.1 mg/kg parathion is reduced 5-10%, well below the 70% threshold necessary for signs of cholinergic hyperstimulation [12]. To avoid the possibility that dams might distinguish between control and parathion-treated pups, all pups in a given litter received the same treatment. Randomization of pup litter assignments within treatment groups was repeated at intervals of several days up until weaning, and in addition, dams were rotated among litters to distribute any maternal caretaking differences randomly across litters and treatment groups. Offspring were weaned on postnatal day 21 and the final litter assignment for each rat was noted; studies were then conducted using one male and one female from each litter. After weaning, animals were separated by sex and housed in groups according to standard guidelines.
Beginning at 15 weeks of age, half the rats were switched to a high-fat diet (OpenSource D12330, Research Diets Inc., New Brunswick, NJ), providing 58% of total calories as fat; 93% of the fat is hydrogenated coconut oil. The remaining rats continued on the standard LabDiet 5001 diet, which provides 13.5% of total calories as fat; with this diet, 27% of the fat is saturated. Although the high-fat diet contains 37% more calories per gram, we found that animals on this diet reduce their food intake by approximately the same proportion [26], so that the total dietary intake is isocaloric; nevertheless, animals gain excess weight because of the higher fat content [26]. During the 24th postnatal week, animals were decapitated and brains were dissected into the frontal/parietal cortex, temporal/occipital cortex, hippocampus, striatum, midbrain and brainstem. Neurochemical determinations were made on regions from 6-12 rats per treatment group for each sex and with each diet, with no more than one male and one female derived from a given litter in each group.
Neurotransmitter concentration and turnover
Tissues were thawed and homogenized in ice-cold 0.1 M perchloric acid and sedimented for 20 min at 40,000 × g. The supernatant solution was collected and aliquots were used for analysis of 5HT, 5-hydroxyindoleacetic acid (5HIAA), DA, dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) by high-performance liquid chromatography with electrochemical detection [47,55]. Concurrently-run standards, containing each of the neurotransmitters and metabolites (Sigma Chemical Co., St. Louis, MO), were used to calculate the regional concentration of each neurochemical. Transmitter turnover was calculated as the ratio of metabolites to transmitter, i.e. 5HIAA/5HT, and either DOPAC/DA or (DOPAC+HVA)/DA; since HVA contributed significantly to total metabolites in only one region (striatum), the ratio was calculated as DOPAC/DA in the other regions.
Ligand binding assays
All of the ligand binding methodologies used in this study have appeared in previous papers [5,38,42,45], so only brief descriptions will be provided here. Tissues were thawed and homogenized (Polytron, Brinkmann Instruments, Westbury, NY) in ice-cold 50 mM Tris (pH 7.4), and the homogenates were sedimented at 40,000 × g for 15 min. The pellets were washed by resuspension (Polytron) in homogenization buffer followed by resedimentation, and were then dispersed with a homogenizer (smooth glass fitted with Teflon pestle) in the same buffer. An aliquot was assayed for measurement of membrane protein [49].
Two radioligands were used to determine 5HTR binding: 1 nM [3H]8-hydroxy-2-(di-n-propylamino)tetralin for the 5HT1AR, and 0.4 nM [3H]ketanserin for the 5HT2R. Binding to the presynaptic 5HTT site was evaluated with 85 pM [3H]paroxetine. For the 5HT1AR and 5HTT sites, specific binding was displaced by addition of 100 μM 5HT; for the 5HT2R, we used 10 μM methylsergide for displacement. The overall strategy was to examine binding at a single ligand concentration in preparations from all regions in every animal, focusing on a concentration above the Kd but below full saturation. We can thus detect changes that originate either in altered Kd or Bmax but can not distinguish between the two possible mechanisms, albeit that a change in Kd would seem highly unlikely. This strategy was necessitated by the amount of tissue available for each determination and technical limitations engendered by the requirement to measure binding in six treatment groups in multiple brain regions, with six animals for each sex. Thus, there were hundreds of separate membrane preparations, each of which had to be evaluated for binding of three different ligands.
Data analysis
Data were compiled as means and standard errors. The experimental design was identical to that in two previous papers using animals from the same treatment cohorts [26,37], so the overall statistical procedures were the same and will be described only briefly here. Each set of determinations began with a global ANOVA incorporating all variables in a single test: neonatal treatment, diet, brain region, sex, and the multiple measurements made for each class of variables, with the latter regarded as repeated measures (since multiple measurements were made from the same tissue sample). When this initial test showed main effects of the contributing variables as well as significant interactions among the variables, we then conducted a series of nested, lower-order ANOVAs separating the measurements according to the interactive variables. The interactions were maintained in the lower-order tests so that we present the data for males and females, separated for each region and measure. Results are then shown with ANOVA followed by Fisher’s Protected Least Significant Difference to establish individual groups that differ from the corresponding control; for concision, the statistical results are shown only for the final subdivisions of the data and the individual differences between groups, but in all cases, these were preceded by significance established with each stage of the nested ANOVAs, essentially as shown in the earlier papers [26,37], and as described in shortened form in the corresponding portions of the Results section, below. For all tests, significance was assumed at p < 0.05.
RESULTS
We reported earlier on the effects of neonatal parathion treatments and dietary manipulations on body weights from the same cohort of animals as used in the present study [26]. Although there were no effects of parathion on body weight during or immediately after the PN1-4 exposure period, small but significant weight differences emerged by weaning, persisting into adulthood. By itself, parathion caused a small (2-3%) but significant elevation in weight at the low dose in males, and reductions of about 4% at either dose in females. Consumption of a high fat diet increased body weights regardless of whether animals were exposed to parathion as neonates, with an average 10% increase in males and 30% in females. Multivariate ANOVA (factors of neonatal treatment, diet, sex, region) for brain region weights did not show any effects of parathion but did indicate a significant diet × region interaction (p < 0.04), reflecting an effect confined to the hippocampus (p < 0.005 for main effect of diet), involving a 5% increase caused by the high fat diet (data not shown).
5HT Concentration and Turnover
For 5HT concentration, the global ANOVA indicated main effects of parathion treatment (p < 0.0002), diet (p < 0.0009), sex (p < 0.05) and region (p < 0.0001), as well as two-, three- and four-factor interactions among all the variables (not shown). The main effect for parathion treatment resulted from a net decrease in 5HT that was significant at both parathion doses (p < 0.0001); the main effect for diet reflected lower 5HT concentrations with the high fat diet as compared to normal diet (p < 0.0001). For 5HT turnover, the global test similarly indicated significant main effects for all variables (p < 0.0001 for parathion treatment, p < 0.0001 for diet, p < 0.05 for sex, p < 0.0001 for region) as well as multiple interactions (not shown). The main effect of treatment resulted from higher turnover in the parathion groups compared to control (p < 0.02 for 0.1 mg/kg, p < 0.0001 for 0.2 mg/kg), and the main effect of diet reflected higher values in the animals consuming a high-fat diet (p < 0.0001).
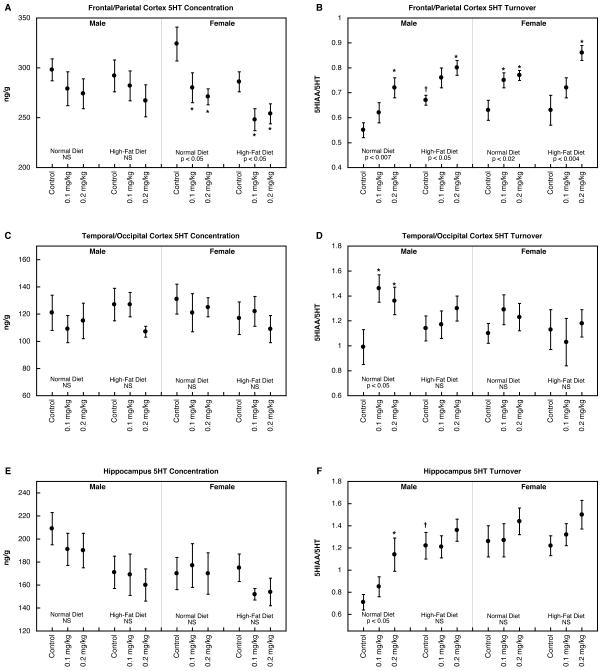
In the frontal/parietal cortex, parathion treatment by itself elicited significant reductions in 5HT concentration in females, an effect that was also present in animals given the high-fat diet (Figure 1A). On the other hand, 5HT turnover was enhanced by parathion exposure in both males and females (Figure 1B); the high-fat diet by itself increased turnover in males (significant difference between normal diet control group and high-fat diet control group) but did not alter the effect of parathion, which still evoked significant increases in both sexes. In the temporal/occipital cortex, neither parathion nor dietary manipulation evoked any significant changes in 5HT concentration (Figure 1C). Nevertheless, males still showed significant increases in 5HT turnover evoked by neonatal parathion exposure (Figure 1D); in this case, animals given the high-fat diet did not show any increase in turnover, nor did females evince any effects of either parathion or diet. Hippocampal 5HT concentrations were not significantly affected by any of the treatment/diet combinations (Figure 1E) and again, 5HT turnover was increased by parathion in males but not females (Figure 1F). Males given the high-fat diet alone displayed significantly higher 5HT turnover than corresponding animals on the normal diet, and did not show any further effects of parathion above that level.
Figure 1.
5HT concentration (A,C,E) and turnover (B,D,F) in frontal/parietal cortex (A,B), temporal/occipital cortex (C,D) and hippocampus (E,F). Data represent means and standard errors obtained from 6-12 animals in each group. ANOVA appears at the bottom of each panel and asterisks denote individual groups that differ significantly from the corresponding control. Daggers indicate significant differences between controls on the high-fat diet vs. normal diet. NS, not significant.
In the striatum, there was an overall decrease in the values for 5HT concentration in animals exposed to parathion (multivariate ANOVA, p < 0.05 for the main effect of treatment, p < 0.04 individually for either dose group vs. control) but none of the values achieved significance individually when subdivided into males and females in the two dietary groups (Figure 2A). Turnover was increased by parathion in both males and females on the normal diet (Figure 2B); for animals given the high-fat diet, the dietary intervention by itself increased 5HT turnover, obtunding any further effect of parathion in males and lessening the difference in females. For midbrain 5HT concentration, parathion exposure had no effect in males with or without the high fat diet (Figure 2C). In females, midbrain 5HT was decreased significantly by either dose of parathion in animals on the normal diet; the high-fat diet by itself reduced 5HT levels, which then lessened the further impact of parathion. Both males and females on the normal diet showed parathion-induced increases in midbrain 5HT turnover (Figure 2D). The high-fat diet raised turnover by itself and accordingly, there were no further differences evoked by parathion exposure. For brainstem 5HT concentration, values were reduced in males exposed to the lower dose of parathion but there were no changes in any other group (Figure 2E). 5HT turnover was increased by parathion in males given the normal diet but not those on the high-fat diet (Figure 2F); again, the reduced effect of parathion resulted from a higher turnover value elicited by the dietary manipulation alone. Females did not show any alterations in brainstem 5HT turnover.
Figure 2.
5HT concentration (A,C,E) and turnover (B,D,F) in striatum (A,B), midbrain (C,D) and brainstem (E,F). Data represent means and standard errors obtained from 6 animals in each group. ANOVA appears at the bottom of each panel and asterisks denote individual groups that differ significantly from the corresponding control. Daggers indicate significant differences between controls on the high-fat diet vs. normal diet. NS, not significant.
5HT Receptors and Transporter
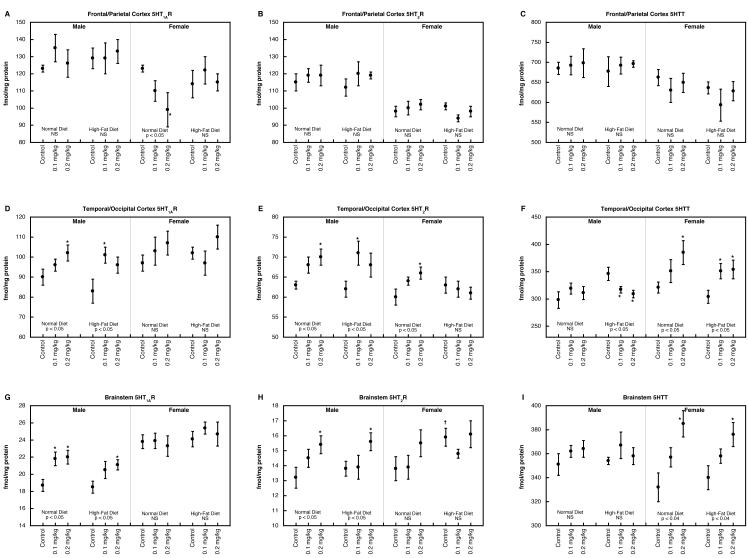
The global ANOVA (neonatal parathion treatment, diet, sex, region, the three 5HT synaptic protein measures) identified main effects for parathion treatment (p < 0.0002), sex (p < 0.03), region (p < 0.0001) and measure (p < 0.0001) as well as two-, three-, four- and five-factor interactions (not shown). The main effect of parathion reflected significantly higher values for either dose group compared to control (p < 0.0005 for 0.1 mg/kg vs. control, p < 0.0002 for 0.2 mg/kg vs. control). This main effect was maintained after subdivision into the three different measures: p < 0.03 for the 5HT1AR (p < 0.03 for 0.1 mg/kg vs. control, p < 0.04 for 0.2 mg/kg vs. control), p < 0.005 for the 5HT2R (p < 0.0002 for 0.2 mg/kg vs. control), p < 0.02 for the 5HTT site (p < 0.05 for 0.1 mg/kg vs. control, p < 0.002 for 0.2 mg/kg vs. control). As before, each main effect also showed interactions of parathion treatment with the other variables (diet, sex, region; not shown), necessitating examination the corresponding data subdivisions.
In the frontal/parietal cortex, males showed no significant alterations in 5HT1AR binding, regardless of neonatal treatment or dietary manipulation, but females evinced a decrease caused by parathion (Figure 3A); this effect was reversed by consumption of a high-fat diet. Within this region, there were no significant effects on either 5HT2R (Figure 3B) or 5HTT binding (Figure 3C).
Figure 3.
5HT1AR concentration (A,D,G), 5HT2R (B,E,H) and 5HTT binding (C,F,I) in frontal/parietal cortex (A,B,C), temporal/occipital cortex (D,E,F) and brainstem (G,H,I). Data represent means and standard errors obtained from 6 animals in each group. ANOVA appears at the bottom of each panel and asterisks denote individual groups that differ significantly from the corresponding control. Daggers indicate significant differences between controls on the high-fat diet vs. normal diet. NS, not significant.
In the temporal/occipital cortex, 5HT1AR binding was augmented by neonatal parathion treatment, an effect that was present regardless of whether animals were given a normal or high-fat diet (Figure 3D); females showed no significant effects. For 5HT2Rs, parathion-induced increases were evident for both males and females on the normal diet (Figure 3E); the high-fat diet obtunded the effect in females but not males. In males consuming a normal diet, neonatal parathion exposure had no effect on 5HTT binding but animals on the high-fat diet showed parathion-evoked decreases (Figure 3F); again, these were associated with generally higher values for the effects of diet alone in the control group. Females displayed significant increases in 5HTT binding after neonatal parathion exposure, regardless of whether they were consuming a normal or high-fat diet.
The brainstem showed strong sex-differences in the effects of parathion exposure by itself on 5HT1AR binding, with significant increases in males but not females (Figure 3G). Addition of a high-fat diet did not change the pattern. For 5HT2R binding, parathion evoked increased values in males on either a normal or high-fat diet (Figure 3H); although females did not show significant increases, the apparent sex difference needs to be viewed with caution because this parameter did not show a significant treatment × sex interaction, indicating that the nonsignificant effect in females was not itself distinguishable from the significant effect in males. For 5HTT binding, females showed significant parathion-induced increases regardless of diet, whereas males were unaffected (Figure 3I).
DA Concentration and Turnover
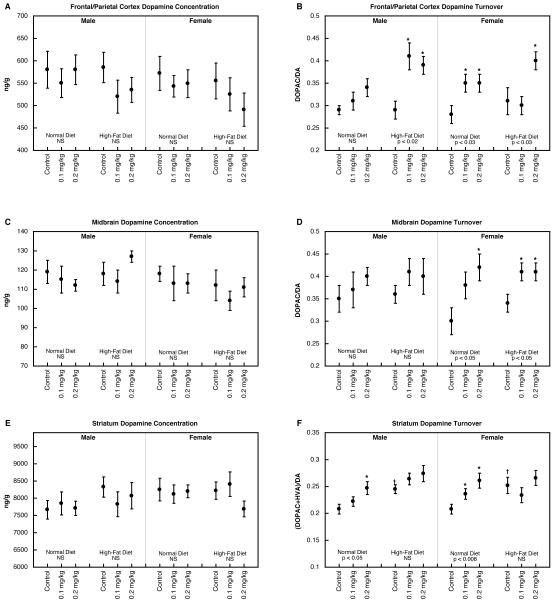
For measurements of the DA concentration, multivariate ANOVA identified only a main effect of region (p < 0.0001), whereas for DA turnover, there were robust main effects of parathion treatment (p < 0.0001), diet (p < 0.007) and region (p < 0.0001), as well as interactions of these three variables with each other and with sex (not shown). The main effect of parathion reflected significant increases (p < 0.0001) for either dose group compared to control and the main effect of diet resulted from higher values overall in the group consuming a high-fat diet (p < 0.004).
In the frontal/parietal cortex, there were no significant changes in DA concentration (Figure 4A) but increases were seen for DA turnover (Figure 4B). For males, there was no significant increase caused by parathion in animals consuming a normal diet, but a marked increase in those on the high-fat diet. In females, parathion evoked increases in DA turnover regardless of diet. The midbrain likewise showed no significant effects on DA concentration (Figure 4C) but increases in DA turnover (Figure 4D). In this region, effects were confined to females, who again displayed significant parathion-evoked increases regardless of diet. The DA concentration in the striatum was unaltered by parathion treatment with or without dietary manipulations (Figure 4E) but turnover was nevertheless enhanced (Figure 4F). Neonatal parathion treatment by itself produced higher turnover values in both males and females; for both, consumption of a high-fat diet evoked increases in the control group, thus obtunding any further effect of parathion.
Figure 4.
DA concentration (A,C,E) and turnover (B,D,F) in frontal/parietal cortex (A,B), midbrain (C,D) and striatum (E,F). Data represent means and standard errors obtained from 6-12 animals in each group. ANOVA appears at the bottom of each panel and asterisks denote individual groups that differ significantly from the corresponding control. Daggers indicate significant differences between controls on the high-fat diet vs. normal diet. NS, not significant.
DISCUSSION
Our results provide some of the first evidence that easily-manipulated factors such as adult diet composition, can influence the outcome of early-life exposure to a developmental neurotoxicant, in this case, the organophosphate pesticide, parathion. In our earlier work with developmental exposure to another organophosphate pesticide, chlorpyrifos, we identified changes in 5HT synaptic activity and receptors indicative of “miswiring” of 5HT circuits [1,2,5,44,45]: 5HT turnover was increased, reflecting presynaptic hyperactivity, yet at the same time, 5HT receptors were supranormal, instead of being downregulated as would be expected from increased 5HT levels in the synapse. 5HT-related behaviors were deficient, despite the enhancement of both presynaptic and postsynaptic components. Here, with parathion, we also found widespread disruption of 5HT systems akin to that seen with chlorpyrifos given during the same developmental period. Neonatal parathion exposure evoked long-term increases in 5HT turnover in multiple brain regions, independent of changes in the 5HT concentration, indicating that the alterations in the index of presynaptic activity were not simply compensatory for reduced transmitter availability. This was further confirmed by the absence of reductions in 5HTT sites, which would have been subnormal if the number of 5HT terminals had been reduced. As with chlorpyrifos, parathion also evoked increases, not decreases in 5HT1AR and 5HT2R binding, indicating again that the presynaptic hyperactivity is accompanied by upregulation of the corresponding postsynaptic sites. Finally, the parathion-exposed animals also show deficient 5HT-related behaviors [52], despite presynaptic 5HT hyperactivity and 5HTR upregulation. Accordingly, it is highly likely that parathion, too, produces miswiring of 5HT circuits. What differs between the two organophosphates, though, is the time course with which these changes emerge. With chlorpyrifos, many of the abnormalities were present as early as adolescence and were fully established by young adulthood [2,5,44]. With parathion, the changes take longer to emerge, with many of the changes seen here at nearly six months of age not yet present or just commencing at three months [39]. There are important ramifications of this dichotomy. First, it indicates that organophosphates do not simply damage 5HT projections, leading to early changes that then persist continually throughout the lifespan. Instead, the exposures alter the trajectory of 5HT synaptic development and function, so that adverse effects can emerge later on, thus interacting with environmental and developmental factors that are expressed only in adolescence and adulthood. Second, this means that interventions later in life may be able to ameliorate, exacerbate, or otherwise alter the outcome of developmental neurotoxicant exposure. As studied here, dietary composition by itself has potent effects on 5HT and DA function, and modulates the net effect of neonatal toxicant exposure. Differences in diet thus represent one way in which subpopulations may be created that are especially vulnerable or resistant to the functional consequences of early-life organophosphate exposure.
There is a specific rationale for exploring the effects of a high-fat diet in models of neurodevelopmental disorders. Alterations in the lipid composition of neural membranes affects neuronal activity, receptor and signaling function, and synaptic transmission [11,20,22,25,32], effects that are likely to contribute to the success of a ketogenic diet in treating intractable epilepsies [8,13,23,24], to pilot studies showing beneficial effects in attentional disorders and autism [13,16,33], and to results in animal models of behavioral deficits [50]. In our earlier work, we found that consumption of a high fat diet in adulthood was able to offset most of the cholinergic synaptic deficits caused by neonatal parathion exposure [37], while at the same time incurring metabolic liabilities [26]. In the present study, our results for the dietary impact on 5HT synaptic defects were more diverse. For a number of the effects of parathion, the dietary intervention did produce amelioration akin to that seen for cholinergic systems (e.g. female frontal/parietal cortex 5HT1AR and temporal/occipital cortex 5HT2R) but for other parameters, the diet had little or no salutary effect (e.g. frontal/parietal cortex 5HT concentration in females and 5HT turnover in both sexes) or, in a few cases, exacerbated the actions of parathion (e.g. DA turnover in male frontal/parietal cortex). Further, for many other variables, the high-fat diet did reduce or eliminate the impact of neonatal parathion exposure, but only by itself causing the same corresponding change in the affected parameter (e.g. 5HT turnover in the hippocampus, striatum, midbrain and brainstem in males; midbrain 5HT turnover in females); this may represent a “ceiling” effect, wherein the actions of the diet by itself already produce maximal changes, thus masking any further effect of parathion exposure. Indeed, this unexpected finding provides important mechanistic clues about both the effects of neonatal parathion exposure and consumption of a high-fat diet in adulthood. If the two interventions targeted totally separate mechanisms and pathways, then we would expect there to be additive effects only; the fact that, when the changes caused by the high-fat diet are in the same direction as those caused by parathion, they obtund the parathion effect, indicates instead that the two interventions are likely to be converging on the same mechanistic processes. In turn, this means that consumption of a high-fat diet may actually produce some aspects of synaptic dysfunction akin to that evoked by early-life parathion exposure; future work should explore whether this prediction holds true for behavioral outcomes as well.
Our studies showed clear-cut differences in the ability of a high-fat diet to reverse parathion-induced deficits in cholinergic function [37], as compared to the more complex interactions seen here for 5HT systems. Accordingly, we also performed comparable work with another monoamine, DA, that subserves separate functions from 5HT and has dissimilar anatomical circuitry. Despite those underlying differences, the net effects of parathion and the interaction with the high-fat diet were distinctly similar to that of 5HT and unlike the effect seen earlier for cholinergic pathways [37]. In earlier work with chlorpyrifos, we also pointed out similar outcomes for 5HT and DA [2,44], as well as shared, direct effects of various organophosphates on gene transcription related to the two neurotransmitters [41,43,46]. These findings point to the potential for comparable effects on DA synaptic function and related behaviors, of both early-life exposure to organophosphates and dietary interventions in adulthood. Given the critical role played by DA in reward, one intriguing possibility is a change in susceptibility to addictive behaviors resulting from either or both interventions. Again, this is an important subject for future work.
In conclusion, our results indicate that neonatal parathion exposure produces lasting alterations in the developmental trajectory of 5HT synaptic function, with effects emerging throughout adolescence [39] and, as seen here, continuing to evolve into adulthood. Unlike the effects on cholinergic systems [37], consumption of a high-fat diet in adulthood did not uniformly offset the effects of parathion but instead, the dietary intervention by itself also produced changes in a number of 5HT synaptic parameters resembling those of neonatal parathion exposure, which then preempted any further effect of the parathion. The convergent actions of neonatal parathion exposure and consumption of a high-fat diet in adulthood point to the real possibility that the explosive increase in neurodevelopmental disorders may reflect not only exposures to neurotoxic chemicals [21], but also the neurobehavioral effects of dietary characteristics that are common to the same, low socioeconomic level, inner-city and agricultural community human subpopulations that are disproportionately exposed to pesticides.
Acknowledgments/disclaimers
The authors thank Jennifer Card for technical assistance. Research was supported by NIH ES10356 and by the Leon Golberg Postdoctoral Fellowship. The study sponsors had no role in the study design; collection, analysis and interpretation of data; the writing of the manuscript; or the decision to submit the manuscript for publication. TAS has provided expert witness testimony in the past three years at the behest of the following law firms: The Calwell Practice (Charleston WV), Frost Brown Todd (Charleston WV), Snyder Weltchek & Snyder (Baltimore MD), Finnegan Henderson Farabow Garrett & Dunner (Washington DC), Frommer Lawrence Haug (Washington DC), Carter Law (Peoria IL), Corneille Law (Madison WI), Angelos Law (Baltimore MD), Kopff, Nardelli & Dopf (New York NY), and Gutglass Erickson Bonville & Larson (Madison WI).
Abbreviations
- 5HIAA
5-hydroxyindoleacetic acid
- 5HT
5-hydroxytryptamine, serotonin
- 5HT1AR
5HT1A receptor
- 5HT2R
5HT2 receptor
- 5HTT
5HT transporter
- ANOVA
analysis of variance
- DA
dopamine
- DOPAC
dihydroxyphenylacetic acid
- HVA
homovanillic acid
- PN
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ Health Perspect. 2005;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ Health Perspect. 2005;113:1027–1031. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Developmental exposure to terbutaline and chlorpyrifos: pharmacotherapy of preterm labor and an environmental neurotoxicant converge on serotonergic systems in neonatal rat brain regions. Toxicol Appl Pharmacol. 2005;203:134–144. doi: 10.1016/j.taap.2004.08.002. [DOI] [PubMed] [Google Scholar]
- [4].Aldridge JE, Seidler FJ, Meyer A, Thillai I, Slotkin TA. Serotonergic systems targeted by developmental exposure to chlorpyrifos: effects during different critical periods. Environ Health Perspect. 2003;111:1736–1743. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJ, Mann JJ. Serotonin-1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- [7].Barr DB, Allen R, Olsson AO, Bravo R, Caltabiano LM, Montesano A, Nguyen J, Udunka S, Walden D, Walker RD. Concentrations of selective metabolites of organophosphorus pesticides in the United States population. Environ Res. 2005;99:314–326. doi: 10.1016/j.envres.2005.03.012. others. [DOI] [PubMed] [Google Scholar]
- [8].Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia. 2007;48:43–58. doi: 10.1111/j.1528-1167.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- [9].Bradman A, Eskenazi B, Barr DB, Bravo R, Castorina R, Chevrier J, Kogut K, Harnly ME, McKone TE. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ Health Perspect. 2005;113:1802–1807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- [11].Clandinin MT, Foot M, Robson L. Plasma membrane: can its structure and function be modulated by dietary fat? Comp Biochem Physiol B. 1983;76:335–339. doi: 10.1016/0305-0491(83)90079-2. [DOI] [PubMed] [Google Scholar]
- [12].Clegg DJ, van Gemert M. Determination of the reference dose for chlorpyrifos: proceedings of an expert panel. J Toxicol Environ Health. 1999;2:211–255. doi: 10.1080/109374099281179. [DOI] [PubMed] [Google Scholar]
- [13].Connolly MB, Hendson G, Steinbok P. Tuberous sclerosis complex: a review of the management of epilepsy with emphasis on surgical aspects. Childs Nervous System. 2006;22:896–908. doi: 10.1007/s00381-006-0130-7. [DOI] [PubMed] [Google Scholar]
- [14].Costa LG, Cole TB, Furlong CE. Polymorphisms of paraoxonase (PON1) and their significance in clinical toxicology of organophosphates. J Toxicol Clin Toxicol. 2003;41:37–45. doi: 10.1081/clt-120018269. [DOI] [PubMed] [Google Scholar]
- [15].Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Evangeliou A, Vlachonikolis I, Mihailidou H, Spilioti M, Skarpalezou A, Makaronas N, Prokopiou A, Christodoulou P, Liapi-Adamidou G, Helidonis E. Application of a ketogenic diet in children with autistic behavior: pilot study. J Child Neurol. 2003;18:113–8. doi: 10.1177/08830738030180020501. others. [DOI] [PubMed] [Google Scholar]
- [17].Fujita M, Charney DS, Innis RB. Imaging serotonergic neurotransmission in depression: hippocampal pathophysiology may mirror global brain alterations. Biol Psychiat. 2000;48:801–812. doi: 10.1016/s0006-3223(00)00960-4. [DOI] [PubMed] [Google Scholar]
- [18].Furlong CE, Cole TB, Jarvik GP, Pettan-Brewer C, Geiss GK, Richter RJ, Shih DM, Tward AD, Lusis AJ, Costa LG. Role of paraoxonase (PON1) status in pesticide sensitivity: genetic and temporal determinants. Neurotoxicology. 2005;26:651–659. doi: 10.1016/j.neuro.2004.08.002. [DOI] [PubMed] [Google Scholar]
- [19].Furlong CE, Holland NT, Richter RJ, Bradman A, Ho A, Eskenazi B. PON1 status of farmworker mothers and children as a predictor of organophosphate sensitivity. Pharmacogen Genomics. 2006;16:183–190. doi: 10.1097/01.fpc.0000189796.21770.d3. [DOI] [PubMed] [Google Scholar]
- [20].Geiser F. Influence of polyunsaturated and saturated dietary lipids on adipose tissue, brain and mitochondrial membrane fatty acid composition of a mammalian hibernator. Biochim Biophys Acta. 1990;1046:159–166. doi: 10.1016/0005-2760(90)90183-x. [DOI] [PubMed] [Google Scholar]
- [21].Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- [22].Gudbjarnason S, Benediktsdottir VE. Regulation of β-adrenoceptor properties and the lipid milieu in heart muscle membranes during stress. Mol Cell Biochem. 1996;164:137–143. doi: 10.1007/978-1-4613-1289-5_16. [DOI] [PubMed] [Google Scholar]
- [23].Hallbook T, Lundgren J, Rosen I. Ketogenic diet improves sleep quality in children with therapy-resistant epilepsy. Epilepsia. 2007;48:59–65. doi: 10.1111/j.1528-1167.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- [24].Hartman AL, Vining EPG. Clinical aspects of the ketogenic diet. Epilepsia. 2007;48:31–42. doi: 10.1111/j.1528-1167.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- [25].Kelly JF, Joseph JA, Denisova NA, Erat S, Mason RP, Roth GS. Dissociation of striatal GTPase and dopamine release responses to muscarinic cholinergic agonists in F344 rats: influence of age and dietary manipulation. J Neurochem. 1995;64:2755–2764. doi: 10.1046/j.1471-4159.1995.64062755.x. [DOI] [PubMed] [Google Scholar]
- [26].Lassiter TL, Ryde IT, MacKillop EA, Brown KK, Levin ED, Seidler FJ, Slotkin TA. Exposure of neonatal rats to parathion elicits sex-selective reprogramming of metabolism and alters the response to a high-fat diet in adulthood. Environ Health Perspect. 2008;116:1456–1462. doi: 10.1289/ehp.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee WJ, Alavanja MCR, Hoppin JA, Rusiecki JA, Kamel F, Blair A, Sandler DP. Mortality among pesticide applicators exposed to chlorpyrifos in the agricultural health study. Environ Health Perspect. 2007;115:528–534. doi: 10.1289/ehp.9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].London L, Flisher AJ, Wesseling C, Mergler D, Kromhout H. Suicide and exposure to organophosphate insecticides: cause or effect? Am J Ind Med. 2005;47:308–321. doi: 10.1002/ajim.20147. [DOI] [PubMed] [Google Scholar]
- [29].Maes M, Meltzer H. The serotonin hypothesis of major depression. In: Bloom FE, Kupfer DJ, Bunney BS, Ciaranello RD, Davis KL, Koob GF, Meltzer HY, Schuster CR, Shader RI, Watson SJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 933–944. [Google Scholar]
- [30].Nemeroff CB. The neurobiology of depression. Sci Am. 1998;278(6):42–49. doi: 10.1038/scientificamerican0698-42. [DOI] [PubMed] [Google Scholar]
- [31].Nutt DJ. The neuropharmacology of serotonin and noradrenaline in depression. Int Clin Psychopharmacol. 2002;17:S1–S12. doi: 10.1097/00004850-200206001-00002. [DOI] [PubMed] [Google Scholar]
- [32].Ponsard B, Durot I, Delerive P, Oudot F, Cordelet C, Grynberg A, Athias P. Cross-influence of membrane polyunsaturated fatty acids and hypoxia-reoxygenation on α- and β-adrenergic function of rat cardiomyocytes. Lipids. 1999;34:457–466. doi: 10.1007/s11745-999-0385-5. [DOI] [PubMed] [Google Scholar]
- [33].Pulsifer MB, Gordon JM, Brandt J, Vining EP, Freeman JM. Effects of ketogenic diet on development and behavior: preliminary report of a prospective study. Dev Med Child Neurol. 2001;43:301–6. doi: 10.1017/s0012162201000573. [DOI] [PubMed] [Google Scholar]
- [34].Rauh VA, Garfinkel R, Perera R, Andrews H, Hoepner L, Barr D, Whitehead D, Tang D, Whyatt RM. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:1845–1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA, Levin ED. Developmental diazinon neurotoxicity in rats: later effects on emotional response. Brain Res Bull. 2008;75:166–172. doi: 10.1016/j.brainresbull.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Slotkin TA. Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. In: Gupta RC, editor. Toxicity of Organophosphate and Carbamate Pesticides. Elsevier Academic Press; San Diego: 2005. pp. 293–314. [Google Scholar]
- [37].Slotkin TA, Lassiter TL, Ryde IT, Wrench N, Levin ED, Seidler FJ. Consumption of a high-fat diet in adulthood ameliorates the effects of neonatal parathion exposure on acetylcholine systems in rat brain regions. Environ Health Perspect. 2009;117:916–922. doi: 10.1289/ehp.0800459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Slotkin TA, Levin ED, Seidler FJ. Comparative developmental neurotoxicity of organophosphate insecticides: effects on brain development are separable from systemic toxicity. Environ Health Perspect. 2006;114:746–751. doi: 10.1289/ehp.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Slotkin TA, Levin ED, Seidler FJ. Developmental neurotoxicity of parathion: progressive effects on serotonergic systems in adolescence and adulthood. Neurotoxicol Teratol. 2009;31:11–17. doi: 10.1016/j.ntt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Slotkin TA, Ryde IT, Levin ED, Seidler FJ. Developmental neurotoxicity of low-dose diazinon exposure of neonatal rats: effects on serotonin systems in adolescence and adulthood. Brain Res Bull. 2008;75:640–647. doi: 10.1016/j.brainresbull.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Slotkin TA, Seidler FJ. Comparative developmental neurotoxicity of organophosphates in vivo: transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res Bull. 2007;72:232–274. doi: 10.1016/j.brainresbull.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Slotkin TA, Seidler FJ. Developmental exposure to terbutaline and chlorpyrifos, separately or sequentially, elicits presynaptic serotonergic hyperactivity in juvenile and adolescent rats. Brain Res Bull. 2007;73:301–309. doi: 10.1016/j.brainresbull.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Slotkin TA, Seidler FJ. Developmental neurotoxicants target neurodifferentiation into the serotonin phenotype: chlorpyrifos, diazinon, dieldrin and divalent nickel. Toxicol Appl Pharmacol. 2008;233:211–219. doi: 10.1016/j.taap.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Slotkin TA, Seidler FJ. Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: critical periods for regional and sex-selective effects. Reprod Toxicol. 2007;23:421–427. doi: 10.1016/j.reprotox.2006.07.010. [DOI] [PubMed] [Google Scholar]
- [45].Slotkin TA, Seidler FJ. The alterations in CNS serotonergic mechanisms caused by neonatal chlorpyrifos exposure are permanent. Dev Brain Res. 2005;158:115–119. doi: 10.1016/j.devbrainres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- [46].Slotkin TA, Seidler FJ. Transcriptional profiles reveal similarities and differences in the effects of developmental neurotoxicants on differentiation into neurotransmitter phenotypes in PC12 cells. Brain Res Bull. 2009;78:211–225. doi: 10.1016/j.brainresbull.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Slotkin TA, Seidler FJ, Ali SF. Cellular determinants of reduced adaptability of the aging brain: neurotransmitter utilization and cell signaling responses after MDMA lesions. Brain Res. 2000;879:163–173. doi: 10.1016/s0006-8993(00)02767-0. [DOI] [PubMed] [Google Scholar]
- [48].Slotkin TA, Tate CA, Ryde IT, Levin ED, Seidler FJ. Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ Health Perspect. 2006;114:1542–1546. doi: 10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- [50].Teegarden SL, Nestler EJ, Bale TL. DeltaFosB-mediated alterations in dopamine signaling are normalized by a palatable high-fat diet. Biol Psychiat. 2008;64:941–950. doi: 10.1016/j.biopsych.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, Levin ED. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicol Teratol. 2008;30:38–45. doi: 10.1016/j.ntt.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Timofeeva OA, Sanders D, Seemann K, Yang L, Hermanson D, Regenbogen S, Agoos S, Kallepalli A, Rastogi A, Braddy D. Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Res Bull. 2008;77:404–411. doi: 10.1016/j.brainresbull.2008.08.019. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol Appl Pharmacol. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
- [54].Whyatt RM, Camann D, Perera FP, Rauh VA, Tang D, Kinney PL, Garfinkel R, Andrews H, Hoepner L, Barr DB. Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicol Appl Pharmacol. 2005;206:246–254. doi: 10.1016/j.taap.2004.11.027. [DOI] [PubMed] [Google Scholar]
- [55].Xu Z, Seidler FJ, Ali SF, Slikker W, Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
- [56].Yatham LN, Liddle PF, Dennie J, Shiah IS, Adam MJ, Lane CJ, Lam RW, Ruth TJ. Decrease in brain serotonin-2 receptor binding in patients with major depression following desipramine treatment: a positron emission tomography study with fluorine-18-labeled setoperone. Arch Gen Psychiat. 1999;56:705–711. doi: 10.1001/archpsyc.56.8.705. [DOI] [PubMed] [Google Scholar]
- [57].Yatham LN, Liddle PF, Shiah IS, Scarrow G, Lam RW, Adam MJ, Zis AP, Ruth TJ. Brain serotonin-2 receptors in major depression: a positron emission tomography study. Arch Gen Psychiat. 2000;57:850–858. doi: 10.1001/archpsyc.57.9.850. [DOI] [PubMed] [Google Scholar]