Abstract
A 30-year-old woman presents with a history of no menses since she stopped taking oral contraceptives 6 months ago in order to conceive. She had undergone puberty that was normal in both timing and development, with menarche at 12 years of age. At 18 years of age, she started taking oral contraceptives for irregular menses. She reports stress at work. Her weight is 59 kg, and her height 1.66 m; her body-mass index (the weight in kilograms divided by the square of the height in meters) is 21.3. There is no galactorrhea, hirsutism, or acne. The pelvic examination is normal, a pregnancy test is negative, the prolactin level is normal, and the follicle-stimulating hormone (FSH) level is in the menopausal range. How should she be evaluated and treated?
THE CLINICAL PROBLEM
The ovary is unique in the endocrine system in that an entirely new secretory structure is developed within it each month — the graafian follicle, which arises from a microscopic primordial follicle. Menopause, defined as the permanent cessation of menses, results from the depletion of potentially functional primordial follicles. The mean (±SD) age at the time of natural menopause is 50±4 years.1 Menopause before the age of 40 years is considered to be premature.
Primary ovarian insufficiency is the preferred term for the condition that was previously referred to as premature menopause or premature ovarian failure; other terms used for this condition include primary ovarian failure and hypergonadotropic hypogonadism, as well as the misnomer, gonadal dysgenesis.2,3 The condition is considered to be present when a woman who is less than 40 years old has had amenorrhea for 4 months or more, with two serum FSH levels (obtained at least 1 month apart) in the menopausal range.4,5 The condition differs from menopause in that there is varying and unpredictable ovarian function in approximately 50% of cases, and about 5 to 10% of women conceive and deliver a child after they have received the diagnosis.4,6–9 Thus, the term “primary ovarian insufficiency,” as originally suggested by Albright, meets the need to describe a continuum of impaired ovarian function rather than a dichotomous state.2,3 This term may also be less stigmatizing than the terms that were used previously.
In 90% of the cases of primary ovarian insufficiency, the cause remains a mystery. Spontaneous 46,XX primary ovarian insufficiency can occasionally occur as part of a syndrome (Table 1). In addition, several single genes (e.g., bone morphogenetic protein 15 [BMP15], diaphanous homolog 2 [DIAPH2], and inhibin alpha subunit [INHA]) have been associated with nonsyndromic primary ovarian insufficiency, but their clinical relevance is not clear. Structural abnormalities in the X chromosome apart from specific gene mutations may also be a cause.
Table 1.
Representative Syndromes with Which Spontaneous 46,XX Primary Ovarian Insufficiency Has Been Associated.*
| Syndrome | Gene | OMIM Number | Prominent Associated Findings |
|---|---|---|---|
| Fragile X–associated disorders | FMR1 (fragile X mental retardation 1) | 309550 | Family history of intellectual disability due to fragile X syndrome or tremor–ataxia disorder |
| Autoimmune polyendocrine syndrome, type 1 | AIRE (autoimmune regulator) | 240300 | Adrenal insufficiency, hypoparathyroidism, chronic mucocutaneous candidiasis |
| Autoimmune polyendocrine syndrome, type 2 | Unknown | 269200 | Adrenal insufficiency, type 1 diabetes mellitus, autoimmune thyroid disease |
| Congenital adrenal hyperplasia due to 17α-hydroxylase deficiency | CYP17A1 (cytochrome P450, family 17, subfamily A, polypeptide 1) | 202110 | Hypertension, hypokalemic alkalosis |
| Lipoid congenital adrenal hyperplasia | STAR (steroidogenic acute regulatory protein) | 600617 | Congenital adrenal insufficiency; testicular function in men more severely affected than ovarian function in women |
| Aromatase deficiency | CYP19A1 (cytochrome P450, family 19, subfamily A, polypeptide 1) | 107910 | Maternal virilization during pregnancy due to absence of placental aromatase |
| Blepharophimosis, ptosis, epicanthus inversus syndrome | FOXL2 (forkhead box L2) | 110100 | Dysmorphic eyelids |
| Progressive external ophthalmoplegia with mitochondrial DNA deletions | POLG (polymerase [DNA directed], gamma) | 157640 | Adult-onset weakness of external eye muscles and exercise intolerance |
| Galactosemia | GALT (galactose-1-phosphate uridylyltransferase) | 230400 | Hepatomegaly, cataracts, intellectual disability |
| Congenital disorder of glycosylation, type 1A | PMM2 (phosphomannomutase 2) | 212065 | Neonatal encephalopathy, hypotonia, psychomotor retardation, cerebellar hypoplasia, retinitis pigmentosa |
| Fanconi’s anemia | FA (Fanconi anemia complementation groups) | 227650 | Anemia, leukopenia, thrombocytopenia; cardiac, renal and limb malformations; dermal pigment changes |
| Ataxia–telangiectasia | ATM (ataxia–telangiectasia mutated) | 208900 | Cerebellar ataxia, telangiectases, immune defects, predisposition to cancer, premature aging, genome instability |
| Bloom’s syndrome | BLM (Bloom syndrome) | 210900 | Premature aging, predisposition to cancer, genome instability |
| Werner’s syndrome | WRN (Werner syndrome) | 277700 | Premature aging, predisposition to cancer, genome instability |
For a complete list of syndromes with which spontaneous 46,XX primary ovarian insufficiency has been associated, see the Supplementary Appendix, available with the full text of this article at NEJM.org. OMIM denotes Online Mendelian Inheritance in Man.
Primary ovarian insufficiency occurs through two major mechanisms: follicle dysfunction and follicle depletion.5 Follicle dysfunction indicates that follicles remain in the ovary, but a pathologic process prevents their normal function (e.g., as a result of an FSH-receptor mutation).10 Follicle depletion indicates that no primordial follicles remain in the ovary. This condition may be due to the failure of an adequate initial pool of primordial follicles to be established in utero, an accelerated expenditure of follicles, or autoimmune or toxic destruction of follicles. Table 2 lists examples of known clinical causes of primary ovarian insufficiency according to the mechanism.
Table 2.
Mechanisms and Causes of Spontaneous Primary Ovarian Insufficiency.
| Mechanism and Cause | Comments |
|---|---|
| Ovarian follicle dysfunction | |
| Signal defect | |
| FSH-receptor mutation | Presence of ovarian follicles confirmed by biopsy; founder effect; rare disorder outside of Finland |
| Luteinizing hormone-receptor mutation | Ovarian follicles present on ultrasound examination; rare disorder |
| G-protein mutation | Secondary amenorrhea, elevated gonadotropin levels, and hypoestrogenemia that responded to gonadotropin therapy developed in patient with pseudohypoparathyroidism11; rare disorder |
| Enzyme deficiency | |
| Isolated 17,20-lyase deficiency | Ovarian follicles present on biopsy, “moderate ovarian enlargement” due to block in estradiol synthesis; rare disorder |
| Aromatase deficiency | Ovarian enlargement or hyperstimulation due to inability of the ovary to aromatize and rostenedione to estradiol; rare disorder |
| Autoimmunity | |
| Autoimmune lymphocytic oophoritis | Antral follicles with lymphocytic infiltration into theca, primordial follicles spared, multifollicular ovaries; accounts for 4% of cases of 46,XX primary ovarian insufficiency; associated with evidence of adrenal autoimmunity |
| Insufficient follicle number | |
| Luteinized graafian follicles | Antral follicles imaged by ultrasonography in 40% of patients with idiopathic spontaneous 46,XX primary ovarian insufficiency; on the basis of histologic findings, at least 60% of antral follicles imaged in these patients are luteinized, a major mechanism of follicle dysfunction in these women7 |
| Ovarian follicle depletion | |
| Insufficient initial follicle number | |
| Blepharophimosis, ptosis, epicanthus inversus syndrome | Mutation in FOXL2 is a mechanism of familial primary ovarian insufficiency, and disruption of the mouse gene causes a pervasive block in primordial follicle development; rare disorder |
| Spontaneous accelerated follicle loss | |
| Turner’s syndrome | Although a normal complement of primordial follicles is established in the ovary during fetal development, follicle loss through apoptosis is accelerated so that the store of primordial follicles is typically depleted before puberty; in oocytes, both X chromosomes must be present and remain active to prevent accelerated follicular atresia; the individual genes responsible for this ovarian syndrome have not been identified |
| Environmental-toxin–induced follicle loss | |
| Industrial exposure to 2-bromopropane | Exposure to cleaning solvent associated with primary ovarian insufficiency in 16 Korean women12 |
This review focuses on spontaneous 46,XX primary ovarian insufficiency, which affects approximately 1 in 100 women by the time they are 40 years of age.13
STRATEGIES AND EVIDENCE
EVALUATION
There is no menstrual history that is characteristic of the development of spontaneous 46,XX primary ovarian insufficiency.5 In most cases, the condition develops after a normal puberty and established regular menses, although primary amenorrhea may be the presenting feature in about 10% of cases.4 Occasionally, menses stop abruptly. In some women, menses fail to resume after a pregnancy or after they have stopped taking hormonal contraceptives. Most commonly, there is a prodrome of oligomenorrhea, polymenorrhea, or dysfunctional uterine bleeding.
Once pregnancy has been ruled out, clinicians evaluating women with secondary amenorrhea should address several questions: Is this condition the earliest manifestation of a decline in general health, such as uncontrolled diabetes mellitus, or of an underlying condition, such as celiac disease?14,15 Is it related to excessive exercise, inadequate caloric intake, or emotional stress? Has the woman undergone prior radiation therapy or chemotherapy? Is there galactorrhea (suggestive of hyperprolactinemia) or are there signs of androgen excess? Although the list of potential causes of secondary amenorrhea is long, the majority of cases are accounted for by four conditions: the polycystic ovary syndrome, hypothalamic amenorrhea, hyperprolactinemia, and primary ovarian insufficiency.14 It is inappropriate to attribute amenorrhea to stress without further evaluation.
Diagnostic criteria have not been established by any professional organization. A commonly used definition requires that there be at least 4 months of amenorrhea.4,16 However, because approximately 50% of women with primary ovarian insufficiency have intermittent ovarian function leading to intermittent and unpredictable menses, rather than complete amenorrhea, a more practical definition is 4 months or more of “disordered” menses (amenorrhea, oligomenorrhea, polymenorrhea, or metrorrhagia) in association with menopausal FSH levels.
Symptoms of estrogen deficiency develop in many, but not all, patients. These symptoms include vasomotor symptoms (hot flashes and night sweats), sleep disturbance, and dyspareunia related to vaginal dryness. However, not all patients have profound estrogen deficiency, and a vaginal examination often shows effects suggesting normal estrogen levels.
Although most cases of primary ovarian insufficiency occur sporadically, there is a positive family history, with an affected first-degree relative, in approximately 10 to 15% of cases.17 Thus, patients should be queried about family history as well as about other autoimmune disorders (including hypothyroidism, adrenal insufficiency, and hypoparathyroidism) that might relate to an autoimmune polyglandular syndrome. The condition may also be associated with the dry-eye syndrome, myasthenia gravis, rheumatoid arthritis, or systemic lupus erythematosus.18,19 A family history of the fragile X syndrome, intellectual disability, dementia, tremor or ataxia, or symptoms similar to those associated with Parkinson’s disease might point to a premutation in the fragile X mental retardation 1 (FMR1) gene.20
The physical examination may reveal evidence of an associated disorder such as hyperpigmentation or vitiligo (which is associated with autoimmune adrenal insufficiency), thyroid enlargement, or stigmata indicative of Turner’s syndrome, such as short stature, webbed neck, and high, arched palate.
After pregnancy is ruled out, the initial evaluation of amenorrhea should include, at a minimum, the measurement of serum prolactin, FSH, and thyrotropin levels.14 In cases of amenorrhea caused by stress (i.e., hypothalamic amenorrhea), the serum FSH level is in the low or normal range. If the FSH level is in the menopausal range, as defined by the reporting laboratory, the test should be repeated in 1 month along with a serum estradiol measurement. A progestin-withdrawal test (in which a progestin is administered and then withdrawn in order to determine whether vaginal bleeding ensues after its withdrawal) was previously used as a diagnostic test of ovarian function, but it is not currently recommended. Nearly 50% of women with primary ovarian insufficiency have withdrawal bleeding in response to the test, despite the presence of menopausal-level gonadotropins, and in the case of these women, relying on this bioassay would delay the diagnosis.4
In cases of primary ovarian insufficiency that are not associated with a syndrome, the laboratory tests that are recommended to determine the cause include a karyotype analysis and testing for an FMR1 premutation and for adrenal antibodies (with the use of indirect immunofluorescence or 21-hydroxylase [CYP21] immunoprecipitation); pelvic ultrasonography should also be performed. Approximately 2% of women with isolated spontaneous 46,XX primary ovarian insufficiency and 14% with familial spontaneous 46,XX primary ovarian insufficiency have an FMR1 premutation, which confers a risk of having a child with fragile X syndrome.20 The results of adrenal antibody testing are positive in approximately 4% of women with primary ovarian insufficiency. These women have steroidogenic cell autoimmunity, and lymphocytic autoimmune oophoritis is the mechanism of the ovarian insufficiency (Fig. 1). Ovarian antibodies lack specificity, and testing for them is not warranted.21 Pelvic ultrasonography identifies cases involving enlarged, multifollicular ovaries, which may undergo torsion, such as in isolated 17,20-lyase deficiency or autoimmune oophoritis (Table 2). An ovarian biopsy does not provide information that is helpful in the management of primary ovarian insufficiency and is therefore not indicated; pregnancy may occur even after examination of a biopsy specimen has shown that follicles are absent.6
Figure 1. Transvaginal Ultrasound Scan from a Patient with Spontaneous 46,XX Primary Ovarian Insufficiency Who Had Follicle Dysfunction Due to Autoimmune Oophoritis.
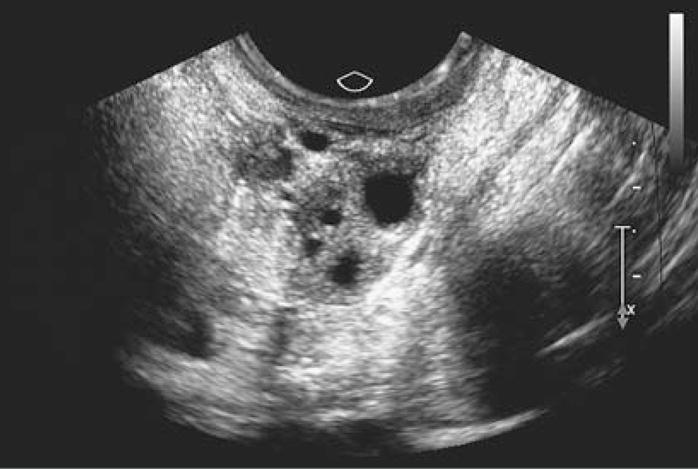
The ovary appears normal, with the presence of multiple follicles, despite amenorrhea, estrogen deficiency, and menopausal-level gonadotropins. Autoimmune oophoritis with thecal infiltration by lymphocytes was confirmed histologically by means of an ovarian biopsy performed when the patient was 26 years of age.9
MANAGEMENT
The diagnosis of primary ovarian insufficiency affects a woman’s physical and emotional well-being, and the management of the condition should address both. Other associated endocrine deficiencies, as well as anxiety, depression, or both, may develop. The presence of an abnormal karyotype, an iatrogenic cause, or a premutation in the FMR1 gene has additional health implications that are beyond the scope of this article.
Emotional Health
Unexpected infertility is a life-altering diagnosis for many women.22 Shyness and social anxiety, impaired self-esteem, and a perceived low level of social support are more frequent among women with spontaneous 46,XX primary ovarian insufficiency than among women who do not have this condition.23,24 Many women report experiencing severe emotional distress25 and want guidance on how to cope with the emotional sequelae, but few ask for it directly. It is best to schedule a return office visit to inform women of this diagnosis; patients should be encouraged to identify sources of emotional support.24
Hormone-Replacement Therapy
Early menopause has been associated with an increased incidence of fractures26 and increased total mortality and mortality due to ischemic heart disease.27–29 In a study of women who were part of the Women’s Health Initiative, combined hormone-replacement therapy (estrogen with progestin) increased the risk of cardiovascular events; however, it is invalid to apply the results of this study, which involved menopausal women who were, on average, 63 years of age,30 to young women with primary ovarian insufficiency. (Menopause is a physiologic condition, whereas primary ovarian insufficiency is a pathologic condition in which women have low serum estradiol levels as compared with other women of similar age.) Although data from randomized, controlled trials are lacking, most experts agree that physiologic estrogen and progestin replacement is reasonable in the case of young women with primary ovarian insufficiency and should be continued until they reach the age when menopause usually occurs.3
The average serum estradiol level during the menstrual cycle in women with a normal menstrual history is approximately 100 pg per milliliter. 31 Although no studies have directly compared various hormonal therapies for women with primary ovarian insufficiency, a dose of 100 μg of estradiol per day, administered by transdermal patch, achieves average serum estradiol levels in this range and effectively treats symptoms. Transdermal estradiol has little effect on hemostatic factors, and in case–control studies, it has been associated with a lower risk of venous thromboembolism than has oral estrogen.32–34 Evidence supports the use of cyclic medroxyprogesterone acetate at a dose of 10 mg per day for 12 days each month as the preferred progestin. This regimen fully induces secretory endometrium and provides protection against endometrial cancer.35,36 Data regarding the effects on the endometrium of oral micronized progesterone when it is given in conjunction with a full replacement dose of estrogen are not available.37 Patients should keep a menstrual calendar and take a pregnancy test if a menstrual period is late. Pregnancy may occur while a woman is taking estrogen and progestin therapy, and the therapy should be stopped if the pregnancy test is found to be positive. Oral contraceptives provide more steroid hormone than is needed for physiologic replacement and are therefore not recommended as first-line management.
Maintaining Bone Health
Women with primary ovarian insufficiency have reduced bone mineral density as compared with controls.38 Thus, bone mineral density should be measured, and women should be educated regarding strategies to maintain bone health. No data are available specifically for these women with regard to the recommended daily intake of calcium and of vitamin D and the recommended frequency and intensity of weight-bearing exercise, but it seems reasonable to follow the guidelines developed for perimenopausal and postmenopausal women by the North American Menopause Society: intake of 1200 mg of elemental calcium per day and maintenance of adequate vitamin D status, which is defined as a serum 25-hydroxyvitamin D level of 30 ng per milliliter (75 nmol per liter) or higher.39 Vitamin D deficiency is common, and it has been recommended that adults with inadequate exposure to the sun take at least 800 to 1000 IU of vitamin D3 per day.40 Women should be encouraged to engage in a variety of exercises, such as jogging, walking, and stair climbing, along with resistance exercises. 41 Bisphosphonates are not advised if pregnancy is possible, since these agents have long skeletal half-lives and the effects on the fetus are uncertain.42
Associated Disorders
There is a 50% risk of the development of adrenal insufficiency in women with adrenal autoimmunity. 43 Patients with positive tests for adrenal antibodies should be evaluated annually with the use of a corticotropin stimulation test. Longitudinal data are lacking to guide the optimal follow-up for patients with negative tests for adrenal antibodies at the initial examination. Theoretically, one would expect adrenal-cell antibodies to be present when the ovarian insufficiency develops if the mechanism is steroidogenic cell autoimmunity. If both tests for adrenal autoimmunity, as measured by indirect immunofluorescence and 21-hydroxylase immunoprecipitation, are negative at the initial examination, a reasonable strategy is not to repeat the testing unless it is otherwise clinically indicated. However, all patients with primary ovarian insufficiency should be educated regarding the symptoms of adrenal insufficiency and should undergo evaluation of adrenal function if such symptoms develop.
There is an increased incidence of the dry-eye syndrome and ocular-surface disease in women with primary ovarian insufficiency, as compared with controls (20% vs. 3%), and women who have either of these disorders benefit from referral to an ophthalmologist.18 Thyroid autoimmune disease, most commonly Hashimoto’s thyroiditis, is present in 14 to 27% of women19,44 at initial diagnosis. It is reasonable to measure thyrotropin levels and test for the presence of thyroid peroxidase antibodies. Other autoimmune disorders, such as myasthenia gravis, rheumatoid arthritis, and systemic lupus erythematosus, have also been reported in association with primary ovarian insufficiency, 19 but such cases are infrequent, and testing for these and other autoimmune conditions should be predicated on symptoms and signs that are suggestive of the condition.
Family Planning
Patients who wish to avoid pregnancy should use a barrier method or possibly an intrauterine device. The effectiveness of oral contraceptives has not been studied in women with primary ovarian insufficiency, and there are anecdotal reports of women who have conceived while complying with the oral contraceptive regimen,45 perhaps because of a failure of the oral contraceptive to suppress the high FSH levels that are characteristic of this condition.
Patients should understand that spontaneous remission resulting in pregnancy occurs in 5 to 10% of cases.8 Generally, remissions are temporary, but they may (although rarely) last for years.5 Currently, there are no known markers that are associated with an increased rate of remission, and there are no therapies that have been shown to restore ovarian function and fertility. Some couples are averse to adoption and to reproductive technologies and are content not to become parents or to accept the low but real chance that the infertility will resolve spontaneously. For couples who decide to pursue parenthood actively, the options are adoption, foster parenthood, egg donation, and embryo donation; ovarian transplantation has been performed in rare cases in which the patient has an identical twin with normal ovarian function.46 There is no medical urgency to proceed to egg donation, because the rates of pregnancy with egg donation appear to be similar among older and younger women.47 Women with primary ovarian insufficiency who become pregnant as a result of oocyte donation may have an increased risk of delivering infants who are small for gestational age and of having pregnancy-induced hypertension and postpartum hemorrhage,48–50 but these findings are controversial. 51
AREAS OF UNCERTAINTY
Studies involving women with the FMR1 premutation have established that this mechanism of primary ovarian insufficiency is associated with a clinical spectrum of impaired ovarian function that involves a continuum of occult, biochemical, and overt ovarian insufficiency; a better understanding is needed of the spectrum of disease associated with other causes of primary ovarian insufficiency (Table 3).3,52–54 In addition, research is needed on strategies to improve fertility for women who have follicles remaining in the ovary. The magnitude of long-term risks associated with the disorder (including cardiovascular disease and osteoporosis) and the optimal means of reducing these risks are uncertain.
Table 3.
Clinical States Included in the Spectrum of Primary Ovarian Insufficiency.*
| Clinical State | Serum FSH Level | Fertility | Menses |
|---|---|---|---|
| Normal | Normal | Normal | Regular |
| Occult | Normal | Reduced | Regular |
| Biochemical | Elevated | Reduced | Regular |
| Overt | Elevated | Reduced | Irregular or absent |
GUIDELINES
Several professional organizations recommend that women with primary ovarian insufficiency undergo testing for a premutation in the FMR1 gene.55–57 The American Society for Reproductive Medicine and the International Menopause Society recommend estrogen-replacement therapy for women with primary ovarian insufficiency.14,58
CONCLUSIONS AND RECOMMENDATIONS
The woman in the vignette has amenorrhea and a menopausal FSH level. Confirmation of the elevated FSH level and a low estradiol level would confirm the diagnosis of primary ovarian insufficiency. This information is highly emotionally charged and should be discussed with the patient at a return visit to the office rather than by telephone, with recognition of the emotional effect of the diagnosis. Patients should understand that remission may occur and that pregnancy, though unlikely, occurs in 5 to 10% of cases. A karyotype analysis, tests for the FMR1 premutation and adrenal autoimmunity, a pelvic ultrasound examination, and measurement of bone mineral density are indicated at the time of diagnosis. Women with primary ovarian insufficiency should be encouraged to maintain a lifestyle that optimizes bone and cardiovascular health, including engaging in regular weight-bearing exercise, maintaining an adequate intake of calcium (1200 mg daily) and vitamin D (at least 800 IU daily), eating a healthy diet to avoid obesity, and undergoing screening for cardiovascular risk factors, with treatment of any identified risk factors. Although there are no data from randomized trials to guide the use of hormonal therapy in women with this condition, a reasonable regimen would be 100 μg of transdermal estradiol and 10 mg of oral medroxyprogesterone acetate daily for the first 12 days of each month. Women should keep a menstrual calendar and have a pregnancy test promptly in the case of late menses.
Supplementary Material
Acknowledgments
I thank my colleague Mary Ryan, M.L.S., for literature searches and helpful discussions and Marcy Lash, M.D., for reviewing the manuscript before submission.
Footnotes
Dr. Nelson reports being an inventor on three United States patents directed to MATER (a potential antigen in autoimmune primary ovarian insufficiency) and its applications (U.S. patent numbers 7,189,812; 7,217,811; and 7,432,067), as well as one pending United States patent application (U.S. patent application number 11/586,160) and foreign counterparts. No other potential conflict of interest relevant to this article was reported.
References
- 1.van Noord PA, Dubas JS, Dorland M, Boersma H, te Velde E. Age at natural menopause in a population-based screening cohort: the role of menarche, fecundity, and lifestyle factors. Fertil Steril. 1997;68:95–102. doi: 10.1016/s0015-0282(97)81482-3. [DOI] [PubMed] [Google Scholar]
- 2.Albright F, Smith PH, Fraser R. A syndrome characterized by primary ovarian insufficiency and decreased stature: report of 11 cases with a digression on hormonal control of axillary and pubic hair. Am J Med Sci. 1942;204:625–48. [Google Scholar]
- 3.Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 2008;68:499–509. doi: 10.1111/j.1365-2265.2007.03073.x. [DOI] [PubMed] [Google Scholar]
- 4.Rebar RW, Connolly HV. Clinical features of young women with hypergonadotropic amenorrhea. Fertil Steril. 1990;53:804–10. [PubMed] [Google Scholar]
- 5.Nelson LM, Anasti JN, Flack MR. Premature ovarian failure. In: Adashi EY, Rock JA, Rosenwaks Z, editors. Reproductive endocrinology, surgery, and technology. Philadelphia: Lippincott–Raven; 1996. pp. 1393–410. [Google Scholar]
- 6.Rebar RW, Erickson GF, Yen SSC. Idiopathic premature ovarian failure: clinical and endocrine characteristics. Fertil Steril. 1982;37:35–41. [PubMed] [Google Scholar]
- 7.Nelson LM, Anasti JN, Kimzey LM, et al. Development of luteinized Graafian follicles in patients with karyotypically normal spontaneous premature ovarian failure. J Clin Endocrinol Metab. 1994;79:1470–5. doi: 10.1210/jcem.79.5.7962345. [DOI] [PubMed] [Google Scholar]
- 8.van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5:483–92. doi: 10.1093/humupd/5.5.483. [DOI] [PubMed] [Google Scholar]
- 9.Bakalov VK, Anasti JN, Calis KA, et al. Autoimmune oophoritis as a mechanism of follicular dysfunction in women with 46,XX spontaneous premature ovarian failure. Fertil Steril. 2005;84:958–65. doi: 10.1016/j.fertnstert.2005.04.060. [DOI] [PubMed] [Google Scholar]
- 10.Aittomäki K, Lucena JLD, Pakarinen P, et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82:959–68. doi: 10.1016/0092-8674(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 11.Wolfsdorf JI, Rosenfield RL, Fang VS, Kobayashi R, Razdan AK, Kim MH. Partial gonadotrophin-resistance in pseudo-hypoparathyroidism. Acta Endocrinol (Copenh) 1978;88:321–8. doi: 10.1530/acta.0.0880321. [DOI] [PubMed] [Google Scholar]
- 12.Koh JM, Kim CH, Hong SK, et al. Primary ovarian failure caused by a solvent containing 2-bromopropane. Eur J Endocrinol. 1998;138:554–6. doi: 10.1530/eje.0.1380554. [DOI] [PubMed] [Google Scholar]
- 13.Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–6. [PubMed] [Google Scholar]
- 14.Practice Committee of the American Society for Reproductive Medicine. Current evaluation of amenorrhea. Fertil Steril. 2004;82(Suppl 1):S33–S39. doi: 10.1016/j.fertnstert.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Stazi AV, Mantovani A. A risk factor for female fertility and pregnancy: celiac disease. Gynecol Endocrinol. 2000;14:454–63. doi: 10.3109/09513590009167719. [DOI] [PubMed] [Google Scholar]
- 16.Nelson LM, Kimzey LM, White BJ, Merriam GR. Gonadotropin suppression for the treatment of karyotypically normal spontaneous premature ovarian failure: a controlled trial. Fertil Steril. 1992;57:50–5. doi: 10.1016/s0015-0282(16)54775-x. [DOI] [PubMed] [Google Scholar]
- 17.van Kasteren YM, Hundscheid RD, Smits AP, Cremers FP, van Zonneveld P, Braat DD. Familial idiopathic premature ovarian failure: an overrated and underestimated genetic disease? Hum Reprod. 1999;14:2455–9. doi: 10.1093/humrep/14.10.2455. [DOI] [PubMed] [Google Scholar]
- 18.Smith JA, Vitale S, Reed GF, et al. Dry eye signs and symptoms in women with premature ovarian failure. Arch Ophthalmol. 2004;122:151–6. doi: 10.1001/archopht.122.2.151. [DOI] [PubMed] [Google Scholar]
- 19.Hoek A, Schoemaker J, Drexhage HA. Premature ovarian failure and ovarian autoimmunity. Endocr Rev. 1997;18:107–34. doi: 10.1210/edrv.18.1.0291. [DOI] [PubMed] [Google Scholar]
- 20.Wittenberger MD, Hagerman RJ, Sherman SL, et al. The FMR1 premutation and reproduction. Fertil Steril. 2007;87:456–65. doi: 10.1016/j.fertnstert.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Novosad JA, Kalantaridou SN, Tong ZB, Nelson LM. Ovarian antibodies as detected by indirect immunofluorescence are unreliable in the diagnosis of autoimmune premature ovarian failure: a controlled evaluation. BMC Womens Health. 2003;3:2. doi: 10.1186/1472-6874-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greil AL. Infertility and psychological distress: a critical review of the literature. Soc Sci Med. 1997;45:1679–704. doi: 10.1016/s0277-9536(97)00102-0. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt PJ, Cardoso GM, Ross JL, Haq N, Rubinow DR, Bondy CA. Shyness, social anxiety, and impaired self-esteem in Turner syndrome and premature ovarian failure. JAMA. 2006;295:1374–6. doi: 10.1001/jama.295.12.1374. [DOI] [PubMed] [Google Scholar]
- 24.Orshan SA, Ventura JL, Covington SN, Vanderhoof VH, Troendle JF, Nelson LM. Women with spontaneous 46,XX primary ovarian insufficiency (hypergonadotropic hypogonadism) have lower perceived social support than control women. Fertil Steril. 2008 September 29; doi: 10.1016/j.fertnstert.2008.07.1718. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groff AA, Covington SN, Halverson LR, et al. Assessing the emotional needs of women with spontaneous premature ovarian failure. Fertil Steril. 2005;83:1734–41. doi: 10.1016/j.fertnstert.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause. 2007;14:567–71. doi: 10.1097/gme.0b013e31804c793d. [DOI] [PubMed] [Google Scholar]
- 27.Jacobsen BK, Knutsen SF, Fraser GE. Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist Health Study. J Clin Epidemiol. 1999;52:303–7. doi: 10.1016/s0895-4356(98)00170-x. [DOI] [PubMed] [Google Scholar]
- 28.de Kleijn MJ, van der Schouw YT, Verbeek AL, Peeters PH, Banga JD, van der Graaf Y. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002;155:339–45. doi: 10.1093/aje/155.4.339. [DOI] [PubMed] [Google Scholar]
- 29.Mondul AM, Rodriguez C, Jacobs EJ, Calle EE. Age at natural menopause and cause-specific mortality. Am J Epidemiol. 2005;162:1089–97. doi: 10.1093/aje/kwi324. [DOI] [PubMed] [Google Scholar]
- 30.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 31.Mishell DR, Jr, Nakamura RM, Crosignani PG, et al. Serum gonadotropin and steroid patterns during the normal menstrual cycle. Am J Obstet Gynecol. 1971;111:60–5. doi: 10.1016/0002-9378(71)90927-6. [DOI] [PubMed] [Google Scholar]
- 32.Scarabin PY, Alhenc-Gelas M, Plu-Bureau G, Taisne P, Agher R, Aiach M. Effects of oral and transdermal estrogen/progesterone regimens on blood coagulation and fibrinolysis in postmenopausal women: a randomized controlled trial. Arterioscler Thromb Vasc Biol. 1997;17:3071–8. doi: 10.1161/01.atv.17.11.3071. [DOI] [PubMed] [Google Scholar]
- 33.Scarabin PY, Oger E, Plu-Bureau G. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362:428–32. doi: 10.1016/S0140-6736(03)14066-4. [DOI] [PubMed] [Google Scholar]
- 34.Canonico M, Oger E, Plu-Bureau G, et al. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840–5. doi: 10.1161/CIRCULATIONAHA.106.642280. [DOI] [PubMed] [Google Scholar]
- 35.Gibbons WE, Moyer DL, Lobo RA, Roy S, Mishell DR., Jr Biochemical and histologic effects of sequential estrogen/progestin therapy on the endometrium of postmenopausal women. Am J Obstet Gynecol. 1986;154:456–61. doi: 10.1016/0002-9378(86)90690-3. [DOI] [PubMed] [Google Scholar]
- 36.Bjarnason K, Cerin A, Lindgren R, Weber T. Adverse endometrial effects during long cycle hormone replacement therapy. Maturitas. 1999;32:161–70. doi: 10.1016/s0378-5122(99)00033-x. [DOI] [PubMed] [Google Scholar]
- 37.PEPI Group. Effects of hormone replacement therapy on endometrial histology in postmenopausal women: the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275:370–5. doi: 10.1001/jama.1996.03530290040035. [DOI] [PubMed] [Google Scholar]
- 38.Anasti JN, Kalantaridou SN, Kimzey LM, Defensor RA, Nelson LM. Bone loss in young women with karyotypically normal spontaneous premature ovarian failure. Obstet Gynecol. 1998;91:12–5. doi: 10.1016/s0029-7844(97)00583-8. [DOI] [PubMed] [Google Scholar]
- 39.North American Menopause Society. The role of calcium in peri- and postmenopausal women: 2006 position statement of the North American Menopause Society. Menopause. 2006;13:862–77. doi: 10.1097/01.gme.0000243566.25205.0b. [DOI] [PubMed] [Google Scholar]
- 40.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 41.Martyn-St James M, Carroll S. A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sports Med. 2008 November 3; doi: 10.1136/bjsm.2008.052704. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 42.Drake MT, Clarke BL, Khosla S. Bis-phosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032–45. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Betterle C, Volpato M, Rees Smith B, et al. I. Adrenal cortex and steroid 21-hydroxylase autoantibodies in adult patients with organ-specific autoimmune diseases: markers of low progression to clinical Addison’s disease. J Clin Endocrinol Metab. 1997;82:932–8. doi: 10.1210/jcem.82.3.3819. [DOI] [PubMed] [Google Scholar]
- 44.Kim TJ, Anasti JN, Flack MR, Kimzey LM, Defensor RA, Nelson LM. Routine endocrine screening for patients with karyotypically normal spontaneous premature ovarian failure. Obstet Gynecol. 1997;89:777–9. doi: 10.1016/s0029-7844(97)00077-x. [DOI] [PubMed] [Google Scholar]
- 45.Alper MM, Jolly EE, Garner PR. Pregnancies after premature ovarian failure. Obstet Gynecol. 1986;67(Suppl):59S–62S. doi: 10.1097/00006250-198603001-00018. [DOI] [PubMed] [Google Scholar]
- 46.Silber SJ, Grudzinskas G, Gosden RG. Successful pregnancy after microsurgical transplantation of intact ovary. N Engl J Med. 2008;359:2617–8. doi: 10.1056/NEJMc0804321. [DOI] [PubMed] [Google Scholar]
- 47.Sauer MV, Paulson RJ, Lobo RA. Reversing the natural decline in human fertility: an extended clinical trial of oocyte donation to women of advanced reproductive age. JAMA. 1992;268:1275–9. doi: 10.1001/jama.268.10.1275. [Erratum, JAMA 1993;269:476.] [DOI] [PubMed] [Google Scholar]
- 48.Abdalla HI, Billett A, Kan AK, et al. Obstetric outcome in 232 ovum donation pregnancies. Br J Obstet Gynaecol. 1998;105:332–7. doi: 10.1111/j.1471-0528.1998.tb10096.x. [DOI] [PubMed] [Google Scholar]
- 49.Söderström-Anttila V, Tiitinen A, Foudila T, Hovatta O. Obstetric and perinatal outcome after oocyte donation: comparison with in-vitro fertilization pregnancies. Hum Reprod. 1998;13:483–90. doi: 10.1093/humrep/13.2.483. [DOI] [PubMed] [Google Scholar]
- 50.Salha O, Sharma V, Dada T, et al. The influence of donated gametes on the incidence of hypertensive disorders of pregnancy. Hum Reprod. 1999;14:2268–73. doi: 10.1093/humrep/14.9.2268. [DOI] [PubMed] [Google Scholar]
- 51.Krieg SA, Henne MB, Westphal LM. Obstetric outcomes in donor oocyte pregnancies compared with advanced maternal age in in vitro fertilization pregnancies. Fertil Steril. 2008;90:65–70. doi: 10.1016/j.fertnstert.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 52.The FMR1 premutation and premature ovarian failure: worldwide community guideline development. New Orleans: October 26, 2006. [Accessed January 12, 2009]. http://fmr1pof.com/pdf/fmr1_meeting_guidelines.pdf. [Google Scholar]
- 53.Platteau P, Sermon K, Seneca S, Van Steirteghem A, Devroey P, Liebaers I. Preimplantation genetic diagnosis for fragile Xa syndrome: difficult but not impossible. Hum Reprod. 2002;17:2807–12. doi: 10.1093/humrep/17.11.2807. [DOI] [PubMed] [Google Scholar]
- 54.Welt CK, Smith PC, Taylor AE. Evidence of early ovarian aging in fragile X premutation carriers. J Clin Endocrinol Metab. 2004;89:4569–74. doi: 10.1210/jc.2004-0347. [DOI] [PubMed] [Google Scholar]
- 55.McConkie-Rosell A, Finucane B, Cronister A, Abrams L, Bennett RL, Pettersen BJ. Genetic counseling for fragile X syndrome: updated recommendations of the National Society of Genetic Counselors. J Genet Couns. 2005;14:249–70. doi: 10.1007/s10897-005-4802-x. [DOI] [PubMed] [Google Scholar]
- 56.Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: diagnostic and carrier testing. Genet Med. 2005;7:584–7. doi: 10.1097/01.GIM.0000182468.22666.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.American College of Obstetricians and Gynecologists Committee on Genetics. ACOG committee opinion. No. 338: screening for fragile X syndrome. Obstet Gynecol. 2006;107:1483–5. doi: 10.1097/00006250-200606000-00059. [DOI] [PubMed] [Google Scholar]
- 58.Pines A, Sturdee DW, Birkhäuser MH, Schneider HP, Gambacciani M, Panay N. IMS updated recommendations on postmenopausal hormone therapy. Climacteric. 2007;10:181–94. doi: 10.1080/13697130701361657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


