Abstract
We have previously reported that a single injection of endotoxin, lipopolysaccharide (LPS, 5 mg/kg, i.p.), causes a delayed and progressive loss of TH-IR neurons in the substantia nigra (SN) in C57BL/six male mice. In this study, we determined sex differences and behavioral deficits accompanying the loss of TH-IR neurons in response to peripheral LPS injection. A single injection of LPS (5 mg/kg, i.p.) failed to produce any loss of TH-IR neurons in the SN of female mice over a 12-month period. To determine if multiple-injections were required, female mice received five injections of LPS (5 mg/kg, i.p.) at either weekly or monthly intervals. Behavioral motor ability and TH-IR neuronal loss were determined after the first injection of LPS. We found significant differences in both behavioral activities and neuronal loss between these two injection paradigms. Between 7 and 20 months after the first injection of LPS, progressive behavioral changes, measured by rotor-rod and open-field activities, and neuronal loss in SN were observed in monthly injected, but not in weekly injected mice. In addition, reduced rotor-rod ability in monthly injected mice were restored following treatment of l-dopa/carbidopa (30 mg/3 mg/kg), i.p.). Approximately 40 and 50% loss of TH-IR neurons at 9 and 20 months, respectively, was observed after exposure to LPS, suggesting that the behavioral deficit is related to loss of dopamine function in the nigrastriatal pathway. More intense immuno-staining of α-synuclein and inflammatory markers were detected in brain sections exposed to LPS. In conclusion, these results show that multi-LPS monthly injections can induce a delayed and progressive loss of TH-IR neurons andmotor deficits which resemble the progressive nature of Parkinson’s disease. Further, the present study reveals a clear sex difference: female mice are more resistant to LPS than male mice. Repeated monthly LPS injections are required to cause both motor behavioral deficits and DA neuronal loss in female mice.
Keywords: Lipopolysaccharide, Neurodegeneration, Parkinson’s disease, TH-IR neurons, Rotarod test
1. Introduction
The hallmark of Parkinson’s disease (PD) is characterized by a progressive loss of dopaminergic (DA) neurons in the substantia nigra (SN). The symptoms of PD include resting tremor, stiffness or rigidity, bradykinesia (Jellinger, 2001; Shulman, 2007). The etiology of PD as well as the precise mechanism underlying the progressive loss of DA neurons has remained unknown. So far, numerous rodent PD models ranging from 6-hydroxydopamine, MPTP, pesticides (Betarbet et al., 2002; Shimohama et al., 2003) such as rotenone (Betarbet et al., 2000; Testa et al., 2005) and paraquat (Dinis-Oliveira et al., 2006) to endotxoins (Ling et al., 2002) have been developed. These models reproduce some key features of PD, and are useful in studying the mechanism underlying the pathogenesis of PD. Few of these models address the progressive nature of the loss of DA neurons and motor function deficits.
To determine the role of microglia in inflammation-related neurodegeneration, we have used lipopolysaccaride (LPS), an endotoxin from the cell walls of gram-negative bacteria, as a tool to create a rodent PD model which shows the characteristic of delayed and progressive nature of neurodegeneration. We previously reported that intra-nigral infusion of LPS through a mini-osmotic pump for 2 weeks produced a delayed, progressive and selective loss of nigral DA neurons(Gao et al.,2002). Our study shows that neurodegeneration is caused by the release of pro-inflammatory factors such as free radicals, cytokines from over-activated microglia by LPS (Gao et al., 2002). We recently reported that a single systemic injection of LPS produced a delayed (7 months after LPS injection) and progressive loss (7–10 months) of nigral DA neurons in mice (Qin et al., 2007). However, mechanistic studies revealed that neurotoxicity was not due to the direct effect of LPS, but rather the toxic effect was mediated through entry of pro-inflammatory cytokines such as TNFα from peripheral blood, which in turn triggers the activation of microglia in the brain. The end results of our systemic LPS study resemble in uterus exposure of developing fetuses to LPS, which results in lesions of nigro-striatal dopaminergic system in neonates (Ling et al., 2002, 2006). Results from the above-mentioned LPS rodent PD models indicate a critical role of inflammation in the delayed and progressive nature of the loss of DA neurons in PD.
In this study, we determined sex differences and behavioral deficits accompanying the loss of TH-IR neurons in response to peripheral LPS injection in mice. We found that female mice are less responsive to LPS injection and repeated injections of LPS were required to produce DA neuron loss in female mice. In addition, we found significant differences in both rotarod motor ability and neuronal loss depending on the injection time intervals. Progressive behavioral changes, measured by rotor-rod and open-field activities, and DA neuronal loss in SN were observed in monthly injected, but not in weekly injected mice.
2. Materials and methods
2.1. Animals
Animal studies were performed in accordance with National Institutes of Health Guidelines, approved by the Institute’s Animal Care and Use Committee, and NIH guidelines were followed.
Eight-week male and female C57BL/six (20–22 g) mice were purchased from Jackson Laboratories (Bar Harbor, ME). The animals were fed on a standard diet and tap water ad libitum for 2 weeks prior to experiments. Environmental conditions were standardized, including a room temperature of 21.8 °C and 12-h artificial lighting cycle.
2.2. Reagents
Lipopolysaccharide (strain O111:B4) was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Rabbit anti-tyrosine hydroxylase polyclonal antibody and rabbit polyclonal antibody against interleukin-6 were from Chemicon (Temecula, CA, USA). Rat anti-mouse CD45 was from Serotec Inc. (Raleigh, NC, USA). α-Synuclein mouse monoclonal antibody was from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). All other reagents came from Sigma Chemical Co. (St. Louis, MO, USA).
2.3. Injection of LPS and l-dopa/carbidopa
We injected three batches of C57BL/six mice. Batch 1, male and female mice were injected with a single dose of LPS (5 mg/kg, i.p.); Batch 2, female mice were weekly injected with five doses of LPS (5 mg/kg, i.p.); Batch 3, female mice were monthly injected with two to five doses of LPS (5 mg/kg, i.p.). At 12 months following the first LPS/saline administration in monthly LPS-injection group, the mice were administered saline (1 ml/kg, i.p.), or l-dopa/carbidopa (30 mg/3 mg/kg, i.p.).
2.4. Rotarod test
The rotorod apparatus (Rotamex, Columbus Instruments, Columbus, OH) was used to evaluate the ability of staying on the rotating rod. Mice were transported (within their home cage) to acclimate to the testing room for 1 h prior to testing started. The parameters of rotor-rod system include start speed, acceleration and highest speed (2 rpm, accelerate 2 rpm/5 s, 40 rpm). Each mouse was placed on the confined section of the rod, turn on the rotarod that begins to rotate with a smooth increase in speed from 2 rpm. Mice received three consecutive trails. The rest period between each trial was 2 min. The mean latency for the three trails was used for the analysis. The latency to fall was measured in seconds. In all trials, if the mouse did not fall from the rod, it was removed from the rod after 2 min.
2.5. Open-field test
Open-field measuring system (Opto-Max, Columbus Instruments, Columbus, OH) was used for measuring animal’s activity, this system can automatically record all activities of the animal. Mice were transported (within their home cage) to acclimate to the testing room for 1 h prior to testing started. Control and five-time monthly LPS injection animals were put in the separated testing field, and 30-min locomotor activity was automatically recorded. Endpoints for analysis are ambulatory activity, total activity, and traveled distance.
2.6. Immunohistochemistry
Mice were perfused transcardially with saline followed by 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4). The brains were removed, postfixed in 4% paraformaldehyde for 24 h, and then transferred to 30% sucrose in 0.1 MPBS for at least 24 h before sectioning for histochemical analysis, according to our standard protocol (Boger et al., 2006). Coronal sections through the striatum and SN (35 µm for TH and α-synuclein; 45 µm for CD45 and IL-6) were cut and processed for free-floating immunohistochemistry using a rabbit polyclonal antibody against tyrosine hydroxylase (TH; 1:1000), a rat anti-mouse antibody against the inflammatory MHC Class II-induced cluster differentiation marker, CD45 (1:1000), a rabbit polyclonal antibody against interleukin-6 (1:10,000) and α-synuclein mouse monoclonal antibody (1:100). Immunodetection was performed using the avidin–biotin–immunoperoxidase method (Boger et al., 2006; Granholm et al., 1997a,b). Briefly, after a 5-min pretreatment with 2% Triton-X to allow penetration into the tissue, a subset of sections from each mouse was incubated in the primary antisera for 24 h at 4 °C. The sections were then rinsed and incubated for 1 h in biotin-conjugated goat anti-rabbit (TH and IL-6) IgG or goat anti-rat (CD45) IgG, or goat anti-mouse (α-synuclein) IgG, rinsed, and incubated for 1 h with avidin–biotin–peroxidase reagents (Elite Vectastain kit, Vector Labs, Burlingame, CA). Diaminobenzidine (DAB, Sigma) was used as a chromagen to develop the reaction using 0.05% of 3% H2O2 and nickel ammonium sulfate (2.5%, Sigma) was used to enhance the reaction. Each of the above steps was separated by 3× 10 min washes in PBS. Sections were mounted on glass slides and coverslipped with DPX.
2.7. Analysis of neurotoxicity
The loss of dopaminergic neurons was assessed by counting the number of TH-IR neurons following immunostaining of brain sections. Twenty-four consecutive brain slices (35 µm), which encompassed the entire SNc, were collected. A normal distribution of the number of TH-IR neurons in the SNc was constructed based on the counts of 24 slices from C57BL/six mice. Eight evenly spaced brain slices from saline or LPS-injected animals were immunos-tained with an antibody against TH and counted. The distribution of the cell numbers from each animal was matched with the normal distribution curve to correct for errors resulting from the cutting. Samples were counted in a double-blind manner with stereology equipment, three individuals, and the CAST system. Data were expressed as percent loss compared to saline-injected controls. Conclusions were drawn only when the difference was within 5%.
2.8. Statistical analysis
The data are expressed as the mean ± S.E.M. and statistical significance was assessed with an ANOVA followed by Bonferroni’s t-test. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Sex differences in TH-IR neuronal loss after a single LPS injection
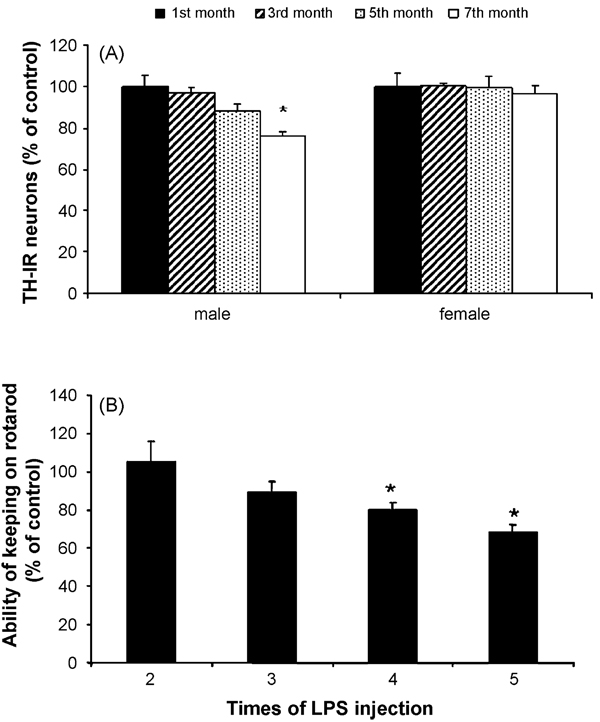
Male or female C57BL/six mice were treated with saline or LPS (5 mg/kg, i.p.) and then sacrificed at the indicated time points. Brain sections (35 µm) were cut through the SN and numbers of TH-IR neurons in the SN were counted. Consistent with our recent report (Qin et al., 2007), the TH-IR neurons decrease at 5 months, and significant loss of TH-IR neurons was observed at 7 months after a systemic single injection of LPS in male mice. In contrast, similar treatment with LPS failed to produce any loss of DA neurons in female mice (Fig. 1A). Thus, further experiments were performed to determine if repeated LPS injections can induce loss of TH-IR neurons or motor deficits in female mice. Two to five consecutive LPS (5 mg/kg, i.p.) or saline injections were given to female mice at monthly intervals and motor deficits by rotarod test were measured at 7 months after the first injection. Fig. 1B shows that two or three injections of LPS failed to alter the duration for mice to stay on the spinning rod, whereas significant decreases in the rotarod ability were observed in mice received four to five LPS injections compared with the corresponding saline-injected controls (Fig. 1B).
Fig. 1.
The different response to LPS injection between male and female mice. Male or female C57BL/six mice were treated with saline or LPS (5 mg/kg, i.p.) and then maintained under normal conditions for the indicated time points. (A) Number of TH-IR neurons in the SN of control and LPS-treated mice at different time points. Single-injected mice (male and female) were sacrificed and brain sections (35 µm) were cut through the nigral complex. After immunostaining with TH antibody, the number of TH-IR neurons in the SN was counted as described in Section 2. Results are expressed as percentage of the corresponding saline controls. (B) Multi-injected (once a month) female mice were made rotarod test at 7 months after the first injection. *P < 0.05 and **P < 0.01 were compared to the saline controls (n = 5–10 per group).
3.2. Motor behavioral changes after repeated LPS injections
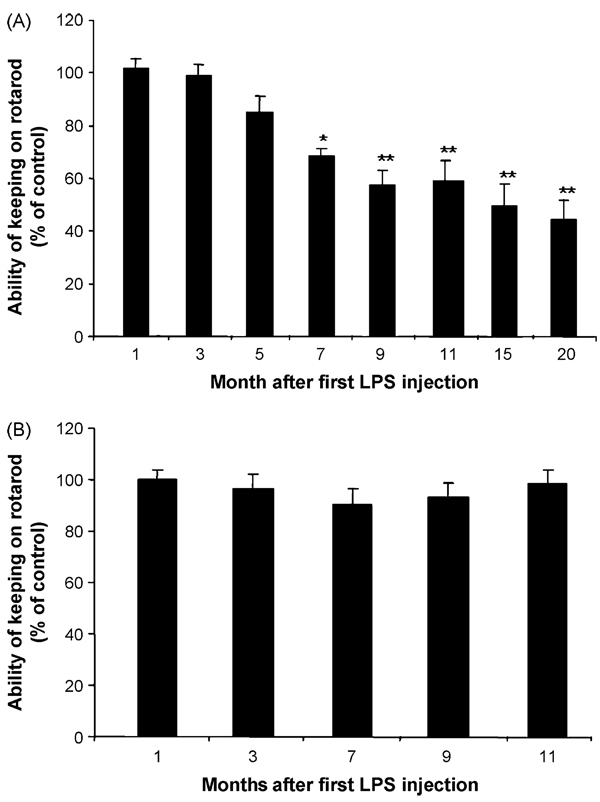
Because four or five repeated LPS injections were required to produce motor function deficits in female mice, another comparison by giving five repeated injections of LPS at either monthly or weekly intervals was performed. The rationale for this study was due to the fact that repeated LPS administration is known to cause tolerance. In monthly LPS-injected mice, rotarod test showed delayed and progressive decreases in the duration in staying on the spinning rod. One to 5 months after the first injection of LPS, there was no significant decrease in the rotarod ability. However, progressive decreases in this motor function were observed 7–20 months after the first injection of LPS (Fig. 2A). In contrast, mice receiving five-repeated weekly injections of LPS failed to show any change in rotarod ability compared with the saline-injected group (Fig. 2B). These data indicate that the time interval between LPS injections is critical for the manifestation of motor behavioral deficits. In addition to rotarod ability measurement, we also determined the locomotor activities in mice 9 months after the first LPS injections. Fig. 3 shows that total open-field activity, ambulatory activity and distance traveled decreased in LPS-treated mice compared with saline-treated controls (Fig. 3).
Fig. 2.
The latency comparison between the monthly and weekly LPS injections in female mice on the rotarod test. The female C57BL/six mice were injected with saline or LPS (5 mg/kg, i.p.) weekly or monthly up to five times. Mice were maintained under normal conditions, and tested on rotarod at different time points: (A) monthly injection group; (B) weekly injection group. **P < 0.01 compared to the saline controls.
Fig. 3.
Decreased locomotor behavior in five monthly LPS-injected mice. Female C57BL/six mice were injected with saline or LPS (5 mg/kg, i.p.) monthly up to five times, maintained under normal conditions, open-field test was performed on the 9th month after the first injection. Total activity, ambulatory activity and distance traveled were recorded in the open-field test. *P < 0.05 compared to the saline controls.
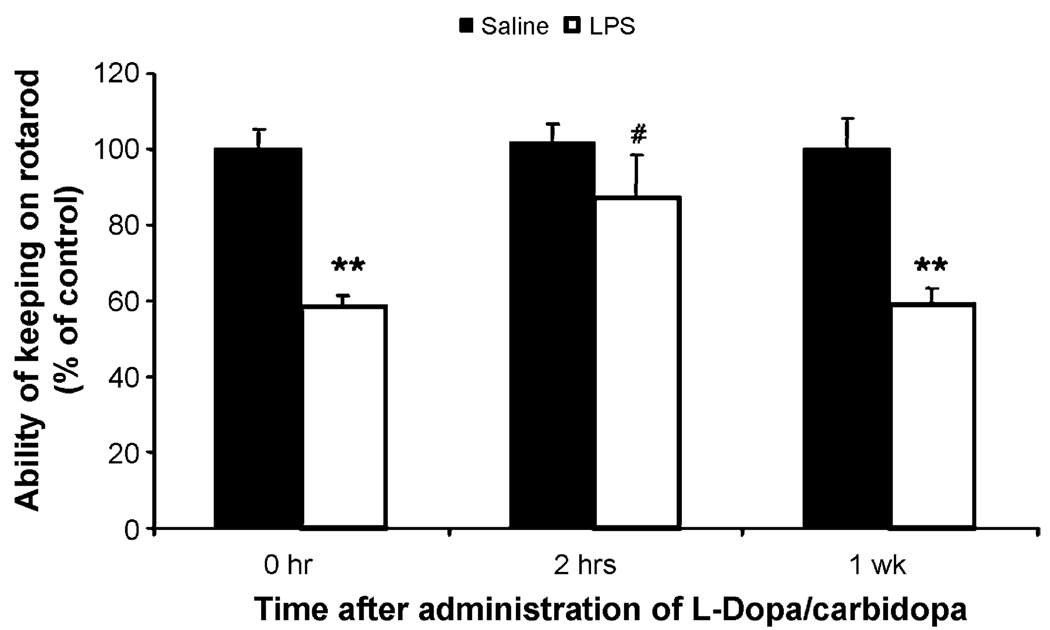
To determine if the loss of motor function in LPS-injected mice was related to impairment in the nigal–striatal dopamine activity, dopamine replacement experiment with l-dopa/carbidopa treatment was conducted. Twelve months after the first LPS injections in five monthly regimen of LPS treatment l-dopa/carbidopa (30 mg/3 mg/kg, i.p.) or saline were administered. Two hours and 1 week after the injection, rotarod ability were measured. Fig. 4 shows that l-dopa/carbidopa treatment restored LPS-induced deficits in rotarod ability at 2 h after the injection. However, 1 week later, the effect of l-dopa/carbidopa was not observed. Results from this replacement experiment suggest that LPS-induced loss of rotarod ability is related to the deficiency in dopamine function in the nigal–striatal pathway. This speculation is supported by the findings that LPS treatment caused progressive loss of nigral DA neurons (see below).
Fig. 4.
l-dopa/carbidopa administration can reverse the reduction of rotarod motor ability. Mice injected with five monthly saline and LPS were administed l-dopa/ carbidopa (30 mg/3 mg/kg, i.p.) 12 months following the first saline or LPS treatment. Rotarod testing was done at 0 h, 2 h and 1 week after l-dopa/carbidopa injection. **P < 0.01 compared to the saline controls. #P < 0.05 compared to the same group at 0 h or 1 week after l-dopa/carbidopa administration.
3.3. Progressive loss of nigral DA neurons after repeated LPS injections
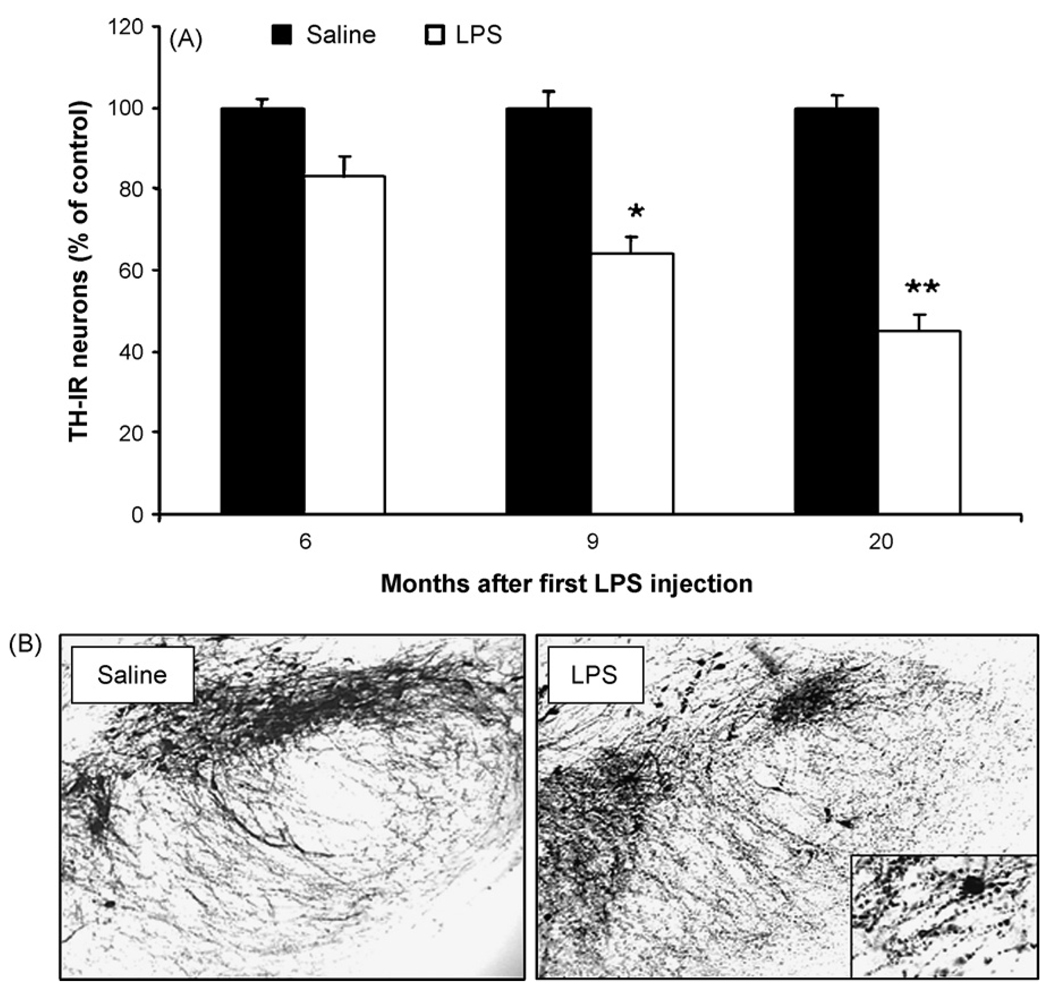
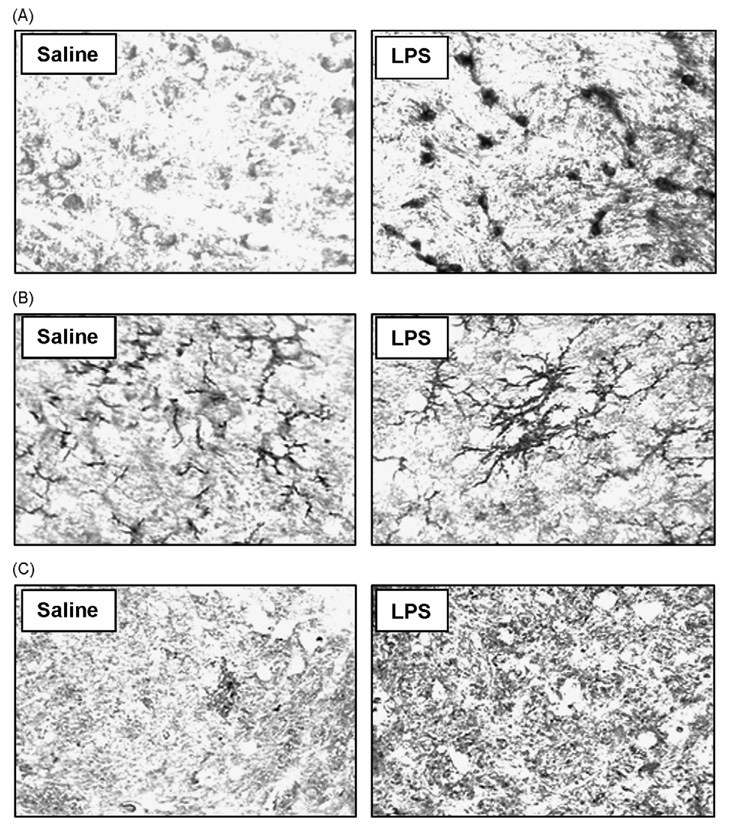
To determine the nature of the nigral DA neuronal loss after five monthly repeated LPS injections, both LPS- and saline-injected mice were sacrificed at different time points after the first injection and TH-IR cell counts were performed. A 37% loss of TH-IR neurons in SNpc was found in the LPS group compared with the vehicle (saline) group at the 9-month time point ( p < 0.05; Fig. 5A). The cell loss was progressive and reached 55% loss at 20 months ( p < 0.001; Fig. 5A). Compared with the saline-injected control mice, five monthly repeated LPS injections induced a significant decrease in the TH immunoreactivity in the neuronal cell bodies and neuronal fibers in the SNpc (Fig. 5B). It is obvious from the micrographs presented in Fig. 5B that there was also a significant degeneratative morphological alteration in the SN neurons and neurites (insert in Fig. 5B). Morphological observation showed greatly enhanced immunostaining of α-synuclein in the LPS-treated group than in the saline-treated group (Fig. 6A). In addition, immunostaining for CD45 and IL-6, both markers for activated microglia, was increased in mice 20 months after the first LPS treatment (Fig. 6B and C). Microglial cells had a branched appearance with increased reactive morphology in LPS-treated animals compared to controls (Fig. 6B). The IL-6 immunohistochemistry is difficult to quantify based on its wide-spread distribution, but future studies utilizing Western blots will determine the extent of IL-6 increase in this brain region following the LPS injections.
Fig. 5.
Delayed loss of nigral DA neurons following systemic LPS injection. Female C57BL/six mice were monthly administered saline or LPS (5 mg/kg, i.p.) for five times, 9 or 20 months after the first LPS injection, mice were sacrificed, brains were harvested and sectioned, and 24 sections (rostral to caudal: 4.52–5.36 mm posterior mm to bregma) were collected as described in methods. Eight evenly spaced brain sections from saline or LPS-injected animals were immunostained with an anti-TH antibody, and the number of TH-IR neurons in the SN was counted. Data were expressed as percent loss compared to saline-injected controls. (A) Number of TH-IR neurons in the SN in control and LPS-treated mice at 9 and 20 months. **P < 0.001, compared with the corresponding control group. (B) Visualization of TH-IR neurons in the substantia nigra and ventral tegmental area in saline and LPS-treated animals at 20 months.
Fig. 6.
Enhanced immunostaining of α-synuclein, CD45 and IL-6 in LPS-treated mice. Female C57BL/six mice were monthly administered saline or LPS (5 mg/kg, i.p.) for five times. At 20 months after the first saline or LPS treatment, brain sections were immunostained with α-synuclein, CD45 and IL-6 antibody as described in methods. Five monthly repeated LPS exposure significantly increased immunostaining of α-synuclein (A), CD45 (B) and IL-6 (C) compared with the saline control group.
4. Discussion
In this study, we have further characterized our previously reported neurodegeneration model after a systemic injection of LPS in mice (Qin et al., 2007). A clear sex difference was found: female mice were more resistant to LPS than male mice. Repeated LPS injections at monthly, but not weekly intervals were required to cause both motor behavioral deficits and DA neuronal loss. Time course studies revealed the time-dependent progressive nature of both rotarod ability and loss of DA neurons. Pharmacological challenge with l-dopa/carbidopa was able to restore the motor function deficits. Markers for α-synuclein, CD45, and IL-6 revealed that the LPS injections gave rise to more wide-spread effects on the nigral region, including both neuronal and glial correlates, and further suggested that the injections gave rise to a chronic increase in neuroinflammation to the nigral region.
A delayed and time-dependent loss of nigral dopaminergic neurons was found in male (Qin et al., 2007), but not in female mice (Fig. 1A), after a single systemic injection of LPS. This finding in sex differences is similar to the previous report indicating that male mice are more sensitive than female mice to MPTP-induced DA neuron degeneration (Miller et al., 1998). These findings from animal studies are in concert with the fact that males outnumber females in PD patients (Shulman, 2007). One of the factors underlying the sex difference in response to LPS is estrogen. It is well known that estrogen protects neurons against a vast variety of toxic insults (Maggi et al., 2004; Wise, 2002). Estrogen has been shown to suppress the LPS-induced activation of microglia by down-regulating inducible nitric oxide synthase (iNOS), nitric oxide (NO), prostaglandin-E2 (PGE2) and matrix metalloproteinase (MMP)-9 activation (Baker et al., 2004; Bruce-Keller et al., 2000; Drew and Chavis, 2000; Vegeto et al., 2000, 2001). Recently, Tenenbaum et al. (2007) reported that 17 beta-estradiol (E2) significantly reduced LPS-induced increase in NO and TNFα (but not PGE2) production in glial cells. Marotta et al. (2006) found that phytoestrogen treatment lowered levels of proinflammatory cytokines including TNFα, IL-β and IL-6 and a higher level of TGF-β. Since female mice are more resistant to LPS exposure, repeated injections of LPS are required to show behavioral deficits and neuronal damage, possibly implying a protective effect of estrogen or other female-specific factors on LPS-induced damages in vivo as well.
It is interesting to note the different responses in behavioral deficits and DA damage in weekly vs. monthly injected paradigms (Fig. 2). These differences can be related to endotoxin tolerance, a phenomenon whereby pre-exposure to endotoxin causes a reduced response to subsequent challenge with endotoxin (Buckley et al., 2006; Munoz et al., 1991; Setrakian et al., 1994). Tolerant animals dramatically reduced cytokine production, including TNFα, IL-1β following an LPS challenge (Cavaillon and Adib-Conquy, 2006). It is likely that LPS injections at weekly interval could elicit tolerance. Thus, in spite of repeated injections, nevertheless, the net result is equivalent to a single injection.
Consistent with our previous report that a single systemic injection of LPS elicited a time-dependent progressive loss of DA neurons in the SN (Qin et al., 2007), five monthly injection of LPS resulted in similar decrease in DA neurons. In this study, we demonstrated that the progressive loss of DA neurons was accompanied by a parallel decrease in rotarod ability in LPS-injected mice. These results suggested that the neurodegeneration of DA neurons is closely associated with the behavioral deficits. This notion was further supported by a pharmacological challenge study using l-dopa/carbidopa. The rotarod ability was restored in LPS-injected mice 2 h, but not 1 week, after l-dopa/carbidopa treatment (Fig. 4). Further, we found that the LPS injection gave rise to a long-term increase in α-synuclein immunoreactivity in the substantia nigra, further demonstrating a wide-spread reduction in DA function in these neurons. Thus, peripheral LPS injection produced a rodent PD model, which reproduced several important features in PD patients, such as a delayed progressive decrease in motor function, loss of DA neurons in the SN and temporal relief of motor deficits after l-dopa/carbidipa replacement therapy.
One of the salient features of systemic LPS PD model is the long-lasting effects of the motor deficits and permanent loss of DA neurons in the SN. Most of the above-mentioned phenomena were still observed 20 months after the first injection of LPS. These long-lasting effects in this model are different from MPTP model in mice, which is known to be reversible 9–12 months after the treatment (Hallman et al., 1985; Mitsumoto et al., 1998). As we previously reported (Qin et al., 2004, 2007), inflammation played a key role in LPS-induced neurodegeneration, which was mediated through cytokines, such as TNFα, produced by macrophages. The entry of these pro-inflammatory cytokines into brain further activates microglia to produce more pro-inflammatory factors, which continue to activate the same or neighboring microglia. Thus, we postulate that once brain inflammation reaches a critical point, due to the dysregulated over-activation of microglia either through the direct action of pro-inflammatory factors or indirect action of reactive microgliosis results in the damage of neurons, a vicious, self-propelling cycle occurs, which perpetuates the progression of neurodegeneration. Consistent with this hypothesis, we observed increase in immunoreactivity of α-synuclein, CD45 and IL-6 even at 20 months after the first LPS injection. In view of the increasing evidence indicating a critical role of inflammation in the pathogenesis of PD (Hong, 2005), results presented in this study suggest that the systemic LPS is a viable rodent PD model for studying the pathogenesis and therapy of neurodegenerative diseases.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Health, the National Institute of Environmental Health Sciences and a Program Project Grant from the National Institutes on Aging (AG023630).
References
- Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology. 2004;145:5021–5032. doi: 10.1210/en.2004-0619. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, Greenamyre JT. Animal models of Parkinson’s disease. Bioessays. 2002;24:308–318. doi: 10.1002/bies.10067. [DOI] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Huang P, Zaman V, Smith AC, Hoffer BJ, et al. A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia migra. Exp Neurol. 2006;202:336–347. doi: 10.1016/j.expneurol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141:3646–3656. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- Buckley JM, Wang JH, Redmond HP. Cellularreprogrammingbygram-positivebacterialcomponents:areview. J Leukoc Biol. 2006;80:731–741. doi: 10.1189/jlb.0506312. [DOI] [PubMed] [Google Scholar]
- Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10:233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinis-Oliveira RJ, Remiao F, Carmo H, Duarte JA, Navarro AS, Bastos ML, et al. Paraquat exposure as an etiological factor of Parkinson’s disease. Neurotoxicology. 2006;27:1110–1122. doi: 10.1016/j.neuro.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Drew PD, Chavis JA. Female sex steroids: effects upon microglial cell activation. J Neuroimmunol. 2000;111:77–85. doi: 10.1016/s0165-5728(00)00386-6. [DOI] [PubMed] [Google Scholar]
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Srivastava N, Bott JL, Henry S, Henry M, Westphal H, et al. Morphological alteration in the peripheral and central nervous systems of mice lacking glial cell line-derived neurotrophic factor (GDNF): immunohistochemical studies. J Neurosci. 1997a;17:1168–1178. doi: 10.1523/JNEUROSCI.17-03-01168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm AC, Mott JL, Bowenkamp K, Eken S, Henry S, Hoffer BJ, et al. Glial cell line-derived neurotrophic factor improves survival of ventral mescencephalic grafts to the 6-hydroxydopamine lesioned striatum. Exp Brain Res. 1997b;116:29–38. doi: 10.1007/pl00005741. [DOI] [PubMed] [Google Scholar]
- Hallman H, Lange J, Olson L, Stromberg I, Jonsson G. Neurochemical and histochemical characterization of neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on brain catecholamine neurones in the mouse. J Neurochem. 1985;44:117–127. doi: 10.1111/j.1471-4159.1985.tb07120.x. [DOI] [PubMed] [Google Scholar]
- Hong JS. Role of inflammation in the pathogenesis of Parkinson’s disease: models, mechanisms, and therapeutic interventions. Ann NY Acad Sci. 2005;1053:151–152. doi: 10.1196/annals.1344.054. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. The pathology of Parkinson’s disease. Adv Neurol. 2001;86:55–72. [PubMed] [Google Scholar]
- Ling Z, Gayle DA, Ma SY, Lipton JW, Tong CW, Hong JS, et al. In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain. Mov Disord. 2002;17:116–124. doi: 10.1002/mds.10078. [DOI] [PubMed] [Google Scholar]
- Ling Z, Zhu Y, Tong C, Snyder JA, Lipton JW, Carvey PM. Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp Neurol. 2006;199:499–512. doi: 10.1016/j.expneurol.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Maggi A, Ciana P, Belcredito S, Vegeto E. Estrogens in the nervous system: mechanisms and nonreproductive functions. Annu Rev Physiol. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- Marotta F, Mao GS, Liu T, Chui DH, Lorenzetti A, Xiao Y, et al. Anti-inflammatory and neuroprotective effect of a phytoestrogen compound on rat microglia. Ann NY Acad Sci. 2006;1089:276–281. doi: 10.1196/annals.1386.033. [DOI] [PubMed] [Google Scholar]
- Miller DB, Ali SF, O’Callaghan JP, Laws SC. The impact of gender and estrogen on striatal dopaminergic neurotoxicity. Ann NY Acad Sci. 1998;844:153–165. [PubMed] [Google Scholar]
- Mitsumoto Y, Watanabe A, Mori A, Koga N. Spontaneous regeneration of nigrostriatal dopaminergic neurons in MPTP-treated C57BL/6 mice. Biochem Biophys Res Commun. 1998;248:660–663. doi: 10.1006/bbrc.1998.8986. [DOI] [PubMed] [Google Scholar]
- Munoz C, Carlet J, Fitting C, Misset B, Bleriot JP, Cavaillon JM. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, et al. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setrakian JC, Yee J, Christou NV. Reduced tumor necrosis factor alpha production in lipopolysaccharide-treated whole blood from patients in the intensive care unit. Arch Surg. 1994;129:187–192. doi: 10.1001/archsurg.1994.01420260083011. [DOI] [PubMed] [Google Scholar]
- Shimohama S, Sawada H, Kitamura Y, Taniguchi T. Disease model: Parkinson’s disease. Trends Mol Med. 2003;9:360–365. doi: 10.1016/s1471-4914(03)00117-5. [DOI] [PubMed] [Google Scholar]
- Shulman LM. Gender differences in Parkinson’s disease. Gend Med. 2007;4:8–18. doi: 10.1016/s1550-8579(07)80003-9. [DOI] [PubMed] [Google Scholar]
- Tenenbaum M, Azab AN, Kaplanski J. Effects of estrogen against LPS-induced inflammation and toxicity in primary rat glial and neuronal cultures. J Endotoxin Res. 2007;13:158–166. doi: 10.1177/0968051907080428. [DOI] [PubMed] [Google Scholar]
- Testa CM, Sherer TB, Greenamyre JT. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res Mol Brain Res. 2005;134:109–118. doi: 10.1016/j.molbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Pollio G, Ciana P, Maggi A. Estrogen blocks inducible nitric oxide synthase accumulation in LPS-activated microglia cells. Exp Gerontol. 2000;35:1309–1316. doi: 10.1016/s0531-5565(00)00161-3. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, et al. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci. 2001;21:1809–1818. doi: 10.1523/JNEUROSCI.21-06-01809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM. Estrogens and neuroprotection. Trends Endocrinol Metab. 2002;13:229–230. doi: 10.1016/s1043-2760(02)00611-2. [DOI] [PubMed] [Google Scholar]