Abstract
A Western-style diet (WD), defined by high-fat, low-calcium, and vitamin D content, is associated with increased risk of human colorectal cancer. Understanding molecular mechanisms altered by the WD is crucial to develop preventive and therapeutic strategies. Effects of a WD on the colonic transcriptome of C57Bl/6J mice, a model for sporadic colon cancer, were studied at endpoints before tumors occur. To assess whether a WD induces inflammatory changes, expression profiles of a broad spectrum of inflammatory proteins were performed and numbers of lamina propria macrophages were determined with semiquantitative morphometry. Transcriptome changes were translated into molecular interaction network maps and pathways. Pathways related to oxidative stress response; lipid, glutathione, and xenobiotic metabolism; and the immune response were perturbed by the WD. Several nuclear factor-erythroid 2-related factor 2- and aryl hydrocarbon receptor-dependent genes, including those coding for enzymes involved in phase 1 and 2 drug metabolism and oxidative stress responses, were induced. Oxidative stress was demonstrated by measurements of endogenous colonic redox-sensitive compound concentrations. Perturbations in immune response-related pathways, expression of inflammatory proteins, and increased numbers of lamina propria macrophages showed that the WD significantly alters the local colonic immune response. Collectively, these data suggest that consumption of a WD interferes with networks of related biological response pathways involving colonic lipid metabolism, oxidative stress, and the immune response. These new findings impact our understanding of links between consumption of WD and colon carcinogenesis, providing additional information for developing preventive means for decreasing colorectal cancer risk.
Introduction
Colorectal cancer is common and often lethal in the Westernized developed world and environmental factors contribute 70% of the risk (1,2). The Western diet (WD)10 comprising excess energy from fat and low fiber, calcium, and cholecalciferol contents is a major contributor (3). The profound effects of a WD with similar nutrient densities has been studied in mice and was able to induce preneoplastic changes (4) and, with methyl donor depletion, tumors in the colon of C57Bl/6 mice without addition of a carcinogen (5). The diet also accelerated tumorigenesis in genetic models of intestinal carcinogenesis (6).
Excess fat and energy and inadequate intake of calcium and vitamin D are risk factors for human colorectal cancers (7) and enhanced calcium intake reduces colon adenoma recurrence (8). These calciotropic effects may have been due to increased vitamin D intake (9). When the rodent WD was supplemented with calcium and cholecalciferol levels comparable to control diets, tumorigenesis was abrogated or diminished (10). Subsequent studies have demonstrated the utility of this mouse model for molecular studies of diet-induced colon carcinogenesis (11).
The original hypothesis for calcium effects on colon carcinogenesis was predicated on lowering bile and fatty acid concentrations by forming insoluble calcium salts in colonic contents (12,13). However, the effects of WD on the transcriptome and cell signaling in the colonic mucosa have been little studied. A recent study showed effects on Wnt signaling in isolated colonic epithelial cells during colorectal carcinogenesis (14).
The present study was designed to explore other molecular changes in the colon associated with WD consumption focusing on the global colonic transcriptome and expression of inflammatory/immune proteins. Our data demonstrate that WD induces numerous genes involved in oxidative stress responses and modest oxidative stress prior to tumorigenesis. Initial WD feeding exerts a proinflammatory stimulus in the colon and results in increased numbers of mature macrophages within the lamina propria. These data offer insight into potential novel mechanisms that direct colonic epithelial cells into a preneoplastic state following exposure to a WD.
Materials and Methods
Mice, diets, and sampling.
Forty male C57Bl/6J mice ∼3–4 wk after weaning (Jackson Laboratory) were housed 5 mice per cage, kept on a 12-:12-h-light/dark cycle in a humidified, temperature-controlled room and maintained under specific pathogen-free conditions (Helicobacter sp. free room). Mice consumed ad libitum a semipurified AIN-76A control diet for 2 wk and were then randomized to either the AIN-76A control diet (control group) containing 5% corn oil, 5 mg/g calcium, and 0.025 μg/g cholecalciferol or a Western-style diet (WD group), a modified AIN-76A diet containing 20% corn oil, 0.5 mg/g calcium, and 0.0125 μg/g cholecalciferol (Research Diets) (14,15) (Supplemental Table 1). This cholecalciferol level was expected to maintain serum levels of vitamin D and normal renal and intestinal synthetic and metabolizing enzymes (16). Some authors suggest that these levels lead to calcium deprivation (17), but mice maintain normal body growth and permit normal pregnancies, making deleterious effects of the diet unlikely. Water and diets were consumed ad libitum, wire-bottom cages were used to prevent coprophagy, and mice were monitored daily and weighed weekly. Diets were fed to 10 mice for 3 or 6 mo and mice were then killed by CO2 asphyxiation. The colon was quickly removed and processed according to requirements of the subsequent assays such as RNA isolation, measurements of inflammatory proteins and redox sensitive component, and F4/80 immunostaining (Supplemental Material). Animal procedures were approved by the Rockefeller University Institutional Animal Care and Use Committee.
Microarray.
Total RNA from colon samples was isolated with an RNeasy Mini kit (Qiagen) then RNA concentrations and purity were determined on a NanoDrop spectrophotometer (NanoDrop Technologies). All samples showed 2.0–2.1 A260:A280 ratios. RNA integrity was checked on an Agilent 2100 Bioanalyzer (Agilent Technologies) and all samples had RNA integrity numbers of 9.0–10.0. Illumina cRNA amplification, hybridization onto Sentrix MouseRef-8 v1 24K Expression BeadChips (Illumina), and scanning were done as described previously (18). Eight biological replicates per dietary group were run on 2 chips with 4 experimental (WD group) and 4 control (AIN-76A) samples per chip, for a total of 16 samples, at each time point.
Analyses and functional interpretation of microarray data.
GeneSifter software (VizX Labs) was used to analyze microarray data between WD and control groups. Array data were normalized to the global mean signal intensity value and values were log base 2 transformed. Data were filtered for spot quality by restricting analysis to genes with signal intensities > 0.95 and further filtered using a 1.2-fold cutoff and pairwise statistical analysis with Student's t test (P < 0.05) and Student's t test with Benjamini-Hochberg multiple testing correction (adjusted P < 0.05). Networks, canonical pathways, and functional analyses were generated on the up, down, and integrated total datasets using Ingenuity Pathways Analysis (Ingenuity Systems) (Supplemental Material) (19). EASE program (NIH, NIAID) (20) was used to determine which Gene Ontology categories were statistically over-represented among differentially expressed genes (Supplemental Material). Differentially expressed data sets [P < 0.05; no false discovery rate (FDR) applied] were used for these analyses. Gene set enrichment analysis (GSEA) was used to identify significant coordinated changes in a defined oxidative stress-related gene set between dietary groups (Supplemental Table 2). Normalized expression values for the whole microarray gene set were extracted from GeneSifter and data were converted from probe sets to gene symbols. GSEA was performed using a desktop application available at Broad Institute (21). Quantitative RT-PCR (qPCR) was used to verify the microarray results. Eleven genes were tested in 4 mice per group using Taqman Gene Expression assays (Applied Biosystems) (Supplemental Table 3).
Immunohistochemistry and computerized histomorphometry analysis.
Semiquantitative comparison of colonic lamina propria macrophages was performed by computerized morphometry analysis (MetaMorph7, MDS Analytical Technologies) of immunostained sections in 7 mice per dietary group with the pathologist unaware of the treatment groups. Indirect immunoperoxidase staining was performed by applying the avidin-biotin based technique and 3′3′diaminobenzidine as a chromogen. Acetone-fixed cryosections of distal colons were stained for mature resident macrophages using rat anti-mouse F4/80 mAb (2 ng/L; Invitrogen) and the Vectastain ABC kit (Vector Laboratories). Digital images of microscopic fields of colonic cross sections (×20 plan objective and ×10 ocular lenses) were acquired with a Zeiss Axioplan2 microscope equipped with a QImaging Retiga Camera system and integrated LCD filter (Supplemental Material). For each mouse, 5–7 images were taken to represent the entire section. Using the line tool of the MetaMorph7 software, lamina propria areas were selected in each image and individual cells or small groups of nonoverlapping cells were manually traced within the selected region and counted. For statistical analysis, cell numbers counted in each image were divided by the area. For area morphometry, the F4/80 stained cells were identified by autothresholding and digitally scored for pixel area. Positive pixel areas were expressed as a percentage of total lamina propria area selected with the software.
Pro- and antiinflammatory protein expression profiling.
Clarified tissue extracts of mid colon from 10 mice/group were analyzed by a Rodent Multi-Analyte Profile testing (Rules-Based Medicine, Rodent MAP version 1.6 antigen panel) (22), a high-density, quantitative immunoassay of pro-and antiinflammatory protein expression in small samples. Tissues were homogenized in 9 volumes of lysis buffer (wt:v; 0.05 mol/L, Tris-HCL with 2 mmol/L EDTA, pH 7.4) containing protease and phosphatase inhibitor cocktails (P 8340 and P 5726, Sigma) and centrifuged for 2 min at 13,000 × g. Supernatant fractions were collected and frozen at −80°C until analysis (Supplemental Material). Total protein concentration in supernatants was measured by the BCA assay (Pierce). Inflammatory protein concentrations in samples were normalized for total protein.
Quantitative analysis of redox active compounds in colon tissues.
Colonic concentrations of redox active compounds were measured in mice fed the WD (n = 6) or the control (n = 6) diet for 6 mo using a Perkin-Elmer HPLC equipped with an 8-channel coulometric array detector (ESA). Concentrations were compared against standards for each derivative [cysteine, cystine, ascorbate, methionine, glutathione (GSH), oxidized glutathione GSSG), and uric acid] and were reported as pmol/g protein (Supplemental Material).
Statistical analysis.
Statistical analysis of microarrays is described above and in detail in the Supplemental Material. Two-tailed Student's t test was used to compare dietary groups for body weight, colonic concentrations of inflammatory proteins, redox active compounds, and lamina propria macrophage numbers. Data are expressed as mean ± SD. Differences were considered significant at P < 0.05.
Results
Body weight
Total body weights of the WD group were higher at 3 mo (31.51 ± 2.65 g; P < 0.001) and 6 mo (38.00 ± 4.58 g; P < 0.005) compared with controls (26.81 ± 2.00 g at 3 mo and 33.38 ± 1.72 g at 6 mo) and more subcutaneous and visceral adipose tissue was observed in the WD groups at each time points. No macroscopic changes were observed in the colon.
WD-induced colonic transcriptome changes
Differentially expressed genes.
Microarray data analysis (P < 0.05; spot quality > 0.95) identified 1147 differentially expressed transcripts at 3 mo and 930 transcripts at 6 mo in the colon of WD-fed mice compared with controls (4.77 and 3.87% of the total > 24K probe sets on the oligoarray; Table 1). Most transcripts showed less than a 2-fold change. Forty-one genes (43 probe sets) were differentially expressed after both 3 and 6 mo, signifying persistent WD-induced expression changes. Sixty-two percent of these genes were related to metabolic processes, primarily lipid metabolism (17%) and GSH metabolism (5%). Genes involved in transcription underlying mammalian circadian rhythm signaling, namely period homolog 2, the D site albumin promoter binding protein and the aryl hydrocarbon receptor nuclear translocator-like, were among these genes (Supplemental Table 4).
TABLE 1.
Numbers of differentially expressed transcripts in the colon of WD-fed C57Bl/6J mice compared with controls1
| Transcripts, n |
|||||
|---|---|---|---|---|---|
| Statistics | Fold cutoff | Time points, mo | Differentially expressed | Up-regulated | Down-regulated |
| t-Test | None | 3 | 1147 | 599 | 548 |
| 6 | 930 | 314 | 616 | ||
| >1.2-fold | 3 | 287 | 160 | 127 | |
| 6 | 406 | 171 | 235 | ||
| t-Test, 5% FDR | >1.2-fold | 3 | 248 | 140 | 108 |
| 6 |
121 |
46 |
75 |
||
1.2-fold cutoff was applied and pairwise statistical analysis was performed using Student's t test (P < 0.05) and Student's t test with Benjamini-Hochberg multiple testing correction (adjusted P < 0.05). n = 10.
Functional analyses of differentially expressed genes
Gene Ontology categorization.
At both time points, major metabolic processes altered included lipid metabolism (P < 0.001) and steroid biosynthesis (P = 0.011). At 3 mo, cell growth (P = 0.007) and GSH metabolism (P < 0.002) were over-represented. Several immune/inflammatory response-associated categories (P < 0.001 to P < 0.05) were enriched at 6 mo, including immune cell chemotaxis (P = 0.042), antigen presentation (P = 0.028), cytokine production (P = 0.014), and humoral defense mechanism (P < 0.006) (Supplemental Table 5).
Canonical pathway and network analysis
Canonical pathways.
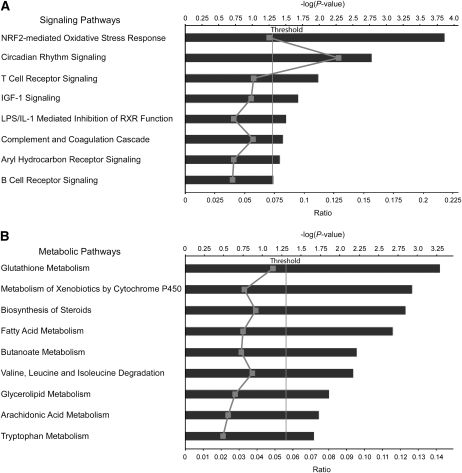
At 3 mo, 11 metabolic and 7 signaling pathways were significantly perturbed by the WD and at 6 mo, 8 signaling (Fig. 1A) and 3 metabolic pathways were significantly altered (Supplemental Table 6). Pathways altered at both time points included nuclear factor-erythroid 2-related factor (Nrf2)-mediated oxidative stress response, aryl hydrocarbon receptor signaling, insulin-like growth factor 1 signaling, lipopolysaccharide /interleukin 1-mediated inhibition of retinoid X receptor function, circadian rhythm signaling, and GSH metabolism. Xenobiotic metabolism signaling at 3 mo, T- and B-cell receptor signaling, and the complement coagulation cascade at 6 mo were altered. Metabolism of xenobiotics by cytochrome P450, butanoate metabolism, and fatty acid metabolism were affected at 3 mo (Supplemental Table 6). Arachidonic acid metabolism was among the significantly upregulated pathways at 6 mo (Fig. 1B). Downregulated pathways at 3 mo included G1/S checkpoint regulation (genes CCND2, E2F2) and nuclear-factor-κB signaling and at 6 mo, T- and B-cell receptor signaling and complement and coagulation cascades (Supplemental Table 6).
FIGURE 1 .
Signaling (A) and metabolic (B) pathways altered by the WD in the colon of C57Bl/6J mice (n = 8) compared with controls (n = 8) at 6 mo analyzed by ingenuity pathways analysis. Significance expressed as P-values that were calculated using the right-tailed Fisher's exact test. Canonical pathways are displayed along the y-axis. The x-axis displays the P-value and ratios. The ratio, indicated as a gray line in the figure, is calculated as the number of differentially expressed genes divided by the total number of genes in the pathway.
Biologically relevant networks.
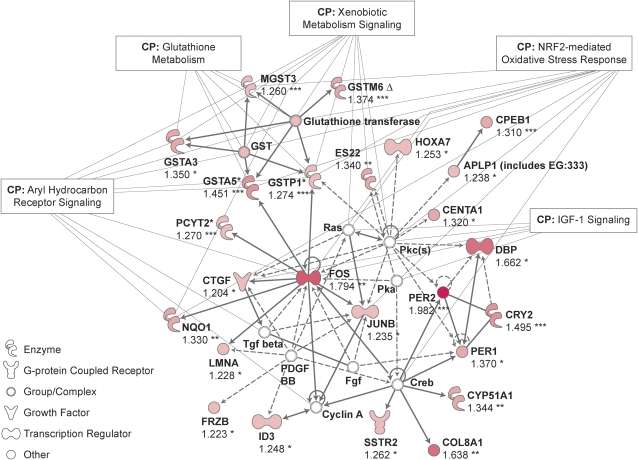
Differentially expressed genes grouped into networks associated with lipid metabolism, cellular compromise such as free radical scavenging, circadian rhythm, cellular growth, and proliferation (Supplemental Table 7). Network representations led to identification of a c-Fos and Jun B proto-oncogene–centered network induced by the WD (Fig. 2).
FIGURE 2 .
Ingenuity network analysis of genes upregulated by the WD in the colon of C57Bl/6J mice (n = 8) compared with controls (n = 8) at 6 mo. Upregulated genes are shown in red; genes that are not user specified but are incorporated into the network through their relationship with other genes are shown in white. Continuous and discontinuous lines represent direct and indirect functional and physical interaction between genes from the literature. Values are fold of control and significance was calculated using the right-tailed Fisher's exact test. Asterisks indicate different from the control, *P < 0.05; **P ≤ 0.01; ***P ≤ 0.001.
GSEA
GSEA, used to identify whether a custom gene set related specifically to oxidative stress was perturbed in response to WD feeding, showed oxidative stress genes (Supplemental Table 7) significantly enriched with the WD feeding at 6 mo. At 3 mo, 19 of 66 (P < 0.062, FDR < 0.13) and at 6 mo, 24 of 66 (P < 0.033) oxidative stress responsive genes were enriched, including NAD(P)H dehydrogenase, quinone 1, superoxide dismutase 1 and 2, catalase, metallothionein 2, heme oxygenase 2, and peroxiredoxin 6, which function to protect cells from oxidative challenge (Supplemental Fig. 1).
The WD increased macrophage numbers and altered inflammatory protein expression
Morphometry analysis of colonic macrophages.
WD-fed mice had more F4/80 stained macrophages in the lamina propria at 3 mo (1.29-fold; P < 0.002) and 6 mo (1.76-fold; P < 0.001). Area morphometry of F4/80 staining confirmed these findings (1.35-fold; P < 0.02), but the difference between groups was not significant at 3 mo (P < 0.10; Supplemental Fig. 2).
Inflammatory protein expression profiling.
Expression profiling of colonic inflammatory proteins of 3 mo WD-fed mice compared with controls showed increased expression of IgA, C-reactive protein (CRP), fibrinogen, myeloperoxidase (MPO), and serum amyloid protein as well as the mouse macrophage/monocyte chemotactic protein-5 (MCP-5) (related to human MCP-1) and macrophage inflammatory protein-1 γ (Table 2). Increased leptin concentrations may have resulted from elevated circulating levels (data not shown). Most changes at 3 mo were no longer apparent at 6 mo (Table 2).
TABLE 2.
Upregulated inflammatory proteins in the colon of C57Bl/6 mice after 3 mo consumption of a WD compared with controls1
| Inflammatory protein, unit/g total protein |
Dietary groups |
|
|---|---|---|
| Control | WD | |
| IgA, mg | 1.36 ± 0.54 | 2.47 ± 0.59*** |
| CRP, μg | 7.08 ± 2.20 | 9.11 ± 0.75* |
| Haptoglobin, μg | 89.77 ± 26.54 | 148.48 ± 99.54 |
| Fibrinogen, mg | 1.39 ± 0.42 | 2.85 ± 0.82*** |
| MPO, ng | 741.95 ± 425.31 | 1109.68 ± 390.39* |
| Osteopontin, ng | 228.65 ± 60.74 | 330.19 ± 59.61** |
| MCP-5, ng | 4.68 ± 1.72 | 8.98 ± 2.08*** |
| MIP-1γ, ng | 685.76 ± 94.22 | 926.64 ± 257.67* |
| Leptin, ng | 49.35 ± 6.44 | 92.16 ± 23.24*** |
| Serum amyloid protein, μg |
48.5 ± 14.54 |
56.29 ± 8.83** |
Values are means ± SD, n = 10. Asterisks indicate different from control, *P < 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Effects of the WD on redox active compound levels
To investigate responses to oxygen stress after 6 mo of WD feeding, we examined colon homogenates of 6 mice from both dietary groups for concentrations of major intracellular redox buffers (Table 3). The cellular redox status is maintained by a balance between intracellular reduced sulfhydryls and oxidized disulfides. Enhanced oxygen stress was evidenced by decreased cysteine:cystine and GSH:GSSG ratios and a 50% decrease in endogenous methionine, a precursor to cysteine. Because mice synthesize ascorbate, the increased levels of ascorbic acid in the WD group may represent a compensatory response to oxidative stress.
TABLE 3.
Redox active compound levels in the colon of C57Bl/6J mice fed the WD or control diet for 6 mo1
| Diet | Cysteine | Cystine | Ascorbate | Methionine | GSH | GSSG | Cysteine:cystine | GSH:GSSG |
|---|---|---|---|---|---|---|---|---|
| pmol/g | ||||||||
| Control | 7.39 ± 1.62 | 2.11 ± 0.61 | 61.76 ± 5.40 | 5.19 ± 1.09 | 87.20 ± 6.60 | 3.56 ± 1.01 | 3.72 ± 1.28 | 26.08 ± 6.75 |
| WD |
5.59 ± 0.90* |
3.21 ± 0.84 |
78.61 ± 8.62* |
2.52 ± 0.88** |
88.09 ± 10.8 |
4.29 ± 0.96 |
1.83 ± 0.51* |
21.62 ± 6.05 |
Values are means ± SD, n = 6. *Different from control, P < 0.05; **P ≤ 0.01.
Discussion
The WD is an accepted risk factor for colorectal cancer development in humans (2). However, WD consumption is accompanied by other lifestyle factors; thus, identification of molecular mechanism(s) underlying carcinogenic effects of the diet is difficult. A rodent WD, high in fat and relatively low in calcium and cholecalciferol content, uniquely induces colonic preneoplastic changes in wild-type C57Bl/6 mice (4) and with additional folate and methionine depletion, adenoma and colon cancer formation within 12–24 mo (5). Our studies show that the WD induces oxidative stress and alters immune responses in the colon of wild-type mice long before tumors occur. Additionally, pathway analysis of colonic transcriptome changes identified other biologically important and related networks altered by the WD, including lipid and xenobiotic metabolism.
The WD-induced colonic transcriptome changes were based upon microarray analysis in 8 mice per dietary group (at 3 and 6 mo) validated by qPCR. The whole colon wall was used, which did not permit separation of effects on epithelial cells, leukocytes, or other cellular components but allowed evaluation of macrophage numbers. The WD, containing 4-fold more lipid than control diets, induced persistent regulation of fatty acid and sterol metabolism and upregulated soluble and mitochondrial HMG CoA reductase, lanosterol synthase, and squalene epoxidase genes. Major stimulation of lipid biosynthesis and metabolism suggest that the WD directly affected the colonic epithelial transcriptome.
WD feeding led to Nrf2-associated oxidative stress responses in the colon. Nrf2, a redox-sensitive basic leucine zipper transcription factor, regulates gene expression through interactions with the antioxidant-response element (ARE) found in the promoter regions of many cellular defense genes (23). During oxidative stress, Nrf2 activates ARE-dependent transcription of phase II and antioxidant defense enzymes and controls expression of γ-glutamylcysteine synthetase, the rate-limiting enzyme for GSH synthesis. Thus, Nrf2-ARE pathway activation is an efficient mechanism increasing cellular detoxification and antioxidant capacity (23–25). Nrf2-knockout mice are more susceptible to chemically induced colitis and carcinogenesis than their corresponding litter mates, emphasizing the importance of Nrf2 in maintaining colonic mucosal integrity (26). Canonical pathway analysis and GSEA showed induction of ARE-regulated cytoprotective genes by the WD, implying increased colonic production of reactive oxygen species (ROS) and responses to oxidative stress mediated presumably by the Nrf2-ARE pathway.
Small coherent changes in expression of functionally related genes may be more important than large differences in small numbers of unrelated genes. We therefore performed GSEA to study expression of many oxidative stress-related genes not restricted to those regulated by Nrf2-ARE, which confirmed induction of ARE-driven genes (e.g. NAD(P)H dehydrogenase, quinone 1, glutathione peroxidase, glutathione S-transferase) and enrichment of antioxidant genes involved in superoxide metabolism (including superoxide dismutase 1, copper chaperone for superoxide dismutase, NADPH oxidase 4, peroxiredoxin), other peroxidases (catalase, prostaglandin endoperoxidase synthase), and metallothionein 2. These observations prompted further studies of oxidative stress responses and changes in redox status in the colon.
Measurement of colonic redox active compounds after 6 mo of WD feeding showed increased cystine and ascorbate concentrations, lower cysteine levels, and highly significant reductions in cysteine:cystine ratios, providing further evidence of colonic oxidative stress. Unlike humans, who are dependent upon dietary ascorbate, mice biosynthesize ascorbate in the liver and kidney (27) and may compensate for oxidative stress by upregulating de novo synthesis in response to WD feeding. Lower colonic methionine levels in the WD group may reflect enhanced scavenging of methionine for cysteine formation in a transulfuration pathway (28). This pattern of changes in redox active components suggests a generalized shift in redox status into a state of oxidative stress despite marginal reduction in the GSH:GSSG ratio (Table 3). De novo synthesis of endogenous GSH may be maintained at the expense of both cysteine and methionine.
Network analysis showed that the WD upregulated pathways centered on c-Jun and Jun B oncogenes (Fig. 2). Members of the Jun and Fos protein family dimerize to form activator protein 1 transcription factor, which regulates genes involved in proliferation, differentiation, inflammation, and defense against environmental stimuli (29), including cytochrome P450, the expression of several of whose members were upregulated by the WD, further suggesting WD induced Nrf2-mediated oxidative stress responses. During oxidative stress, ROS-activated protein kinase C and mitogen-activated protein kinase pathways lead to nuclear translocation of cytosolic Nrf2, which interacts with transcription factors in the bZIP family, including Fos or Jun, and regulates genes involved in drug metabolism, detoxification, and antioxidant defense through their ARE (23).
The WD is rich in linoleic acid, which is converted to arachidonic acid and can be metabolized by cyclooxygenases and lipoxygenases to eiconsanoids such as the proinflammatory and procarcinogenic prostaglandin E2. WD induced cyclooxygenase-2 (prostaglandin endoperoxidase synthase 2) (Supplemental Fig. 1) could generate prostaglandins important in colorectal carcinogenesis (30).
About 15% of all cancers are linked to inflammation (31). Our hypothesis that WD could affect colonic immune responses was confirmed by increased inflammatory protein levels at 3 mo, including IgA, CRP, MPO, and fibrinogen, as well as macrophage chemokines. These changes were largely absent after 6 mo, suggesting that the proinflammatory stimulus was short lived. Because WD induced murine macrophage chemokines MCP-5 and MIP-1γ, we measured F4/80 glycoprotein expressing macrophages in the colonic lamina propria. MCP-5 (CCL12) is a major mouse macrophage chemotactic protein similar to the human chemokine MCP-1. MIP-1γ (CCL9) is a potent suppressor of transcriptional activation of the tumor suppressor gene P53 and thereby could enhance carcinogenesis (32). WD feeding increased numbers of colonic macrophages, suggesting enhanced macrophage recruitment or effects on macrophage differentiation. Activated macrophages could release ROS and reactive nitrogen species, causing modification of proteins, lipids, and DNA and enhancing carcinogenesis (33,34).
Effects of dietary fat intake could not be distinguished from that of energy intake by the WD. However, equal caloric WD feeding still increased tumor formation (Lipkin M., personal communication). WD-fed mice were heavier and had more intraperitoneal fat than controls. Obesity is associated with increases in many cancers, including colorectal cancer (35). Possible mechanisms include insulin resistance and failure to control plasma glucose levels (36), resulting in increased proliferation of colonic epithelial cells (37). Obesity also is associated with chronic inflammation in adipose tissue, coronary arterioles (38), and possibly in the colon, and results in hyperleptinemia, which can increase colon carcinogenesis (39). This study shows increased colonic and circulating leptin levels in WD-fed mice. Besides being a growth factor for colonic epithelial cells (40), leptin also promotes inflammation (41) and generation of ROS by increasing fatty acid oxidation (42), all factors enhancing tumor formation.
In summary, our studies show that a WD feeding for 3 or 6 mo, known to enhance colon carcinogenesis, principally induces oxidative stress-responsive genes and decreases colonic cysteine:cystine ratios. The WD stimulated expression of lipid metabolism genes, suggesting direct effects upon colonic epithelial cells. The WD also increased macrophage numbers in the colonic lamina propria. Combining these data suggests that macrophage recruitment and oxidative stress is a potential early mechanism underlying the carcinogenic effect of the WD.
Supplementary Material
Acknowledgments
We thank Connie Zao at the Genomics Resource Center at Rockefeller University for technical assistance with the Illumina Genearray and Jenny Z. Xiang at the Microarray Core Facility of the Weill Cornell Medical College for the qPCR. I.E., M.L., F.W.Q, J.T.P., and P.R.H. designed research; I.E., E.Y.L., and J.T.P. conducted research; I.E., N.L., and J.T.P. analyzed data; I.E. and P.R.H. wrote the paper. I.E. had primary responsibility for final content. All authors read and approved the final manuscript.
Supported by grants from the NIH (NCI 5 R25 CA105012) and the Irving Weinstein Foundation, Inc. to I.E. and from Center for Clinical and Translational Science (UL1RR024143) to N.L.
Author disclosures: I. Erdelyi, N. Levenkova, E. Y. Lin, J. T. Pinto, T. Liu, M. Lipkin, F. W. Quimby, and P. R. Holt, no conflicts of interest.
Supplemental Tables1–7, Supplemental Figures 1 and 2, and Supplemental Text are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: ARE, antioxidant response element; CRP, C-reactive protein; FDR, false discovery rate; GSEA, gene set enrichment analysis; GSH, glutathione; GSSG; oxidized glutathione; MCP, monocyte chemotactic protein; MPO, myeloperoxidase; Nrf2, nuclear factor-erythroid 2-related factor; qPCR, quantitative RT-PCR; ROS, reactive oxygen species; WD, Western-style diet.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. [DOI] [PubMed] [Google Scholar]
- 2.Wiseman M. The Second World Cancer Research Fund/American Institute for Cancer Research Expert Report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253–6. [DOI] [PubMed] [Google Scholar]
- 3.Lipkin M, Reddy B, Newmark H, Lamprecht SA. Dietary factors in human colorectal cancer. Annu Rev Nutr. 1999;19:545–86. [DOI] [PubMed] [Google Scholar]
- 4.Newmark HL, Lipkin M, Maheshwari N. Colonic hyperproliferation induced in rats and mice by nutritional-stress diets containing four components of a human Western-style diet (series 2). Am J Clin Nutr. 1991;54:S209–14. [DOI] [PubMed] [Google Scholar]
- 5.Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki HA. Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis. 2001;22:1871–5. [DOI] [PubMed] [Google Scholar]
- 6.Yang WC, Mathew J, Velcich A, Edelmann W, Kucherlapati R, Lipkin M, Yang K, Augenlicht LH. Targeted inactivation of the p21(WAF1/cip1) gene enhances Apc-initiated tumor formation and the tumor-promoting activity of a Western-style high-risk diet by altering cell maturation in the intestinal mucosal. Cancer Res. 2001;61:565–9. [PubMed] [Google Scholar]
- 7.Cho E, Smith-Warner SA, Spiegelman D, Beeson WL, van den Brandt PA, Colditz GA, Folsom AR, Fraser GE, Freudenheim JL, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst. 2004;96:1015–22. [DOI] [PubMed] [Google Scholar]
- 8.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, Rothstein R, Summers RW, Snover DC, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340:101–7. [DOI] [PubMed] [Google Scholar]
- 9.Grau MV, Baron JA, Sandler RS, Haile RW, Beach ML, Church TR, Heber D. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003;95:1765–71. [DOI] [PubMed] [Google Scholar]
- 10.Xue L, Lipkin M, Newmark H, Wang J. Influence of dietary calcium and vitamin D on diet-induced epithelial cell hyperproliferation in mice. J Natl Cancer Inst. 1999;91:176–81. [DOI] [PubMed] [Google Scholar]
- 11.Lipkin M. New rodent models for studies of chemopreventive agents. J Cell Biochem Suppl. 1997;67:28–29:144–47. [PubMed] [Google Scholar]
- 12.Newmark HL, Wargovich MJ, Bruce WR. Colon cancer and dietary fat, phosphate, and calcium: a hypothesis. J Natl Cancer Inst. 1984;72:1323–5. [PubMed] [Google Scholar]
- 13.Govers MJ, Termont DS, Lapre JA, Kleibeuker JH, Vonk RJ, Van der Meer R. Calcium in milk products precipitates intestinal fatty acids and secondary bile acids and thus inhibits colonic cytotoxicity in humans. Cancer Res. 1996;56:3270–5. [PubMed] [Google Scholar]
- 14.Yang K, Lipkin M, Newmark H, Rigas B, Daroqui C, Maier S, Augenlicht L. Molecular targets of calcium and vitamin D in mouse genetic models of intestinal cancer. Nutr Rev. 2007;65:S134–7. [DOI] [PubMed] [Google Scholar]
- 15.Yang K, Yang W, Mariadason J, Velcich A, Lipkin M, Augenlicht L. Dietary components modify gene expression: implications for carcinogenesis. J Nutr. 2005;135:2710–4. [DOI] [PubMed] [Google Scholar]
- 16.Hunt JR, Hunt CD, Zito CA, Idso JP, Johnson LK. Calcium requirements of growing rats based on bone mass, structure, or biomechanical strength are similar. J Nutr. 2008;138:1462–8. [DOI] [PubMed] [Google Scholar]
- 17.Vieth R, Milojevic S, Peltekova V. Improved cholecalciferol nutrition in rats is noncalcemic, suppresses parathyroid hormone and increases responsiveness to 1, 25-dihydroxycholecalciferol. J Nutr. 2000;130:578–84. [DOI] [PubMed] [Google Scholar]
- 18.Protiva P, Cross HS, Hopkins ME, Kallay E, Bises G, Dreyhaupt E, Augenlicht L, Lipkin M, Lesser M, et al. Chemoprevention of colorectal neoplasia by estrogen: potential role of vitamin D activity. Cancer Prev Res. 2009;2:43–51. [DOI] [PubMed] [Google Scholar]
- 19.Ingenuity Pathway Analysis Software. Complete Pathway Database [cited 2009 Apr]. Available from: http://www.ingenuity.com/.
- 20.DAVID. 2008 Functional annotation bioinformatics microarray analysis [cited 2008 Dec]. Available from: http://david.abcc.ncifcrf.gov/.
- 21.Gene Set Enrichment Analysis [cited 2008 Dec]. Available from: http://www.broad.mit.edu/gsea/.
- 22.Rules-Based Medicine, Inc. The Biomarker Testing Laboratory [cited 2008 Jun]. Available from: http://www.rulesbasedmedicine.com.
- 23.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–207. [DOI] [PubMed] [Google Scholar]
- 24.Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen T, Sherratt PJ, Nioi P, Yang CS, Pickett CB. Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. J Biol Chem. 2005;280:32485–92. [DOI] [PubMed] [Google Scholar]
- 26.Khor TO, Huang MT, Prawan A, Liu Y, Hao X, Yu S, Cheung WK, Chan JY, Reddy BS, et al. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prevention Research. 2008;1:187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banhegyi G, Braun L, Csala M, Puskas F, Mandl J. Ascorbate metabolism and its regulation in animals. Free Radic Biol Med. 1997;23:793–803. [DOI] [PubMed] [Google Scholar]
- 28.Kato A, Ogura M, Suda M. Control mechanism in the rat liver enzyme system converting L-methionine to L-cystine. 3. Noncompetitive inhibition of cystathionine synthetase-serine dehydratase by elemental sulfur and competitive inhibition of cystathionase-homoserine dehydratase by L-cysteine and L-cystine. J Biochem. 1966;59:40–8. [DOI] [PubMed] [Google Scholar]
- 29.Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117:5965–73. [DOI] [PubMed] [Google Scholar]
- 30.Kawamori T, Uchiya N, Sugimura T, Wakabayashi K. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis. 2003;24:985–90. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- 32.Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999;190:1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91:3652–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. [DOI] [PubMed] [Google Scholar]
- 35.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–25. [DOI] [PubMed] [Google Scholar]
- 36.Wei EK, Ma J, Pollak MN, Rifai N, Fuchs CS, Hankinson SE, Giovannucci E. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2005;14:850–5. [DOI] [PubMed] [Google Scholar]
- 37.Tran TT, Medline A, Bruce WR. Insulin promotion of colon tumors in rats. Cancer Epidemiol Biomarkers Prev. 1996;5:1013–5. [PubMed] [Google Scholar]
- 38.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–3. [DOI] [PubMed] [Google Scholar]
- 39.Stattin P, Lukanova A, Biessy C, Soderberg S, Palmqvist R, Kaaks R, Olsson T, Jellum E. Obesity and colon cancer: does leptin provide a link? Int J Cancer. 2004;109:149–52. [DOI] [PubMed] [Google Scholar]
- 40.Attoub S, Noe V, Pirola L, Bruyneel E, Chastre E, Mareel M, Wymann MP, Gespach C. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, rho-, and rac-dependent signaling pathways. FASEB J. 2000;14:2329–38. [DOI] [PubMed] [Google Scholar]
- 41.Sitaraman S, Liu X, Charrier L, Gu LH, Ziegler TR, Gewirtz A, Merlin D. Colonic leptin: source of a novel proinflammatory cytokine involved in IBD. FASEB J. 2004;18:696–8. [DOI] [PubMed] [Google Scholar]
- 42.Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276:25096–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.