Introduction
Since the publication of our previous Perspective dealing with polyamine drug discovery,1 the field has undergone numerous changes. Most prominent among these changes, from a medicinal chemistry point of view, is that polyamine drug discovery efforts have partially shifted away from an emphasis on inhibitors of polyamine biosynthetic and catabolic enzymes. This shift is in part due to the fact that multiple compensatory mechanisms are available that are able to maintain homeostasis in cellular polyamine pools,2 and thus the utility of specific enzyme inhibitors as drugs is limited. Specific inhibitors are available for every enzyme in the polyamine biosynthetic pathway,1, 3-6 and for the polyamine transport system.7-9 Although these inhibitors have significant effects on their respective target enzymes, only one inhibitor, α-difluoromethylornithine (DFMO) has reached the market. DFMO was originally designed as an antitumor agent, but the drug was not effective enough to warrant continued Phase II trials. However, it has been shown to be an effective cure for infection caused by Trypanosoma brucei gambiense (West African Sleeping Sickness),10, 11 and has recently shown considerable potential as a cancer chemopreventive agent (see below).12-14 Although no other polyamine biosynthesis inhibitor has been advanced to the market, the ubiquitous nature of the natural polyamines would lead one to conclude that these molecules have numerous cellular effector sites that are frequently dysregulated in cancer, and as such should provide a target rich environment for therapeutic intervention. Recent medicinal chemistry efforts in the polyamine field have focused on the discovery of compounds that produce cellular effects that are either independent of, or in addition to the polyamine metabolic enzymes. In addition, polyamine chains have been used to make hybrid drug molecules in order to improve cellular import, increase affinity for chromatin or to serve as carriers. This Perspective will focus on developments in polyamine drug discovery since our previous article.1
Polyamine Metabolism as a Drug Target
The role of natural polyamines in cellular homeostasis
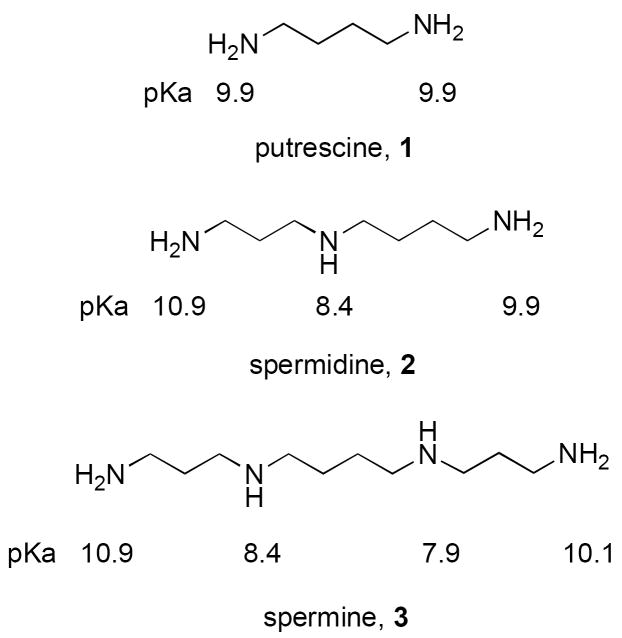
The polyamines putrescine (1,4-diaminobutane, 1), spermidine (1,8-diamino-4-azaoctane, 2) and spermine (1,12-diamino-4,9-diazadodecane, 3) (Figure 1) are ubiquitous polycationic compounds that are found in significant amounts in nearly every prokaryotic and eukaryotic cell type. Spermidine and spermine primarily exist in aqueous solution at pH 7.4 as fully protonated polycations, and possess the pKa values indicated in Figure 1.15 This high degree of positive charge is an important factor in the biological functions of these molecules, and as will be discussed below, alterations in the pKa of polyamine nitrogens can affect and disrupt their cellular function. Polyamines are widely distributed in nature, and are known to be required in micromolar to millimolar concentrations to support a wide variety of cellular functions. However, data that establishes the precise role of the polyamines and their analogues in cellular processes remains incomplete. The ongoing identification of new functions for the polyamines ensures that new avenues for research are arising continuously in an extremely diverse set of disciplines. The human and mammalian pathways for polyamine metabolism have been extensively studied, and analogous pathways have been elucidated for many organisms. There are important interspecies differences in polyamine metabolism, especially within eukaryotic cells (plant versus animal) and between higher eukaryotes, bacteria, and protozoa. In some prokaryotes, only putrescine and spermidine are synthesized, while in other cases, such as certain thermophilic bacteria, polyamines with chains longer than spermine are found. In some parasitic organisms, there are additional enzymes that are not present in the host cell, and as such provide a target for the design of specific antiparasitic agents. The enzymes involved in human and other mammalian polyamine metabolism are reasonably similar, and inhibitors targeted to these enzymes rely on the observation that polyamine metabolism is accelerated, and polyamines are required in higher quantities, in target cell types. It is reasonable to assume that carefully designed polyamine analogues could have the potential to selectively disrupt polyamine metabolism, and thus such agents have been investigated as potential therapeutic agents in vitro and in vivo. Depletion of polyamines results in the disruption of a variety of cellular functions, and may in specific cases result in cytotoxicity.1, 16, 17 Comprehensive reviews of polyamine biochemistry, polyamine biosynthesis inhibitors and the role of polyamines in normal and tumor cell metabolism have recently been published.1, 16-22
Figure 1.
Structures and pKa values of the polyamines putrescine, spermidine and spermine.
DFMO in cancer chemoprevention
DFMO was originally evaluated as an antitumor agent in the early 1980’s, with limited success. Phase I studies suggested a dose of 2.25 g/m2 every 6 hours for patients with advanced solid tumors or lymphomas.23 Phase II studies were conducted in patients with melanoma,24 small cell lung carcinoma,25 colon cancer25 and prostate cancer,26 among others. The drug was generally well tolerated, although significant but infrequent adverse effects including thrombocytopenia, transient hearing loss and osmotic diarrhea were noted. The results of these studies did not warrant continued evaluation of the drug as an antitumor agent.25 DFMO was eventually approved for use in Pneumocystis carinii infection in AIDS patients.27 In 1985, it was discovered that DFMO was curative for infections with Trypanosoma brucei gambiense (West African Sleeping Sickness).28 It was also curative in a mouse model for Trypanosoma brucei rhodesiense,29 but the same effect was not observed in humans. Reports outlining the use of DFMO as a chemopreventative agent began to appear after 1985, and the first report of chemoprevention by DFMO in combination with a non-steroidal anti-inflammatory agent (piroxicam) appeared shortly thereafter.30 DFMO has been shown to suppress skin carcinogenesis in patients with moderate to severe actinic keratosis with no systemic toxicities.31, 32 More recently, DFMO dosed at 500 mg per day in a one-year randomized trial reduced prostate putrescine content and slowed prostate growth in men with a family history of prostate cancer.33 In this study, no grade 3 or 4 toxicities, including ototoxicity, were observed. In a landmark study, Meyskens et al. demonstrated that 500 mg of DFMO in combination with 150 mg of sulindac daily over a 3 year period led to a dramatic reduction in the recurrance of colon adenomas in patients with prior disease, with remarkably few toxicities.34 Subsequent studies analyzing the potential toxicities associated with long-term DFMO/non-steroidal anti-inflammatory agent combination treatment suggest minimal risk for the development of ototoxicity35 and adverse cardiovascular events.36 It is clear from these data that DFMO has considerable potential for use as a cancer chemopreventative agent.
Analogues of the Natural Polyamines Spermidine and Spermine
Symmetrical Terminally Alkylated Polyamine Analogues
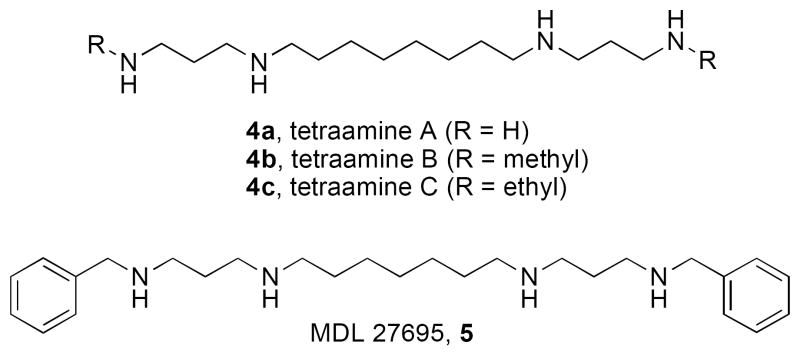
The development of analogues of spermidine and spermine as potential antitumor agents was first attempted in the late 1980’s. Initially, these analogues were structurally similar to the natural polyamines, in that they had terminal primary amine groups, with variations in the length of the intermediate carbon chains.37 Compounds with eight carbons between the central nitrogens, such as tetramines A-C (4a, b and c, Figure 2), generally showed significant antitumor activity. In a related study, a series of (bis)benzyl analogues related to MDL 27695 (5, Figure 2), and an additional series of substituted tetraamines were evaluated for the ability to inhibit proliferation of HeLa cells.38 Compounds 4a and 5 were active antiproliferative compounds, exhibiting IC50 values of 5 and 50 μM, respectively. Interestingly, no correlation between the DNA binding properties and antitumor activity of these analogues was detected.
Figure 2.
Early examples of polyamine analogues with biological activity in vitro.
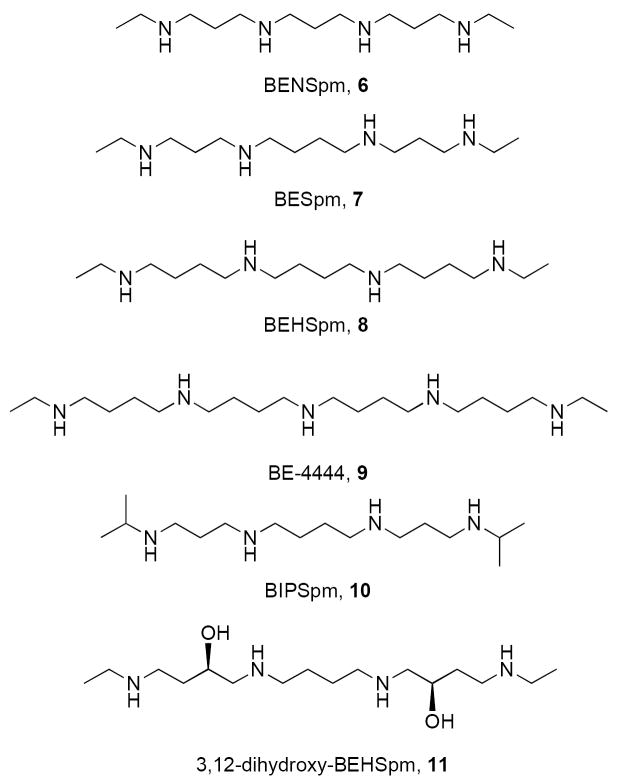
Subsequent attempts to develop polyamine analogues as potential modulators of polyamine function focused on the synthesis of symmetrical, terminally-substituted bis(alkyl)polyamines. These analogues were designed in response to the finding that natural polyamines utilize several feed-back mechanisms which autoregulate their synthesis,2, 39, 40 and that they can be taken into cells by the energy dependent polyamine transport system. A variety of symmetrically substituted polyamine analogues have been synthesized which enter the cell using the polyamine transport system, including analogues 6-11 (Figure 3). These analogues specifically slow the synthesis of polyamines through down regulation of the biosynthetic enzymes ornithine decarboxylase (ODC) and S-adenosylmethionine decarboxylase (AdoMet-DC), but cannot substitute for the natural polyamines in terms of their cell growth and survival functions.1, 41 As will be discussed below, some but not all alkylpolyamine analogues are potent inducers of SSAT in cultured tumor cells, an effect that leads to the induction of apoptosis. The most successful of the symmetrically substituted polyamines analogues to date are the N,N’-bis (ethyl)polyamines shown in Figure 3: bis(ethyl)norspermine (BENSpm, 6), bis(ethyl)spermine (BESpm, 7),41-43 bis(ethyl)homospermine (BEHSpm, 8)41-43 and 1,20-(ethylamino)-5,10,15-triazanonadecane (BE-4444, 9).44, 45 These compounds have been shown to possess a wide variety of therapeutic effects, and illustrate the fact that small structural changes in alkylpolyamine analogues can result in surprisingly significant changes in biological activity.
Figure 3.
Structures of bis(ethyl)polyamines 6-11.
A major advantage of the bis(ethyl)polyamines lies in the fact that their synthesis is extremely straightforward, and depends only on the availability of the appropriate parent polyamine backbone.41, 46,47 These syntheses can also be readily scaled up to pilot-plant quantities. The N,N’-bis(ethyl)polyamines are readily transported into mammalian cells by the same transport mechanism as the natural polyamines.40 Treatment of mammalian cells with these analogues leads to a reduction of putrescine, spermidine and spermine, downregulation of ODC and AdoMetDC, and depending on the cell lines utilized, cytostasis or cytotoxicity.39, 41 These effects are often accompanied by a tremendous induction of SSAT activity, in some cases as much as 1000-fold. However, these data were collected using only symmetrically substituted (bis)alkylpolyamines, and as a result, only bis(ethyl)-substituted analogues were advanced to clinical trials. Among these analogues, the most promising were 6, which has a 3-3-3 backbone, 8, which has a 4-4-4 carbon skeleton and 9, which has a 4-4-4-4 architecture. Interestingly, BEHSpm proved to be useful as an antidiarrheal agent,48-50 and was advanced to clinical trials for this indication. Phase I and II clinical studies involving 6 revealed that it was safe for administration, and had minimal toxicity, but the compound did not show any significant clinical effects in patients with breast or lung malignancies.51-53 However, compelling pre-clinical data suggested that the study of additional polyamine analogues was warranted. It should be noted that the possibility of combination trials using 6 with standard chemotherapeutic agents is being considered to demonstrate synergy in in vitro models.54 Phase I clinical studies involving 8 were discontinued due to neuro- and hepatotoxicity issues. However, the toxicities observed with 8 could be reduced by the introduction of hydroxyl groups into the intermediate chain, such as in 11, probably due to the fact that these hydroxylated analogues are more rapidly cleared through phase 2 metabolism.48, 55
Conformationally Restricted Symmetrically Alkylated Polyamine Analogues
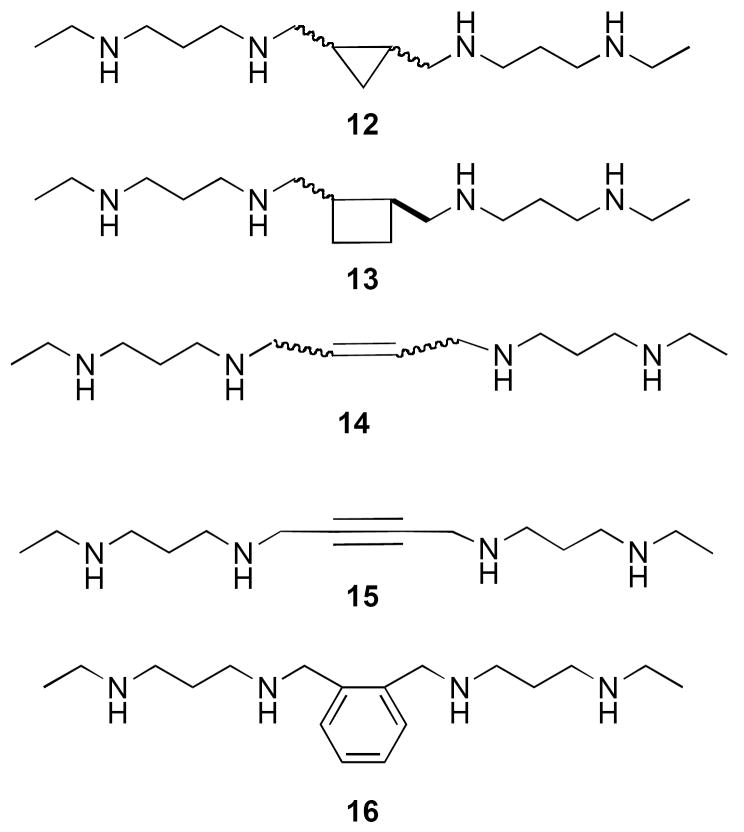
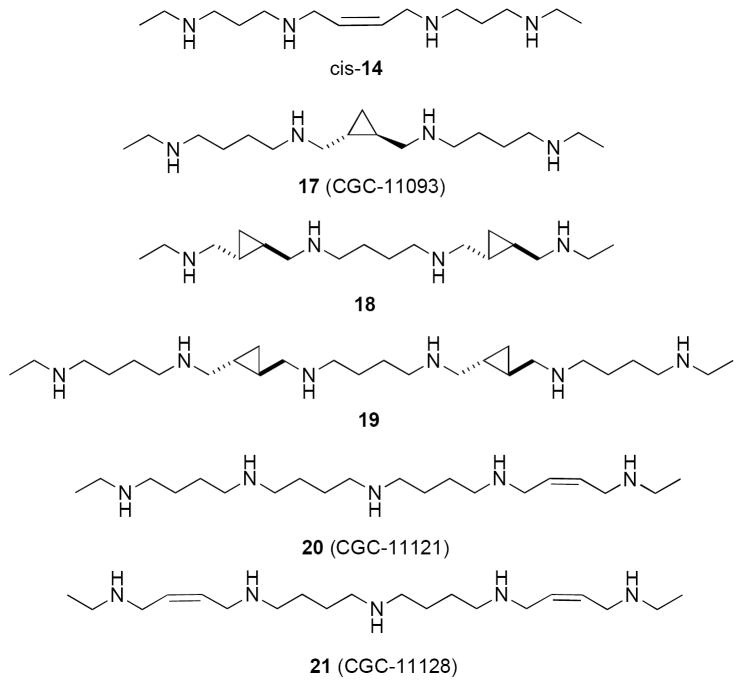
Specific alterations to the polyamine backbone structure of 6-11 has resulted in a series of “second generation” bis(ethyl)polyamines (Figure 4) with impressive antitumor and antiparasitic activity. Restriction of rotation in the central region of the polyamine chain in 8 by including a cis- and trans- cyclopropyl (12) or -cyclobutyl (13) ring, a cis- and trans double bond (14), a triple bond (15), and a 1,2-disubstituted aromatic ring (16) produced analogues with varying antitumor activity in a panel of human tumor cell lines (A549, HT-29, U251MG, DU145, PC-3 and MCF7).56 By contrast, insertion of a central dimethylsilane group resulted in a significant decrease in growth inhibition when compared to the bis(ethyl)polyamine analogues.57 It was later found that the cis-14 (CGC-11047, Figure 5) was an effective treatment for Cryptosporidum parvum infections, producing cures in a murine model.58 By contrast, the 4-4-4 (homospermine) analogue CGC-11093 (17, Figure 5), which contains a trans-cyclopropyl moiety in the central region, was an effective antitumor agent in vitro, and in vivo against DU-145 nude mouse xenografts.59 Compound 17 was recently found to be effective in combination with the proteosome inhibitor bortezomib for the treatment of myeloma. Compounds with a 4-4-4 or 4-4-4-4-4 backbone that featured trans-cyclopropyl or a trans-cyclobutyl moieties in non-central regions of the chain, were more active in vitro against prostate tumor cell lines (LnCap, DU145, DUPRO and PC-3), and inclusion of a cis unsaturation in one of the terminal aminobutyl groups also enhanced activity,60 presumably by enhancing DNA binding.61 The trans-bis-cyclopropyl analogues 18 and 19 (Figure 5) were effective antitumor agents against Du-Pro and DU-145 prostate tumor cells in vitro.62 In general, structural modifications to homospermine-like backbones that are analogous to those made to the backbone of 7 (i.e. cis-and trans-cyclopropyl, -cyclobutyl and double bond moieties) afforded analogues with enhanced antitumor activity and diminished systemic toxicity. In addition, insertion of a cis double bond into the terminal aminobutyl moieties of 9 (i.e. as in CGC-11121, 20 and CGC-11128, 21, Figure 5) also affords analogues that are equipotent to 9 with respect to ID50 values, but that are an order of magnitude more cytotoxic in a dose-response study.63 Recently, cis-14 was shown to effectively inhibit the growth of both small cell (NCI H82 and H69) and non-small cell (NCI A549 and H157) lung cancer cells in vitro.64 In the non-small cell lung cancer cells, a profound down regulation of ODC activity was observed, along with a significant increase in polyamine catabolism, polyamine pool depletion and an accumulation of cis-14. These effects were significantly less prominent in the small cell lung tumor lines. Importantly, cis-14 was found to significantly delay the progression of established tumors in an in vivo A549 xenograft model. Finally, 14 and 17 were found to cause significant suppression and regression of laser-induced choroidal neovascularization following periocular injection, most likely by initiating apoptosis in proliferating vascular cells.65 These data suggest a potential role for these analogues in the treatment of macular degeneration. Thus, cis-14 represents a promising new polyamine analogue that warrants further clinical evaluation.
Figure 4.
Conformationally restricted analogues of BESpm.
Figure 5.
Conformationally restricted bis(ethyl) polyamine analogues with enhanced antitumor activity against prostate tumor cells in culture.
Symmetrically Substituted Oligamines and Macrocyclic Polyamine Analogues
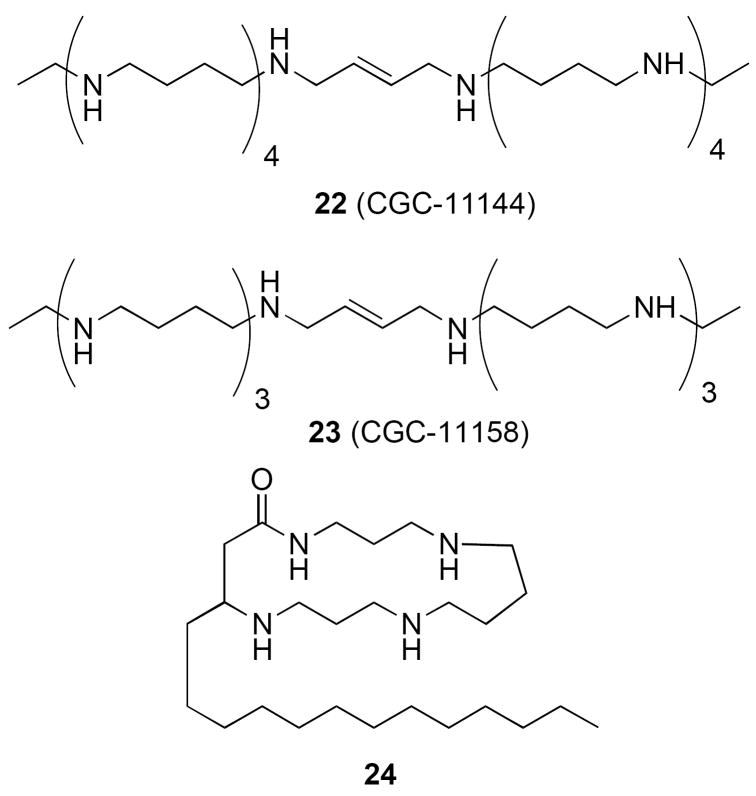
Recently, a series of bis(ethyl)oligamine analogues have been described that show promise as potential chemotherapeutic agents. In the limited series of oligamines that were evaluated, the decamine CGC-11144 (22) and the octamine CG-11158 (23) (Figure 6) proved to be most growth inhibitory against a panel of prostate tumor cells in vitro (LnCap, DU-145, DuPro and PC-3). These analogues exhibited IC50 values that were an order of magnitude lower than previously studies polyamine analogues. Not surprisingly, their activity roughly correlated with their ability to aggregate DNA.66 Compound 22 was also found to have effective antineoplastic action against human breast cancer cells in vitro and in vivo, and it was shown that multiple apoptotic mechanisms are associated with its cytotoxic effect in specific human breast cancer cell lines.67 These oligoamines significantly inhibit cell growth and activate AP-1 members c-Jun and c-Fos in MDA-MB-435cells,68 suggesting that c-Jun plays a protective role in oligoamine-induced cell death. In addition, 22 and two related oligamines specifically suppressed the mRNA and protein expression of estrogen receptor α and estrogen receptor target genes in the estrogen receptor-positive MCF-7 and T47D human breast cancer cell lines,whereas neither estrogen receptor β nor other steroid hormonal receptors were affected. These results suggest a novel antiestrogenic mechanism for specific polyamine analogues in human breast cancer cells.69 In addition to antitumor effects, compound 23 and related analogues have been shown to have significant inhibitory activity against Enterocytozoon bieneusi, the most common cause of chronic diarrhea in HIV/AIDS, and for which there is no effective therapy.70
Figure 6.
Structures of oligamines and macrocyclic polyamines with antitumor activity.
It has been shown that macrocyclic polyamines known as budmunchiamines71 act as potent antitumor agents by virtue of their ability to selectively deplete ATP. Based on this observation, a series of 5 macrocyclic polyamines with the representative structure 24 (Figure 6) were synthesized and evaluated as antitumor agents in the DuPro and PC-3 prostate cell lines.72 All 5 of these analogues were readily imported by cells, and caused a dramatic depletion of cellular polyamines. These compounds also proved to be cytotoxic in the tumor lines tested, and the degree of cytotoxicity roughly correlated to their ability to deplete ATP.
A relatively large number of symmetrically substituted alkylpolyamines have been synthesized, and a number of these were advanced to pre-clinical trials, and in some cases human clinical trials. A subset of these agents are continuing to be developed, and are now being evaluated in Phase I clinical trials. In these clinical trials, some adverse effects have been noted. Patients with advanced malignancies who were treated with 6 at a dose of 83 mg/kg/m2 developed a complex of unusual neurological symptoms that included unilateral weakness, dysphagia, dysarthria, numbness, paresthesias, and ataxia after treatment had been completed.73 In a similar study, doses of 6 that exceeded 94 mg/kg/m2 produced CNS dose-limiting toxicities characterized by aphasia, ataxia, dizziness, vertigo and slurred speech.53 A later Phase I study in patients with non-small cell lung cancer showed that 6 at 25-231 mg/m2 could be administered safely, and dose-limiting toxicities were mainly gastrointestinal.52 Clinical trials involving 8 were discontinued due to grade 3 or 4 adverse effects included nausea, vomiting, constipation, ileus, elevations of aspartate aminotransferase (AST) and alkaline phosphatase, hyperbilirubinemia, and ventricular bigeminy.74 Despite these findings, human clinical evaluation of second generation bis(ethyl) polyamine analogues such as 17 and 21-23 is warranted. A summary of the antitumor effects of symmetrically substituted polyamine analogues 4-24 appears in Table 1.
Table 1.
Summary of the antitumor effects of symmetrically substituted polyamine analogues 4-24. References for these data can be found in the text.
| Compound | Target | IC50 (μM) | Biological Effect | Cell Line(s) | Therapeutic Area | Status |
|---|---|---|---|---|---|---|
| 4c | unknown | not determined | cytotoxicity | L 1210 | antleukemic | abandoned |
| 5 | unknown | 3.0 | antiparasitic | Plasmodium falciparum | antimalarial | abandoned |
| 6 | SSAT, mitochondrial DNA synthesis | 0.2 – 1.0 | cytotoxicity; apoptosis | L 1210; MALME-3M | lung tumor; breast tumor; melanoma; antidiarrheal | Phase II trials terminated (lack of efficacy) |
| 7 | SSAT, mitochondrial DNA synthesis | 0.5 – 1.0 | cytotoxicity; apoptosis | L1210 | lung tumor; melanoma; antidiarrheal | abandoned |
| 8 | SSAT, mitochondrial DNA synthesis | 1.0 – 5.0 | cytotoxicity; apoptosis | MALME-3M | lung tumor; melanoma; antidiarrheal | abandoned |
| 9 | DNA | 0.1 – 1.0 | cytotoxicity; apoptosis | DU145; LnCaP; PC3 | prostate tumor; brain tumor | abandoned |
| 10 | SSAT, mitochondrial DNA synthesis | 3.0 – 5.0 | cytotoxicity; apoptosis | MALME-3M | lung tumor; melanoma; antidiarrheal | abandoned |
| 11 | SSAT, mitochondrial DNA synthesis | 0.6 | cytotoxicity | L 1210 | lung tumor; melanoma; antidiarrheal | abandoned |
| 12 | DNA | 0.3 - > 30 | cytotoxicity | A549, HT-29, U251MG, DU145, PC-3 and MCF7 | prostate tumor; breast tumor | abandoned |
| 13 | DNA | 0.1 - > 30 | cytotoxicity | A549, HT-29, U251MG, DU145, PC-3 and MCF7 | prostate tumor; breast tumor | abandoned |
| cis-14 | DNA | 0.25 – 3.6 (prostate) and 9.25 (breast) | cytotoxicity | A549, HT-29, U251MG, DU145, PC-3 and MCF7; Cryptosporidium parvum | prostate tumor; breast tumor, lung tumor; antiparasitic macular degeneration |
Phase I trials |
| 15 | DNA | > 30 | growth inhibition | A549, HT-29, U251MG, DU145, PC-3 and MCF7 | prostate tumor; breast tumor | abandoned |
| 16 | DNA | > 30 | growth inhibition | A549, HT-29, U251MG, DU145, PC-3 and MCF7 | prostate tumor; breast tumor | abandoned |
| 17 | DNA | 0.25 – 12.4 (prostate) and > 30 (breast) | cytotoxicity; apoptosis | A549, HT-29, U251MG, DU145, PC-3 and MCF7 | lung tumor; prostate tumor; myeloma; macular degeneration | Phase I trials |
| 18 | DNA | 0.06 – 0.26 | cytotoxicity; apoptosis | DuPro; DU145 | prostate tumor | Phase I trials |
| 19 | DNA | 0.12 – 0.38 | cytotoxicity; apoptosis | DuPro; DU145 | prostate tumor | Phase I trials |
| 20 | DNA | 0.08 - 0.52 | cytotoxicity; apoptosis | LnCaP; DU145; DuPro; PC-3 | prostate tumor | Phase I trials |
| 21 | DNA | 0.14 – 0.5 | cytotoxicity; apoptosis | LnCaP; DU145; DuPro; PC-3 | prostate tumor | Phase I trials |
| 22 | DNA | 0.09 – 0.47 | cytotoxicity; DNA aggregation | LnCaP; DU145; DuPro; PC-3; MDA-MB-435 | prostate tumor | advanced pre-clinical trials |
| 23 | DNA | 0.13 – 0.41 | cytotoxicity; DNA aggregation | LnCaP; DU145; DuPro; PC-3; MDA-MB-435; Enterocytozoon bieneusi | prostate tumor, chronic diarrhea | advanced pre-clinical trials |
| 24 | ATP | 0.6 – 0.83 | ATP depletion | DuPro; PC-3 | prostate tumor | unknown |
Unsymmetrical, Terminally Alkylated Polyamine Analogues
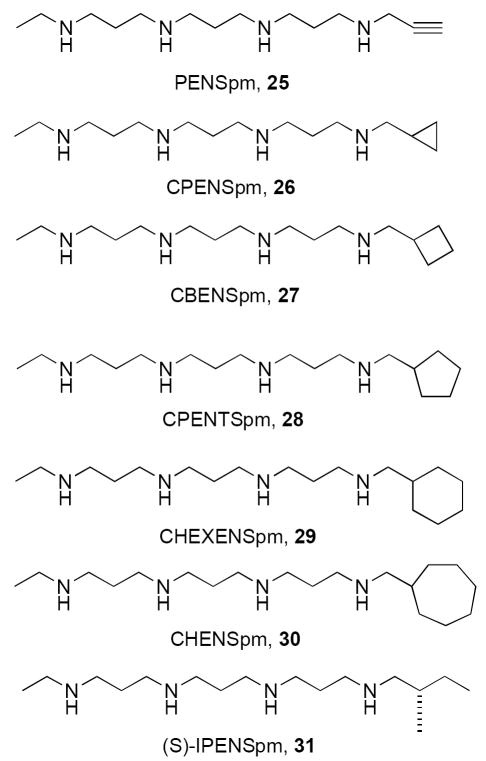
Structure activity studies involving the bis(ethyl)polyamines revealed much about the role of charge and flexibility in the polyamine backbone structure. However, the most useful compounds were symmetrically substituted with ethyl groups at the terminal nitrogens, and it was erroneously concluded that substituents of greater size than ethyl would render a molecule inactive. It was clear that unsymmetrically substituted alkylpolyamines needed to be synthesized to determine the optimal substituent pattern for the terminal nitrogens, and to explore the chemical space surrounding the terminal alkyl groups. The first examples in this series, N1-propargyl-N11-ethylnorspermine (PENSpm, 25) and N1-cyclopropylmethyl-N11-ethylnorspermine (CPENSpm, 26), which possess unsymmetrically substituted terminal nitrogens, were described in 1993 (Figure 7).75 In general, the synthesis of these and other unsymmetrically substituted analogues is more difficult, since it requires selective protection and deprotection of the internal and external nitrogens. Studies involving unsymmetrically substituted alkylpolyamine analogues such as 25-31 (Figure 7) were extensively discussed in earlier publications,76-86 and in our previous Perspective.1 The data described in these manuscripts clearly support the contention that there are at least two mechanisms by which unsymmetrically substituted alkylpolyamines produce cytotoxicity in NCI H157 non-small cell lung carcinoma cells, and that each of these mechanisms appears to lead to apoptosis as a common endpoint.81, 85 Similar results were obtained in the DU145 and PC-3 androgen receptor-negative prostate cell lines, and the LnCap androgen receptor-positive prostate cell lines,84 as well as in several breast tumor cell lines.79, 83 The selective cytotoxicity of alkylpolyamines in these cell lines may be due to the fact that the analogues are concentrated in tumor cells, wherein the polyamine transport system is significantly up regulated.82
Figure 7.
The unsymmetrically substituted alkylpolyamines PENSpm, CPENSpm, CBENSpm, CPENTSpm, CHEXENSpm, CHENSpm and IPENSpm.
In addition to production of apoptosis by at least 2 mechanisms, unsymmetrically substituted alkylpolyamines can have dramatically different effects on the cell cycle.80 Symmetrically substituted alkylpolyamines such as 6-11, and unsymmetricl alkylpolyamines with small substituents such as 25 and 26, either produce no effect on cell cycle, or induce a G1 cell cycle arrest, while analogues with one large substituent (e.g. 30 and 31) produce a G2/M cell cycle arrest.86
Chemical Diversity in Unsymmetrical Alkylpolyamine Analogues
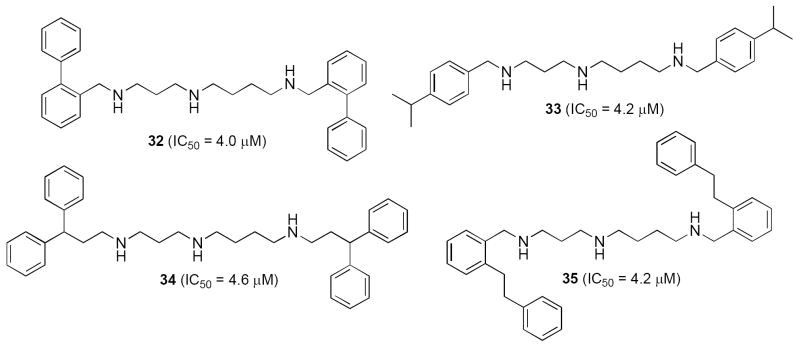
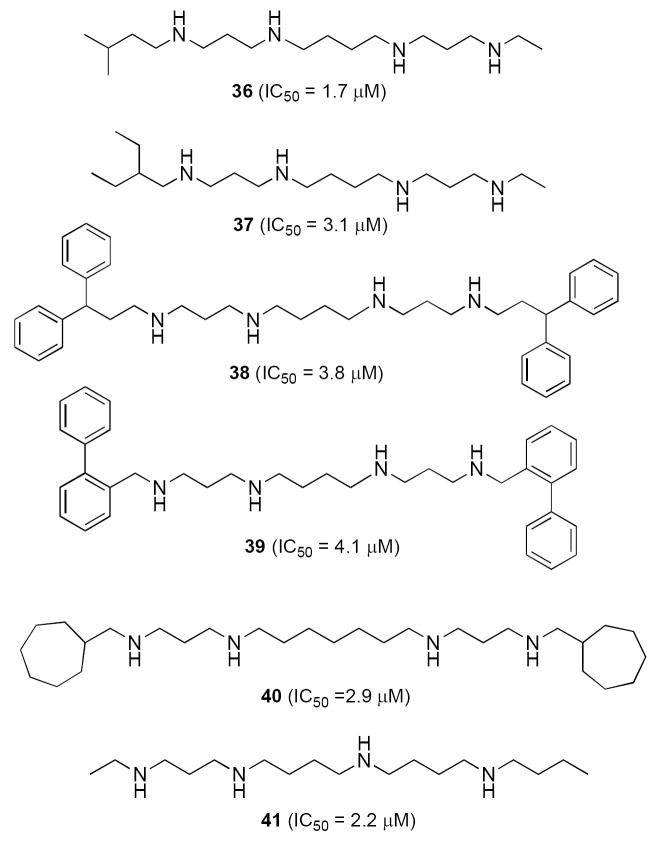
The symmetrically and unsymmetrically substituted alkylpolyamines described above have been of great value in determining the mechanisms of analogue-induced cytotoxicity. However, the alkyl substituents in these molecules are representative of only a minute portion of the available chemical diversity for the terminal alkyl substituents. Recently, more than 120 alkylpolyamines were synthesized and evaluated as antitumor agents in an effort to develop an SAR model for alkylpolyamine analogues with antitumor activity. Preliminary biological evaluation of these analogues was conducted using a high throughput screen based on MTT cell viability determination in NCI H157 lung tumor cells. These analogues were designed with varying polyamine backbone structure (3-4, 3-3-3, 3-4-4 and 3-7-3), and variation of the terminal alkyl substituents has been attempted to determine the optimal overall structure for antitumor effects. As previously described,75, 79, 83-88 alkylpolyamine analogues have a variety of cellular effects, including cytotoxicity, induction of apoptosis, disruption of tubulin polymerization, induction of spermidine/spermine-N1-acetyltransferase (SSAT) and spermine oxidase (SMO), and inhibition of trypanothione reductase. To date, 27 alkylpolyamine analogues have been identified that exhibit IC50 values of 5.0 μM or less against the NCI H157 non-small cell lung tumor line in vitro, as shown in Figures 8-10. Many of these analogues share the properties of 6, in that they are taken up into tumor cells by the polyamine transporter, where they deplete polyamines and down regulate polyamine biosynthetic enzymes, but do not substitute for the natural polyamines in terms of their cellular function. Analogues of spermidine exemplified by compounds 32-35 (3-4 architecture, Figure 8) are capable of producing potent antitumor effects, although in this series symmetrically substituted compounds with terminal aralkyl substituents were much more potent than symmetrically substituted alkylpolyamines or unsymmetrically substituted alkyl- or aralkylpolyamines. Six compounds based on the natural polyamine spermine with a 3-4-3, 3-5-3 or 3-4-4 polyamine backbone were also shown to possess IC50 values less than 5 μM, as seen in Figure 9 (36-41).
Figure 8.
Alkypolyamine analogues with a 3-4 architecture that possess antitumor activity in vitro.
Figure 10.
Alkypolyamine analogues with a 3-3-3 architecture that possess antitumor activity in vitro.
Figure 9.
Alkypolyamine analogues with a 3-4-3, 3-5-3 or 3-4-4 architecture that possess antitumor activity in vitro.
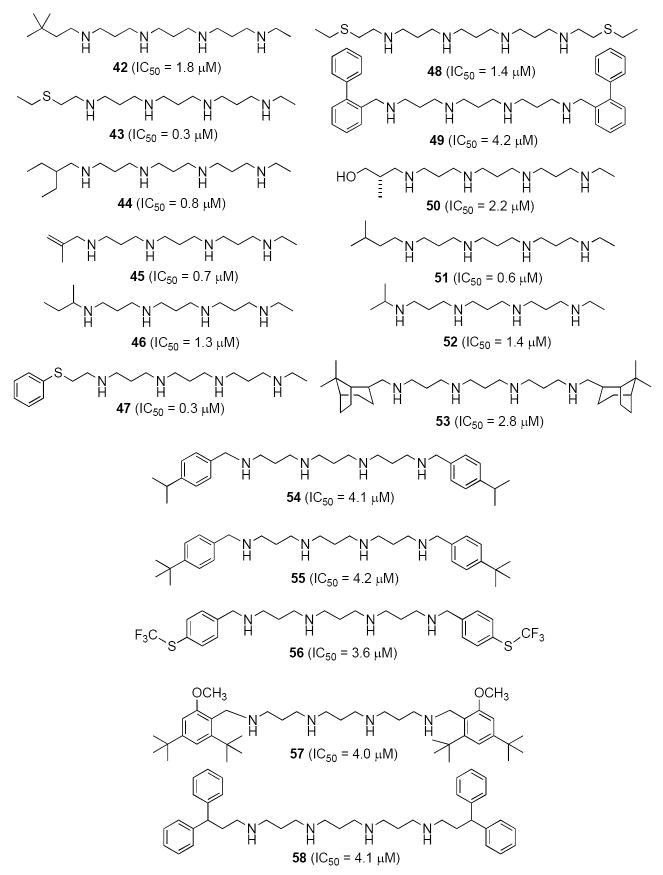
A primary synthetic goal in this area has been to produce a large number of norspermine (3-3-3) analogues related to the parent compound 6. Numerous symmetrically- and unsymmetrically substituted derivatives have been produced that incorporate chemical diversity into the terminal alkyl substituent as a means to elucidate the structure-activity relationships for alkylpolamine analogues with antitumor activity. As shown in Figure 10, seventeen 3-3-3 derivatives (compounds 42-58) with IC50 values of 5 μM of less have been discovered. Subsequent studies have shown that these analogues produce cellular effects (cytotoxocity, apoptosis, induction of SSAT and SMO, and alteration of cellular polyamine levels) in varying degrees. It was also observed that slight changes in chemical structure often effect large changes in biological response in tumor cells in vitro. It is noteworthy that the antitumor alkylpolyamine analogues shown in Figure 10 display a variety of functionalities that have not previously been reported in analogues of this type. Active analogues were identified possessing aralkyl and alkyl substituents on the terminal nitrogens, both symmetrical and unsymmetrical, that in some cases contain sulfur, oxygen or fluorine atoms. A full description of the cellular effects of all of these analogues is not included here, but will be reported in an upcoming manuscript.
Based on a number of factors, including IC50, effects on SSAT and SMO levels and other parameters, three of these analogues, compounds 42 (PG11400), 46 (PG11401) and 50 (PG11402) were selected for scale-up and more extensive pre-clinical trials in vivo. Despite having similar structures, 42, 46 and 50 show different dose-response profiles against four lung tumor cell lines (H157, H82, H69 and A549, data not shown) over a range of doses between 0.1 and 100 μM. Importantly, 42 does not produce significant SSAT induction in any of these lung tumor cell lines, while 46 and 50 produce SSAT induction in the H157 line of the same magnitude as 14. By contrast, 42 initiates a 30-fold induction of SMO, and is 3-6 times more effective in this regard than 46, 50, 14 and 17. The in vivo implications of the variation in these cellular effects between analogues and among different cell lines remains to be determined. Compounds 42, 46 and 50 have recently been evaluated in an A549 human lung tumor xenograft model in athymic nu/nu Fox Chase mice. Mice were treated with either 75, 100 or 150 mg/kg of each agent twice a week over a period of 70 days. Compounds 42 and 50 proved to be most effective at inhibiting tumor growth, although 42 produced toxicity at the 100 mg/kg dose. In all three cases, mean body weights of the animals were comparable to control animals. The toxicity observed during treatment with 42 was not observed during treatment with 50, and as shown in Figure 11, 50 was effective in limiting tumor growth by 65% at both the 75 and 150 mg/kg doses. The reduced in vivo toxicity observed during treatment with 50 may be due to the presence of a hydroxyl moiety in one terminal alkyl substituent, consistent with what was observed for compound 11.55 Additional in vivo experiments are being conducted using compounds 42, 46 and 50, and additional analogues in this series are being synthesized and used to determine the structural requirements for binding at the various alkylpolyamine effector sites. A summary of the antitumor effects of unsymmetrically substituted polyamine analogues selected for pre-clinical studies appears in Table 2.
Figure 11.
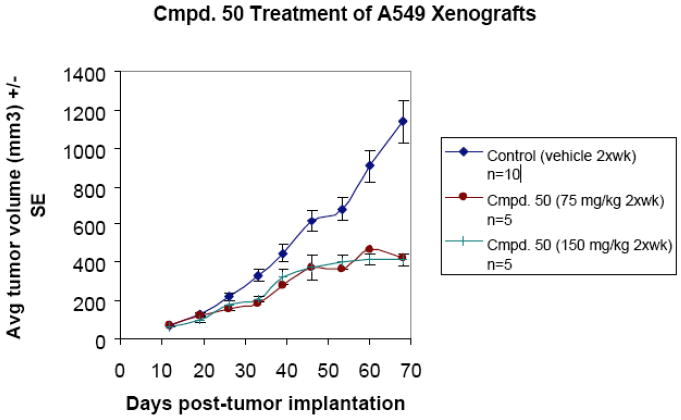
Antitumor effects of compound 50 in an A549 human lung tumor xenograft model. Animals were given either 75 or 150 mg/kg of 50 twice weekly by i.p. injection.
Table 2.
Summary of the antitumor effects of selected unsymmetrically substituted polyamine analogues. References for these data can be found in the text.
| Compound | Target | IC50 (μM) | Biological Effect | Cell Line(s) | Therapeutic Area | Status |
|---|---|---|---|---|---|---|
| 26 | SSAT | 0.2 - 1.3 | SSAT induction, production of peroxide, apoptosis | H157; MCF-7; T47D; ZR-75-1; MDA-MDB-468; MDA-MB-231; Hs578T | lung tumor; breast tumor | advanced pre-clinical trials |
| 30 | unknown; polyamine metabolic enzymes | 0.7 | apoptosis; disruption of tubulin depolymerization | H157; H82 | lung tumor | not currently in development |
| (S)-31 | unknown; polyamine metabolic enzymes | 1.3 | apoptosis; disruption of tubulin depolymerization | H157; H82 | lung tumor | not currently in development |
| 32 | polyamine metabolic enzymes | 4.0 | apoptosis | H157 | lung tumor | not currently in development |
| 33 | polyamine metabolic enzymes | 4.2 | apoptosis | H157 | lung tumor | not currently in development |
| 34 | polyamine metabolic enzymes | 4.6 | apoptosis | H157 | lung tumor | not currently in development |
| 35 | Polyamine metabolic enzymes | 4.2 | apoptosis | H157 | lung tumor | not currently in development |
| 36 | polyamine metabolic enzymes | 1.7 | apoptosis | H157 | lung tumor | not currently in development |
| 37 | polyamine metabolic enzymes | 3.1 | apoptosis | H157 | lung tumor | not currently in development |
| 38 | polyamine metabolic enzymes | 3.8 | apoptosis | H157 | lung tumor | not currently in development |
| 39 | polyamine metabolic enzymes | 4.1 | apoptosis | H157 | lung tumor | not currently in development |
| 40 | polyamine metabolic enzymes | 2.9 | apoptosis | H157 | lung tumor | not currently in development |
| 41 | polyamine metabolic enzymes | 2.2 | apoptosis | H157 | lung tumor | not currently in development |
| 42 | polyamine metabolic enzymes | 0.9 – 9.1 | induction of SMO (A549); polyamine depletion; apoptosis | H157; A549; H69; H82 | lung tumor | pre-clinical |
| 46 | polyamine metabolic enzymes | 0.8 – 3.6 | induction of SSAT (H157); polyamine depletion; apoptosis | H157; A549; H69; H82 | lung tumor | pre-clinical |
| 50 | polyamine metabolic enzymes | 0.7 – 7.1 | induction of SSAT (H157); polyamine depletion; apoptosis | H157; A549; H69; H82 | lung tumor | pre-clinical |
| 58 | unknown; polyamine metabolic enzymes | 4.1 | apoptosis; anoikis | H157 | lung tumor | abandoned |
Structure/Activity Relationships for Alkylpolyamine Analogues
Early structure/activity correlations for alkylpolyamines were based on data from symmetrically alkylated polyamine analogues such as 6-11 (Figure 3). These studies suggested that monoalkylation at both terminal nitrogens of spermidine or spermine was important for optimal antiproliferative activity, and that alkylation at an internal nitrogen reduced in vitro activity.41 It was further determined that the greatest induction of SSAT was dependent on the presence of “protected” aminopropyl or aminobutyl moieties.40, 78, 89, 90 Terminal nitrogen bis(alkyl) substituents larger than ethyl led to analogues exhibiting a dramatic reduction in antitumor activity.43 These studies also suggested that compounds with a 3-3-3 carbon skeleton are more effective that the corresponding 3-4-3 analogues, and that spermine-like compounds are more effective that spermidine-like analogues. Hydroxylation of the central carbon in the terminal aminopropyl chain of spermine analogues afforded alkylpolyamine analogues that retained potency, but had reduced toxicity, presumably due to more rapid clearance.48, 55 The introduction of conformationally restricted bis(ethyl)polyamines demonstrated the importance of flexibility in the central butane moiety, wherein highly rigid analogues such as 15 and 16 had poor activity.56 The somewhat more flexible cis/trans isomeric pairs resulting from central cyclopropane- cyclobutane- or ethylene moieties in the central chain were more active than the unrestricted parent compounds, but there was no significant difference in potency between the isomers.56, 61 Inclusion of multiple cis unsaturations in the terminal aminobutyl chains of longer polyamines such as 21-23 (Figures 5 and 6) also produced active analogues.
The structure-activity relationships for unsymmetrically substituted alkylpolyamines appear to be more complex than those for the symmetrically-substituted analogues. This complexity most likely arises from the fact that not all of the effector sites for these analogues have been identified. The result is that seemingly small changes in structure can have dramatic effects of antiproliferative activity, induction of SSAT, down regulation of polyamine biosynthetic enzymes, and other parameters.1 Unsymmetrically substituted alkylpolyamines can exhibit varying degrees of SSAT and/or SMO induction based on the size of their terminal alkyl substituents, and these analogues can induce these enzymes as well or better than the parent analogues 6-8. An SAR model for SSAT inducers and non-inducers has been described.1 Among SSAT inducers, terminal substituents can vary in size from small (e.g. ethyl, cyclopropylmethyl) to medium size, however, the size of only one of the substituents can be increased beyond ethyl. The central nitrogens are seperated by 5.0-5.8 angstroms, and each terminal nitrogen is 5.0 angstroms from the adjacent central nitrogen. The cytotoxicity of agents (e.g. 25 and 26, Figure 7) that fit these criteria can be directly related to the superinduction of SSAT, which induces apoptosis through the overproduction of hydrogen peroxide. Among SSAT non-inducers, the requirement for bis-alkyl substitution remains, but the binding pockets for the terminal alkyl substituents seem to be less restrictive. The most active analogues have one small (ethyl) or medium sized alkyl substituent, and one large terminal substituent. Agents with two large terminal alkyl substituents may possess activity, but are not likely to superinduce SSAT. In active analogues, the central nitrogens are separated by 5-10 angstroms, and the terminal nitrogens are roughly 5 angstroms away from the respective internal nitrogens. Steric bulk on the intermediate chain is not well tolerated. Agents that fit these criteria (e.g. 5, Figure 2 and 30 and 31, Figure 7) produce apoptosis in the same time frame as the SSAT inducers, but through as yet undetermined pathways. Importantly, expansion of the central carbon chain to a 3-7-3 or 3-8-3 architecture can act as antiparasitic agents that are effective against trypanosomes and Microsporidia.87, 88, 91
As outlined above, a large number of symmetrically- and unsymmetrically substituted alkylpolyamine analogues for evaluation as antitumor and/or antiparasitic agents. These analogues were designed with varying polyamine backbone structure (3-4, 3-3-3, 3-4-4 and 3-7-3),75, 79, 83-88 and have a variety of cellular effects, including cytotoxicity, induction of apoptosis, disruption of tubulin polymerization, induction of SSAT and SMO, and inhibition of trypanothione reductase. Analogues of spermidine (3-4 architechture) are capable of producing potent antitumor effects, such as compounds 32-35 shown in Figure 8, although in this series the symmetrically substituted compounds with aralkyl substituents on the terminal nitrogens were much more potent than symmetrically substituted alkylpolyamines or unsymmetrically substituted alkyl- or aralkylpolyamines. Compounds based on the natural polyamine spermine with a 3-4-3, 3-5-3 or 3-4-4 polyamine backbone were also shown to possess IC50 values less than 5 μM, as seen in Figures 9 and 10. The SAR for these analogues conforms to that observed for SSAT inducers and non-inducers. It is noteworthy that these antitumor alkylpolyamine analogues display a variety of functionalities that have not previously been reported in analogues of this type. Active analogues were identified possessing aralkyl and alkyl substituents on the terminal nitrogens, both symmetrical and unsymmetrical, that in some cases contain sulfur, oxygen or fluorine atoms. Additional SAR studies are required to determine the optimal structures for interaction with various known and unknown polyamine effector sites.
Polyamine/Metal Complexes
Polyamine/Metal Complexes as Cancer Chemotherapeutics
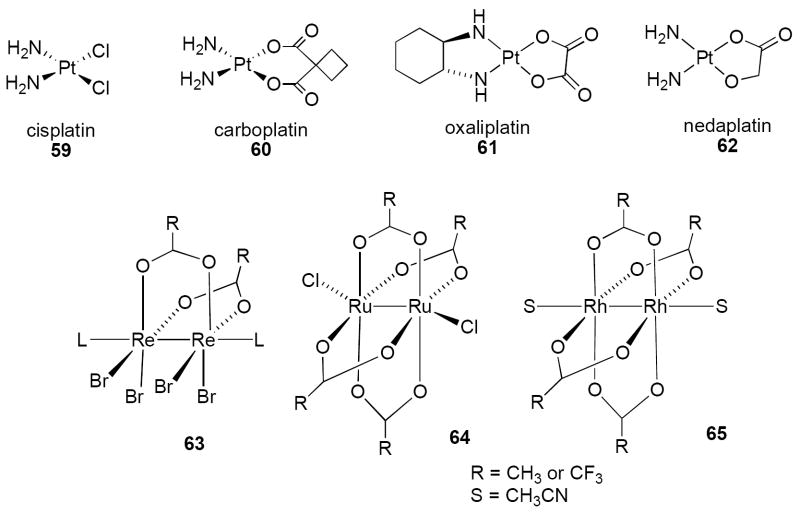
The serendipitous discovery of cisplatin by Rosenberg in 196992 opened a new era for metallopharmaceuticals. The antitumor agents cisplatin 59 and carboplatin 60 (Figure 12) exhibit broad-spectrum activity against epithelial cancers, testicular and ovarian cancers, and cancers of the lung, head and neck, esophagus, bladder, cervix, and endometrium. Compound 59 forms a variety of stable bifunctional adducts with DNA that block replication and inhibit transcription, and treated cells undergo apoptosis93 characterized by cell volume reduction and chromatin condensation.94 It is well established that Pt-containing compounds exhibit antitumor activity by forming specific adducts with DNA,94, 95 binding preferentially to sequences containing two or more adjacent guanosine residues, and form 1,2 or 1,3 intrastrand substitution adducts of the type (NH3)2-Pt-GpG, (NH3)2-Pt-ApG, or (NH3)2-Pt-GpXpG, where A=adenosine, G=guanine and X= any nucleotide residue.94, 95 Unfortunately, cisplatin, and to a lesser degree carboplatin, have substantial renal, neurologic, and emetogenic toxicities, and resistance to these agents develops through reduction in cellular import, enhanced cytoplasmic detoxification and enhanced repair of DNA adducts. Second generation Pt(IV) and Pt(II) drugs exhibit high therapeutic activity and have somewhat reduced toxicity, but only two additional Pt drugs have joined 59 and 60 in the clinic: oxaliplatin 61 and nedaplatin 62 (Figure 12).96 More recently, the nucleotide binding properties of transition metal complexes including rhenium (Re), rhodium (Rh) and ruthenium (Ru) that assume a “lantern-type” geometry, such as the dinuclear compounds 63, 64 and 65 (Figure 12), were shown to possess significant antitumor activity in vitro and in vivo. These compounds form unique bonds involving substitutions at the N7 position of purines, the O6 position of guanine and the N6 position of adenine.97, 98 More recently, it has been demonstrated that dirhodium complexes related to 7 form unprecedented intrastrand DNA adducts, with the dirhodium unit cross-linking in the major groove at residues C5 and A6.99 Based in part on these findings, there has been renewed interest in the development of metal-based therapy for human cancer.96
Figure 12.
Platinum, rhenium, rhodium and ruthenium compounds with demonstrated antitumor activity.
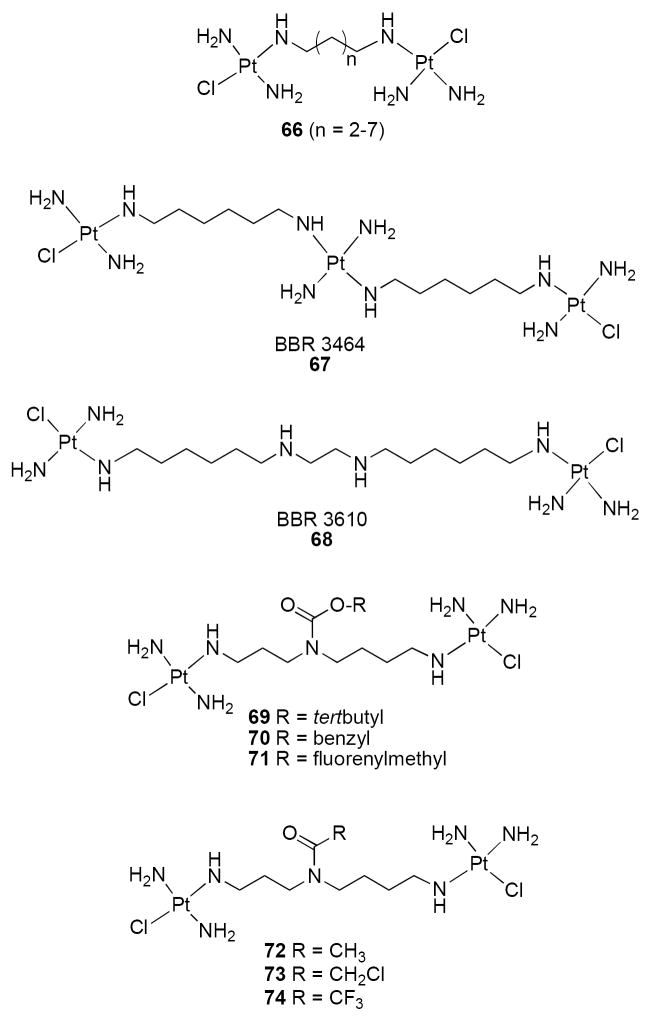
Early studies indicated that dinuclear bis(platinum) complexes in which the metal centers were separated by diaminoalkanes (compounds 66, Figure 13) were more potent than 59 against murine leukemia cells, murine solid tumor cells and four human tumor cells in vitro, and had good activity against 59-resistant cell lines.100 In the corresponding xenografts, the version of 66 in which n = 3 showed greater activity in the HCT-8 colon adenocarcinoma and H23 non-small cell lung tumor lines, but diminished potency in the AH125 and H520 non-small cell lung tumor lines, when compared to 59. Complexes between Pt and spermidine were subsequently described101, 102 that were shown to alter the secondary structure of DNA, but that had unremarkable antitumor effects.101, 103 However, the trinuclear Pt compound known as BBR 3464 (67, Figure 13), in which three Pt centers are separated by diaminohexyl spacers,104, 105 was shown to produce cytotoxicity in L1210 mouse leukemia cells that was 30 times greater than 59 in vitro, and to bind to DNA in a time-dependent manner104 that was distinct from the binding pattern of 59.105 Continuing studies confirm that 67 is more potent than 59, forms distinct bifunctional DNA adducts,106 and induces cell cycle arrest in tumor cells.107, 108 Clinical trials have now been initiated to evaluate 67 as a treatment for gastric or gastroesophageal adenocarcinoma109 and non-small cell lung carcinoma.109, 110 Recently, the compound BBR3610 68, a cogener of 67, was shown to produce cytotoxicity superior to 67 through a caspase 8-dependent mechanism that is enhanced by ERBB1/PI3K inhibitors that activate BAX and caspase 9.111 In order to increase the therapeutic index of Pt/spermidine complexes, three carbamate prodrugs (69-71, Figure 13) were synthesized and evaluated for antitumor activity;112 only the Fmoc-protected analogue 71 showed any enhanced cytotoxicity. This approach was later extended to acetyl-protected spermidine-bridged dinuclear platinum complexes 72-74 (Figure 13).113 The rate of hydrolysis of these prodrugs corresponded to the electronegativity of the amide functional group, and thus 74 (R = trifluoromethyl) produced the greatest effect in vitro. The fact that 74 produces cytotoxicity at micromolar levels suggests that the prodrug approach is valid.
Figure 13.
Dinuclear and trinuclear Pt/polyamine complexes with significant antitumor activity.
Drugs containing Pt or other transition metals have not found widespread utility in the treatment of advanced breast cancer, but recent data indicate that Pt compounds can be useful. Preclinical studies indicate that a mouse antibody against HER2 synergizes with 59 through a mechanism involving inhibition of the repair of Pt-induced DNA damage.114, 115 Subsequent phase II clinical trials in patients with HER2-positive breast cancer combining the anti-HER2 humanized antibody trastuzumab in combination with 59 or 60 and docetaxel have shown promising results.116 In addition, Pt analogues might be particularly useful in breast cancers harboring BRCA1 or BRCA2 mutations, since mouse-derived BRCA1- negative cell lines have been shown to be five-fold more sensitive to 59 when compared to wild-type cells.117
Polyamine/Metal Complexes with Pt, Re, Rh and Ru
As outlined above, there are compelling reasons to pursue the discovery and development of new antitumor therapies containing Pt or other transition metals. To date, the structure/activity relationships of polyamine/Pt complexes have not been adequately studied. Further, polyamine metal complexes of Re, Rh and Ru have not been synthesized, and the utility of these metals in cancer chemotherapy is largely unexplored. Based on the promise of the polyamine/Pt complexes described above and the promising antitumor activity of Re, Rh and Ru complexes, compounds containing a metal complex with known DNA binding and antitumor activity, combined with a polyamine sidechain such as those found in 25-58 with demonstrated pro-apoptotic antitumor effects in breast tumor cells,67, 79, 83, 84, 118, 119 would be expected to be a highly effective for the treatment of breast cancer. It is hypothesized that these complexes would retain the antitumor activity of a mono- or dinuclear metal complex, as well as the pro-apoptotic antitumor effects of the polyamine side chain, as outlined above. In addition, such complexes could, in theory, have three distinct advantages over the individual constituents. Specifically, the polycationic polyamine sidechain could increase the water solubility of the metal complexes, which is usually limited, and could potentially enhance the affinity of the metal for DNA.
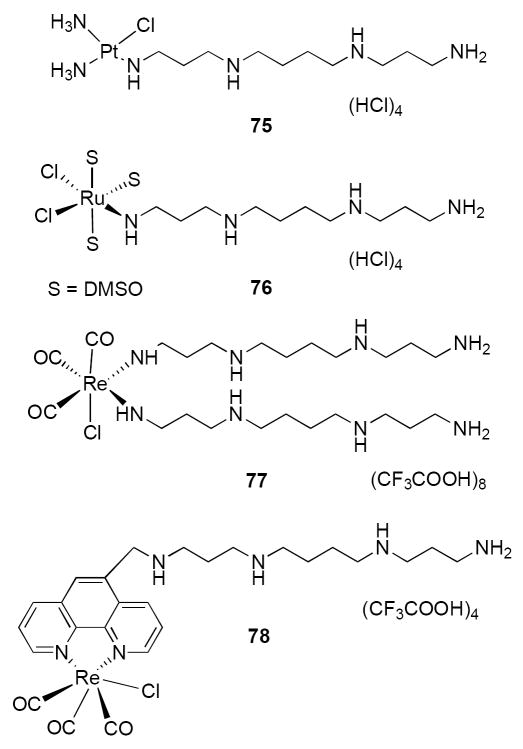
In order to test the hypothesis outlined above, an initial library of 25 polyamine/metal complexes containing Pt, Re, Rh or Ru centers was synthesized. These analogues were synthesized from commercially available metal complexes and polyamine fragments that have been previously described.75, 86, 87, 120, 121 Eleven of these analogues possess IC50 values of <10.0 μM in an H157 non-small cell lung cancer MTT assay (see below), with 3 of the 11 having IC50 values of <1.0 μM. Four of these analogues, Pt compound 75, Ru compound 76 and Re compounds 77 and 78 (Figure 14), were selected for dose-response studies using an MTT assay in the MCF7 breast tumor cell line. All compounds were evaluated over a range of concentrations between 1 and 100 μM. The results of these studies are shown in Figure 15. Polyamine metal complexes 75, 76, 77 and 78 exhibited IC50 values of 6.6, 3.8, 9.3 and 6.9 μM, respectively, while the IC50 value for 59 could not be determined under these conditions (59 produced 56% inhibition at 300 μM, data not shown). Importantly, these results demonstrate that polyamine complexes containing 3 distinct (Pt, Ru and Re) metal centers possess antitumor activity against MCF7 breast tumor cells in vitro.
Figure 14.
Representative polyamine/metal complexes with antitumor activity in vitro. S = solvent (DMSO).
Figure 15.
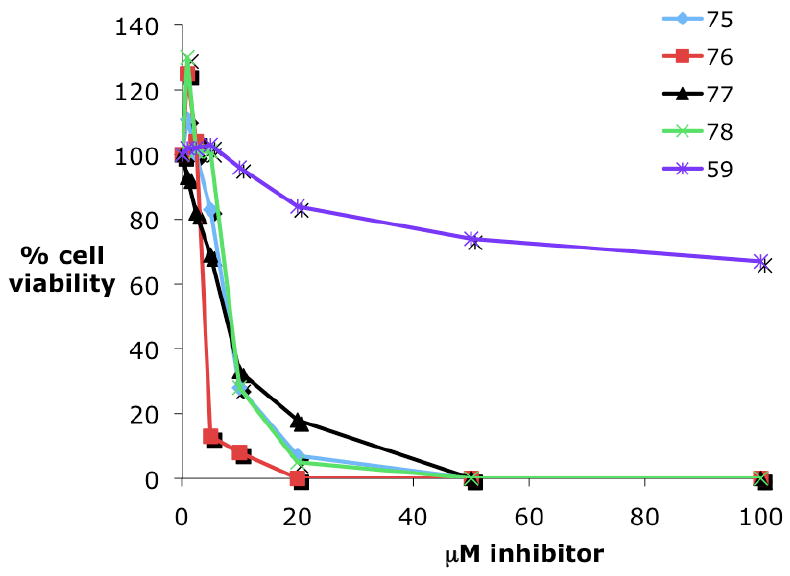
MTS assay demonstrating growth inhibition caused by 75, 76, 77 and 78 in cultured MCF7 breast tumor cells.
Polyamine metal complexes 75, 76, 77 and 78 were next evaluated for their ability to bind to isolated DNA, as shown in Figure 16. Compound 75 formed more extensive crosslinks with plasmid DNA than cisplatin (Figure 16, Panel A). This DNA binding was found to be concentration dependent between 3 and 300 μM (Figure 16, Panel B) and time dependent over a period of 6 hours (Figure 16, Panel C). Compounds 76, 77 and 78 produced similar results (not shown) and were more effective than cisplatin with respect to DNA crosslink formation. In each case, the observed DNA binding was concentration- and time-dependent with kinetics similar to 75. These data clearly demonstrate that polyamine metal complexes represent antitumor agents that are more potent than the cisplatin, and show a greater ability to bind to DNA. It should be noted that 4 reasonable lead compounds for drug discovery have been identified from a library of only 25 analogues, and that active analogues can be identified that incorporate transition metals (Ru, Re) not previously used for cancer chemotherapy.
Figure 16.
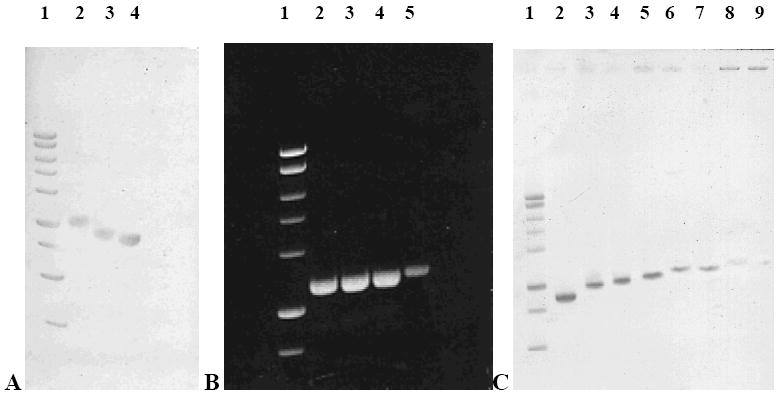
DNA binding characteristics of compound 75. Linearized plasmid DNA (1.5μg) was incubated with appropriate concentrations of metal complexes at 37°C for the indicated time. Samples were analyzed on 0.8% agarose gel. Panel A. Comparison of Lane 1: MW standard; Lane 2: DNA + 30 μM 75; Lane 3: DNA + 30 μM cisplatin; Lane 4: plasmid DNA control. Panel B. Concentration dependence of DNA binding to 75. A 1.5μ G sample of plasmid DNA was treated with 3, 10, 30, 100 and 300 μM 75 at 37°C for 4 hours. Lane 1: MW standard; Lane 2: 10 μM 75; Lane 3: 30 μM 75; Lane 4: 100 μM 75; Lane 5: 300 μM 75. Panel C. Time-dependence of DNA binding to 75. From left to right - Lane 1: MW standard; Lane 2: Plasmid DNA control; Lane 3: 5 min; Lane 4: 15 min; Lane 5: 30 min; Lane 6: 1 h; Lane 7: 2 h; Lane 8: 4 h; Lane 9: 6 h.
Polyamine-based Histone Deacetylase (HDAC) Inhibitors
HDACs and HDAC Inhibitors in Normal and Transformed Cells
Chromatin architecture is a key determinant in the regulation of gene expression, and this architecture is strongly influenced by post–translational modifications of histones.122, 123 Histone protein tails, consisting of up to 40 amino acid residues, contain lysine residues that protrude through the DNA strand, and these residues act as a site for post-translational modification of chromatin, allowing alteration of higher order nucleosome structure.124 Multiple post-translational modifications of histones125, 126 mediate epigenetic remodeling of chromatin, with the dynamic process of acetylation/deacetylation being one of the best-characterized processes.122, 127 Transcriptional repression dominates at times when specific DNA promoter CpG-island methylation occurs, and HDAC activity recruited by specific repressor complexes, which are recruited to these promoters, is an important event in epigenetic silencing.126, 128 The acetylation status of histones is controlled by a balance between two enzymes, histone acetyltransferase (HAT), which promotes histone hyperacetylation, and the histone deacetylases (HDACs) which catalyze acetyl group cleavage.127, 129 Acetylation of the ε-amino groups of lysine residues located at N-termini removes the positive charge and reduces the affinity of the histone proteins/nucleosome for the DNA,124, 127 allowing greater access to DNA by transcription factors and RNA polymerase, thus promoting gene expression.
Normal mammalian cells exhibit an exquisite level of control of chromatin architecture by maintaining a balance between HAT and HDAC activity.130 There are 11 known zinc-dependent HDAC isoforms131-133 that can be divided into 4 structural classes: class I (isoforms 1,2,3 and 8),134 class IIa (isoforms 4, 5, 7 and 9),131 class IIb (isoforms 6 and 10)131 and class IV (HDAC 11).131 An additional class of HDAC, termed Class III, is comprised of enzymes similar to the NAD-dependent yeast transcriptional repressor protein Sir2.135, 136 These HDACs do not require zinc, and are unresponsive to known Class I/II HDAC inhibitors. Despite extensive study, it is still uncertain which HDAC isoforms play the most important roles in human cancer.
In some tumor cells, hypoacetylation of histones results in aberrant gene silencing leading to the under-expression of growth regulatory proteins such as the cyclin dependent kinase inhibitor p21Waf1, and this scenario contributes to the development of cancer. 122, 127 Histone hyperacetylation caused by HDAC inhibitors such as trichostatin A (TSA, 79, Figure 17), N-(2- aminophenyl)-4-[N-(pyridin-3-ylmethoxycarbonyl)-aminomethyl]benzamide (MS-275, 80, Figure 17) and suberoylanilide hydroxamic acid (SAHA, 81, Figure 17) can cause growth arrest in a wide range of transformed cells, and can inhibit the growth of human tumor xenografts.127, 129, 134, 137 HDAC inhibitors restore quasi-normal cell cycle and apoptotic functions in human tumor cells. Clinical studies indicate that HDAC inhibitors such as 80 and 81 are effective therapies for human cancer,138 but dose-limiting toxicity remains a problem, and step-down dosing of the HDAC inhibitor is often required to minimize toxicity.139 Known HDAC inhibitors fall into several structural classes including short chain carboxylic acids (valproate, butyrate), hydroxamic acids (79, 81), benzamides (e.g. 80) and cyclic peptide inhibitors such as apicidin and depsipeptide.137 Recent studies have identified isoform-selective inhibitors of HDACs 1, 3 and 8140 and HDAC 6,141 but do not provide structural information that can aid in the design of more specific inhibitors. Such molecules would be of great value in elucidating the roles of HDAC isoforms in both normal and cancer cells. In vitro models and phase I clinical trials have demonstrated that synergistic re-expression of genes that are silenced through promoter methylation can be achieved by the sequential application of DNA methyltransferase (DNMT) inhibitors and HDAC inhibitors,142-145 demonstrating a correlation between chromatin remodeling and clinical efficacy. Although they are effective both in vitro and in vivo, HDAC inhibitors typified by 79-81 suffer from lack of specificity among the various forms of HDAC, including deacetylases that target non-histone proteins, and can produce unacceptable toxicity in non-cancerous cells. Effective HDAC inhibitors with minimal inherent toxicity would be useful for combination therapy with DNA methyltransferase inhibitors such as 5-azacytidine (5-AC) and 5-aza-2’-deoxycytidine.
Figure 17.
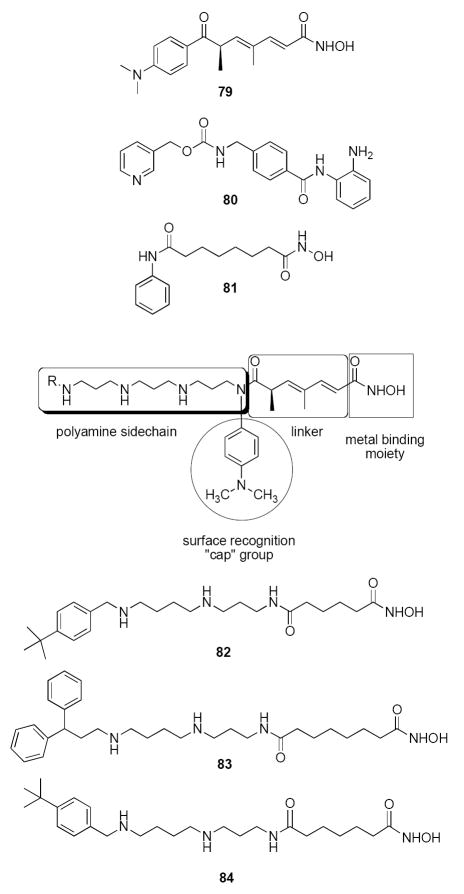
Structures of the classical HDAC inhibitors 79-81, the general structure for polyamine-based HDAC inhibitors, and PAHAs 82-84.
Polyaminohydroxamic Acid Analogues
HDAC inhibitors such as 79-81 possess three structural features that are required for optimal activity: an aromatic cap group, an aliphatic chain and a metal binding functional group (Figure 17). Structure/activity studies involving analogues of 79-81 have focused largely on modifications to the aromatic ring moiety and aliphatic linker region present in these molecules.137 Structurally, there is a high degree of sequence homology between Class I and II HDACs in the channel and surface recognition portion of the proteins. However, there is reduced sequence homology in the rim area that lies outside of the binding pocket.134 One strategy for targeting specific HDAC isoforms is to design molecules that exploit the variation in structure in this rim area. We and others have reported alkylpolyamine analogues related to 2 and 3 (see Figures 1 and 7-10) that enter cells using a specific polyamine transporter1, 7 and exert antitumor effects due to their high affinity for DNA and other effector sites.1, 7, 59, 66, 75, 86, 87, 146, 147 Thus, a series of polyaminohydroxamic acid (PAHA) derivatives was proposed that incorporate structural features of alkylpolyamines such as 4-58, and the metal binding moiety, linker and cap groups found in molecules such as 80 and 81. It was postulated that these compounds could enter cells using the polyamine cellular transporter,and be selectively directed to DNA and the associated HDACs by the positively charged polyamine portion of the structure. An additional advantage to this approach is that it may ultimately be possible to produce isoform specific inhibitors for individual HDACs by altering the polyamine chain composition and the terminal alkyl group on that chain, taking advantage of the fact that histone deacetylases differ in primary sequence at specific residues in the rim region.134
An initial series of 16 PAHA analogues was synthesized and screened for inhibitory activity against a mixture of HDACs derived from HeLa cell lysates.120 Two of these compounds, 82 and 83 (Figure 17), inhibited HDAC in this assay by 75% at a 1.0 μM concentration. Compound 83 at 1.0 μM was subsequently found to produce significantly greater induction of acetylated histone H3 (AcH3) and acetylated histone H4 (AcH4), and was markedly more effective at promoting re-expression of the cyclin dependent kinase inhibitor p21Waf1, in the ML-1 mouse leukemia cell line. However, 83 exhibited an IC50 value of 10 μM in ML-1 leukemia cells following a 7-day treatment, as compared to 0.25 μM and 0.75 μM for 79 and 80, respectively. Following the synthesis of additional PAHAs, 6 new analogues were found that inhibited HDACs in a HeLa cell lysate by 50% or greater. Among these derivatives, compound 84 (Figure 17) inhibited the HDAC mixture by 51.5%, but caused a 253-fold induction of acetylated α-tubulin while only causing a minimal increase in AcH3, AcH4 and p21Waf1.148 These data strongly suggest that 84 shows a marked selectivity towards HDAC6, which is known to deacetylate α-tubulin.149
Polyaminobenzamide Analogues
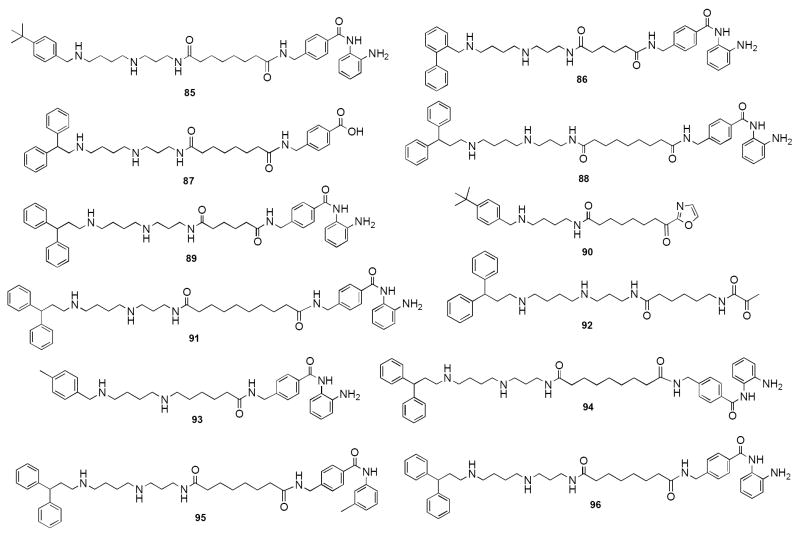
Using the same design strategy, a series of 40 polyaminobenzamides (PABAs) and their homologues were synthesized and evaluated as inhibitors of HDAC.148 Representative structures for this class are shown in Figure 18. Compound 85, one of the first entries in the series, exhibits an IC50 value against global HDAC from the HeLa cell lysate of 4.9 μM, which is comparable to the reported IC50 value for 80 (4.8 μM).150 When the purified HDAC isoforms became available, 85 was evaluated as an inhibitor of the class I HDAC 3, and the class II HDAC 6. Although 85 was an effective inhibitor of global HDAC, it had little activity against either HDAC 3 or 6, suggesting that the observed inhibition of global HDAC was due to inhibition of one or more of the remaining HDACs. Additional analogues in the PABA series (for example 86-96) were evaluated against 4 HDAC isoforms representing Class I (HDAC 1, 3 and 8) and Class II (HDAC 6). In these analogues, structural modifications were made in the linker chain length, in the polyamine substituent and in some cases in the metal binding moiety (87, 90, 92 and 95). As can be seen in Table 3, isoform selectivity among the 4 HDACs evaluated varied significantly, demonstrating that global percent HDAC inhibition is a composite of strong and weak inhibition at different isoforms. For example, 87 produced a 72.6% inhibition of global HDAC at a 5 μM concentration, and inhibited the Class II HDAC 6 by 97.9%, but acted as a significantly weaker inhibitor against the Class I HDACs 1, 3 and 8. By contrast, 91, which inhibited global HDAC by a moderate 42.9%, was selective for HDAC 1 (78.7% inhibition), but has almost no effect against HDACs 3, 6 and 8. In our hands, the traditional HDAC inhibitor 81 at 5μM produced a 97.1% inhibition of global HDAC (Table 3), but exhibited no selectivity for HDACs 1, 3, 6 or 8. These data suggest that the observed HDAC selectivity with PAHAs such as 84, and PABAs such as 87 and 91 may be in part due to structural variations in their polyamine side chains. The synthesis of additional analogues and accompanying molecular modeling studies are being conducted to determine if interaction of the polyamine side chain with the rim region of HDAC 8151 provides a basis for isoform selectivity.
Figure 18.
Structures of PABA analogue inhibitors of HDAC 85-95.
Table 3.
IC50 values (MCF 7 and MCF10A breast tumor cells) and percent inhibition of global HDAC, and of HDAC 1,3,6 and 8 by compounds 81 and 85-96. ND = not determined
| Compound | IC50 in MCF7 cells (μM) | IC50 in MCF10A cells (μM) | Global HDAC % inhibition | HDAC 1 % inhibition | HDAC 3 % inhibition | HDAC 6 % inhibition | HDAC 8 % inhibition | Status |
|---|---|---|---|---|---|---|---|---|
| 81 | 8.5 | 31 | 97.2 | 91.40 | 97.0 | 97.20 | 94.20 | marketed (vorinostat) |
| 85 | ND | ND | 51.2 | ND | 1.3 | 11.7 | ND | pre-clinical trials |
| 86 | > 30 | > 30 | 60.1 | 68.6 | 72.9 | 3.8 | 14.8 | not currently under development |
| 87 | > 30 | > 30 | 72.6 | 45.6 | 35.4 | 97.9 | 48.6 | not currently under development |
| 88 | > 30 | > 30 | 47.0 | 79.9 | 25.0 | 17.5 | 13.4 | not currently under development |
| 89 | > 30 | > 30 | 48.4 | 61.9 | 0.3 | 3.6 | 10.4 | not currently under development |
| 90 | 27 | > 30 | 43.7 | 19.5 | 0.3 | 9.7 | 18.9 | not currently under development |
| 91 | > 30 | > 30 | 42.9 | 78.7 | 13.2 | 0.3 | 11.9 | not currently under development |
| 92 | > 30 | > 30 | 30.2 | 0.5 | ND | ND | 0.00 | not currently under development |
| 93 | 0.9 | 24.0 | 48.1 | 73.5 | ND | ND | 8.6 | pre-clinical trials |
| 94 | > 30 | > 30 | 47.0 | 79.9 | 25.0 | 17.5 | 13.4 | pre-clinical trials |
| 95 | > 30 | > 30 | 42.6 | 0.0 | 3.0 | 3.0 | 0.0 | pre-clinical trials |
| 96 | > 30 | > 30 | 66.9 | 65.2 | 18.6 | 15.0 | 13.9 | pre-clinical trials |
As mentioned above, one of the potential advantages of incorporating polyamine side chains into PAHA and PABA HDAC inhibitors was that the resulting molecules could utilize the polyamine transport system. Cellular uptake of polyamine-based drug molecules presents a particular advantage in most tumor cells, where the polyamine transport system is highly up regulated as compared to normal cells.8 Although polyamine transport proteins have been characterized for bacteria, yeast and protozoans, an analogous transport system in mammalian cells has not been identified. There is recent evidence that mammalian polyamine transport is mediated through caveolin 1-dependent endocytosis,152, 153 and that this process may involve electrostatic attraction of polyamines by the glypican subfamily of glycosylphosphatidylinositol-anchoredcell-surface heparan sulfate proteoglycans (HSPG).152 These data are consistent with the finding that hydroxamic acid-containing PAHAs, with pKa values of approximately 9, are not substrates for the mammalian transporter, while PABAs such as 85 are effectively imported using the polyamine transport system.148
PABA Analogues in Breast Cancer
The most active global HDAC inhibitors in the PABA series were evaluated against MCF7 wild type tumor cells in vitro, and variable growth inhibitory activity was observed. Although PABA analogues 85-96 show interesting selectivity among HDAC isoforms 1, 3, 6 and 8, only 2 of these analogues produced significant cytotoxicity in tumor cells in vitro. The antitumor activity of compounds 91, 92 and 93 are shown in Figure 19. Over a range of concentrations between 0.3 and 30 μM, benzamide 91 appeared to stimulate tumor cell growth, while 92, which features a shorter linker chain region and an α-ketoamide metal-binding moiety, was cytostatic. However, benzamide 93, featuring a shorter polyamine substituent, was cytotoxic in the MCF7 cell line, with an IC50 of 0.9 μM. In the MCF10A normal breast epithelial cell line, 93 exhibited an IC50 of 24 μM, and thus demonstrated significant selectivity for tumor cells. Under the same conditions, the marketed inhibitor 81 (also known as vorinostat) exhibited IC50 values of 8.5 μM (MCF7) and 31 μM (MCF10A), and thus 93 compares favorably to known HDAC inhibitors that are currently in use in the clinic. Microscopic examination of treated cells revealed that cytotoxicity was mediated by apoptosis in MCF7 cells treated with 93 (data not shown). Subsequent experiments showed that 93, but not 91, promoted the induction of annexin A1 (Figure 20), which is considered an early intermediate in the apoptosis pathway.154 HDAC inhibitors have been shown to promote apoptosis through induction of annexin A1, and recent studies suggest that breast tumors expressing high levels of annexin I are more likely to respond to neoadjuvant chemotherapy than cells with low levels of the protein.155
Figure 19.
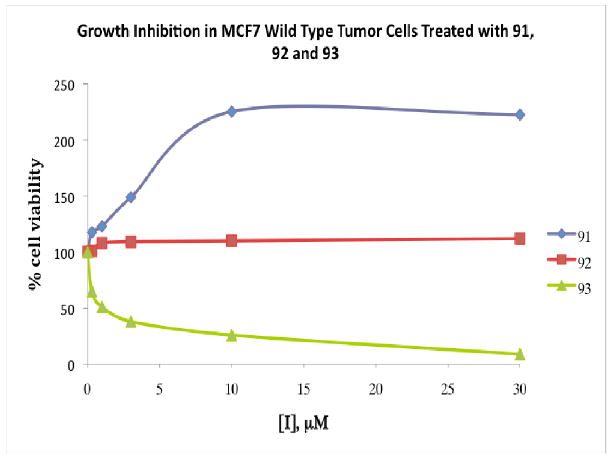
Dose-response curves following 96 hour treatment of MCF7 wild type tumor cells with 91, 92 or 93. Data points are the average of three determinations that in all cases differ by < 5%.
Figure 20.
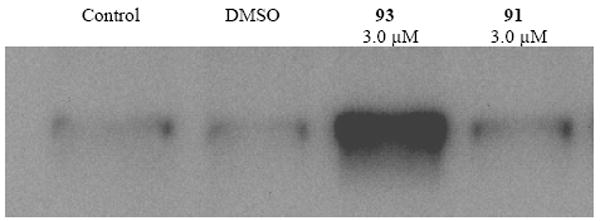
Induction of Annexin I by PABA HDAC inhibitors 91 and 93.
Polyamine-based HDAC Inhibitors in Other Diseases
It is tempting to assume that HDAC inhibitors that do not produce frank cytotoxicity cannot be used clinically, but such is not the case. In cancer chemotherapy, traditional HDAC inhibitors are used clinically in combination with other classes of antitumor compounds that promote apoptosis, and thus their tumor cell cytotoxicity is acceptable. Combination therapy is based on the supposition that inhibition of HDAC restores tumor cells to a pseudo-normal cell cycle, making them susceptible to agents that cause apoptosis. However, aberrant HDAC activity plays a role in other diseases where a non-toxic HDAC inhibitor would be of great value. Recent studies have suggested that histone acetylation status plays a role in the development of diabetes mellitus,156, 157 and that HDAC inhibitors may serve as a novel class of agents for the treatment of diabetes and its complications.158, 159 As described above, a number of PAHA and PABA analogues produce significant epigenetic effects in cells (e.g. re-expression of tumor suppressor factors and increased acetylation of histones) with little or no cytotoxicity. We thus evaluated PABA HDAC inhibitors for their cytoprotective effects against IL-1β-induced damage to isolated β-cells. Three PABAs, 94-96 (Figure 18) significantly inhibited HDAC activity and increased the acetylation of histone H4 in isolated β-cells. These compounds further exerted no toxic effects on metabolic cell viability in these cells. However, among the three compounds tested only 94 protected against IL-1β-mediated loss in β-cell viability.160 Remarkably, compound 94 differs from 96, which does not protect against this loss in β-cell viability, by a single carbon in the linker chain region. Compound 94 was also able to attenuate IL-1β-induced iNOS expression and subsequent NO release. These data suggest that the cytoprotective properties of 94 against IL-1β-mediated effects may, in part, be due to inhibition of IL-1β-induced transactivation of NFκB in these cells. A novel HDAC inhibitor such as 94 that can prevent IL-1β -mediated effects on isolated β-cells supports the development of non-toxic PABAs to prevent deleterious effects of cytokines and the onset of autoimmune diabetes.
Polyamines and LSD1 Inhibition
Epigenetic Control of Gene Expression by Lysine-Specific Demethylase 1
As stated above, in cancer, DNA promoter hypermethylation in combination with other chromatin modifications, including decreased activating marks and increased repressive marks on histone proteins 3 and 4, have been associated with the silencing of tumor suppressor genes.161 The important role of promoter CpG island methylation and its relationship to covalent histone modifications has recently been reviewed.162 As was mentioned above, the N-terminal lysine tails of histones can undergo numerous post-translational modifications, including phosphorylation, ubiquitination, acetylation and methylation.127, 163, 164 To date, 17 lysine residues and 7 arginine residues on various histone proteins have been shown to undergo methylation,165 and lysine methylation on histones can signal transcriptional activation or repression, depending on the specific lysine residue involved.166-168 All known histone lysine methyltransferases contain a conserved SET methyltransferase domain, and it has been shown that aberrant methylation of histones due to SET domain deregulation is linked to carcinogenesis.169 Histone methylation, once thought to be an irreversible process, has recently been shown to be a dynamic process regulated by the addition of methyl groups by histone methyltransferases and removal of methyl groups from mono- and dimethyllysines by lysine specific demethylase 1 (LSD1), and from trimethyllysines by specific Jumonji C (JmjC) demethylases.163, 164, 170, 171 Additional demethylases in the JmjC methylase class are continuing to be identified. It has recently been demonstrated that LSD1 demethylates K370me1 and K370me2 on the p53 tumor suppressor and transcriptional activator protein, indicating that LSD1 also plays a role in demethylation of non-histone proteins.172 A key positive chromatin mark found associated with promoters of active genes is histone 3 dimethyllysine 4 (H3K4me2).173, 174 LSD1, also known as BHC110,163, 175 catalyzes the oxidative demethylation of histone 3 methyllysine 4 (H3K4me1) and H3K4me2, and thus is associated with transcriptional repression. H3K4me2 is a transcription-activating chromatin mark at gene promoters, and demethylation of this mark by LSD1 may prevent expression of tumor suppressor genes important in human cancer.176 Thus, LSD1 is emerging as an important new target for the development of specific inhibitors as a new class of antitumor drugs.177
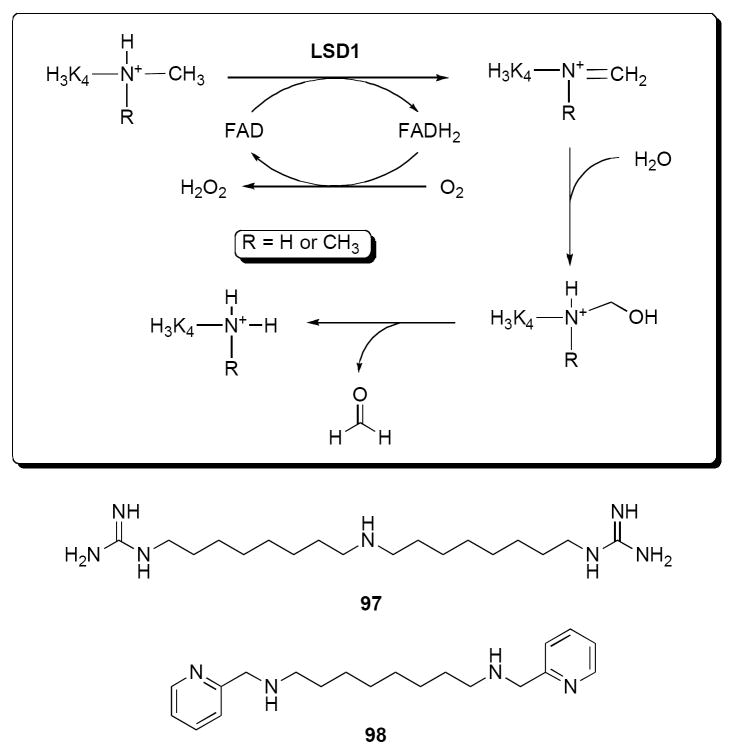
The X-ray structure and mechanism of LSD1 have recently been reported. As shown in Figure 21, the LSD1 reaction is an FAD-dependent oxidative demethylation that most likely proceeds through a protonated imine intermediate.178 The Km for H3K4me2 has been determined to be 30 μM,163 which is comparable to substrate Km values for other histone-modifying enzymes. To date, only a few compounds have been shown to act as inhibitors of LSD1. The active site structure of LSD1 has considerable sequence homology to monoamine oxidases A and B (MAO A and B), and to acetylpolyamine oxidase (APAO) and spermine oxidase (SMO).163, 179, 180 The MAO inhibitors nialamide, clorgyline, deprenyl, and pargyline were devoid of any inhibitory activity, while phenelzine and tranylcypromine effectively inhibited LSD1 at 1 mM. The most potent inhibitor, tranylcypromine was titrated to reveal an IC50 of < 2 μM. LSD1 inhibition by tranylcypromine was subsequently shown to be irreversible and mechanism-based, and it increased global levels of H3K4me2 in the P19 cell line. However, no evidence was presented to show whether tranylcypromine caused the re-expression of silenced tumor suppressor genes. It has recently been demonstrated that the polyaminoguanidine guazatine (97, Figure 21) is a non-competitive inhibitor of maize polyamine oxidase.181 Importantly, guazatine can discriminate between purified recombinant murine APAO and SMO, suggesting that it is possible to selectively inhibit these enzymes.182 Recently, a series of N,N’-bis[(pyridinyl) methyl]-diamines typified by BPOD (98, Figure 21) were shown to produce competitive inhibition of purified plant polyamine oxidase (PAO) in vitro.183
Figure 21.
The catalytic mechanism of LSD1, and the amine oxidase inhibitors 97 and 98.
Polyamine Analogues, LSD1 Inhibition and Re-expression of Silenced Gene Products
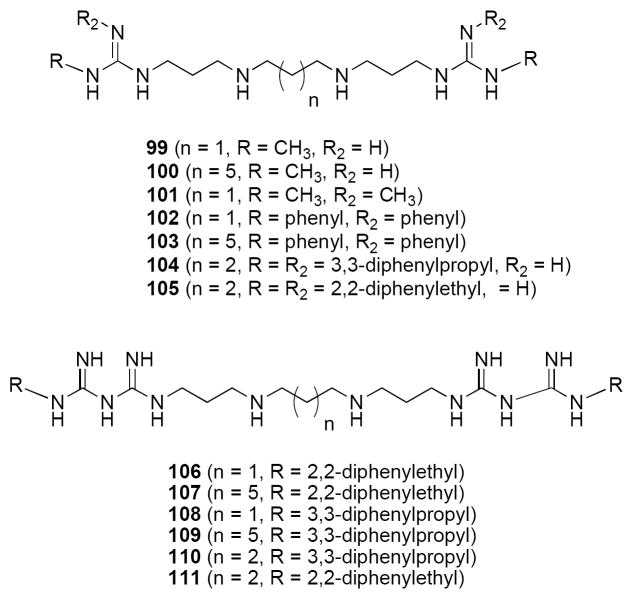
We reported the synthesis of a novel series of polyamino(bis)guanidines and polyaminobiguanides with potent trypanocidal activity in vitro,88 and subsequently found these analogues to be effective inhibitors of APAO and SMO (unpublished observations). Because LSD1 is a similar flavin-dependent oxidase, we evaluated small library of these bisguanidine (99-105, Figure 22) and biguanide (106-111, Figure 22) polyamine analogues for the ability to inhibit recombinant LSD1 in vitro. Nine of the 13 compounds tested were found to non-competitively inhibit recombinant LSD1 activity by >50% at 1 μM.176 Although these analogues also inhibit APAO and SMO to some degree, they are significantly less potent in that regard. The most potent LSD1 inhibitor, 109 (90.9% inhibition), was chosen for further study. In many cancer cell types, H3K4me2 is depleted in the promoters of several epigenetically silenced, and aberrantly DNA-hypermethylated genes that are important in tumorigenesis.184 In HCT116 human colon tumor cells, exposure to 1 μM 109 produced significant increases in both H3K4me1 and H3K4me2, while not affecting the global histone 3 dimethyllysine 9 (H3K9me2) levels. Following 48-hour treatment of HCT116 cells with 1.0 μM 109, four members of the secreted frizzle-related protein family (SFRP1, SFRP2, SFRP4, and SFRP5)185 and two GATA family transcription factors (GATA4 and GATA5)186 were re-expressed. Compound 109 produced similar effects on re-expression of SFRP4 and SFRP5, and global H3K4me2 levels in RKO colon cancer cells. Treatment with 109 resulted in ~20-35% of the gene re-expression produced by 1 μM of the DNA methyltransferase inhibitor 5-AC, whereas no measurable expression was seen after treatment with the HDAC inhibitor 79 (300 nM), or 1 μM of the related analogues 102 or 107. These results demonstrate that 109, although not as potent as 5-AC, is effective at re-expressing epigenetically silenced genes. Importantly, compound 109 and its cogeners are the first examples of synthetic LSD1 inhibitors that produce epigenetic changes leading to re-expression of silenced tumor suppressor genes. Promising in vivo effects of treatment with 109 alone and in combination with 5-AC have recently been observed in an HCT166 human colon tumor xenograft model in athymic nu/nu Fox Chase mice. The results of these studies are being reported in an upcoming primary publication. Taken together, these data support the contention that LSD1 inhibitors that promote re-expression of aberrantly silenced tumor suppressor genes can be of benefit when used in combination with established antitumor agents. Additional analogues in this series are being synthesized and evaluated as a means to develop structure/activity relationships for this novel class of LSD1 inhibitors.
Figure 22.
Structures of polyamino(bis)guanidines 99-105 and polyaminobiguanides 106-111.
Conclusion
Prior to 1990, antitumor drug discovery research in the polyamine area was characterized by the design, synthesis and development of polyamine biosynthesis inhibitors. Despite a great deal of research, development of specific inhibitors as antitumor agents has only resulted in advancing one compound to the market. The discovery of the bis(ethyl)polyamines in the early 1990’s made it clear that the natural polyamines have numerous effector sites in tumor cells, and that therapeutic agents designed to interact with effector sites distinct from the biosynthetic enzymes can be developed for use in chemotherapy. The latest trend, the inclusion of polyamine-like features in drug candidates for the purpose of enhancing transport or DNA affinity, has opened the door to new targets and new avenues of investigation. Thus, polyamine research in drug development remains a robust area of exploration, with great potential for the identification of new antitumor agents.
Acknowledgments
Portions of the work described in this manuscript were supported by NIH RO1 CA85509 (PMW), and by a generous gift from Progen Pharmaceuticals, Toowong, Queensland, Australia (PMW, RAC).
Abbreviations
- DFMO
α-difluoromethylornithine
- ODC
ornithine decarboxylase
- AdoMet-DC
S-adenosylmethionine decarboxylase
- BENSpm
bis(ethyl)norspermine
- BESpm
bis(ethyl)spermine
- BEHSpm
bis(ethyl)homospermine
- BE-4444
1,20-(ethylamino)-5,10,15-triazanonadecane
- PENSpm
N1-propargyl-N11-ethylnorspermine
- CPENSpm
N1-cyclopropylmethyl-N11-ethylnorspermine
- MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- SSAT
spermidine/spermine-N1-acetyltransferase
- SMO
spermine oxidase
- HDAC
histone deacetylase
- HAT
histone acetyltransferase
- SAHA
suberoylanilide hydroxamic acid
- DNMT
DNA methyltransferase
- 5-AC
5-azacytidine
- PAHA
polyaminohydroxamic acid
- PABA
polyaminobenzamide
- HSPG
heparan sulfate proteoglycans
- LSD1
lysine-specific demethylase 1
- JmjC
jumonji C demethylases
- H3K4me1
histone 3 methyllysine 4
- H3K4me2
histone 3 dimethyllysine 4
- MAO
monoamine oxidase
- APAO
acetylpolyamine oxidase
- SFRP
secreted frizzle-related protein
- SAR
structure/activity relationships
- CNS
central nervous system
Biographies
Robert A. Casero, Jr., Ph.D. is a Professor of Oncology in the Johns Hopkins University School of Medicine. Dr. Casero is a molecular pharmacologist who has spent most of the last 30 years studying the role of polyamines in normal and tumour cell growth, and devising strategies to target polyamine function and metabolism for therapeutic benefit. His laboratory was responsible for cloning several genes involved in human polyamine catabolism; genes whose expression are thought to play a role in determining cellular responses to specific polyamine analogues. Dr. Casero is also a scientific advisor to Progen Pharmaceuticals.
Patrick M. Woster, Ph.D. is Professor of Pharmaceutical Sciences in the Eugene Applebaum College of Pharmacy and Health Sciences at Wayne State University. Dr. Woster is a medicinal chemist with an interest in the synthesis of molecules that modulate polyamine metabolism as potential antitumor agents. He has produced a number of enzyme inhibitors that target enzymes in the polyamine biosynthetic pathway, and synthesized the first unsymmetrically substituted alkylpolyamine analogues. Molecules developed in the Woster laboratory have been shown to produce dramatic effects on a variety of tumor cells by initiating apoptosis, binding to DNA and by producing epigenetic changes in gene expression. Dr. Woster currently serves as a consultant to Progen Pharmaceuticals.
Footnotes
This manuscript is dedicated to a mentor and friend, Professor Edward B. Roche, in honor of his 70th birthday.
References
- 1.Casero RA, Woster PM. Terminally alkylated polyamine analogues as chemotherapeutic agents. J Med Chem. 2001;44(1):1–26. doi: 10.1021/jm000084m. [DOI] [PubMed] [Google Scholar]
- 2.Porter CW, Sufrin JR. Interference with polyamine biosynthesis and/or function by analogs of polyamines or methionine as a potential anticancer chemotherapeutic strategy. Anticancer Res. 1986;6(4):525–542. [PubMed] [Google Scholar]
- 3.Mamont PS, Duchesne MC, Grove J, Bey P. Anti-proliferative properties of DL-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978;81(1):58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]
- 4.Danzin C, Marchal P, Casara P. Irreversible inhibition of rat S-adenosylmethionine decarboxylase by 5’-([(Z)-4-amino-2-butenyl]methylamino)-5’-deoxyadenosine. Biochem Pharmacol. 1990;40(7):1499–1503. doi: 10.1016/0006-2952(90)90446-r. [DOI] [PubMed] [Google Scholar]
- 5.Tang KC, Mariuza R, Coward JK. Synthesis and evaluation of some stable multisubstrate adducts as specific inhibitors of spermidine synthase. J Med Chem. 1981;24(11):1277–1284. doi: 10.1021/jm00143a003. [DOI] [PubMed] [Google Scholar]
- 6.Woster PM, Black AY, Duff KJ, Coward JK, Pegg AE. Synthesis and biological evaluation of S-adenosyl-1,12-diamino-3-thio-9-azadodecane, a multisubstrate adduct inhibitor of spermine synthase. J Med Chem. 1989;32(6):1300–1307. doi: 10.1021/jm00126a026. [DOI] [PubMed] [Google Scholar]
- 7.Cullis PM, Green RE, Merson-Davies L, Travis NG. Chemical highlights of polyamine transport. Biochem Soc Trans. 1998;26(4):595–601. doi: 10.1042/bst0260595. [DOI] [PubMed] [Google Scholar]
- 8.Seiler N, Delcros JG, Moulinoux JP. Polyamine transport in mammalian cells. An update. Int J Biochem Cell Biol. 1996;28(8):843–861. doi: 10.1016/1357-2725(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 9.Weeks RS, Vanderwerf SM, Carlson CL, Burns MR, O’Day CL, Cai F, Devens BH, Webb HK. Novel lysine-spermine conjugate inhibits polyamine transport and inhibits cell growth when given with DFMO. Exp Cell Res. 2000;261(1):293–302. doi: 10.1006/excr.2000.5033. [DOI] [PubMed] [Google Scholar]
- 10.Bacchi CJ, Garofalo J, Mockenhaupt D, McCann PP, Diekema KA, Pegg AE, Nathan HC, Mullaney EA, Chunosoff L, Sjoerdsma A, Hutner SH. In vivo effects of alpha-DL-difluoromethylornithine on the metabolism and morphology of Trypanosoma brucei brucei. Mol Biochem Parasitol. 1983;7(3):209–225. doi: 10.1016/0166-6851(83)90022-1. [DOI] [PubMed] [Google Scholar]
- 11.Bacchi CJ, Nathan HC, Livingston T, Valladares G, Saric M, Sayer PD, Njogu AR, Clarkson AB., Jr Differential susceptibility to DL-alpha-difluoromethylornithine in clinical isolates of Trypanosoma brucei rhodesiense. Antimicrob Agents Chemother. 1990;34(6):1183–1188. doi: 10.1128/aac.34.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Feith DJ, Welsh P, Coleman CS, Lopez C, Woster PM, O’Brien TG, Pegg AE. Studies of the mechanism by which increased spermidine/spermine N1-acetyltransferase activity increases susceptibility to skin carcinogenesis. Carcinogenesis. 2007;28(11):2404–2411. doi: 10.1093/carcin/bgm162. [DOI] [PubMed] [Google Scholar]
- 13.Meyskens FL, Jr, Gerner EW. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin Cancer Res. 1999;5(5):945–951. [PubMed] [Google Scholar]
- 14.Raul F, Gosse F, Osswald AB, Bouhadjar M, Foltzer-Jourdainne C, Marescaux J, Soler L. Follow-up of tumor development in the colons of living rats and implications for chemoprevention trials: assessment of aspirin-difluoromethylornithine combination. Int J Oncol. 2007;31(1):89–95. [PubMed] [Google Scholar]
- 15.Bergeron RJ, McManis JS, Weimar WR, Schreier KM, Gao F, Wu Q, Ortiz-Ocasio J, Luchetta GR, Porter C, Vinson JR. The role of charge in polyamine analogue recognition. J Med Chem. 1995;38(13):2278–2285. doi: 10.1021/jm00013a003. [DOI] [PubMed] [Google Scholar]
- 16.Casero RA, Jr, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov. 2007;6(5):373–390. doi: 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- 17.Wallace HM, Niiranen K. Polyamine analogues - an update. Amino Acids. 2007;33(2):261–265. doi: 10.1007/s00726-007-0534-z. [DOI] [PubMed] [Google Scholar]
- 18.Casero RA, Jr, Frydman B, Stewart TM, Woster PM. Significance of targeting polyamine metabolism as an antineoplastic strategy: unique targets for polyamine analogues. Proc West Pharmacol Soc. 2005;48:24–30. [PubMed] [Google Scholar]
- 19.Boncher T, Bi X, Varghese S, Casero RA, Jr, Woster PM. Polyamine-based analogues as biochemical probes and potential therapeutics. Biochem Soc Trans. 2007;35(Pt 2):356–363. doi: 10.1042/BST0350356. [DOI] [PubMed] [Google Scholar]
- 20.Heby O, Persson L, Rentala M. Targeting the polyamine biosynthetic enzymes: a promising approach to therapy of African sleeping sickness, Chagas’ disease, and leishmaniasis. Amino Acids. 2007;33(2):359–366. doi: 10.1007/s00726-007-0537-9. [DOI] [PubMed] [Google Scholar]
- 21.Wallace HM. Targeting polyamine metabolism: a viable therapeutic/preventative solution for cancer? Expert Opin Pharmacother. 2007;8(13):2109–2116. doi: 10.1517/14656566.8.13.2109. [DOI] [PubMed] [Google Scholar]
- 22.Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4(10):781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 23.Abeloff MD, Slavik M, Luk GD, Griffin CA, Hermann J, Blanc O, Sjoerdsma A, Baylin SB. Phase I trial and pharmacokinetic studies of alpha-difluoromethylornithine--an inhibitor of polyamine biosynthesis. J Clin Oncol. 1984;2(2):124–130. doi: 10.1200/JCO.1984.2.2.124. [DOI] [PubMed] [Google Scholar]
- 24.Meyskens FL, Kingsley EM, Glattke T, Loescher L, Booth A. A phase II study of alpha-difluoromethylornithine (DFMO) for the treatment of metastatic melanoma. Invest New Drugs. 1986;4(3):257–262. doi: 10.1007/BF00179593. [DOI] [PubMed] [Google Scholar]
- 25.Abeloff MD, Rosen ST, Luk GD, Baylin SB, Zeltzman M, Sjoerdsma A. Phase II trials of alpha-difluoromethylornithine, an inhibitor of polyamine synthesis, in advanced small cell lung cancer and colon cancer. Cancer Treat Rep. 1986;70(7):843–845. [PubMed] [Google Scholar]
- 26.Herr HW, Warrel RP, Burchenal JH. Phase I trial of alpha-difluoromethyl ornithine (DFMO) and methylglyoxal bis (guanylhydrazone) (MGBG) in patients with advanced prostatic cancer. Urology. 1986;28(6):508–511. doi: 10.1016/0090-4295(86)90154-8. [DOI] [PubMed] [Google Scholar]
- 27.Paulson YJ, Gilman TM, Heseltine PN, Sharma OP, Boylen CT. Eflornithine treatment of refractory Pneumocystis carinii pneumonia in patients with acquired immunodeficiency syndrome. Chest. 1992;101(1):67–74. doi: 10.1378/chest.101.1.67. [DOI] [PubMed] [Google Scholar]
- 28.Van Nieuwenhove S, Schechter PJ, Declercq J, Bone G, Burke J, Sjoerdsma A. Treatment of gambiense sleeping sickness in the Sudan with oral DFMO (DL-alpha-difluoromethylornithine), an inhibitor of ornithine decarboxylase; first field trial. Trans R Soc Trop Med Hyg. 1985;79(5):692–698. doi: 10.1016/0035-9203(85)90195-6. [DOI] [PubMed] [Google Scholar]
- 29.Bitonti AJ, Byers TL, Bush TL, Casara PJ, Bacchi CJ, Clarkson AB, Jr, McCann PP, Sjoerdsma A. Cure of Trypanosoma brucei brucei and Trypanosoma brucei rhodesiense infections in mice with an irreversible inhibitor of S-adenosylmethionine decarboxylase. Antimicrob Agents Chemother. 1990;34(8):1485–1490. doi: 10.1128/aac.34.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy BS, Nayini J, Tokumo K, Rigotty J, Zang E, Kelloff G. Chemoprevention of colon carcinogenesis by concurrent administration of piroxicam, a nonsteroidal antiinflammatory drug with D,L-alpha-difluoromethylornithine, an ornithine decarboxylase inhibitor, in diet. Cancer Res. 1990;50(9):2562–2568. [PubMed] [Google Scholar]
- 31.Alberts DS, Dorr RT, Einspahr JG, Aickin M, Saboda K, Xu MJ, Peng YM, Goldman R, Foote JA, Warneke JA, Salasche S, Roe DJ, Bowden GT. Chemoprevention of human actinic keratoses by topical 2-(difluoromethyl)-dl-ornithine. Cancer Epidemiol Biomarkers Prev. 2000;9(12):1281–1286. [PubMed] [Google Scholar]
- 32.Stratton SP, Dorr RT, Alberts DS. The state-of-the-art in chemoprevention of skin cancer. Eur J Cancer. 2000;36(10):1292–1297. doi: 10.1016/s0959-8049(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 33.Simoneau AR, Gerner EW, Nagle R, Ziogas A, Fujikawa-Brooks S, Yerushalmi H, Ahlering TE, Lieberman R, McLaren CE, Anton-Culver H, Meyskens FL., Jr The effect of difluoromethylornithine on decreasing prostate size and polyamines in men: results of a year-long phase IIb randomized placebo-controlled chemoprevention trial. Cancer Epidemiol Biomarkers Prev. 2008;17(2):292–299. doi: 10.1158/1055-9965.EPI-07-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyskens FL, Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ, Kidao J, McCracken J, Albers CG, Ahnen DJ, Turgeon DK, Goldschmid S, Lance P, Hagedorn CH, Gillen DL, Gerner EW. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila Pa) 2008;1(1):32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLaren CE, Fujikawa-Brooks S, Chen WP, Gillen DL, Pelot D, Gerner EW, Meyskens FL., Jr Longitudinal assessment of air conduction audiograms in a phase III clinical trial of difluoromethylornithine and sulindac for prevention of sporadic colorectal adenomas. Cancer Prev Res (Phila Pa) 2008;1(7):514–521. doi: 10.1158/1940-6207.CAPR-08-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zell JA, Pelot D, Chen WP, McLaren CE, Gerner EW, Meyskens FL. Risk of cardiovascular events in a randomized placebo-controlled, double-blind trial of difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas. Cancer Prev Res (Phila Pa) 2009;2(3):209–212. doi: 10.1158/1940-6207.CAPR-08-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards ML, Prakash NJ, Stemerick DM, Sunkara SP, Bitonti AJ, Davis GF, Dumont JA, Bey P. Polyamine analogues with antitumor activity. J Med Chem. 1990;33(5):1369–1375. doi: 10.1021/jm00167a014. [DOI] [PubMed] [Google Scholar]
- 38.Edwards ML, Snyder RD, Stemerick DM. Synthesis and DNA-binding properties of polyamine analogues. J Med Chem. 1991;34(8):2414–2420. doi: 10.1021/jm00112a016. [DOI] [PubMed] [Google Scholar]
- 39.Porter CW, Berger FG, Pegg AE, Ganis B, Bergeron RJ. Regulation of ornithine decarboxylase activity by spermidine and the spermidine analogue N1N8-bis(ethyl)spermidine. Biochem J. 1987;242(2):433–440. doi: 10.1042/bj2420433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porter CW, Ganis B, Libby PR, Bergeron RJ. Correlations between polyamine analogue-induced increases in spermidine/spermine N1-acetyltransferase activity, polyamine pool depletion, and growth inhibition in human melanoma cell lines. Cancer Res. 1991;51(14):3715–3720. [PubMed] [Google Scholar]
- 41.Bergeron RJ, Neims AH, McManis JS, Hawthorne TR, Vinson JR, Bortell R, Ingeno MJ. Synthetic polyamine analogues as antineoplastics. J Med Chem. 1988;31(6):1183–1190. doi: 10.1021/jm00401a019. [DOI] [PubMed] [Google Scholar]
- 42.Bergeron RJ, Feng Y, Weimar WR, McManis JS, Dimova H, Porter C, Raisler B, Phanstiel O. A comparison of structure-activity relationships between spermidine and spermine analogue antineoplastics. J Med Chem. 1997;40(10):1475–1494. doi: 10.1021/jm960849j. [DOI] [PubMed] [Google Scholar]
- 43.Bergeron RJ, McManis JS, Liu CZ, Feng Y, Weimar WR, Luchetta GR, Wu Q, Ortiz-Ocasio J, Vinson JR, Kramer D, et al. Antiproliferative properties of polyamine analogues: a structure- activity study. J Med Chem. 1994;37(21):3464–3476. doi: 10.1021/jm00047a004. [DOI] [PubMed] [Google Scholar]
- 44.Jeffers L, Church D, Basu H, Marton L, Wilding G. Effects of the polyamine analogues BE-4-4-4-4, BE-3-7-3, and BE-3-3-3 on the proliferation of three prostate cancer cell lines. Cancer Chemother Pharmacol. 1997;40(2):172–179. doi: 10.1007/s002800050643. [DOI] [PubMed] [Google Scholar]
- 45.Bergeron CJ, Basu HS, Marton LJ, Deen DF, Pellarin M, Feuerstein BG. Two polyamine analogs (BE-4-4-4 and BE-4-4-4-4) directly affect growth, survival, and cell cycle progression in two human brain tumor cell lines. Cancer Chemother Pharmacol. 1995;36(5):411–417. doi: 10.1007/BF00686190. [DOI] [PubMed] [Google Scholar]
- 46.Roemmele RC, Rappoport H. Removal of N-Arylsulfonyl Groups from Hydroxy alpha-Amino Acids. J Org Chem. 1988;53:2367–2371. [Google Scholar]
- 47.Yajima H, Takeyama M, Kanaki J, Nishimura O, Fujino M. Studies on Peptides. LXXX. NG-Mesitylene-2-sulfonylarginine. Chem Pharm Bull. 1978;26:3752–3757. [Google Scholar]
- 48.Bergeron RJ, Muller R, Huang G, McManis JS, Algee SE, Yao H, Weimar WR, Wiegand J. Synthesis and evaluation of hydroxylated polyamine analogues as antiproliferatives. J Med Chem. 2001;44(15):2451–2459. doi: 10.1021/jm000532q. [DOI] [PubMed] [Google Scholar]
- 49.Bergeron RJ, Wiegand J, Fannin TL. Control of irritable bowel syndrome with polyamine analogs: a structure-activity study. Dig Dis Sci. 2001;46(12):2615–2623. doi: 10.1023/a:1012750723644. [DOI] [PubMed] [Google Scholar]
- 50.Bergeron RJ, Wiegand J, McManis JS, Weimar WR, Smith RE, Algee SE, Fannin TL, Slusher MA, Snyder PS. Polyamine analogue antidiarrheals: a structure-activity study. J Med Chem. 2001;44(2):232–244. doi: 10.1021/jm000277+. [DOI] [PubMed] [Google Scholar]
- 51.Wolff AC, Armstrong DK, Fetting JH, Carducci MK, Riley CD, Bender JF, Casero RA, Jr, Davidson NE. A Phase II study of the polyamine analog N1,N11-diethylnorspermine (DENSpm) daily for five days every 21 days in patients with previously treated metastatic breast cancer. Clin Cancer Res. 2003;9(16 Pt 1):5922–5928. [PubMed] [Google Scholar]
- 52.Hahm HA, Ettinger DS, Bowling K, Hoker B, Chen TL, Zabelina Y, Casero RA., Jr Phase I study of N(1),N(11)-diethylnorspermine in patients with non-small cell lung cancer. Clin Cancer Res. 2002;8(3):684–690. [PubMed] [Google Scholar]
- 53.Streiff RR, Bender JF. Phase 1 study of N1-N11-diethylnorspermine (DENSPM) administered TID for 6 days in patients with advanced malignancies. Invest New Drugs. 2001;19(1):29–39. doi: 10.1023/a:1006448516938. [DOI] [PubMed] [Google Scholar]
- 54.Hector S, Tummala R, Kisiel ND, Diegelman P, Vujcic S, Clark K, Fakih M, Kramer DL, Porter CW, Pendyala L. Polyamine catabolism in colorectal cancer cells following treatment with oxaliplatin, 5-fluorouracil and N1, N11 diethylnorspermine. Cancer Chemother Pharmacol. 2008;62(3):517–527. doi: 10.1007/s00280-007-0633-2. [DOI] [PubMed] [Google Scholar]
- 55.Bergeron RJ, Yao GW, Yao H, Weimar WR, Sninsky CA, Raisler B, Feng Y, Wu Q, Gao F. Metabolically programmed polyamine analogue antidiarrheals. J Med Chem. 1996;39(13):2461–2471. doi: 10.1021/jm950827h. [DOI] [PubMed] [Google Scholar]
- 56.Reddy VK, Valasinas A, Sarkar A, Basu HS, Marton LJ, Frydman B. Conformationally restricted analogues of 1N,12N-bisethylspermine: synthesis and growth inhibitory effects on human tumor cell lines. J Med Chem. 1998;41(24):4723–4732. doi: 10.1021/jm980172v. [DOI] [PubMed] [Google Scholar]
- 57.Seiler N, Delcros JG, Vaultier M, Le Roch N, Havouis R, Douaud F, Moulinoux JP. Bis(7-amino-4-azaheptyl)dimethlysilane and bis(7-ethylamino-4-azaheptyl)dimethylsilane: inhibition of tumor cell growth in vitro and in vivo. Cancer Res. 1996;56(24):5624–5630. [PubMed] [Google Scholar]
- 58.Waters WR, Frydman B, Marton LJ, Valasinas A, Reddy VK, Harp JA, Wannemuehler MJ, Yarlett N. [(1)N,(12)N]Bis(Ethyl)-cis-6,7-dehydrospermine: a new drug for treatment and prevention of Cryptosporidium parvum infection of mice deficient in T-cell receptor alpha. Antimicrob Agents Chemother. 2000;44(10):2891–2894. doi: 10.1128/aac.44.10.2891-2894.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frydman B, Porter CW, Maxuitenko Y, Sarkar A, Bhattacharya S, Valasinas A, Reddy VK, Kisiel N, Marton LJ, Basu HS. A novel polyamine analog (SL-11093) inhibits growth of human prostate tumor xenografts in nude mice. Cancer Chemother Pharmacol. 2003;51(6):488–492. doi: 10.1007/s00280-003-0598-8. [DOI] [PubMed] [Google Scholar]
- 60.Carew JS, Nawrocki ST, Reddy VK, Bush D, Rehg JE, Goodwin A, Houghton JA, Casero RA, Jr, Marton LJ, Cleveland JL. The novel polyamine analogue CGC-11093 enhances the antimyeloma activity of bortezomib. Cancer Res. 2008;68(12):4783–4790. doi: 10.1158/0008-5472.CAN-07-6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valasinas A, Sarkar A, Reddy VK, Marton LJ, Basu HS, Frydman B. Conformationally restricted analogues of 1N,14N-bisethylhomospermine (BE-4-4-4): synthesis and growth inhibitory effects on human prostate cancer cells. J Med Chem. 2001;44(3):390–403. doi: 10.1021/jm000309t. [DOI] [PubMed] [Google Scholar]
- 62.Frydman B, Blokhin AV, Brummel S, Wilding G, Maxuitenko Y, Sarkar A, Bhattacharya S, Church D, Reddy VK, Kink JA, Marton LJ, Valasinas A, Basu HS. Cyclopropane-containing polyamine analogues are efficient growth inhibitors of a human prostate tumor xenograft in nude mice. J Med Chem. 2003;46(21):4586–4600. doi: 10.1021/jm030175u. [DOI] [PubMed] [Google Scholar]
- 63.Reddy VK, Sarkar A, Valasinas A, Marton LJ, Basu HS, Frydman B. cis-Unsaturated analogues of 3,8,13,18,23-pentaazapentacosane (BE-4-4-4-4): synthesis and growth inhibitory effects on human prostate cancer cell lines. J Med Chem. 2001;44(3):404–417. doi: 10.1021/jm000310s. [DOI] [PubMed] [Google Scholar]
- 64.Hacker A, Marton LJ, Sobolewski M, Casero RA., Jr In vitro and in vivo effects of the conformationally restricted polyamine analogue CGC-11047 on small cell and non-small cell lung cancer cells. Cancer Chemother Pharmacol. 2008 doi: 10.1007/s00280-008-0706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lima e Silva R, Kachi S, Akiyama H, JiKui S, Hatara MC, Aslam S, Gong YY, Khu NH, Lauer TW, Hackett SF, Marton LJ, Campochiaro PA. Trans-scleral delivery of polyamine analogs for ocular neovascularization. Experimental Eye Research. 2006;83(5):1260–1267. doi: 10.1016/j.exer.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 66.Valasinas A, Reddy VK, Blokhin AV, Basu HS, Bhattacharya S, Sarkar A, Marton LJ, Frydman B. Long-chain polyamines (oligoamines) exhibit strong cytotoxicities against human prostate cancer cells. Bioorg Med Chem. 2003;11(18):4121–4131. doi: 10.1016/s0968-0896(03)00453-x. [DOI] [PubMed] [Google Scholar]
- 67.Huang Y, Hager ER, Phillips DL, Dunn VR, Hacker A, Frydman B, Kink JA, Valasinas AL, Reddy VK, Marton LJ, Casero RA, Jr, Davidson NE. A novel polyamine analog inhibits growth and induces apoptosis in human breast cancer cells. Clin Cancer Res. 2003;9(7):2769–2777. [PMC free article] [PubMed] [Google Scholar]
- 68.Huang Y, Keen JC, Hager E, Smith R, Hacker A, Frydman B, Valasinas AL, Reddy VK, Marton LJ, Casero RA, Jr, Davidson NE. Regulation of polyamine analogue cytotoxicity by c-Jun in human MDA-MB-435 cancer cells. Mol Cancer Res. 2004;2(2):81–88. [PubMed] [Google Scholar]
- 69.Huang Y, Keen JC, Pledgie A, Marton LJ, Zhu T, Sukumar S, Park BH, Blair B, Brenner K, Casero RA, Jr, Davidson NE. Polyamine analogues down-regulate estrogen receptor alpha expression in human breast cancer cells. J Biol Chem. 2006;281(28):19055–19063. doi: 10.1074/jbc.M600910200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feng X, Reddy VK, Mayanja-Kizza H, Weiss LM, Marton LJ, Tzipori S. Therapeutic evaluation of polyamine analogue drug candidates against Enterocytozoon bieneusi in the SCID mouse model. Antimicrob Agents Chemother. 2009 doi: 10.1128/AAC.01113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rukunga GM, Waterman PG. New macrocyclic spermine (budmunchiamine) alkaloids from Albizia gummifera: with some observations on the structure--activity relationships of the budmunchiamines. J Nat Prod. 1996;59(9):850–853. doi: 10.1021/np960397d. [DOI] [PubMed] [Google Scholar]
- 72.Frydman B, Bhattacharya S, Sarkar A, Drandarov K, Chesnov S, Guggisberg A, Popaj K, Sergeyev S, Yurdakul A, Hesse M, Basu HS, Marton LJ. Macrocyclic polyamines deplete cellular ATP levels and inhibit cell growth in human prostate cancer cells. J Med Chem. 2004;47(4):1051–1059. doi: 10.1021/jm030437s. [DOI] [PubMed] [Google Scholar]
- 73.Creaven PJ, Perez R, Pendyala L, Meropol NJ, Loewen G, Levine E, Berghorn E, Raghavan D. Unusual central nervous system toxicity in a phase I study of N1N11 diethylnorspermine in patients with advanced malignancy. Invest New Drugs. 1997;15(3):227–234. doi: 10.1023/a:1005827231849. [DOI] [PubMed] [Google Scholar]
- 74.Wilding G, King D, Tutsch K, Pomplun M, Feierabend C, Alberti D, Arzoomanian R. Phase I trial of the polyamine analog N1,N14-diethylhomospermine (DEHSPM) in patients with advanced solid tumors. Invest New Drugs. 2004;22(2):131–138. doi: 10.1023/B:DRUG.0000011789.79368.ae. [DOI] [PubMed] [Google Scholar]
- 75.Saab NH, West EE, Bieszk NC, Preuss CV, Mank AR, Casero RA, Jr, Woster PM. Synthesis and evaluation of unsymmetrically substituted polyamine analogues as modulators of human spermidine/spermine-N1- acetyltransferase (SSAT) and as potential antitumor agents. J Med Chem. 1993;36(20):2998–3004. doi: 10.1021/jm00072a020. [DOI] [PubMed] [Google Scholar]
- 76.Casero RA, Jr, Celano P, Ervin SJ, Porter CW, Bergeron RJ, Libby PR. Differential induction of spermidine/spermine N1-acetyltransferase in human lung cancer cells by the bis(ethyl)polyamine analogues. Cancer Res. 1989;49(14):3829–3833. [PubMed] [Google Scholar]
- 77.Casero RA, Jr, Celano P, Ervin SJ, Wiest L, Pegg AE. High specific induction of spermidine/spermine N1-acetyltransferase in a human large cell lung carcinoma. Biochem J. 1990;270(3):615–620. doi: 10.1042/bj2700615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Casero RA, Jr, Ervin SJ, Celano P, Baylin SB, Bergeron RJ. Differential response to treatment with the bis(ethyl)polyamine analogues between human small cell lung carcinoma and undifferentiated large cell lung carcinoma in culture. Cancer Res. 1989;49(3):639–643. [PubMed] [Google Scholar]
- 79.Davidson NE, Hahm HA, McCloskey DE, Woster PM, Casero RA., Jr Clinical aspects of cell death in breast cancer: the polyamine pathway as a new target for treatment. Endocr Relat Cancer. 1999;6(1):69–73. doi: 10.1677/erc.0.0060069. [DOI] [PubMed] [Google Scholar]
- 80.Ha HC, Woster PM, Casero RA., Jr Unsymmetrically substituted polyamine analogue induces caspase- independent programmed cell death in Bcl-2-overexpressing cells. Cancer Res. 1998;58(13):2711–2714. [PubMed] [Google Scholar]
- 81.Ha HC, Woster PM, Yager JD, Casero RA., Jr The role of polyamine catabolism in polyamine analogue-induced programmed cell death. Proc Natl Acad Sci U S A. 1997;94(21):11557–11562. doi: 10.1073/pnas.94.21.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu RH, Pegg AE. Rapid induction of apoptosis by deregulated uptake of polyamine analogues. Biochem J. 1997;328(Pt 1):307–316. doi: 10.1042/bj3280307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McCloskey DE, Casero RA, Jr, Woster PM, Davidson NE. Induction of programmed cell death in human breast cancer cells by an unsymmetrically alkylated polyamine analogue. Cancer Res. 1995;55(15):3233–3236. [PubMed] [Google Scholar]
- 84.McCloskey DE, Woster PM, Casero RA, Jr, Davidson NE. Effects of the polyamine analogues N1-ethyl-N11-((cyclopropyl)methyl)- 4,8-diazaundecane and N1-ethylN-11-((cycloheptyl)methyl)-4,8- diazaundecane in human prostate cancer cells. Clin Cancer Res. 2000;6(1):17–23. [PubMed] [Google Scholar]
- 85.McCloskey DE, Yang J, Woster PM, Davidson NE, Casero RA., Jr Polyamine analogue induction of programmed cell death in human lung tumor cells. Clin Cancer Res. 1996;2(3):441–446. [PubMed] [Google Scholar]
- 86.Webb HK, Wu Z, Sirisoma N, Ha HC, Casero RA, Jr, Woster PM. 1-(N-alkylamino)-11-(N-ethylamino)-4,8-diazaundecanes: simple synthetic polyamine analogues that differentially alter tubulin polymerization. J Med Chem. 1999;42(8):1415–1421. doi: 10.1021/jm980603+. [DOI] [PubMed] [Google Scholar]
- 87.Bellevue FH, Iii, Boahbedason M, Wu R, Woster PM, Casero JRA, Rattendi D, Lane S, Bacchi CJ. Structural comparison of alkylpolyamine analogues with potent in vitro antitumor or antiparasitic activity. Bioorganic & Medicinal Chemistry Letters. 1996;6(22):2765. [Google Scholar]
- 88.Bi X, Lopez C, Bacchi CJ, Rattendi D, Woster PM. Novel alkylpolyaminoguanidines and alkylpolyaminobiguanides with potent antitrypanosomal activity. Bioorg Med Chem Lett. 2006;16(12):3229–3232. doi: 10.1016/j.bmcl.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 89.Bergeron RJ, Hawthorne TR, Vinson JR, Beck DE, Jr, Ingeno MJ. Role of the methylene backbone in the antiproliferative activity of polyamine analogues on L1210 cells. Cancer Res. 1989;49(11):2959–2964. [PubMed] [Google Scholar]
- 90.Casero RA, Jr, Mank AR, Xiao L, Smith J, Bergeron RJ, Celano P. Steady-state messenger RNA and activity correlates with sensitivity to N1,N12-bis(ethyl)spermine in human cell lines representing the major forms of lung cancer. Cancer Res. 1992;52(19):5359–5363. [PubMed] [Google Scholar]
- 91.Bacchi CJ, Yarlett N, Faciane E, Bi X, Rattendi D, Weiss LM, Woster PM. Metabolism of an Alkyl Polyamine Analog by a Polyamine Oxidase from the Microsporidian Encephalitozoon cuniculi. Antimicrob Agents Chemother. 2009 doi: 10.1128/AAC.00267-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosenberg B, Vancamp L, Krigas T. Inhibition of Cell Division in Escherichia Coli by Electrolysis Products from a Platinum Electrode. Nature. 1965;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- 93.Barry MA, Behnke CA, Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990;40(10):2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- 94.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4(4):307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 95.Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33(1):9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7(8):573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 97.Asara JM, Hess JS, Lozada E, Dunbar KR, Allison J. Evidence for Binding of Dirhodium Bis-Acetate Units to Adjacent GG and AA Sites on Single-Stranded DNA. J Am Chem Soc. 2000;122(1):8–13. [Google Scholar]
- 98.Catalan KV, Mindiola DJ, Ward DL, Dunbar KR. A Novel Dirhodium Compound with Neutral, Bridging 9-Ethyladenine Ligands. Inorg Chem. 1997;36(11):2458–2460. doi: 10.1021/ic960682k. [DOI] [PubMed] [Google Scholar]
- 99.Kang M, Chifotides HT, Dunbar KR. 2D NMR study of the DNA duplex d(CTCTC*A*ACTTCC).d(GGAAGTTGAGAG) cross-linked by the antitumor-active dirhodium(II,II) unit at the cytosine-adenine step. Biochemistry. 2008;47(8):2265–2276. doi: 10.1021/bi701901c. [DOI] [PubMed] [Google Scholar]
- 100.Kraker AJ, Hoeschele JD, Elliott WL, Showalter HD, Sercel AD, Farrell NP. Anticancer activity in murine and human tumor cell lines of bis(platinum) complexes incorporating straight-chain aliphatic diamine linker groups. J Med Chem. 1992;35(24):4526–4532. doi: 10.1021/jm00102a003. [DOI] [PubMed] [Google Scholar]
- 101.Navarro-Ranninger C, Ochoa PA, Perez JM, Gonzalez VM, Masaguer JR, Alonso C. Platinum (II) and (IV) spermidine complexes. Synthesis, characterization, and biological studies. J Inorg Biochem. 1994;53(3):177–190. doi: 10.1016/0162-0134(94)80003-0. [DOI] [PubMed] [Google Scholar]
- 102.Rauter H, DiDomenico R, Menta E, Oliva A, Qu Y, Farrell N. Selective platination of biologically relevant polyamines. Linear coordinating spermidine and spermine as amplifying linkers in dinuclear platinum complexes. Inorganic Chemistry. 1997;36(18):3919–3927. [Google Scholar]
- 103.Amo-Ochoa P, Gonzalez VM, Perez JM, Masaguer JR, Alonso C, Navarro-Ranninger C. Cytotoxicity, DNA binding, and reactivity against nucleosides of platinum (II) and (IV) spermine compounds. J Inorg Biochem. 1996;64(4):287–299. doi: 10.1016/s0162-0134(96)00082-7. [DOI] [PubMed] [Google Scholar]
- 104.Di Blasi P, Bernareggi A, Beggiolin G, Piazzoni L, Menta E, Formento ML. Cytotoxicity, cellular uptake and DNA binding of the novel trinuclear platinun complex BBR 3464 in sensitive and cisplatin resistant murine leukemia cells. Anticancer Res. 1998;18(4C):3113–3117. [PubMed] [Google Scholar]
- 105.Brabec V, Kasparkova J, Vrana O, Novakova O, Cox JW, Qu Y, Farrell N. DNA Modifications by a Novel Bifunctional Trinuclear Platinum Phase I Anticancer Agent. Biochemistry. 1999;38(21):6781–6790. doi: 10.1021/bi990124s. [DOI] [PubMed] [Google Scholar]
- 106.McGregor TD, Hegmans A, Kasparkova J, Neplechova K, Novakova O, Penazova H, Vrana O, Brabec V, Farrell N. A comparison of DNA binding profiles of dinuclear platinum compounds with polyamine linkers and the trinuclear platinum phase II clinical agent BBR3464. J Biol Inorg Chem. 2002;7(45):397–404. doi: 10.1007/s00775-001-0312-4. [DOI] [PubMed] [Google Scholar]
- 107.Billecke C, Finniss S, Tahash L, Miller C, Mikkelsen T, Farrell NP, Bogler O. Polynuclear platinum anticancer drugs are more potent than cisplatin and induce cell cycle arrest in glioma. Neuro Oncol. 2006;8(3):215–226. doi: 10.1215/15228517-2006-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chvalova K, Kasparkova J, Farrell N, Brabec V. Deoxyribonuclease I footprinting reveals different DNA binding modes of bifunctional platinum complexes. Febs J. 2006;273(15):3467–3478. doi: 10.1111/j.1742-4658.2006.05356.x. [DOI] [PubMed] [Google Scholar]
- 109.Jodrell DI, Evans TR, Steward W, Cameron D, Prendiville J, Aschele C, Noberasco C, Lind M, Carmichael J, Dobbs N, Camboni G, Gatti B, De Braud F. Phase II studies of BBR3464, a novel tri-nuclear platinum complex, in patients with gastric or gastro-oesophageal adenocarcinoma. Eur J Cancer. 2004;40(12):1872–1877. doi: 10.1016/j.ejca.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 110.Hensing TA, Hanna NH, Gillenwater HH, Gabriella Camboni M, Allievi C, Socinski MA. Phase II study of BBR 3464 as treatment in patients with sensitive or refractory small cell lung cancer. Anticancer Drugs. 2006;17(6):697–704. doi: 10.1097/01.cad.0000215054.62942.7f. [DOI] [PubMed] [Google Scholar]
- 111.Mitchell C, Kabolizadeh P, Ryan J, Roberts JD, Yacoub A, Curiel DT, Fisher PB, Hagan MP, Farrell NP, Grant S, Dent P. Low-dose BBR3610 toxicity in colon cancer cells is p53-independent and enhanced by inhibition of epidermal growth factor receptor (ERBB1)-phosphatidyl inositol 3 kinase signaling. Mol Pharmacol. 2007;72(3):704–714. doi: 10.1124/mol.107.038406. [DOI] [PubMed] [Google Scholar]
- 112.Hegmans A, Qu Y, Kelland LR, Roberts JD, Farrell N. Novel approaches to polynuclear platinum pro-drugs. Selective release of cytotoxic platinum-spermidine species through hydrolytic cleavage of carbamates. Inorg Chem. 2001;40(24):6108–6114. doi: 10.1021/ic010509a. [DOI] [PubMed] [Google Scholar]
- 113.Hegmans A, Kasparkova J, Vrana O, Kelland LR, Brabec V, Farrell NP. Amide-based prodrugs of spermidine-bridged dinuclear platinum. Synthesis, DNA binding, and biological activity. J Med Chem. 2008;51(7):2254–2260. doi: 10.1021/jm070813z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pegram MD, Slamon DJ. Combination therapy with trastuzumab (Herceptin) and cisplatin for chemoresistant metastatic breast cancer: evidence for receptor-enhanced chemosensitivity. Semin Oncol. 1999;26(4 Suppl 12):89–95. [PubMed] [Google Scholar]
- 115.Pietras RJ, Fendly BM, Chazin VR, Pegram MD, Howell SB, Slamon DJ. Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene. 1994;9(7):1829–1838. [PubMed] [Google Scholar]
- 116.Hurley J, Doliny P, Reis I, Silva O, Gomez-Fernandez C, Velez P, Pauletti G, Powell JE, Pegram MD, Slamon DJ. Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. J Clin Oncol. 2006;24(12):1831–1838. doi: 10.1200/JCO.2005.02.8886. [DOI] [PubMed] [Google Scholar]
- 117.Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275(31):23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 118.Hegardt C, Johannsson OT, Oredsson SM. Rapid caspase-dependent cell death in cultured human breast cancer cells induced by the polyamine analogue N(1),N(11)-diethylnorspermine. Eur J Biochem. 2002;269(3):1033–1039. doi: 10.1046/j.0014-2956.2001.02744.x. [DOI] [PubMed] [Google Scholar]
- 119.Huang Y, Hager ER, Phillips DL, Hacker A, Frydman B, Valasinas AL, Reddy VK, Marton LJ, Casero RA, Davidson NE. Conformationally Constrained Polyamine Analogues and Oligoamines Inhibit Growth and Induce Apoptosis in Human Breast Cancer Cells. Pro Amer Assoc Cancer Res. 2002;43:90. [Google Scholar]
- 120.Varghese S, Gupta D, Baran T, Jiemjit A, Gore SD, Casero RA, Jr, Woster PM. Alkyl-substituted polyaminohydroxamic acids: a novel class of targeted histone deacetylase inhibitors. J Med Chem. 2005;48(20):6350–6365. doi: 10.1021/jm0505009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zou Y, Wu Z, Sirisoma N, Woster PM, Casero RA, Jr, Weiss LM, Rattendi D, Lane S, Bacchi CJ. Novel alkylpolyamine analogues that possess both antitrypanosomal and antimicrosporidial activity. Bioorg Med Chem Lett. 2001;11(12):1613–1617. doi: 10.1016/s0960-894x(01)00315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marks PA, Richon VM, Breslow R, Rifkind RA. Histone deacetylase inhibitors as new cancer drugs. Curr Opin Oncol. 2001;13(6):477–483. doi: 10.1097/00001622-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 123.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 124.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 125.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349(21):2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 126.Robertson KD. DNA methylation, methyltransferases, and cancer. Oncogene. 2001;20(24):3139–3155. doi: 10.1038/sj.onc.1204341. [DOI] [PubMed] [Google Scholar]
- 127.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1(4):287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 128.Shogren-Knaak M, Ishii H, Sun J-M, Pazin MJ, Davie JR, Peterson CL. Histone H4-K16 Acetylation Controls Chromatin Structure and Protein Interactions. Science. 2006;311(5762):844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- 129.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1(3):194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 130.Shogren-Knaak M, Ishii H, Sun J-M, Pazin MJ, Davie JR, Peterson CL. Histone H4-K16 Acetylation Controls Chromatin Structure and Protein Interactions. Science. 2006;311(5762):844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- 131.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10(1):32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gray SG, Ekstrom TJ. The human histone deacetylase family. Exp Cell Res. 2001;262(2):75–83. doi: 10.1006/excr.2000.5080. [DOI] [PubMed] [Google Scholar]
- 133.Taunton J, Hassig CA, Schreiber SL. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science. 1996;272(5260):408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- 134.Grozinger CM, Schreiber SL. Deacetylase enzymes: biological functions and the use of small-molecule inhibitors. Chem Biol. 2002;9(1):3–16. doi: 10.1016/s1074-5521(02)00092-3. [DOI] [PubMed] [Google Scholar]
- 135.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 136.Marmorstein R, Roth SY. Histone acetyltransferases: function, structure, and catalysis. Curr Opin Genet Dev. 2001;11(2):155–161. doi: 10.1016/s0959-437x(00)00173-8. [DOI] [PubMed] [Google Scholar]
- 137.Weinmann H, Ottow E. Recent advances in the medicinal chemistry of histone deacetylase inhibitors. Ann Rep Med Chem. 2004;39:185–196. [Google Scholar]
- 138.Kouraklis G, Theocharis S. Histone deacetylase inhibitors: a novel target of anticancer therapy (review) Oncol Rep. 2006;15(2):489–494. [PubMed] [Google Scholar]
- 139.Ryan QC, Headlee D, Acharya M, Sparreboom A, Trepel JB, Ye J, Figg WD, Hwang K, Chung EJ, Murgo A, Melillo G, Elsayed Y, Monga M, Kalnitskiy M, Zwiebel J, Sausville EA. Phase I and pharmacokinetic study of MS-275, a histone deacetylase inhibitor, in patients with advanced and refractory solid tumors or lymphoma. J Clin Oncol. 2005;23(17):3912–3922. doi: 10.1200/JCO.2005.02.188. [DOI] [PubMed] [Google Scholar]
- 140.Hu E, Dul E, Sung C-M, Chen Z, Kirkpatrick R, Zhang G-F, Johanson K, Liu R, Lago A, Hofmann G, Macarron R, De Los Frailes M, Perez P, Krawiec J, Winkler J, Jaye M. Identification of Novel Isoform-Selective Inhibitors within Class I Histone Deacetylases. J Pharmacol Exp Ther. 2003;307(2):720–728. doi: 10.1124/jpet.103.055541. [DOI] [PubMed] [Google Scholar]
- 141.Glaser KB, Li J, Pease LJ, Staver MJ, Marcotte PA, Guo J, Frey RR, Garland RB, Heyman HR, Wada CK. Differential protein acetylation induced by novel histone deacetylase inhibitors. Biochemical and Biophysical Research Communications. 2004;325(3):683. doi: 10.1016/j.bbrc.2004.10.082. [DOI] [PubMed] [Google Scholar]
- 142.Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21(1):103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 143.Herman JG, Civin CI, Issa JP, Collector MI, Sharkis SJ, Baylin SB. Distinct patterns of inactivation of p15INK4B and p16INK4A characterize the major types of hematological malignancies. Cancer Res. 1997;57(5):837–841. [PubMed] [Google Scholar]
- 144.Herman JG, Jen J, Merlo A, Baylin SB. Hypermethylation-associated inactivation indicates a tumor suppressor role for p15INK4B. Cancer Res. 1996;56(4):722–727. [PubMed] [Google Scholar]
- 145.Corn PG, Smith BD, Ruckdeschel ES, Douglas D, Baylin SB, Herman JG. E-cadherin expression is silenced by 5’ CpG island methylation in acute leukemia. Clin Cancer Res. 2000;6(11):4243–4248. [PubMed] [Google Scholar]
- 146.Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA., Jr The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci U S A. 1998;95(19):11140–11145. doi: 10.1073/pnas.95.19.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ha HC, Yager JD, Woster PA, Casero RA., Jr Structural specificity of polyamines and polyamine analogues in the protection of DNA from strand breaks induced by reactive oxygen species. Biochem Biophys Res Commun. 1998;244(1):298–303. doi: 10.1006/bbrc.1998.8258. [DOI] [PubMed] [Google Scholar]
- 148.Varghese S, Senanayake T, Murray-Stewart T, Doering K, Fraser A, Casero RA, Woster PM. Polyaminohydroxamic Acids and Polyaminobenzamides as Isoform Selective Histone Deacetylase Inhibitors. Journal of Medicinal Chemistry. 2008;51(8):2447–2456. doi: 10.1021/jm701384x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417(6887):455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 150.Suzuki T, Ando T, Tsuchiya K, Fukazawa N, Saito A, Mariko Y, Yamashita T, Nakanishi O. Synthesis and histone deacetylase inhibitory activity of new benzamide derivatives. J Med Chem. 1999;42(15):3001–3003. doi: 10.1021/jm980565u. [DOI] [PubMed] [Google Scholar]
- 151.Dowling DP, Gantt SL, Gattis SG, Fierke CA, Christianson DW. Structural Studies of Human Histone Deacetylase 8 and Its Site-Specific Variants Complexed with Substrate and Inhibitors. Biochemistry. 2008;47(51):13554–13563. doi: 10.1021/bi801610c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Belting M, Mani K, Jonsson M, Cheng F, Sandgren S, Jonsson S, Ding K, Delcros JG, Fransson LA. Glypican-1 is a vehicle for polyamine uptake in mammalian cells: a pivital role for nitrosothiol-derived nitric oxide. J Biol Chem. 2003;278(47):47181–47189. doi: 10.1074/jbc.M308325200. [DOI] [PubMed] [Google Scholar]
- 153.Uemura T, Yerushalmi HF, Tsaprailis G, Stringer DE, Pastorian KE, Hawel L, 3rd, Byus CV, Gerner EW. Identification and characterization of a diamine exporter in colon epithelial cells. J Biol Chem. 2008;283(39):26428–26435. doi: 10.1074/jbc.M804714200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tabe Y, Jin L, Contractor R, Gold D, Ruvolo P, Radke S, Xu Y, Tsutusmi-Ishii Y, Miyake K, Miyake N, Kondo S, Ohsaka A, Nagaoka I, Andreeff M, Konopleva M. Novel role of HDAC inhibitors in AML1/ETO AML cells: activation of apoptosis and phagocytosis through induction of annexin A1. Cell Death Differ. 2007;14(8):1443–1456. doi: 10.1038/sj.cdd.4402139. [DOI] [PubMed] [Google Scholar]
- 155.Chuthapisith S, Bean BE, Cowley G, Eremin JM, Samphao S, Layfield R, Kerr ID, Wiseman J, El-Sheemy M, Sreenivasan T, Eremin O. Annexins in human breast cancer: Possible predictors of pathological response to neoadjuvant chemotherapy. European Journal of Cancer. doi: 10.1016/j.ejca.2008.12.026. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 156.Gowri PM, Yu JH, Shaufl A, Sperling MA, Menon RK. Recruitment of a repressosome complex at the growth hormone receptor promoter and its potential role in diabetic nephropathy. Mol Cell Biol. 2003;23(3):815–825. doi: 10.1128/MCB.23.3.815-825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Miao F, Gonzalo IG, Lanting L, Natarajan R. In vivo chromatin remodeling events leading to inflammatory gene transcription under diabetic conditions. J Biol Chem. 2004;279(17):18091–18097. doi: 10.1074/jbc.M311786200. [DOI] [PubMed] [Google Scholar]
- 158.Gray SG, De Meyts P. Role of histone and transcription factor acetylation in diabetes pathogenesis. Diabetes Metab Res Rev. 2005;21(5):416–433. doi: 10.1002/dmrr.559. [DOI] [PubMed] [Google Scholar]
- 159.Lee HB, Noh H, Seo JY, Yu MR, Ha H. Histone deacetylase inhibitors: a novel class of therapeutic agents in diabetic nephropathy. Kidney Int Suppl. 2007;106:S61–66. doi: 10.1038/sj.ki.5002388. [DOI] [PubMed] [Google Scholar]
- 160.Susick L, Senanayake T, Veluthakal R, Woster PM, Kowluru A. A novel histone deacetylase inhibitor prevents IL-1beta-induced metabolic dysfunction in pancreatic beta-cells. J Cell Molec Med. 2009 doi: 10.1111/j.1582-4934.2008.00672.x. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6(2):107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 162.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119(7):941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 164.Whetstine JR, Nottke A, Lan F, Huarte M, Smolikov S, Chen Z, Spooner E, Li E, Zhang G, Colaiacovo M, Shi Y. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell. 2006;125(3):467–481. doi: 10.1016/j.cell.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 165.Bannister AJ, Kouzarides T. Reversing histone methylation. Nature. 2005;436(7054):1103–1106. doi: 10.1038/nature04048. [DOI] [PubMed] [Google Scholar]
- 166.Kouzarides T. Histone methylation in transcriptional control. Curr Opin Genet Dev. 2002;12(2):198–209. doi: 10.1016/s0959-437x(02)00287-3. [DOI] [PubMed] [Google Scholar]
- 167.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005;6(11):838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 168.Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15(18):2343–2360. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- 169.Schneider R, Bannister AJ, Kouzarides T. Unsafe SETs: histone lysine methyltransferases and cancer. Trends Biochem Sci. 2002;27(8):396–402. doi: 10.1016/s0968-0004(02)02141-2. [DOI] [PubMed] [Google Scholar]
- 170.Huarte M, Lan F, Kim T, Vaughn MW, Zaratiegui M, Martienssen RA, Buratowski S, Shi Y. The fission yeast JMJ2 reverses histone H3 lysine 4 tri-methylation. J Biol Chem. 2007 doi: 10.1074/jbc.M703897200. [DOI] [PubMed] [Google Scholar]
- 171.Tsukada Y, Zhang Y. Purification of histone demethylases from HeLa cells. Methods. 2006;40(4):318–326. doi: 10.1016/j.ymeth.2006.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Huang J, Sengupta R, Espejo AB, Lee MG, Dorsey JA, Richter M, Opravil S, Shiekhattar R, Bedford MT, Jenuwein T, Berger SL. p53 is regulated by the lysine demethylase LSD1. Nature. 2007;449(7158):105–108. doi: 10.1038/nature06092. [DOI] [PubMed] [Google Scholar]
- 173.Liang G, Klose RJ, Gardner KE, Zhang Y. Yeast Jhd2p is a histone H3 Lys4 trimethyl demethylase. Nat Struct Mol Biol. 2007;14(3):243–245. doi: 10.1038/nsmb1204. [DOI] [PubMed] [Google Scholar]
- 174.Schneider R, Bannister AJ, Myers FA, Thorne AW, Crane-Robinson C, Kouzarides T. Histone H3 lysine 4 methylation patterns in higher eukaryotic genes. Nat Cell Biol. 2004;6(1):73–77. doi: 10.1038/ncb1076. [DOI] [PubMed] [Google Scholar]
- 175.Lee MG, Wynder C, Schmidt DM, McCafferty DG, Shiekhattar R. Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications. Chem Biol. 2006;13(6):563–567. doi: 10.1016/j.chembiol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 176.Huang Y, Greene E, Murray Stewart T, Goodwin AC, Baylin SB, Woster PM, Casero RA., Jr Inhibition of lysine-specific demethylase 1 by polyamine analogues results in reexpression of aberrantly silenced genes. Proc Natl Acad Sci U S A. 2007;104(19):8023–8028. doi: 10.1073/pnas.0700720104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stavropoulos P, Hoelz A. Lysine-specific demethylase 1 as a potential therapeutic target. Expert Opin Ther Targets. 2007;11(6):809–820. doi: 10.1517/14728222.11.6.809. [DOI] [PubMed] [Google Scholar]
- 178.Culhane JC, Szewczuk LM, Liu X, Da G, Marmorstein R, Cole PA. A mechanism-based inactivator for histone demethylase LSD1. J Am Chem Soc. 2006;128(14):4536–4537. doi: 10.1021/ja0602748. [DOI] [PubMed] [Google Scholar]
- 179.Lee MG, Wynder C, Cooch N, Shiekhattar R. An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature. 2005;437(7057):432–435. doi: 10.1038/nature04021. [DOI] [PubMed] [Google Scholar]
- 180.Schmidt DM, McCafferty DG. trans-2-Phenylcyclopropylamine is a mechanism-based inactivator of the histone demethylase LSD1. Biochemistry. 2007;46(14):4408–4416. doi: 10.1021/bi0618621. [DOI] [PubMed] [Google Scholar]
- 181.Cona A, Manetti F, Leone R, Corelli F, Tavladoraki P, Polticelli F, Botta M. Molecular basis for the binding of competitive inhibitors of maize polyamine oxidase. Biochemistry. 2004;43(12):3426–3435. doi: 10.1021/bi036152z. [DOI] [PubMed] [Google Scholar]
- 182.Bianchi M, Polticelli F, Ascenzi P, Botta M, Federico R, Mariottini P, Cona A. Inhibition of polyamine and spermine oxidases by polyamine analogues. Febs J. 2006;273(6):1115–1123. doi: 10.1111/j.1742-4658.2006.05137.x. [DOI] [PubMed] [Google Scholar]
- 183.Stranska J, Sebela M, Tarkowski P, Rehulka P, Chmelik J, Popa I, Pec P. Inhibition of plant amine oxidases by a novel series of diamine derivatives. Biochimie. 2007;89(1):135–144. doi: 10.1016/j.biochi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 184.McGarvey KM, Fahrner JA, Greene E, Martens J, Jenuwein T, Baylin SB. Silenced tumor suppressor genes reactivated by DNA demethylation do not return to a fully euchromatic chromatin state. Cancer Res. 2006;66(7):3541–3549. doi: 10.1158/0008-5472.CAN-05-2481. [DOI] [PubMed] [Google Scholar]
- 185.Fahrner JA, Eguchi S, Herman JG, Baylin SB. Dependence of histone modifications and gene expression on DNA hypermethylation in cancer. Cancer Res. 2002;62(24):7213–7218. [PubMed] [Google Scholar]
- 186.Akiyama Y, Watkins N, Suzuki H, Jair KW, van Engeland M, Esteller M, Sakai H, Ren CY, Yuasa Y, Herman JG, Baylin SB. GATA-4 and GATA-5 transcription factor genes and potential downstream antitumor target genes are epigenetically silenced in colorectal and gastric cancer. Mol Cell Biol. 2003;23(23):8429–8439. doi: 10.1128/MCB.23.23.8429-8439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]