Abstract
Persons with schizophrenia experience subjective sensory anomalies and objective deficits on assessment of sensory function. Such deficits could be produced by abnormal signaling in the sensory pathways and sensory cortex or later stage disturbances in cognitive processing of such inputs. Steady state responses (SSRs) provide a noninvasive method to test the integrity of sensory pathways and oscillatory responses in schizophrenia with minimal task demands. SSRs are electrophysiological responses entrained to the frequency and phase of a periodic stimulus. Patients with schizophrenia exhibit pronounced auditory SSR deficits within the gamma frequency range (35–50 Hz) in response to click trains and amplitude-modulated tones. Visual SSR deficits are also observed, most prominently in the alpha and beta frequency ranges (7–30 Hz) in response to high-contrast, high-luminance stimuli. Visual SSR studies that have used the psychophysical properties of a stimulus to target specific visual pathways predominantly report magnocellular-based deficits in those with schizophrenia. Disruption of both auditory and visual SSRs in schizophrenia are consistent with neuropathological and magnetic resonance imaging evidence of anatomic abnormalities affecting the auditory and visual cortices. Computational models suggest that auditory SSR abnormalities at gamma frequencies could be secondary to γ-aminobutyric acid–mediated or N-methyl-D-aspartic acid dysregulation. The pathophysiological process in schizophrenia encompasses sensory processing that probably contributes to alterations in subsequent encoding and cognitive processing. The developmental evolution of these abnormalities remains to be characterized.
Keywords: entrainment, EEG, neural synchrony, schizophrenia, steady state
Introduction
Unusual sensory experiences are common in schizophrenia, from visual and auditory distortions in the prodromal phase to the vivid hallucinations often reported by those with chronic schizophrenia.1–4 These subjective reports are paralleled by objective testing.5 Visual psychophysical measures have documented deficits affecting motion perception,6–10 form perception,8,11 low spatial frequency discrimination,12 location discrimination,9,11 perceptual organization,13,14 and backward masking performance.15–18 Within the auditory domain, patients with schizophrenia exhibit deficits on behavioral measures of tone matching,19 temporal discrimination,20 and pitch discrimination.21
The neurophysiological basis for these perceptual abnormalities is not well characterized. One promising approach is the utilization of steady state responses (SSRs) of the electroencephalogram (EEG) to probe the integrity of the networks necessary for accurate sensory processing. The SSR is generated by synchronous activity of large populations of neurons to a temporally modulated stimulus. SSRs have several advantages for the study of sensory processing in schizophrenia. SSRs have been used extensively to investigate electrophysiological responses to sensory stimulation in both healthy children and adults.22,23 In these studies, the effects of stimulus properties such as temporal frequency, spatial frequency, and contrast on visual SSRs have been well characterized. SSRs can be obtained noninvasively with minimal task demands from psychiatric patients. SSRs can also be recorded in intracranial animal paradigms, allowing cross-species comparison of responses, identification of neural generators and use in animal models of human disorders. Finally, SSRs can test the capacity of neural circuits to support oscillatory activity at a range of frequencies. The functional capacity of these circuits may inform theories about the neural mechanisms associated with schizophrenia.24,25 This review will describe methods of analysis of SSRs, human and animal data regarding the generators of this activity, findings in the visual and auditory modalities in patients with schizophrenia, and the possible neurophysiological mechanisms underlying abnormal SSRs in schizophrenia.
Signal Analysis of SSRs: Measures of Power and Phase
EEG activity evoked by external stimuli, response generation, or internal events can be analyzed in either the time or frequency domain. Transient evoked or event-related potentials (ERPs) are elicited by a single event and consist of a series of deflections in the EEG that usually return to baseline prior to the next stimulus. Transient ERPs are typically obtained by segmenting the continuous EEG at the onset of the stimulus and averaging these trials in the time domain to extract only the stimulus-locked activity (as seen with SSR data in figure 1a). Typical variables derived from this time domain analysis include peak latency, amplitude, and topography. A variety of transient ERPs are impaired in schizophrenia and are among the best validated neurobiological endophenotypes for the illness.26
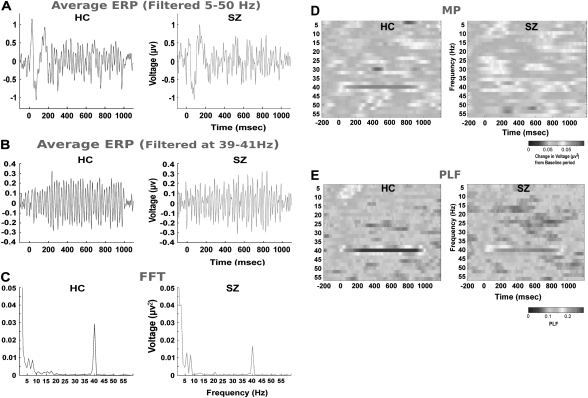
Fig. 1.
Different Signal Processing Techniques Applied to the Auditory Steady State Response at Electrode Site Cz in Response to a 40-Hz Amplitude-Modulated Tone (1000 Hz Carrier Frequency) Presented for 1000 ms in a Healthy Control Group (N = 21) and in Patients With Schizophrenia (N = 21). Panel A represents the waveform averaged across trials in the time domain to show both the transient event-related potential and the steady state response. Panel B represents the averaged waveform that has been filtered between 39 and 41 Hz to show only the steady state response at 40 Hz. Panel C represents the magnitude of response in the frequency domain by using a fast Fourier transform, showing neural entrainment at the frequency of stimulation. Panel D represents mean power, obtained by using a Hilbert transform on individual trials, indicating the average change in power at a given frequency from the mean baseline power. The x-axis represents time in milliseconds, the y-axis represents frequency in Hertz, and the colors represent the magnitude of power with warmer colors associated with higher power and cooler colors associated with less power. Panel E represents the phase locking factor or averaged normalized phase across trials, obtained by using a Hilbert transform on individual trials. The x-axis represents time in milliseconds, the y-axis represents frequency in Hertz, and the colors represent phase reproducibility across trials ranging from 0 (absence of synchronization) to 1 (perfect synchronization).
SSRs, on the other hand, are usually analyzed in the frequency domain and have seen extensive use in studies of sensory processes.27 The traditional approach to frequency domain analysis relies on the application of the Fourier transform to convert a time domain waveform into a sum of sinusoidal waveforms differing in power and phase. The power spectrum derived from Fourier coefficients displays EEG power (usually in microvolts2) in a segment of EEG as a function of frequency. Averaging across trials is done prior to the Fourier transform to isolate phase-locked activity to the stimulus, similar to the ERP analysis. The averaged SSR (figure 1a and 1b) and corresponding power spectrum (figure 1c) are shown in figure 1 for a steady state–evoked waveform elicited by an amplitude-modulated tone at 40 Hz (1000 Hz carrier frequency). Each frequency is associated with a phase value indicating the phase of that frequency component relative to the onset of the stimulus.
Oscillations that vary in amplitude over time like the SSR, otherwise known as nonstationary signals, are not appropriate for the traditional Fourier analysis because such signals violate several assumptions behind Fourier transforms.28 Further, while use of the fast Fourier transform (FFT) is commonly used to estimate the Fourier transform for discrete time series, using this transform on the whole trial period of SSR does not provide information on how the SSR evolves over the trial (figure 1c). Therefore, identification and characterization of the temporal dynamics of EEG responses have motivated the development and application of other signal analysis techniques including short-time window Fourier transform, multitaper Fourier transform, wavelet analysis, and Hilbert transforms.28–30
Another advantage of analysis in the frequency domain is the ability to derive statistical estimates of phase and power in single trials. Measures of mean power difference from baseline (also called event-related spectral perturbation [ERSP]) and mean normalized phase (also called intertrial coherence [ITC] or phase locking factor [PLF]) are relatively easy to compute and provide information about the magnitude and consistency of the entrained response. The ERSP or mean power is obtained by first subtracting the power from a baseline period and then averaging across trials. Thus, this measure represents the average change in power at a given frequency from the mean baseline power and so can detect changes in power that are induced by, but are not necessarily phase locked to, stimulus onset31 (figure 1d).
The ITC or PLF is an estimate of mean normalized phase across trials. First, a phasor (or the normalized complex number) is obtained from the complex output of the frequency transformation by dividing by its complex norm for each trial. The phasor is then averaged across trials, and a complex norm is taken to obtain the PLF. The PLF values can range from 0 (absence of synchronization) to 1 (perfect synchronization or phase reproducibility across trials at a given latency). Figure 1e shows the PLF plot for a 40-Hz signal. Both mean power and PLF are statistical measures and thus can be calculated for each frequency and time period for which the frequency transformation was applied. The interested reader is encouraged to examine several excellent reviews of EEG signal processing methods.32,33
Auditory Steady State Response
The auditory steady state response (ASSR) is usually elicited by amplitude-modulated tones or click trains presented at a given frequency. The ASSR in humans is largest in response to stimuli in the gamma (30–50 Hz) frequency range34 and may reflect the driving of smaller populations of neurons compared with larger network functioning associated with lower frequency activity.35 The simplest theory regarding the generation of the 40-Hz ASSR is that it is composed of a superposition of midlatency ERPs and auditory brain stem activity, that can be revealed using a specialized deconvolution algorithm.36–38 However, several findings indicate that the ASSR in the gamma range represents a resonance response or oscillation, rather than a simple superposition of discrete evoked potentials to individual clicks. The 40-Hz SSR in magnetoencephalograph (MEG) evolves during the 200-millisecond interval after stimulus onset,39 phase delay shortens with repeated stimuli, and phase synchronization continues after offset of a stimulus.24,40 Additionally, the 40-Hz SSR can be disrupted by a brief noise pulse,41,42 and this effect persists longer than the offset of the pulse. Such a prolonged disruption of the 40-Hz oscillation cannot easily be simulated by superposition of discrete responses and may indicate an inhibitory process that disrupts ongoing perceptual or binding processes.42 Therefore, it is possible that the ASSR reflects a combination of the initial response to the individual stimulus and the output of a resonance response. Finally, phase locking of the 40-Hz SSR has been reported to be enhanced by selective attention, whereas the 20-Hz ASSR was not,43 suggesting differential sensitivity of 40 Hz to attentional modulation. Similarly, Ross and Pantev44 found increased mean sustained amplitude MEG activity during a stimulus discrimination task that required differentiation of modulation rates within the gamma frequency range. While Linden et al45 found no effect of selective attention to intensity variations on the ASSR, Rockstroh et al46 found decreased SSR amplitude to 40-Hz stimuli when participants performed a concurrent auditory oddball task.
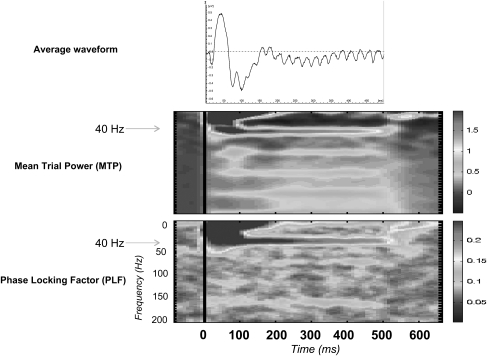
Animal studies have helped delineate the generators of the ASSRs. ASSRs have been elicited from many nonhuman mammalian species, including primates,47–50 cats,49,51–53 rabbits,54,55 and rodents56–60 (figure 2). As with human recordings, there is growing evidence that the ASSR in animals is primarily generated in auditory cortex and may be related to local field potentials. Kuwada et al54 investigated neural generators of the ASSR using behavioral, pharmacological, and cortical inactivation techniques in unanesthetized rabbits. Their data suggest that while the cortex is optimally activated by low frequencies (∼20 Hz) of stimulation, subcortical structures are likely involved in generating responses at higher frequencies of stimulation (∼90 Hz).54 This pattern has been supported by source localization analysis of EEG data in humans.61 However, in cats, ablation of lower auditory structures, such as the inferior colliculus, has been shown to decrease the phase synchrony of the ASSRs to frequencies of stimulation within the gamma range (20–80 Hz) leading the authors to suggest that this structure is a primary generator of the response.49 Using 40-Hz trains of clicks, Tsuzuku62 found that focal lesioning of the auditory cortices decreased 40-Hz ASSRs but to a lesser extent than ablation of the inferior colliculi. The role of the cortex is greatly diminished under anesthesia compared with that of the inferior colliculus.59 These data indicate that ASSRs, especially at frequencies below 80 Hz, likely reflect contributions from multiple brain generators with the primary auditory cortex and the inferior colliculus playing important roles.
Fig. 2.
Auditory Steady State Response in Healthy Sprague-Dawley Rats (N = 10) in Response to a 40-Hz Click Train Presented for 500 ms. Panel A represents the average waveform in the time domain. Panel B represents the mean power of the data. Panel C represents phase locking factor of the data. Stimulus onset occurred at 0 ms and ended at 500 ms, all axes labels are the same as corresponding analyses in figure 1.
ASSR Abnormalities in Schizophrenia
Patients with schizophrenia exhibit deficits in auditory steady state entrainment, particularly in the gamma (30–50 Hz) frequency range. In the first study to examine the ASSR in schizophrenia, Kwon et al24 found that patients exhibited reduced power and delayed phase synchrony to a 40-Hz click train. Similarly, Light et al63 found reduced power and intertrial phase coherence in response to 30- and 40-Hz clicks but not to 20-Hz click trains in those with schizophrenia. Krishnan et al41 also found reductions in measures of phase locking and in overall power in patients with chronic schizophrenia. A left hemisphere phase locking deficit to 40-Hz click trains has been found in patients experiencing their first episode,64 but such lateralization is not always found in chronic schizophrenia.65,66 Studies using amplitude-modulated tones show the largest reductions proximal to 40 Hz, but lower and higher frequencies appear to be affected as well.41,67 Because attention can affect the 40-Hz SSR, Krishnan et al41 required that subjects perform a visual discrimination task while ASSRs were recorded. Despite the absence of attention to the auditory stimuli, the ASSR deficit at gamma frequency was still evident in the schizophrenia patients.
While the reduction in 40 Hz has been replicated by all studies except one,68 changes in other stimulus frequencies are less consistent and may interact with stimulus characteristics. Vierling-Classen et al69 found an increase in 20 Hz in MEG for click stimuli in schizophrenia subjects, and Light et al63 reported decreased 30-Hz SSRs using EEG. Neither finding has been replicated in other studies using click stimuli. Changes in harmonics and subharmonics have also been inconsistent. Vierling-Classen et al69 found an increased subharmonic of 40 Hz (20 Hz) and a reduction of harmonic of 20-Hz (40-Hz) click stimuli in schizophrenia, but these phenomena were not observed by Krishnan et al.41 It should also be noted that all but 2 studies (Brenner et al67 and Krishnan et al41) measured responses only in 3 stimuli frequencies (20, 30, and 40 Hz). The changes in SSR to frequencies other than 40 Hz are crucial for identification of the mechanisms that result in the changes in ASSR. Further, differences in the type of stimuli (click- or amplitude-modulated tones) are likely to influence the response at the stimulus frequency and harmonics, and this issue requires more systematic investigation.
Another issue that remains unclear is whether the gamma-range ASSR deficit is a trait marker of risk for schizophrenia or a measure sensitive to the expression of psychosis. Hong et al68 reported that medicated patients with schizophrenia did not show a 40-Hz deficit but that first-degree relatives did show a deficit, suggestive of a vulnerability biomarker. On the other hand, Brenner et al67 found that ASSR power in the gamma range was not affected in subjects with schizotypal personality disorder. In summary, reduced ASSR power and phase locking is a robust finding in schizophrenia for both click- and amplitude-modulated tones and appears early in the course of the illness. Genetic risk for schizophrenia may also be associated with this deficit, but it does not appear in schizotypal personality disorder. The effect of medication on the deficit remains to be experimentally evaluated.
Scalp-recorded EEG and MEG activity (especially in the gamma band) is associated with primary auditory cortex generators including the superior temporal plane bilaterally,70–72 though some have found additional sources in the right anterior cingulate.73 These findings are consistent with the 40-Hz ASSR deficits exhibited by patients with schizophrenia because reduced gray matter volume in the superior temporal gyrus, where the primary and secondary auditory cortices are located, is one of the most consistent structural magnetic resonance imaging (MRI) findings in schizophrenia research.74 Neuropathological studies have identified a spectrum of cellular abnormalities in the auditory cortex as well. Sweet et al75,76 showed that the volume of pyramidal neurons in deep layer 3 of primary and secondary auditory cortices is reduced in schizophrenia. In contrast, there were no changes in layer 5 in secondary auditory cortex. These findings suggest a specific reduction in pyramidal cell volume in schizophrenia only in layer 3 of auditory cortex. Neuroanatomical studies of auditory cortex suggest that feed-forward connections arise from layer 3 and terminate in layer 4 while feedback connections arise from layer 3/4 and terminate in layer 1.76 Based on these anatomical studies and neuropathological findings in schizophrenia, Sweet et al77 suggested an abnormality in the feed-forward auditory processing circuit in schizophrenia. Moreover, decreased gray matter volume of left auditory cortex in patients with schizophrenia has been linked with P300 auditory ERP deficits.78 In summary, the ASSR deficit probably reflects alterations in auditory cortex gray matter volume and cellular abnormalities. Slice studies and computational modeling may provide a method to relate SSR deficits to specific cellular abnormalities.69,79
Visual SSR
The visual SSR is synchronized in frequency and phase to a temporally modulated visual stimulus80 and may be used as a tool to evaluate the integrity of visual pathways (figure 3). As with ASSRs, visual SSRs can be obtained with minimal task demands and can be elicited by a wide range of stimulation frequencies, from 1 to 100 Hz in humans27,81 and in animals.82 Visual SSRs show a more complex modulation transfer function than the ASSR. In humans, the strongest entrainment of visual SSRs to an unpatterned temporally modulated stimulus have been reported at 10, 16, 20, 40, and 80 Hz, indicative of frequency tuning or resonance in the underlying networks that generate these responses.80,81 Herrmann (2001) suggested that the 10-Hz peak may relate to mechanisms producing alpha activity while the 40-Hz peak may reflect a neural network responsible for gamma activity. As with the auditory domain, attention appears to enhance the visual SSR.83–85
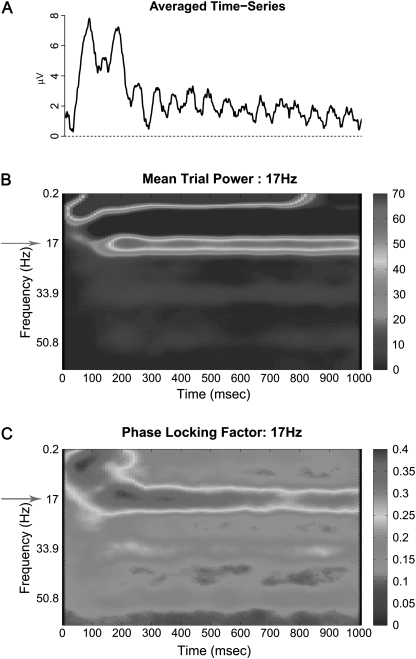
Fig. 3.
Averaged Visual Steady State Response Elicited by Flickering White-Black Visual Stimuli, Presented at a Flicker Rate of 17 Hz and Presented for 1000 ms in 46 Healthy Participants. Panel A represents the averaged time series waveform. Panel B represents the time-frequency spectrogram of mean power. Panel C represents the time-frequency spectrogram of phase locking factor. All axes labels are the same as corresponding analyses in figure 1.
Source analysis and imaging studies suggest that the scalp recorded visual SSR peaks at lower frequencies than in the auditory domain and is primarily generated by the occipital cortex. Emir et al86 presented visual photic stimuli at 1–44 Hz and found a peak blood oxygen level–dependent response at 8 Hz, with secondary peaks at 16 and 24 Hz in the primary visual cortex. Zhang et al87 found increased activity in area V1 during high-contrast pattern perception vs low-contrast pattern perception in a binocular rivalry paradigm. Muller et al88 used MEG to investigate the neural generation of visual SSR and found that 6.0- and 11.2-Hz activity was located in the posterior occipital cortex near the calcarine fissure, whereas the 15.2-Hz response was more anterior and ventromedially located in the lingual gyrus of the occipital cortex. These studies used dipole source estimation techniques to identify the neural generators of scalp-recorded activity; however, other techniques indicate that visual SSRs can activate long-range networks reaching the medial frontal lobes and may also be affected by abnormalities in the retinostriate projections.27,89–93
Visual SSR Abnormalities in Schizophrenia
Most visual SSR studies in schizophrenia have used unpatterned visual stimuli at different temporal frequencies at high contrast. Rice et al94 reported that subjects with schizophrenia exhibited reduced power at frontal sites in the alpha frequency range (7.2–9.6 Hz) and in 4.8, 7.2, and 9.6 Hz at parietal sites. Patients also showed reduced alpha activity (7–13 Hz) during resting period, but the reduction in alpha activity was larger during photic stimulation compared with the resting period.94 A series of studies by Jin et al95 showed that visual SSR reduction in schizophrenia occurred at higher alpha frequencies (12.5 Hz) and not at lower alpha frequencies (9.375 Hz). They later showed that schizophrenia subjects showed reduced power at 10, 11, and 12 Hz when evaluating the harmonics in the alpha frequency range.96 While the previous studies focused on a narrow range of temporal frequencies, Krishnan et al97 evaluated the visual SSR for a wide range of frequencies (4, 8, 17, 20, 23, 30, and 40 Hz) using a sinusoidally modulated high-luminance stimulus. Krishnan et al97 found that the visual SSR was reduced in schizophrenia at 17-, 23-, and 30-Hz stimulation rates. There was also an increase in background (or nonphase-locked) activity from 4 to 20 Hz in schizophrenia subjects. In summary, for high-contrast and high-luminance periodic stimulation, individuals with schizophrenia have reduced visual SSRs across alpha frequencies (from 9 to 12 Hz) and for frequencies in the beta and gamma range. EEG activity that is not phase locked to the stimulus may actually be higher at some frequencies, similar to findings in resting EEG.98 These findings indicate that the reduced capacity for neural entrainment found in patients with schizophrenia may be superimposed on resting state abnormalities. Delta and theta frequencies have received limited investigation but appear relatively intact.
Some visual SSR studies have used the psychophysical properties of a stimulus to differentially test the magno (M) and parvo (P) visual pathways in schizophrenia. Butler and colleagues99–101 used the differential sensitivity of the M and P pathways to luminance and chromatic contrast to evaluate these pathways in schizophrenia. Butler et al99,104 showed that the visual SSR was reduced in schizophrenia when the stimuli were presented at low-luminance contrast levels but not for high-luminance contrast levels. Visual SSRs were also reduced in schizophrenia subjects for low spatial frequency stimuli presented in the low-contrast condition. There were no differences in the chromatic visual SSRs. The selective reduction of SSRs at low-luminance contrast and relatively spared SSRs for high-luminance contrast and chromatic contrast provides neurophysiological evidence for a selective magnocellular pathway abnormality in schizophrenia. In support of this relationship, Kim et al101 reported that velocity discrimination thresholds on psychophysical tests were correlated with visual SSRs for magnocellular but not parvocellular stimulus conditions. They also found that patients with schizophrenia exhibited reduced visual SSR at the second harmonic for low-contrast stimuli, further supporting the role of a magnocellular pathway deficit in this population.101
In one of the only studies to evaluate attentional effects on the visual SSR, Clementz et al102 presented a visual target detection task and found that patients with schizophrenia showed enhanced visual SSR to attended vs unattended flickering stimuli similar to that of healthy controls. However, patient's evoked potentials and behavioral performance in response to visual targets (change in flickering bar width) were reduced. Because the flickering stimuli were thought to activate simple cells in area V1 and the detection of changes in bar width was thought to activate complex cells in higher cortical areas of V1, they interpreted their results to indicate that visual deficits exhibited by patients with schizophrenia may be related to supragranular integration deficits.
These findings of functional abnormalities in visual processing in schizophrenia are consistent with structural changes associated with the disorder. At first episode, gray matter thickness measured by MRI is reduced in the occipital cortex.103 In patients with chronic schizophrenia, Onitsuka et al104 found reduced gray matter volumes in the visual association areas, though not in the primary visual cortex. Butler et al105 reported an association between visual SSR to magnocellular biased stimuli and white matter tract integrity in the optic radiations. Finally, postcmortem studies have found reduced volume and density in BA17 in schizophrenia.106,107 It is likely that these structural aberrations of occipital cortex contribute to the psychophysical and visual SSR abnormalities observed in early stage visual processing.
Cellular Abnormalities and Gamma-Range Deficits in Schizophrenia
The selective disturbances of SSRs in the gamma frequency range in the auditory modality may be due to specific cellular neuropathology affecting γ-aminobutyric acid–mediated (GABAergic) and glutamatergic networks in the auditory cortex. Studies of cellular mechanisms subserving synchronization and oscillations among populations of neurons have implicated a central role of GABAergic modulation of glutamatergic neruons within the cortex. Interconnected pyramidal cell networks appear necessary for the generation of oscillations, while GABAergic inhibitory interneuron networks fire in rapid pairs to act as an oscillatory “pace maker” for these larger pyramidal cell networks.108–110 Several in vitro studies indicate that oscillatory activity can be induced by γ-aminobutyric acid (GABA) agonists and disrupted by GABA antagonists, underscoring the importance of GABA synapses in the modulation of gamma oscillations. More specifically, it is likely that parvalbumin-expressing chandelier and basket cells are the type of GABAergic interneurons involved with the modulation of oscillatory activity because these neurons form far-reaching networks consisting of both other interneurons and pyramidal cell nodes.111 In addition, chandelier cells form synapses on the axon hillock of pyramidal cells and are therefore structurally able to influence pyramidal cell firing.112 In regard to pyramidal cell specificity, neurons in laminar layer 3 also form far-reaching networks to other regions of the cortex, and they receive projections from the medial dorsal thalamus, making them structurally capable of influencing oscillatory brainwave activity.113
The finding that GABA- and glutamatergic mediated neurons interact to generate oscillatory activity in the cortex has important implications on the pathophysiology of schizophrenia. Lewis and colleagues reported that although the density of chandelier neurons was unaffected in postmortem tissue in schizophrenia, the parvalbumin messenger RNA (mRNA) expression of chandelier neurons may be decreased in the prefrontal cortex. In addition, the alpha 2 subunit of GABA receptor A (GABAA) found in chandelier interneurons are selectively upregulated in the prefrontal cortex in schizophrenia. These interneuron abnormalities may affect glutamate transmission in schizophrenia as well, given that the decreased levels of parvalbumin mRNA expression was highly correlated with decreased density of neurons containing detectable levels of GABA decarboxylase GAD67 mRNA.114–118 Ketamine, an N-methyl-D-aspartic acid (NMDA) receptor antagonist, induces symptoms similar to the positive, negative, and cognitive symptoms of schizophrenia and leads to reduced theta activity (that can be reversed by haloperidol administration) in response to repetitive auditory stimuli in mice.119 Morrow et al120 showed that repeated administration of an NMDA antagonist resulted in a reduction in the number of parvalbumin-containing axoaxonic cartridges, which may represent the axon terminals formed by chandelier cells. Reduced glutamatergic input on parvalbumin-containing interneurons via the NMDA receptor may mediate the downregulation of GAD67 and parvalbumin-containing neurons, which may in turn affect the oscillatory capabilities of the system. If GABAergic neurons are responsible for modulating pyramidal firing, then the decreased cartridge density or irregular mRNA expression of certain GABAergic neurons in schizophrenia may also contribute to the aberrant synchrony found in schizophrenia patients.
However, aberrant cellular morphology in schizophrenia is not confined to interneurons. Lewis and colleagues also found reduced spine density of pyramidal neurons in layer 3 of the cortex in schizophrenia. Pyramidal neurons in this layer undergo significant pruning in the dorsolateral prefrontal cortex (DLPFC) during late adolescence (a common age of onset for symptoms of schizophrenia) and have a smaller somal size in both the DLPFC as well as primary auditory cortex in schizophrenia.121,122 Together, these findings indicate that just as both excitatory pyramidal cell and inhibitory interneuron activity are necessary to generate sustained oscillatory activity in the cortex at high frequencies, both are vulnerable to structural and functional instability in schizophrenia.
Computational Models of SSR
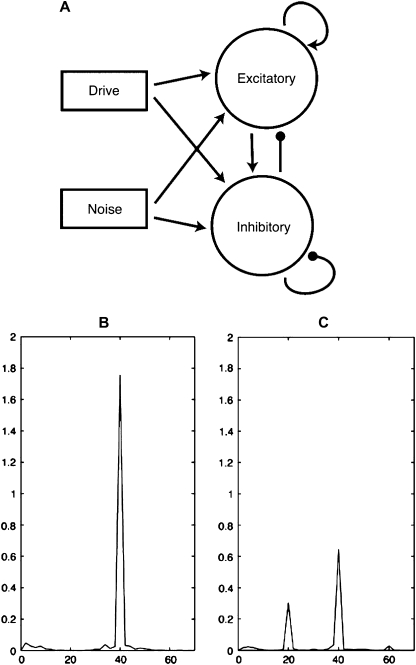
Human imaging data cannot provide direct measures of cellular abnormalities that produce SSR deficits in humans, but computational neuroscience can use cell and circuit simulations to test the effect of neuropathological changes on oscillatory responses. Computational models of SSRs are consistent with hypotheses of GABAergic neural network dysfunction in schizophrenia, which may be secondary to NMDA hypofunction.123 These models suggest that gamma-range synchronization (30–70 Hz) may support local circuit interactions in the cortex and that beta-range synchronization (13–29 Hz) may support long-distance integration across cortical regions.124,125 Vierling-Claassen et al69 created 2 computational models of the auditory cortex MEG response to 20-, 30-, and 40-Hz click trains in healthy controls and schizophrenia patients. They simulated time-locked frequency responses in which schizophrenia patients exhibited less than 40-Hz power and greater than 20-Hz power in response to both 40- and 20-Hz driving, respectively, by increasing the time constant of GABAA synaptic activity for both chandelier and basket cell networks onto the pyramidal neuron (figure 4). The increase in the time constant was based on the postmortem evidence for reduced GABA transporter in schizophrenia. Other postmortem findings also suggest reduced number of interneurons and GAD67 enzyme that is involved in manufacturing of GABA. Interestingly, simulating a reduction in available GABA by decreasing the strength of inhibitory-to-inhibitory and inhibitory-to-excitatory activity only reduced 40-Hz power in response to a 40-Hz stimulus. Increasing the time constant of the inhibitory interneuron networks was necessary to introduce the 20-Hz activity seen in the experimental data.69 Increasing the decay time constant at the GABAA synapse from interneuron to pyramidal neuron results in longer “inhibition” and a reduced probability of pyramidal cell spiking for a longer duration. These results indicate that variability in the timing of interneuron activity prevents precise entrainment of pyramidal networks. These findings are supported by the model by Traub et al126 that demonstrated that pyramidal cell activity is sufficient to produce gamma oscillations but that dendritic gap junctions (found in interneuron activity) are required to sharpen them. Computer simulations of cortical oscillations demonstrate the importance of interneuron activity in modulating the precision firing of large-scale networks necessary for steady state entrainment.
Fig. 4.
Representations of the Model Developed by Vierling-Claassen et al69 (used with Permission from J Neurophysiol 2008;99:2660,2663). Panel A reflects a network diagram of their simplified model, composed of 20 excitatory and 20 inhibitory cells (parvalbumin-containing fast-spiking interneurons). Poisson noise and drive input at 40 Hz were received by every cell. Panel B reflects the simulation output for a healthy group in response to 40-Hz drive. Panel C reflects the simulation output for a schizophrenic group to 40-Hz drive, where the only difference is a change in the decay time of the inhibitory postsynaptic currents that represent γ-aminobutyric acid receptor A synaptic decay.
Conclusions and Future Directions
Schizophrenia is associated with both ASSR and visual SSR abnormalities indicative of disrupted sensory processing. The ASSR demonstrates reduced power, delayed onset and termination, and lower phase locking values that are most apparent to stimuli modulated within the gamma frequency range. The visual SSR shows a more complex pattern of disturbances that are influenced by stimulus characteristics. For high-contrast, unpatterned stimuli, decreased power is most consistently found for temporal frequencies of 8 Hz and above. When contrast gain is manipulated, deficits are most apparent at low levels of contrast, suggestive of a magnocellular pathway disturbance. Frequency- and feature-specific deficits in the SSR may be indicative of cellular abnormalities that affect synchronized activity within sensory cortices.
ASSRs within the 1–60 Hz range have been linked to generators within the auditory cortex. It is possible that auditory pathways in the brain stem or the lateral geniculate nucleus in the thalamus could produce altered ASSRs, but there have been no reports to our knowledge of neuropathology in these nuclei. In contrast, abnormalities in the auditory cortex have been observed both in structural MRI and neuropathological studies. This evidence therefore suggests neural network dysfunction within the auditory cortex that may result in faulty generation and unreliable maintenance of oscillatory activity.
Most visual SSR deficits have been reported in the alpha (9 Hz) and beta (17–30 Hz) ranges in response to unpatterned suprathreshold stimuli. While the neural generation of visual SSRs is thought to originate in the primary visual cortex, several studies suggest long-range network activation, including thalamocortical circuits and inferior colliculus involvement, as well. Visual SSR studies that manipulate the psychophysical properties (luminance and contrast) to probe the different visual systems generally report magnocellular pathway–mediated disruption. A variety of psychophysical and electrophysiological findings suggest that visual processes associated with the magnocellular visual pathways may be more affected than those subserved by the parvocellular pathways.100,127 Psychophysical studies of contrast sensitivity, however, often suggest involvement of parvocellular pathway as well.128 With respect to neuropathology, cellular abnormalities have not been found in magnocellular or parvocellular populations in the lateral geniculate nucleus.129,130 This suggests that the visual cortex, which is the primary generator of the visual SSR, is most likely responsible for visual SSR disturbances in schizophrenia. This conclusion is consistent with MRI and cellular findings of occipital pathology. In summary, visual SSRs are likely affected by neuroanatomic disturbances in the visual cortices, but the mechanisms responsible for specific deficits remain to be characterized.
At the cellular level, oscillatory activity within the beta and gamma frequency ranges appears to be maintained by the interaction of GABAergic interneurons and excitatory pyramidal networks. Functional abnormalities in schizophrenia (especially those mediated by high frequency oscillatory activity within discrete cortical areas) may arise from aberrant glutamatergic input (via NMDA receptors) on specific parvalbumin-containing interneurons, which in turn may lead to the decreased cellular density and mRNA expression of the GABAergic neurons responsible for maintaining synchronous activation.
These functional impairments in neurotransmission likely interact with structural abnormalities seen in schizophrenia, such as reduced spine density and abnormal laminal organization. These region-specific deficits in the integrity of oscillatory activity can have far-reaching consequences throughout the cortex. Roopun et al131 have suggested that reduced power within a local network may lead to phase delays between oscillating networks across the cortex. Therefore, the integrity of local circuits during a simple entrainment paradigm may be associated with alterations in long-range synchrony that is required for integrative cognitive functioning.
While ASSR and visual SSR in schizophrenia have been established, major interpretative issues remain to be addressed. Because data suggest that attention modifies the SSR, those contemplating an SSR paradigm should consider controlling for selective attention in future experimental paradigms using patients. Furthermore, the relationship between SSR abnormalities and the course of schizophrenia is not known. Neither ASSR nor visual SSR have been tested in the premorbid or prodromal phases of the illness or evaluated in a longitudinal study. The relationship of SSRs to genetic risk factors merits further investigation on the basis of the finding of disturbed ASSRs in family members of probands with schizophrenia by Hong et al.68 SSRs are well suited for animal models and provide a useful vehicle for assessing pharmacologic and genetic effects on electrophysiological measures linked to schizophrenia.
Funding
Indiana University College of Arts and Sciences (Dissertation Award to G.P.K.); the National Institutes of Mental Health (1 RO1 MH62150 to B.F.O., R01 MH074983 to W.P.H.); National Alliance for Research on Schizophrenia and Depression (to C.A.B.); Indiana Clinical and Translational Sciences Institute Predoctoral Training Award (TL1RR02575 to J.L.V.).
Acknowledgments
We would like to thank Misty Bodkins, Colleen Merrill, and Jennifer Boggs for their invaluable help with data collection and Indiana University for their continued support of translational science.
References
- 1.Bunney WE, Hetrick WP, Bunney BG, et al. Structured interview for assessing perceptual anomalies. Schizophr Bull. 1999;25:577–592. doi: 10.1093/oxfordjournals.schbul.a033402. [DOI] [PubMed] [Google Scholar]
- 2.McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 3.Phillipson OT, Harris JP. Perceptual changes in schizophrenia: a questionnaire survey. Psychol Med. 1985;15:859–866. doi: 10.1017/s0033291700005092. [DOI] [PubMed] [Google Scholar]
- 4.Cutting J, Dunne F. The nature of the abnormal perceptual experiences at the onset of schizophrenia. Psychopathology. 1986;19:347–352. doi: 10.1159/000284459. [DOI] [PubMed] [Google Scholar]
- 5.Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Palafax GP, Nakayama K, Levy DL, Matthysse S, Holzman PS. Motion perception in schizophrenia. Arch Gen Psychiatry. 1999;56:149–154. doi: 10.1001/archpsyc.56.2.149. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Nakayama K, Levy D, Matthysse S, Holzman P. Processing of global, but not local, motion direction is deficient in schizophrenia. Schizophr Res. 2003;61:215–227. doi: 10.1016/s0920-9964(02)00222-0. [DOI] [PubMed] [Google Scholar]
- 8.Brenner CA, Wilt MA, Lysaker PH, Koyfman A, O'Donnell BF. Psychometrically matched visual processing tasks in schizophrenia spectrum disorders. J Abnorm Psychol. 2003;112:28–37. [PubMed] [Google Scholar]
- 9.O'Donnell BF, Swearer JM, Smith LT, Nestor PG, Shenton ME, McCarley RW. Selective deficits in visual perception and recognition in schizophrenia. Am J Psychiatry. 1996;153:687–692. doi: 10.1176/ajp.153.5.687. [DOI] [PubMed] [Google Scholar]
- 10.Stuve TA, Friedman L, Jesberger JA, Gilmore GC, Strauss ME, Meltzer HY. The Relationship between smooth pursuit performance, motion perception and sustained visual attention in patients with schizophrenia and normal controls. Psychol Med. 1997;27:143–152. doi: 10.1017/s0033291796004230. [DOI] [PubMed] [Google Scholar]
- 11.Tek C, Gold J, Blaxton T, Wilk C, McMahon RP, Buchanan RW. Visual perceptual and working memory impairments in schizophrenia. Arch Gen Psychiatry. 2002;59:146–153. doi: 10.1001/archpsyc.59.2.146. [DOI] [PubMed] [Google Scholar]
- 12.O'Donnell BF, Potts GF, Nestor PG, Stylianopoulos KC, Shenton ME, McCarley RW. Spatial frequency discrimination in schizophrenia. J Abnorm Psychol. 2002;111:620–625. doi: 10.1037//0021-843x.111.4.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Place EJS, Gilmore GC. Perceptual organization in schizophrenia. J Abnorm Psychol. 1980;89:409–418. doi: 10.1037//0021-843x.89.3.409. [DOI] [PubMed] [Google Scholar]
- 14.Silverstein SM, Knight RA, Schwarzkopf SB, West LL, Osborn LM, Kamin D. Stimulus configuration and context effects in perceptual organization in schizophrenia. J Abnorm Psychol. 1996;105:410–420. doi: 10.1037//0021-843x.105.3.410. [DOI] [PubMed] [Google Scholar]
- 15.Braff DL. Impaired speed of information processing in nonmedicated schizotypal patients. Schizophr Bull. 1981;7:499–508. doi: 10.1093/schbul/7.3.499. [DOI] [PubMed] [Google Scholar]
- 16.Cadenhead KS, Serper Y, Braff DL. Transient versus sustained visual channels in the visual backward masking deficits of schizophrenia patients. Biol Psychiatry. 1998;43:132–138. doi: 10.1016/S0006-3223(97)00316-8. [DOI] [PubMed] [Google Scholar]
- 17.Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Backward masking in unmedicated schizophrenic patients in psychotic remission: possible reflection of aberrant cortical oscillation. Am J Psychiatry. 1999;156:1367–1373. doi: 10.1176/ajp.156.9.1367. [DOI] [PubMed] [Google Scholar]
- 18.Rund BR. Backward-masking performance in chronic and nonchronic schizophrenics, affectively disturbed patients, and normal control subjects. J Abnorm Psychol. 1993;102:74–81. doi: 10.1037//0021-843x.102.1.74. [DOI] [PubMed] [Google Scholar]
- 19.Strous RD, Cowan N, Ritter W, Javitt DC. Auditory sensory (“echoic”) memory dysfunction in schizophrenia. Am J Psychiatry. 1995;152:1517–1510. doi: 10.1176/ajp.152.10.1517. [DOI] [PubMed] [Google Scholar]
- 20.Todd J, Michie PT, Jablensky AV. Association between reduced duration mismatch negativity (MMN) and raised temporal discrimination thresholds in schizophrenia. Clin Neurophysiol. 2003;114:2061–2070. doi: 10.1016/s1388-2457(03)00246-3. [DOI] [PubMed] [Google Scholar]
- 21.Leitman DI, Laukka P, Juslin PN, Saccente E, Butler P, Javitt DC. Getting the cue: sensory contributions to auditory emotion recognition impairments in schizophrenia. Schizophr Bull. 2008 doi: 10.1093/schbul/sbn115. doi:10.1093/schbul/sbn115. Advance Access publication on September 12, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picton TW, John MS, Dimitrijevic A, Purcell D. Human auditory steady-state responses. Int J Audiol. 2003;42:177–219. doi: 10.3109/14992020309101316. [DOI] [PubMed] [Google Scholar]
- 23.Regan D. Human Brain Electrophysiology: Evoked Potentials and Evoked Magnetic Fields in Science and Medicine. New York, NY: Elsevier; 1989. [Google Scholar]
- 24.Kwon JS, O'Donnell BF, Wallenstein GV, et al. Gamma frequency range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regan D. Human Brain Electrophysiology. New York, NY: Elsevier; 1989. [Google Scholar]
- 28.Mitra PP, Pesaran B. Analysis of dynamic brain imaging data. Biophys J. 1999;76:691–708. doi: 10.1016/S0006-3495(99)77236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Le Van Quyen M, Foucher J, Lachaux J, et al. Comparison of Hilbert transform and wavelet methods for the analysis of neuronal synchrony. J Neurosci Methods. 2001;111:83–98. doi: 10.1016/s0165-0270(01)00372-7. [DOI] [PubMed] [Google Scholar]
- 31.Makeig S. Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalogr Clin Neurophysiol. 1993;86:283–293. doi: 10.1016/0013-4694(93)90110-h. [DOI] [PubMed] [Google Scholar]
- 32.Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Handy T, editor. Event-Related Potentials: A Methods Handbook. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- 34.Azzena GB, Conti G, Santarelli R, Ottaviani F, Paludetti G, Maurizi M. Generation of human auditory steady-state responses (SSRs). I: stimulus rate effects. Hear Res. 1995;83:1–8. doi: 10.1016/0378-5955(94)00184-r. [DOI] [PubMed] [Google Scholar]
- 35.Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci. 2005;6:285–296. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- 36.Bohorquez J, Ozdamar O. Generation of the 40-Hz auditory steady-state response (ASSR) explained using convolution. Clin Neurophysiol. 2008;119:2598–2607. doi: 10.1016/j.clinph.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Delgado RE, Ozdamar O. Deconvolution of evoked responses obtained at high stimulus rates. J Acoust Soc Am. 2004;115:1242–1251. doi: 10.1121/1.1639327. [DOI] [PubMed] [Google Scholar]
- 38.Galambos R, Makeig S. Physiological studies of central masking in man. I: the effects of noise on the 40-Hz steady-state response. J Acoust Soc Am. 1992;92:2683–2690. doi: 10.1121/1.404383. [DOI] [PubMed] [Google Scholar]
- 39.Ross B, Picton TW, Pantev C. Temporal integration in the human auditory cortex as represented by the development of the steady-state magnetic field. Hear Res. 2002;165:68–84. doi: 10.1016/s0378-5955(02)00285-x. [DOI] [PubMed] [Google Scholar]
- 40.Santarelli R, Conti G. Generation of auditory steady-state responses: linearity assessment. Scand Audiol Suppl. 1999;51:23–32. [PubMed] [Google Scholar]
- 41.Krishnan GP, Hetrick WP, Brenner CA, Shekhar A, Steffens AN, O'Donnell BF. Steady state and induced auditory gamma deficits in schizophrenia. NeuroImage. 2009;47:1711–1719. doi: 10.1016/j.neuroimage.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross B, Herdman AT, Pantev C. Stimulus induced desynchronization of human auditory 40-Hz steady-state responses. J Neurophysiol. 2005;94:4082–4093. doi: 10.1152/jn.00469.2005. [DOI] [PubMed] [Google Scholar]
- 43.Skosnik PD, Krishnan GP, O'Donnell BF. The effect of selective attention on the gamma-band auditory steady-state response. Neurosci Lett. 2007;420:223–228. doi: 10.1016/j.neulet.2007.04.072. [DOI] [PubMed] [Google Scholar]
- 44.Ross B, Pantev C. Auditory steady-state responses reveal amplitude modulation gap detection thresholds. J Acoust Soc Am. 2004;115(pt 1):2193–2206. doi: 10.1121/1.1694996. [DOI] [PubMed] [Google Scholar]
- 45.Linden RD, Picton TW, Hamel G, Campbell KB. Human auditory steady-state evoked potentials during selective attention. Electroencephalogr Clin Neurophysiol. 1987;66:145–159. doi: 10.1016/0013-4694(87)90184-2. [DOI] [PubMed] [Google Scholar]
- 46.Rockstroh B, Muller MM, Heinz A, Wagner M, Berg P, Elbert T. Modulation of auditory responses during oddball tasks. Biol Psychiatry. 1996;43:41–55. doi: 10.1016/0301-0511(95)05175-9. [DOI] [PubMed] [Google Scholar]
- 47.Burton MJ, Cohen LT, Rickards FW, McNally KI, Clark GM. Steady-state evoked potentials to amplitude modulated tones in the monkey. Acta Otolaryngol. 1992;112:745–751. doi: 10.3109/00016489209137469. [DOI] [PubMed] [Google Scholar]
- 48.Steinschneider M, Reser DH, Fishman YI, Schroeder CE, Arezzo JC. Click train encoding in primary auditory cortex of the awake monkey: evidence for two mechanisms subserving pitch perception. J Acoust Soc Am. 1998;104:2935–2955. doi: 10.1121/1.423877. [DOI] [PubMed] [Google Scholar]
- 49.Kiren T, Aoyagi M, Furuse H, Koike Y. An experimental study on the generator of amplitude-modulation following response. Acta Otolaryngol. 1994;511:28–33. [PubMed] [Google Scholar]
- 50.Lu T, Liang L, Wang X. Temporal and rate representations of time-varying signals in the auditory cortex of awake primates. Nat Neurosci. 2001;4:1131–1138. doi: 10.1038/nn737. [DOI] [PubMed] [Google Scholar]
- 51.Karmos G, Lakatos P, Pincze Z, Rajkai C, Ulbert I. Frequency of gamma activity is modulated by motivation in the auditory cortex of cat. Acta Biol Hung. 2002;53:473–483. doi: 10.1556/ABiol.53.2002.4.8. [DOI] [PubMed] [Google Scholar]
- 52.Makela JP, Karmos G, Molnar M, Csepe V, Winkler I. Steady-state responses from the cat auditory cortex. Hear Res. 1990;45:41–50. doi: 10.1016/0378-5955(90)90181-n. [DOI] [PubMed] [Google Scholar]
- 53.Eggermont JJ. Temporal modulation transfer functions in cat primary auditory cortex: separating stimulus effects from neural mechanisms. J Neurophysiol. 2002;87:305–321. doi: 10.1152/jn.00490.2001. [DOI] [PubMed] [Google Scholar]
- 54.Kuwada S, Anderson JS, Batra R, Fitzpatrick DC, Teissier N, D'Angelo WR. Sources of the scalp-recorded amplitude-modulation following response. J Am Acad Audiol. 2002;13:188–204. [PubMed] [Google Scholar]
- 55.Ottaviani F, Paludetti G, Grassi S, et al. Auditory steady-state responses in the rabbit. Audiology. 1990;29:212–218. doi: 10.3109/00206099009072852. [DOI] [PubMed] [Google Scholar]
- 56.Conti G, Santarelli R, Grassi S, Ottaviani F, Azzena GB. Auditory steady-state responses to click trains from the rat temporal cortex. Clin Neurophysiol. 1999;110:62–70. doi: 10.1016/s0168-5597(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 57.Franowicz MN, Barth DS. Comparison of evoked potentials and high-frequency (gamma-band) oscillating potentials in rat auditory cortex. J Neurophysiol. 1995;74:96–112. doi: 10.1152/jn.1995.74.1.96. [DOI] [PubMed] [Google Scholar]
- 58.Santarelli R, Carraro L, Conti G, Capello M, Plourde G, Arslan E. Effects of isoflurane on auditory middle latency (MLRs) and steady-state (SSRs) responses recorded from the temporal cortex of the rat. Brain Res. 2003;973:240–251. doi: 10.1016/s0006-8993(03)02520-4. [DOI] [PubMed] [Google Scholar]
- 59.Szalda K, Burkard R. The effects of nembutal anesthesia on the auditory steady-state response (ASSR) from the inferior colliculus and auditory cortex of the chinchilla. Hear Res. 2005;203:32–44. doi: 10.1016/j.heares.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 60.Dolphin WF, Mountain DC. The envelope following response: scalp potentials elicited in the Mongolian gerbil using sinusoidally AM acoustic signals. Hear Res. 1992;58:70–78. doi: 10.1016/0378-5955(92)90010-k. [DOI] [PubMed] [Google Scholar]
- 61.Herdman AT, Lins O, Van Roon P, Stapells DR, Scherg M, Picton TW. Intracerebral sources of human auditory steady-state responses. Brain Topogr. 2002;15:69–86. doi: 10.1023/a:1021470822922. [DOI] [PubMed] [Google Scholar]
- 62.Tsuzuku T. 40-Hz steady state response in awake cats after bilateral chronic lesions in auditory cortices or inferior colliculi. Auris Nasus Larynx. 1993;20:263–274. doi: 10.1016/s0385-8146(12)80118-0. [DOI] [PubMed] [Google Scholar]
- 63.Light GA, Hsu JL, Hsieh MH, et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 64.Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64:369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teale P, Carlson J, Rojas D, Reite M. Reduced laterality of the source locations for generators of the auditory steady-state field in schizophrenia. Biol Psychiatry. 2003;54:1149–1153. doi: 10.1016/s0006-3223(03)00411-6. [DOI] [PubMed] [Google Scholar]
- 66.Teale P, Collins D, Maharajh K, Rojas DC, Kronberg E, Reite M. Cortical source estimates of gamma band amplitude and phase are different in schizophrenia. NeuroImage. 2008;42:1481–1489. doi: 10.1016/j.neuroimage.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brenner CA, Sporns O, Lysaker PH, O'Donnell BF. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder and schizotypal personality disorder. Am J Psychiatry. 2003;160:2238–2240. doi: 10.1176/appi.ajp.160.12.2238. [DOI] [PubMed] [Google Scholar]
- 68.Hong LE, Summerfelt A, McMahon R, et al. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res. 2004;70:293–302. doi: 10.1016/j.schres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 69.Vierling-Claassen D, Siekmeier P, Stufflebeam S, Kopell N. Modeling GABA alterations in schizophrenia: a link between impaired inhibition and altered gamma and beta range auditory entrainment. J Neurophysiol. 2008;99:2656–2671. doi: 10.1152/jn.00870.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pastor MA, Artieda J, Arbizu J, Marti-Climent JM, Penuelas I, Masdeu JC. Activation of human cerebral and cerebellar cortex by auditory stimulation at 40 Hz. J Neurosci. 2002;22:10501–10506. doi: 10.1523/JNEUROSCI.22-23-10501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schoonhoven R, Boden CJ, Verbunt JP, de Munck JC. A whole head MEG study of the amplitude-modulation-following response: phase coherence, group delay and dipole source analysis. Clin Neurophysiol. 2003;114:2096–2106. doi: 10.1016/s1388-2457(03)00200-1. [DOI] [PubMed] [Google Scholar]
- 72.Simpson MI, Hadjipapas A, Barnes GR, Furlong PL, Witton C. Imaging the dynamics of the auditory steady-state evoked response. Neurosci Lett. 2005;385:195–197. doi: 10.1016/j.neulet.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 73.Reyes SA, Salvi RJ, Burkard RF, et al. PET imaging of the 40 Hz auditory steady state response. Hear Res. 2004;194:73–80. doi: 10.1016/j.heares.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 74.McCarley RW, Wible CG, Frumin M, et al. MRI anatomy of schizophrenia. Biol Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sweet RA, Pierri JN, Auh S, Sampson AR, Lewis DA. Reduced pyramidal cell somal volume in auditory association cortex of subjects with schizophrenia. Neuropsychopharmacology. 2003;28:599–609. doi: 10.1038/sj.npp.1300120. [DOI] [PubMed] [Google Scholar]
- 76.Sweet RA, Bergen SE, Sun Z, Sampson AR, Pierri JN, Lewis DA. Pyramidal cell size reduction in schizophrenia: evidence for involvement of auditory feedforward circuits. Biol Psychiatry. 2004;55:1128–1137. doi: 10.1016/j.biopsych.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Sweet RA, Bergen SE, Sun Z, Marcsisin MJ, Sampson AR, Lewis DA. Anatomical evidence of impaired feedforward auditory processing in schizophrenia. Biol Psychiatry. 2007;61:854–864. doi: 10.1016/j.biopsych.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 78.O'Donnell BF, McCarley RW, Potts GF, et al. Identification of neural circuits underlying P300 abnormalities in schizophrenia. Psychophysiology. 1999;36:388–398. doi: 10.1017/s0048577299971688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Whittington MA, Faulkner HJ, Doheny HC, Traub RD. Neuronal fast oscillations as a target site for psychoactive drugs. Pharmacol Ther. 2000;86:171–190. doi: 10.1016/s0163-7258(00)00038-3. [DOI] [PubMed] [Google Scholar]
- 80.Regan MP, Regan D. Objective investigation of visual function using a nondestructive zoom-FFT technique for evoked potential analysis. Can J Neurol Sci. 1989;16:168–179. doi: 10.1017/s0317167100028845. [DOI] [PubMed] [Google Scholar]
- 81.Herrmann CS, Mecklinger A. Gamma activity in human EEG is related to high-speed memory comparisons during object selective attention. Vis Cogn. 2001;8:593–608. [Google Scholar]
- 82.Rager G, Singer W. The response of cat visual cortex to flicker stimuli of variable frequency. Eur J Neurosci. 1998;10:1856–1877. doi: 10.1046/j.1460-9568.1998.00197.x. [DOI] [PubMed] [Google Scholar]
- 83.Fuchs S, Andersen SK, Gruber T, Muller MM. Attentional bias of competitive interactions in neuronal networks of early visual processing in the human brain. NeuroImage. 2008;41:1086–1101. doi: 10.1016/j.neuroimage.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 84.Ding J, Sperling G, Srinivasan R. Attentional modulation of SSVEP power depends on the network tagged by the flicker frequency. Cereb Cortex. 2006;16:1016–1029. doi: 10.1093/cercor/bhj044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Srinivasan R, Bibi FA, Nunez PL. Steady-state visual evoked potentials: distributed local sources and wave-like dynamics are sensitive to flicker frequency. Brain Topogr. 2006;18:167–187. doi: 10.1007/s10548-006-0267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Emir UE, Bayraktaroglu Z, Ozturk C, Ademoglu A, Demiralp T. Changes in BOLD transients with visual stimuli across 1-44 Hz. Neurosci Lett. 2008;436:185–188. doi: 10.1016/j.neulet.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Y, Wang R, Hong B, Gao X, Gao S. Source estimation of contrast-related perception based on frequency-tagged binocular rivalry. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1177–1180. doi: 10.1109/IEMBS.2006.260094. [DOI] [PubMed] [Google Scholar]
- 88.Muller MM, Teder W, Hillyard SA. Magnetoencephalographic recording of steady-state visual evoked cortical activity. Brain Topogr. 1997;9:163–168. doi: 10.1007/BF01190385. [DOI] [PubMed] [Google Scholar]
- 89.Pastor MA, Artieda J, Arbizu J, Valencia M, Masdeu JC. Human cerebral activation during steady-state visual-evoked responses. J Neurosci. 2003;23:11621–11627. doi: 10.1523/JNEUROSCI.23-37-11621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burkitt GR, Silberstein RB, Cadusch PJ, Wood AW. Steady-state visual evoked potentials and travelling waves. Clin Neurophysiol. 2000;111:246–258. doi: 10.1016/s1388-2457(99)00194-7. [DOI] [PubMed] [Google Scholar]
- 91.Srinivasan R, Fornari E, Knyazeva MG, Neuli R, Maeder P. fMRI responses in medial frontal cortex that depend on the temporal frequency of visual input. Exp Brain Res. 2007;180:677–679. doi: 10.1007/s00221-007-0886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thorpe SG, Nunez PL, Srinivasan R. Identification of wave-like spatial structure in the SSVEP: comparison of simultaneous EEG and MEG. Stat Med. 2007;26:3911–3926. doi: 10.1002/sim.2969. [DOI] [PubMed] [Google Scholar]
- 93.Weinberg H, Johnson B, Cohen P, Crisp D, Robertson A. Functional imaging of brain responses to repetitive sensory stimulation: sources estimated from EEG and SPECT. Brain Topogr. 1989;2:171–180. doi: 10.1007/BF01128854. [DOI] [PubMed] [Google Scholar]
- 94.Rice DM, Potkin SG, Jin Y, et al. EEG alpha photic driving abnormalities in chronic schizophrenia. Psychiatr Res. 1989;30:313–324. doi: 10.1016/0165-1781(89)90022-x. [DOI] [PubMed] [Google Scholar]
- 95.Jin Y, Sandman CA, Wu JC, Bernat J, Potkin SG. Topographic analysis of EEG photic driving in normal and schizophrenic subjects. Clin Electroencephalogr. 1995;26:102–107. doi: 10.1177/155005949502600207. [DOI] [PubMed] [Google Scholar]
- 96.Jin Y, Castellanos A, Solis ER, Potkin SG. EEG resonant responses in schizophrenia: a photic driving study with improved harmonic resolution. Schizophr Res. 2000;44:213–220. doi: 10.1016/s0920-9964(99)00211-x. [DOI] [PubMed] [Google Scholar]
- 97.Krishnan GP, Vohs JL, Hetrick WP, et al. Steady state visual evoked potential abnormalities in schizophrenia. Clin Neurophysiol. 2005;116:614–624. doi: 10.1016/j.clinph.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 98.Sponheim SR, Clementz BA, Iacono WG, Beiser M. Clinical and biological concomitants of resting state EEG power abnormalities in schizophrenia. Biol Psychiatry. 2000;48:1088–1097. doi: 10.1016/s0006-3223(00)00907-0. [DOI] [PubMed] [Google Scholar]
- 99.Butler PD, Schechter I, Zemon V, et al. Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- 100.Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry. 2005;18:151–157. doi: 10.1097/00001504-200503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim D, Zemon V, Saperstein A, Butler PD, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia: harmonic analysis. Schizophr Res. 2005;76:55–65. doi: 10.1016/j.schres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 102.Clementz BA, Wang J, Keil A. Normal electrocortical facilitation but abnormal target identification during visual sustained attention in schizophrenia. J Neurosci. 2008;28:13411–13418. doi: 10.1523/JNEUROSCI.4095-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Narr KL, Toga AW, Szeszko P, et al. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 2005;58:32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 104.Onitsuka T, McCarley RW, Kuroki N, et al. Occipital lobe gray matter volume in male patients with chronic schizophrenia: a quantitative MRI study. Schizophr Res. 2007;92:197–206. doi: 10.1016/j.schres.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Butler PD, Zemon V, Schechter I, et al. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dorph-Petersen KA, Pierri JN, Sun Z, Sampson AR, Lewis DA. Stereological analysis of the mediodorsal thalamic nucleus in schizophrenia: volume, neuron number, and cell types. J Comp Neurol. 2004;472:449–462. doi: 10.1002/cne.20055. [DOI] [PubMed] [Google Scholar]
- 107.Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- 108.Traub R, Whittington MA, Stanford IM, Jefferys JGR. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature. 1996;383:621–624. doi: 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- 109.Traub RD, Cunningham MO, Gloveli T, et al. GABA-enhanced collective behavior in neuronal axons underlies persistent gamma-frequency oscillations. Proc Natl Acad Sci U S A. 2003;100:11047–11052. doi: 10.1073/pnas.1934854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Whittington MA, Traub R, Jefferys JGR. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 111.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 112.Coyle JT, Tsai G. NMDA receptor function, neuroplasticity, and the pathophysiology of schizophrenia. Int Rev Neurobiol. 2004;59:491–515. doi: 10.1016/S0074-7742(04)59019-0. [DOI] [PubMed] [Google Scholar]
- 113.Lewis DA, Cruz D, Eggan S, Erickson S. Postnatal development of prefrontal inhibitory circuits and the pathophysiology of cognitive dysfunction in schizophrenia. Ann N Y Acad Sci. 2004;1021:64–76. doi: 10.1196/annals.1308.008. [DOI] [PubMed] [Google Scholar]
- 114.Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 115.Hashimoto T, Volk DW, Eggan SM, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lewis DA. GABAergic local circuit neurons and prefrontal cortical dysfunction in schizophrenia. Brain Res Rev. 2000;31:270–276. doi: 10.1016/s0165-0173(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 117.Lewis DA, Gonzalez-Burgos G. Intrinsic excitatory connections in the prefrontal cortex and the pathophysiology of schizophrenia. Brain Res Bull. 2000;52:309–317. doi: 10.1016/s0361-9230(99)00243-9. [DOI] [PubMed] [Google Scholar]
- 118.Lewis DA, Volk DW, Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology. 2004;174:143–150. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- 119.Ehrlichman RS, Gandal MJ, Maxwell CR, et al. N-methyl-D-aspartic acid receptor antagonist-induced frequency oscillations in mice recreate pattern of electrophysiological deficits in schizophrenia. Neuroscience. 2009;158:705–712. doi: 10.1016/j.neuroscience.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 120.Morrow BA, Elsworth JD, Roth RH. Repeated phencyclidine in monkeys results in loss of parvalbumin-containing axo-axonic projections in the prefrontal cortex. Psychopharmacology. 2007;192:283–290. doi: 10.1007/s00213-007-0708-0. [DOI] [PubMed] [Google Scholar]
- 121.Lewis DA, Glantz LA, Pierri JN, Sweet RA. Altered cortical glutamate neurotransmission in schizophrenia: evidence from morphological studies of pyramidal neurons. Ann N Y Acad Sci. 2003;1003:102–112. doi: 10.1196/annals.1300.007. [DOI] [PubMed] [Google Scholar]
- 122.Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex. Arch Gen Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- 123.Grunze HC, Rainnie DG, Hasselmo ME, et al. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bibbig A, Traub RD, Whittington MA. Long-range synchronization of gamma and beta oscillations and the plasticity of excitatory and inhibitory synapses: a network model. J Neurophysiol. 2002;88:1634–1654. doi: 10.1152/jn.2002.88.4.1634. [DOI] [PubMed] [Google Scholar]
- 125.Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A. 1999;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Traub RD, Pais I, Bibbig A, et al. Contrasting roles of axonal (pyramidal cell) and dendritic (interneuron) electrical coupling in the generation of neuronal network oscillations. Proc Natl Acad Sci U S A. 2003;100:1370–1374. doi: 10.1073/pnas.0337529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Keri S, Kelemen O, Benedek G, Janka Z. Vernier threshold in patients with schizophrenia and their unaffected siblings. Neuropsychology. 2004;18:537–542. doi: 10.1037/0894-4105.18.3.537. [DOI] [PubMed] [Google Scholar]
- 128.Skottun BC, Skoyles JR. Contrast sensitivity and magnocellular functioning in schizophrenia. Vision Res. 2007;47:2923–2933. doi: 10.1016/j.visres.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 129.Selemon LD, Begovic A. Stereologic analysis of the lateral geniculate nucleus of the thalamus in normal and schizophrenic subjects. Psychiatr Res. 2007;151:1–10. doi: 10.1016/j.psychres.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dorph-Petersen KA, Caric D, Saghafi R, Zhang W, Sampson AR, Lewis DA. Volume and neuron number of the lateral geniculate nucleus in schizophrenia and mood disorders. Acta Neuropathol. 2009;117:364–384. doi: 10.1007/s00401-008-0410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr Bull. 2008;34:962–973. doi: 10.1093/schbul/sbn059. [DOI] [PMC free article] [PubMed] [Google Scholar]