Abstract
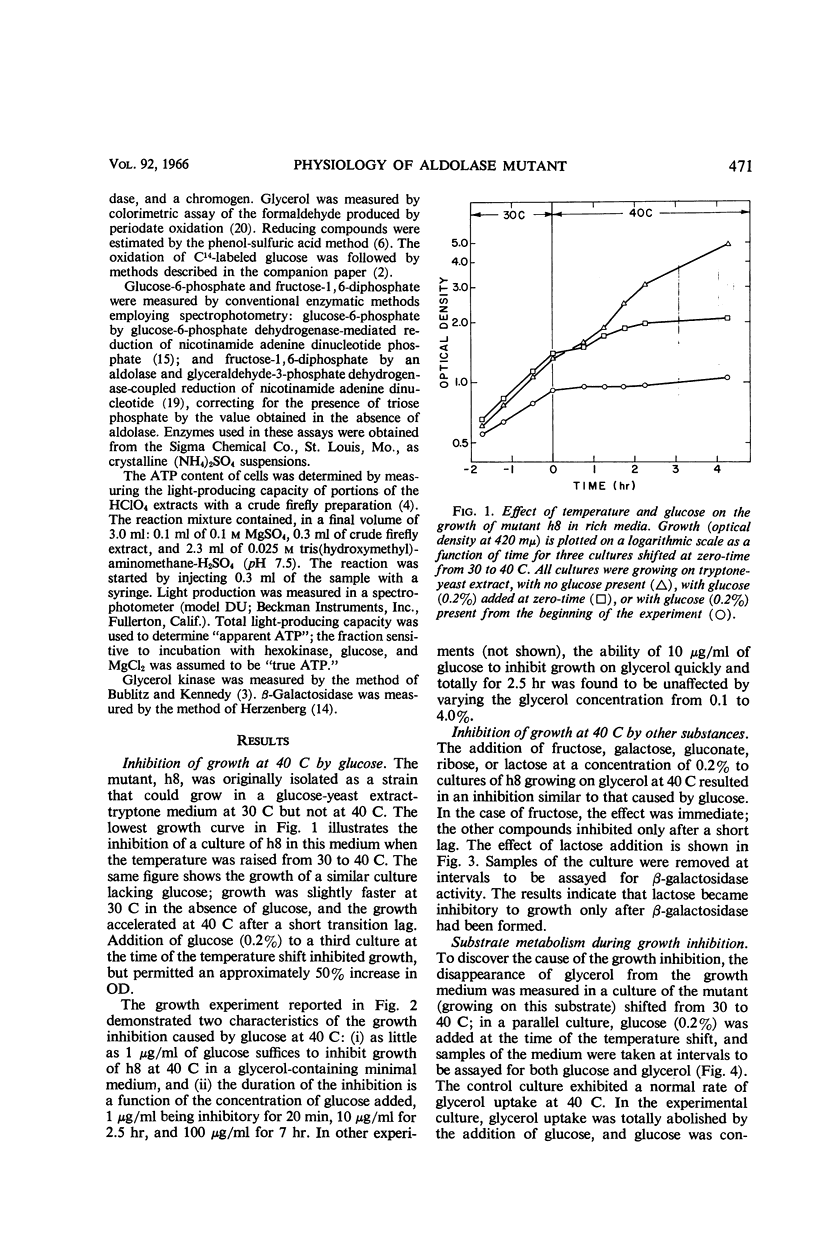
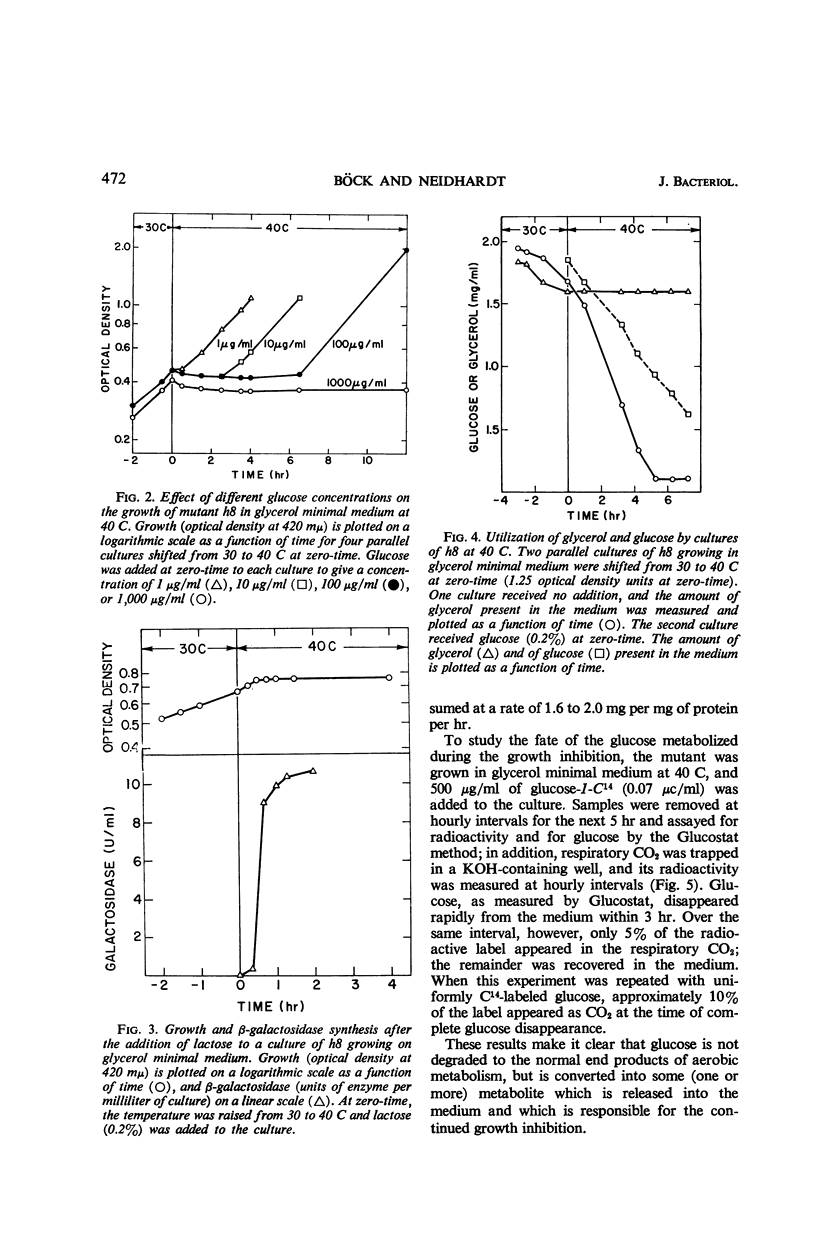
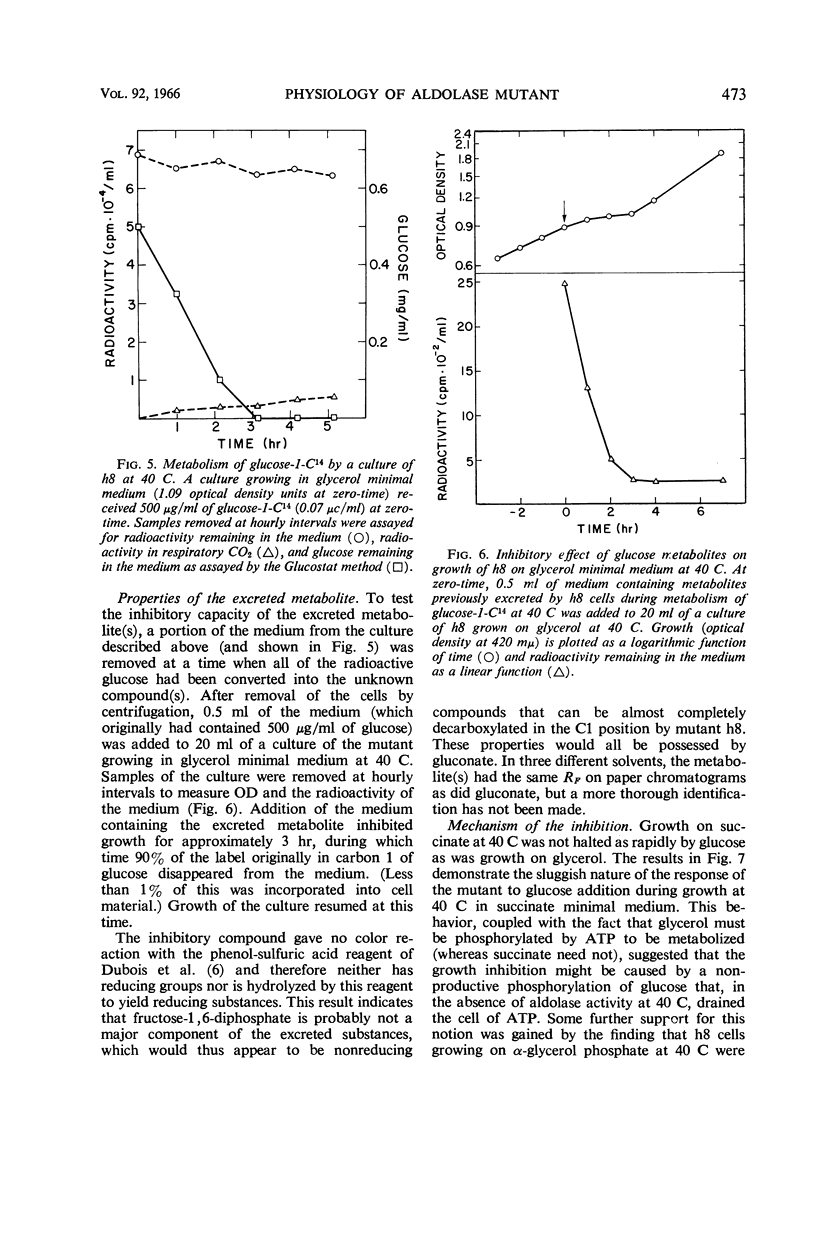
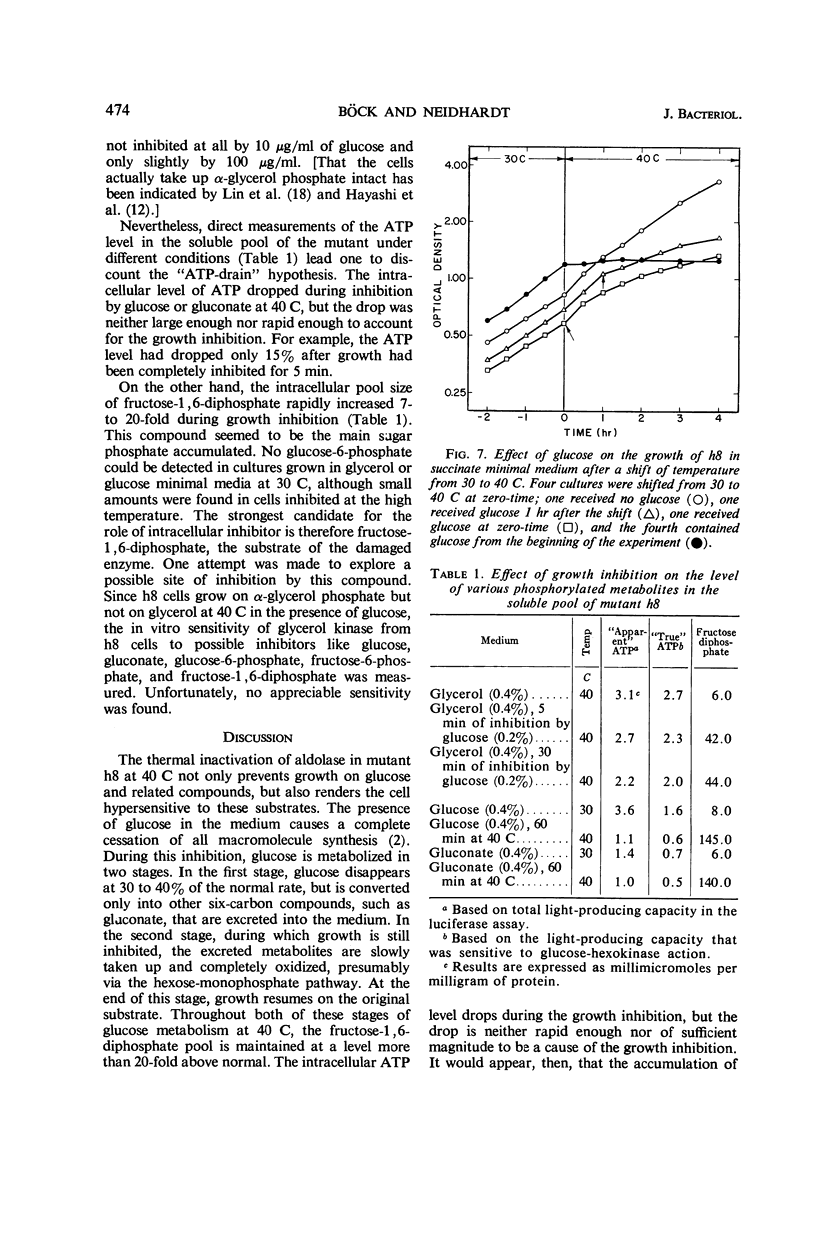
Böck, August (Purdue University, Lafayette, Ind.), and Frederick C. Neidhardt. Properties of a mutant of Escherichia coli with a temperature-sensitive fructose-1,6-diphosphate aldolase. J. Bacteriol. 92:470–476. 1966.—A mutant of Escherichia coli in which fructose-1,6-diphosphate aldolase functions at 30 C but not at 40 C was used to study the physiological effect of a specific block in the Embden-Meyerhof glycolytic pathway. Growth of the mutant at 40 C was found to be inhibited by the presence of glucose or certain related compounds in the medium. At 40 C, glucose was metabolized at 30 to 40% of the control rate and was abnormal in that glucose was converted into other six-carbon substances (probably gluconate, in large part) that were released into the culture medium. The inhibition was complete, but transient; its duration depended upon the initial amount of inhibitor added. The resumption of growth at 40 C was correlated with the further catabolism of the excreted compounds. When glycerol was used to grow the mutant at 40 C, the growth inhibition by glucose was accompanied by cessation of glycerol metabolism. Growth on α-glycerol phosphate was not inhibited under these conditions, implicating glycerol kinase as a possible site of inhibition; no inhibition of glycerol kinase by sugar phosphates, however, could be detected in vitro. The inhibitory effect of glucose on growth at 40 C is not caused by a deficit of intracellular adenosine triphosphate, but may be the result of a generalized poisoning of many cell processes by a greatly increased intracellular concentration of fructose-1,6-diphosphate, the substrate of the damaged enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUBLITZ C., KENNEDY E. P. Synthesis of phosphatides in isolated mitochondria. III. The enzymatic phosphorylation of glycerol. J Biol Chem. 1954 Dec;211(2):951–961. [PubMed] [Google Scholar]
- Böck A., Neidhardt F. C. Isolation of a Mutant of Escherichia coli with a Temperature-sensitive Fructose-1,6-Diphosphate Aldolase Activity. J Bacteriol. 1966 Aug;92(2):464–469. doi: 10.1128/jb.92.2.464-469.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHASE A. M. The measurement of luciferin and luciferase. Methods Biochem Anal. 1960;8:61–117. doi: 10.1002/9780470110249.ch2. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Koch J. P., Hayashi S., Lin E. C. Growth stasis by accumulated L-alpha-glycerophosphate in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1325–1329. doi: 10.1128/jb.90.5.1325-1329.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. PROTEIN AND NUCLEIC ACID SYNTHESIS IN TWO MUTANTS OF ESCHERICHIA COLI WITH TEMPERATURE-SENSITIVE AMINOACYL RIBONUCLEIC ACID SYNTHETASES. J Bacteriol. 1965 Mar;89:706–711. doi: 10.1128/jb.89.3.706-711.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., ANDERSON R. L., WEINBERG R., LEE N., HOFFEE P., HUTTENHAUER G., BOYER H. L-Arabinose-sensitive, L-ribulose 5-phosphate 4-epimerase-deficient mutants of Escherichia coli. J Bacteriol. 1962 Jul;84:137–146. doi: 10.1128/jb.84.1.137-146.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., BARON L. S. Mutation to L-rhamnose resistance and transduction to L-rhamnose utilization in Salmonella typhosa. J Bacteriol. 1959 Nov;78:675–686. doi: 10.1128/jb.78.5.675-686.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL D. G., HORECKER B. L. PATHWAYS OF D-GLUCOSE METABOLISM IN SALMONELLA TYPHINMURIUM. A STUDY OF A MUTANT LACKING PHOSPHOGLUCOSE ISOMERASE. J Biol Chem. 1964 Sep;239:2765–2771. [PubMed] [Google Scholar]
- GOLDBERG E. B., NITOWSKY H. M., COLOWICK S. P. THE ROLE OF GLYCOLYSIS IN THE GROWTH OF TUMOR CELLS. IV. THE BASIS OF GLUCOSE TOXICITY IN OXAMATE-TREATED, CULTURED CELLS. J Biol Chem. 1965 Jul;240:2791–2796. [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- HAYASHI S., LIN E. C. CAPTURE OF GLYCEROL BY CELLS OF ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Mar 29;94:479–487. doi: 10.1016/0926-6585(65)90056-7. [DOI] [PubMed] [Google Scholar]
- HERZENBERG L. A. Studies on the induction of beta-galactosidase in a cryptic strain of Escherichia coli. Biochim Biophys Acta. 1959 Feb;31(2):525–538. doi: 10.1016/0006-3002(59)90029-0. [DOI] [PubMed] [Google Scholar]
- IMSANDE J., PRESTIDGE L. S. A CROSS-LINKED CONTROL SYSTEM. II. CONTROL OF PYRIDINE NUCLEOTIDE FORMATION IN VIVO. Biochim Biophys Acta. 1964 May 4;85:265–273. doi: 10.1016/0926-6569(64)90247-0. [DOI] [PubMed] [Google Scholar]
- KURAHASHI K., WAHBA A. J. Interference with growth of certain Escherichia coli mutants by galactose. Biochim Biophys Acta. 1958 Nov;30(2):298–302. doi: 10.1016/0006-3002(58)90054-4. [DOI] [PubMed] [Google Scholar]
- LIN E. C., KOCH J. P., CHUSED T. M., JORGENSEN S. E. Utilization of L-alpha-glycerophosphate by Escherichia coli without hydrolysis. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2145–2150. doi: 10.1073/pnas.48.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARZ V., GOLBERG L., KOMROWER G. M., HOLZEL A. Some disturbances of erythrocyte metabolism in galactosaemia. Biochem J. 1956 Jan;62(1):34–40. doi: 10.1042/bj0620034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLS A., CADENAS E., ALVARADO F. Enzymatic basis of mannose toxicity in honey bees. Science. 1960 Jan 29;131(3396):297–298. doi: 10.1126/science.131.3396.297. [DOI] [PubMed] [Google Scholar]
- SUNDARARAJAN T. A., RAPIN A. M., KALCKAR H. M. Biochemical observations on E. coli mutants defective in uridine diphosphoglucose. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2187–2193. doi: 10.1073/pnas.48.12.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky M. B., Wiesmeyer H., Kalckar H. M., Jordan E. HEREDITARY DEFECTS IN GALACTOSE METABOLISM IN ESCHERICHIA COLI MUTANTS, II. GALACTOSE-INDUCED SENSITIVITY. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1786–1791. doi: 10.1073/pnas.45.12.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]


