Abstract
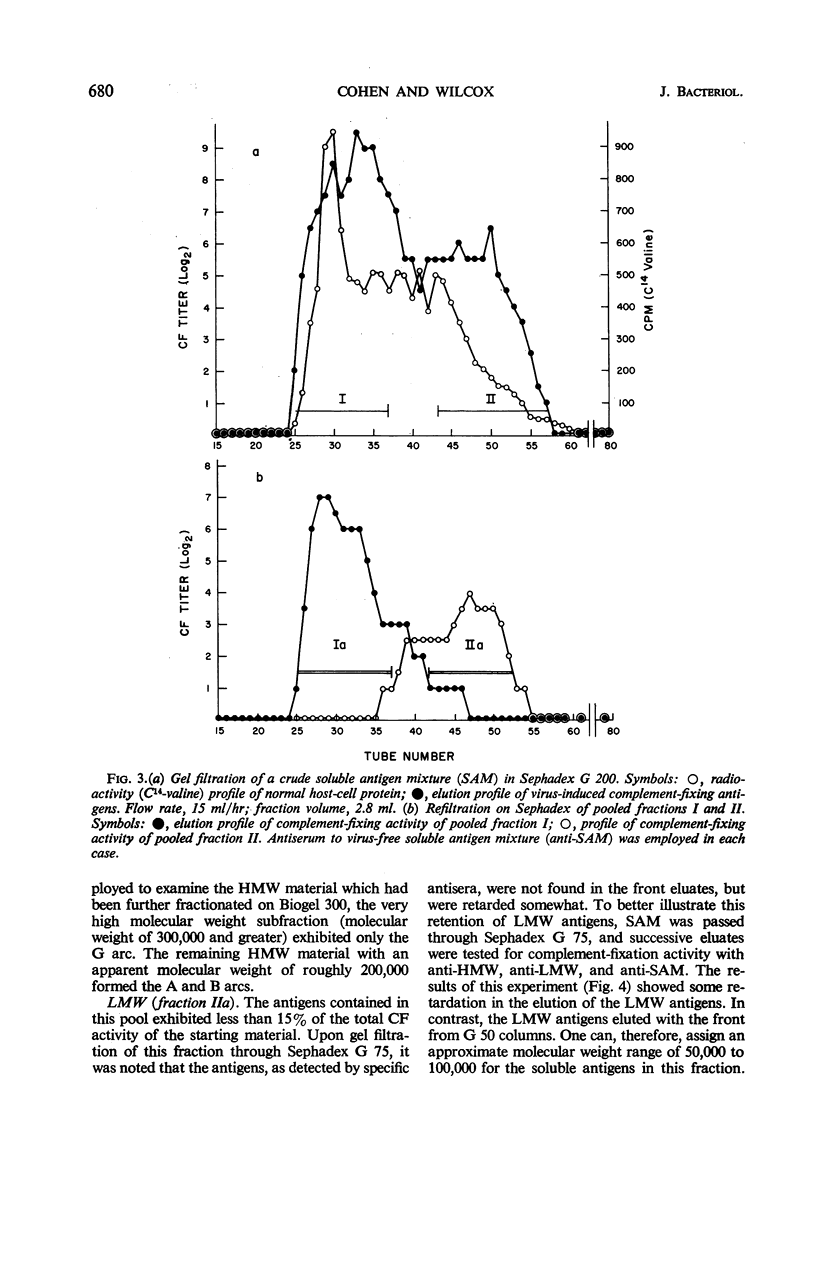
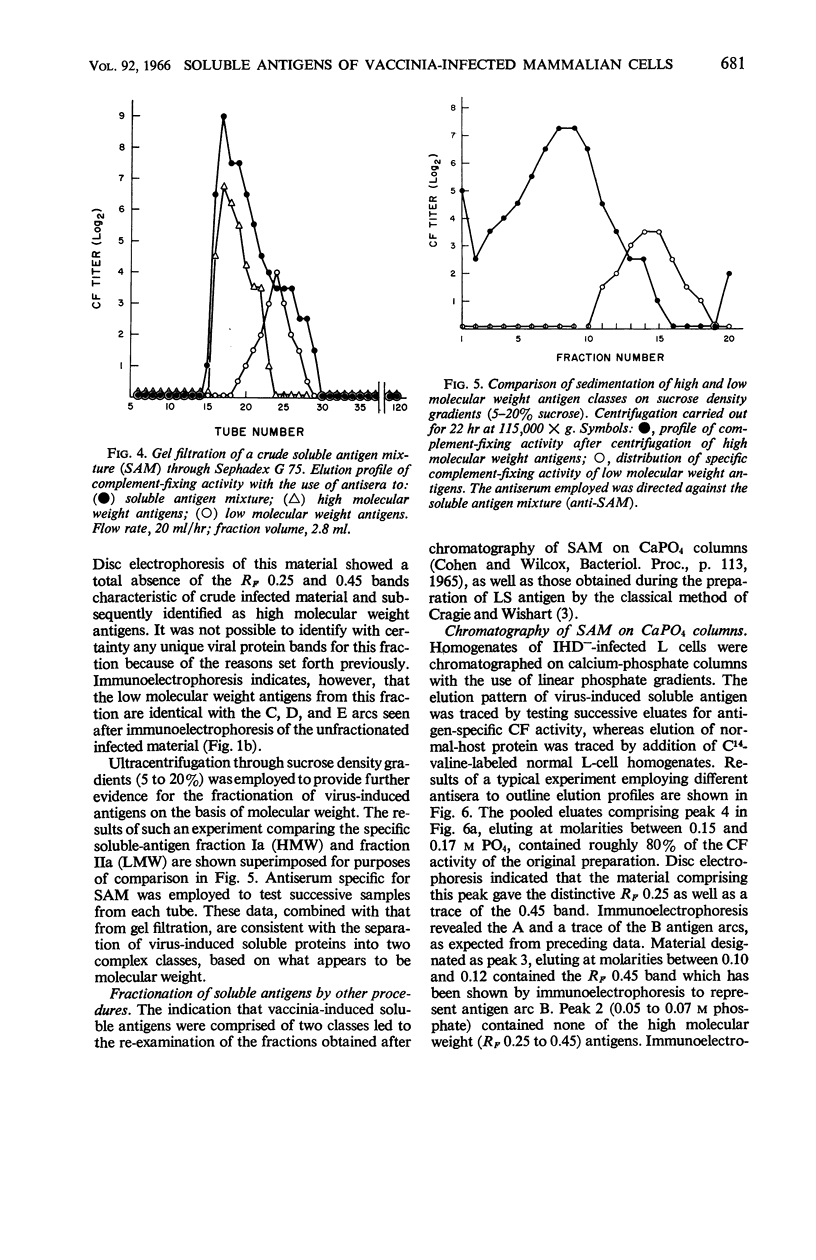
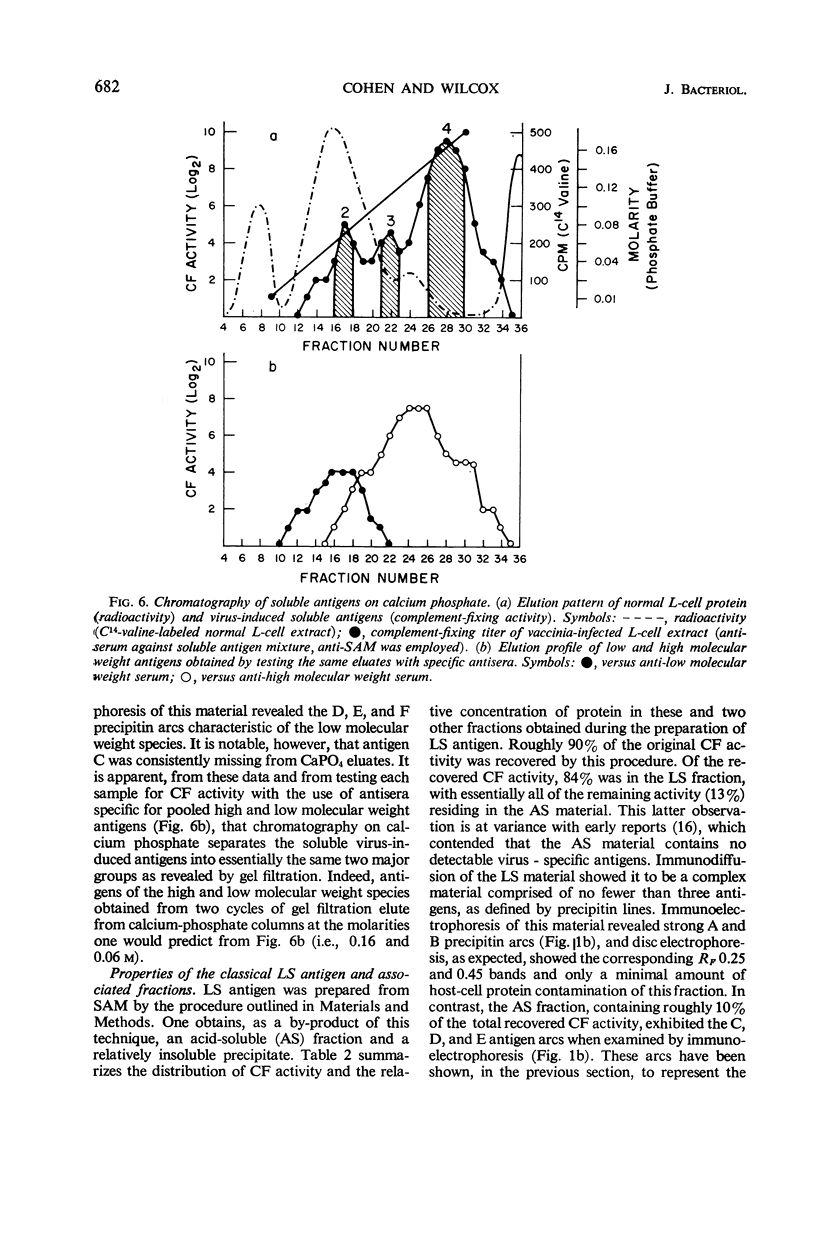
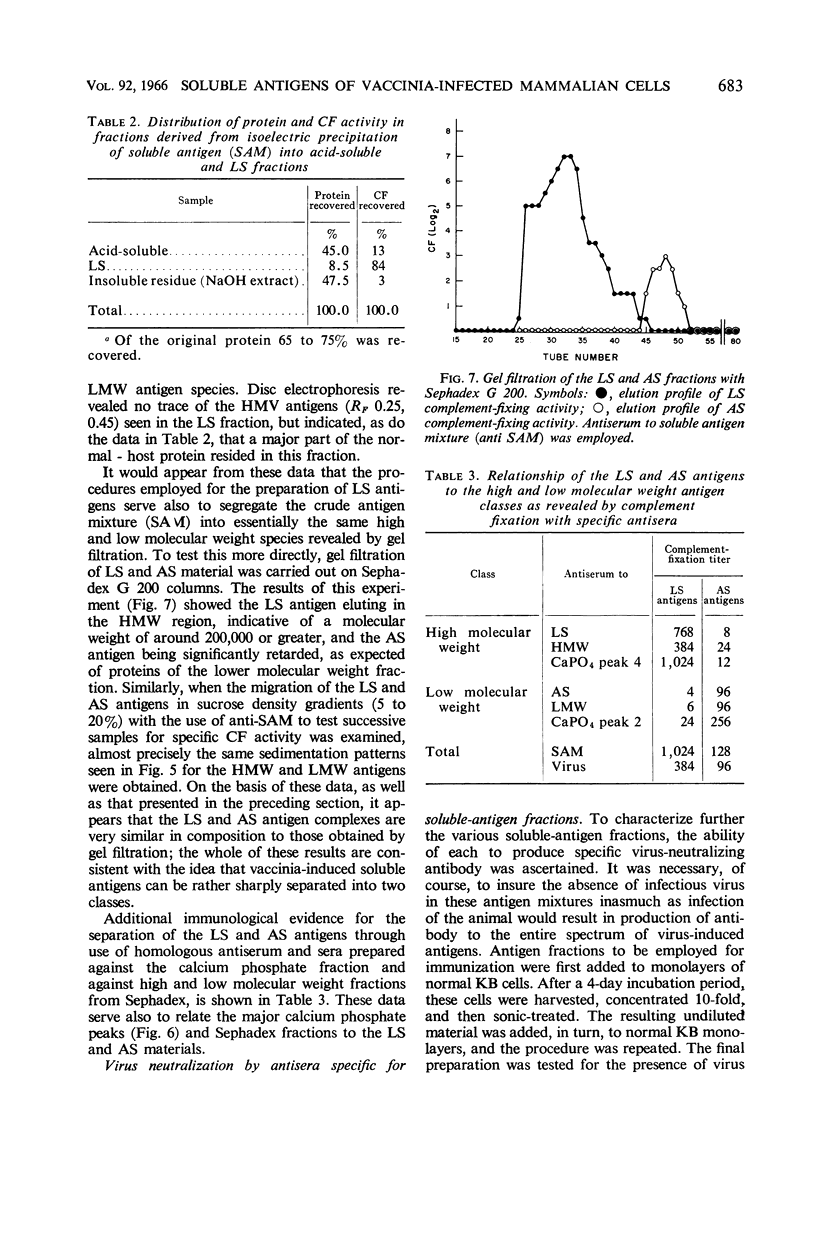
Cohen, Gary H. (University of Pennsylvania, Philadelphia), and Wesley C. Wilcox. Soluble antigens of vaccinia-infected mammalian cells. I. Separation of virus-induced soluble antigens into two classes on the basis of physical characteristics. J. Bacteriol. 92:676–686. 1966—Infection of mammalian cells with members of the poxvirus group elicits production of a number of virus-induced, soluble antigens. Immunoelectrophoresis and immunodiffusion techniques employing soluble antigen preparations obtained from vaccinia virus-infected KB cells revealed at least seven well-defined immunoprecipitin bands. On the basis of fractionation and subsequent characterization of the soluble antigen mixture by gel filtration, calcium phosphate chromatography, isoelectric precipitation, disc electrophoresis, and ultracentrifugation studies, two distinct classes of virus-induced antigens differing markedly in molecular weight were recognized. A high molecular weight class (200,000 and greater) contained at least three virus-induced antigens; a low molecular weight class (50,000 to 100,000 range) contained at least four immunoprecipitins. Further separation of the antigens within the two groups was accomplished. The two classes were distinguished also by their ability to stimulate synthesis of virus-neutralizing antibody. Antisera prepared against the high molecular weight class proved effective in neutralizing vaccinia virus. In contrast, the low molecular weight antigens showed little, if any, ability to induce formation of neutralizing antibody.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEYARD G., WESTWOOD J. C. THE GROWTH OF RABBITPOX VIRUS IN TISSUE CULTURE. J Gen Microbiol. 1964 Dec;37:391–401. doi: 10.1099/00221287-37-3-391. [DOI] [PubMed] [Google Scholar]
- APPLEYARD G., ZWARTOUW H. T., WESTWOOD J. C. A PROTECTIVE ANTIGEN FROM THE POX-VIRUSES. I. REACTION WITH NEUTRALIZING ANTIBODY. Br J Exp Pathol. 1964 Apr;45:150–161. [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J. Enhanced thymidine phosphorylating activity of mouse fibroblasts (strain LM) following vaccinia infection. Biochem Biophys Res Commun. 1962 Jun 19;8:72–75. doi: 10.1016/0006-291x(62)90238-3. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. PROPERTIES OF DEOXYTHYMIDINE KINASE PARTIALLY PURIFIED FROM NONINFECTED MOUSE FIBROBLAST CELLS. Virology. 1965 May;26:16–27. doi: 10.1016/0042-6822(65)90021-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marquardt J., Holm S. E., Lycke E. Immunoprecipitating factors of vaccinia virus. Virology. 1965 Oct;27(2):170–178. doi: 10.1016/0042-6822(65)90156-x. [DOI] [PubMed] [Google Scholar]
- SALZMAN N. P., SHATKIN A. J., SEBRING E. D. Viral protein and DNA synthesis in vaccinia virus-infected HeLacell cultures. Virology. 1963 Apr;19:542–550. doi: 10.1016/0042-6822(63)90049-7. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- SWINGLE S. M., TISELIUS A. Tricalcium phosphate as an adsorbent in the chromatography of proteins. Biochem J. 1951 Feb;48(2):171–174. doi: 10.1042/bj0480171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODROOFE G. M., FENNER F. Serological relationships within the poxvirus group: an antigen common to all members of the group. Virology. 1962 Mar;16:334–341. doi: 10.1016/0042-6822(62)90255-6. [DOI] [PubMed] [Google Scholar]


