Abstract
Background
Inducible nitric oxide synthase (iNOS) is an obligatory mediator of the late phase of ischemic preconditioning (PC) but the mechanisms of its cardioprotective actions are unknown. In addition, it remains unclear whether sustained elevation of iNOS in myocytes provides chronic protection against ischemia-reperfusion injury.
Methods and Results
Constitutive overexpression of iNOS in transgenic (TG) mice (α-myosin heavy chain promoter) did not induce contractile dysfunction and did not affect mitochondrial respiration or biogenesis, but profoundly decreased infarct size in mice subjected to 30 min of coronary occlusion and 24 h of reperfusion. In comparison with wild-type (WT) hearts, isolated iNOS-transgenic (TG) hearts subjected to ischemia for 30 min followed by 40 min of reperfusion displayed better contractile recovery, smaller infarct size, and less mitochondrial entrapment of 2-deoxy-[3H]-glucose (DOG). Reperfusion-induced loss of NAD+ and mitochondrial release of cytochrome c were attenuated in iNOS-TG hearts, indicating reduced mitochondrial permeability transition (MPT). The NO donor NOC-22 prevented permeability transition in isolated mitochondria, and MPT-induced NAD+ loss was decreased in WT but not iNOS-null mice treated with the NO donor DETA/NO, 24 h before ischemia and reperfusion ex vivo. iNOS-mediated cardioprotection was not abolished by atractyloside. Reperfusion-induced production of oxygen-derived free radicals (measured by electron paramagnetic resonance spectroscopy) was attenuated in iNOS-TG hearts and was increased in WT hearts treated with the MPT inhibitor cyclosporin A.
Conclusions
Cardiomyocyte-restricted expression of iNOS provides sustained cardioprotection. This is associated with a decrease in reperfusion-induced oxygen radicals and inhibition of mitochondrial swelling and permeability transition.
Keywords: ischemia, reperfusion, nitric oxide, EPR, and free radicals
INTRODUCTION
Myocardial ischemic injury is attenuated in hearts subjected to brief bouts of ischemia before the onset of sustained ischemia (ischemic preconditioning, [PC]). Both early and delayed phases of ischemic PC have been described1–3. The early phase occurs immediately after the PC stimulus but disappears within 1–2 h. The protective effects of early PC have been attributed to post-translational modification of proteins, particularly kinase-mediated protein phosphorylation events3. Late PC becomes manifest 12–24 h after ischemia, lasts 3–4 days, and is associated with increased synthesis of cardioprotective proteins1. Although the signaling pathways involved in triggering cardioprotection have been extensively characterized1–3, the metabolic basis for the anti-ischemic phenotype of the preconditioned heart remains obscure.
Mechanistic studies of late PC have demonstrated that NO plays a central role in mediating cardioprotection. According to the NO hypothesis of late PC, increased generation of NO from endothelial nitric oxide synthase (eNOS) on day 1 triggers multiple signaling pathways1;4. These lead to the upregulation of a number of proteins, including inducible NOS (iNOS), which in turn mediate the cardioprotective effects of late PC 24 h later (day 2)1;4. The postulated dual role of NO – both, as a trigger of late PC on day 1 and as a mediator on day 2 - is based on the observations that pretreatment with NO donors protects the heart from ischemia (24 h later). NOS inhibitors given on day 1 abolish the development of delayed cardioprotection, and iNOS inhibitors given on day 2 abrogate the infarct sparing effect of late PC1;4. The obligatory role of iNOS is also supported by the observation that targeted deletion of the iNOS gene abrogates late PC induced by a variety of stimuli, including ischemia, adenosine A1 agonists, opioid δ1 agonists, endotoxin derivatives, and exercise, suggesting that iNOS is the final common effector of cardioprotection1;4;5. Collectively, these data support a key role of iNOS-derived NO in mitigating ischemic injury. Nevertheless, it is unclear whether this protection is mediated by a myocyte-specific increase in iNOS and whether continuous expression of iNOS can confer chronic protection against I/R injury. These issues are important considerations in developing therapeutically viable anti-ischemic strategies and in understanding the mechanisms underlying the cardioprotective effects of iNOS.
Several mechanisms could account for the anti-ischemic actions of NO. These include regulation of mitochondrial respiration6, antioxidant protection7, activation of the mitochondrial KATP channels8, and inhibition of cell death pathways9;10. Because of the central role of mitochondria in governing cell death/survival decisions11;12, it seems likely that the mechanism of NO protection may be related to mitochondrial injury. Indeed, mitochondrial swelling is the first sign of irreversible ischemic damage13 and multiple cardioprotective signaling pathways converge on the mitochondria14. Induction of mitochondrial permeability transition (MPT), in particular, has been suggested to be the defining event in myocardial reperfusion injury15–17 and activation of cell death pathways11;12. Therefore, we hypothesized that the beneficial actions of iNOS stem from mitochondrial protection. To test this hypothesis, we investigated whether chronic cardiomyocyte-specific expression of iNOS affects mitochondrial permeability and the generation of oxygen-derived free radicals in hearts subjected to I/R. The results demonstrate, for the first time, that cardiomyocyte-restricted expression of iNOS is sufficient to confer chronic cardioprotection and that transgenic upregulation of cardiac iNOS decreases free radical generation, which in turn prevents mitochondrial permeability transition and swelling and chronically protects the heart against I/R injury. Preliminary findings of this study have been reported18.
MATERIALS AND METHODS
Detailed methodology is provided in the Data Supplement. Adult C57BL/6 mice were purchased from The Jackson Laboratory. The iNOS-TG mice express the iNOS gene specifically in cardiomyocytes under the control of the α-myosin heavy chain promoter19. These mice are healthy and their cardiovascular function is normal19. Baseline two-dimensional echocardiography (Toshiba T380 Powervision) was performed as previously described20.
Ischemic preconditioning and acute myocardial infarction (MI) in vivo
The murine model of ischemic PC and infarction has been described previously21. Briefly, mice were preconditioned with a sequence of six 4-min occlusion/4-min reperfusion cycles. Control mice were subjected to sham operation. Myocardial infarction was produced by subjecting mice to a 30-min coronary occlusion followed by 24 h of reperfusion.
MPT measurement
Induction of MPT was assessed by the mitochondrial uptake of 2-deoxy-[3H]-glucose (DOG)16;22, NAD+ measurement15, and appearance of cytochrome c in the cytosol12. Hearts were excised and perfused in the Langendorff mode at constant flow (3 ml/min) to ensure consistent perfusion. After 20 min of equilibration, the hearts were perfused in the recirculating mode with 50 ml of modified Krebs-Henseleit (KH) buffer containing 0.5 mmol/l 2-[3H]-DOG (0.1 μCi/ml) for 20 min. Perfusion was then returned to normal for 10 min and the hearts were subjected to 30 min of ischemia. After 15 min of reperfusion, mitochondria were isolated and the radioactivity associated with the mitochondrial fraction was measured by scintillation counting. To ensure equal yield of mitochondria, citrate synthase activity was measured by colorimetric assay (Sigma-Aldrich, USA). Retention of 3H-DOG was calculated by dividing the radioactivity in the mitochondria by the total radioactivity recovered from the tissue.
Electron Paramagnetic Resonance (EPR) spin trapping measurement of oxygen-radicals
After intraperitoneal induction of anesthesia (pentobarbital sodium, Abbott Laboratories, USA, 5 mg) the heart was excised and the ascending aorta was cannulated and perfused at a constant flow (2 ml/min) with KH buffer. After ischemia the spin trap 5,5-dimethyl-1-pyrroline-N-oxide (1 M DMPO, Dojindo Laboratories, Japan) in buffer containing 100 μM diethylenetriaminepentaacetic acid, (Sigma Aldrich, USA) was administered immediately upon reperfusion with the trap infused at a rate of 100 μl/min through a side arm located close to the heart. During reperfusion, periodic collections of the effluent were made till 5 min of reperfusion at indicated intervals. Upon sample collection, each tube was immediately frozen in liquid nitrogen. EPR spectra were recorded as described previously23;24.
Statistical analysis
Data are reported as mean ± SEM. Comparisons between two groups were performed with unpaired Student’s t-tests. Comparisons among multiple groups or between two groups at multiple time-points were performed by either one-way or two-way ANOVA, as appropriate, followed by paired or unpaired Student’s t-tests with the Bonferroni correction. For comparing WT and iNOS-TG mice at multiple time points a repeated measures ANOVA was used.
Statement of Responsibility
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
RESULTS
Constitutive expression of iNOS protects against I/R injury
Previous studies have shown that the late phase of ischemic PC is associated with selective upregulation of iNOS and that targeted disruption of iNOS abrogates the infarct sparing effect of late PC1;4;5. Nevertheless, it remains unclear whether continuous expression of iNOS in cardiac myocytes can establish a state of chronic cardioprotection and enhance ischemic tolerance. Accordingly, to test the duration and cellular dependence of iNOS-mediated cardioprotection, we utilized 12–20 week old iNOS-TG mice (20–30 g) in which the iNOS gene is under the control of the cardiac myocyte-specific α-myosin heavy chain promoter. Despite an increase in cardiac iNOS, these mice are healthy and breed normally19. Heger et al. have also reported that cardiomyocyte-specific iNOS expression does not affect the LV/body weight ratio or the heart rate, and although cardiac output and mean arterial pressures were mildly depressed, there was no overt hypertrophy or heart failure19. In agreement with these observations, we also found no significant differences in echocardiographically-assessed cardiac function between hearts of iNOS-TG and WT mice (Table 1). The iNOS-TG hearts, however, displayed a slight concentric hypertrophy as evidenced by increased anterior and posterior wall thickness and elevated LV mass with no changes in LV diastolic or systolic dimensions (Table 1).
Table 1.
Cardiac parameters in iNOS-TG and WT mice
| M-mode Echocardiogram Parameter | WT | iNOS-TG |
|---|---|---|
| Left ventricular end-diastolic diameter (mm) | 3.8 ± 0.1 | 3.9 ± 0.1 |
| Left ventricular end-systolic diameter (mm) | 2.2 ± 0.1 | 2.3 ± 0.1 |
| Fractional shortening (%) | 44 ± 14 | 41 ± 15 |
| Septal wall thickness (mm) | 0.73 ± 0.01 | 0.84 ± 0.01* |
| Posterior wall thickness | 0.78 ± 0.01 | 0.83 ± 0.01* |
| LV mass (mg) | 100 ± 2.6 | 116 ± 3.4* |
| LV/body weight | 3.95± 0.10 | 4.64 ±0.17* |
| HR (beats/min) | 488 ± 11 | 485 ± 12 |
| Vcf (circumferences/s) | 8.7 ± 0.4 | 8.3 ± 0.4 |
P<0.05 versus WT; n =12–14
Cardiac homogenates from iNOS-TG mice displayed a ~400-fold increase in iNOS protein expression and a ~3-fold increase in NOx content when compared with WT littermates. Although it has been reported that NO triggers mitochondrial biogenesis25, we found no difference in the size-distribution or the number of mitochondria in electron micrographs from WT and iNOS-TG hearts (Supplemental Fig. 1). The expression of cytochrome c and cytochrome c oxidase subunit IV was also similar between WT and iNOS-TG hearts (Supplemental Fig. 2) and there was no difference in the ratio of the mitochondrial and nuclear DNA content between WT and iNOS-TG hearts (Supplemental Fig. 2), indicating that mitochondrial synthesis is not stimulated by a chronic increase in iNOS abundance and activity. Rates of State 3 and State 4 respiration and the ADP:O ratios measured in mitochondria isolated from iNOS-TG and WT mice were similar (Supplemental Fig. 3) and no difference was observed in basal ROS production or ROS production after inhibition of complexes I and III (Supplemental Fig. 5). Small increases in the abundance of complex II and complex IV, but not complex I, III, and V, were observed (Supplemental Fig. 3). The reasons for the increase in complex II and IV are unclear, but because no changes in respiration and ROS generation were observed, it appears unlikely that these changes significantly affect mitochondrial function.
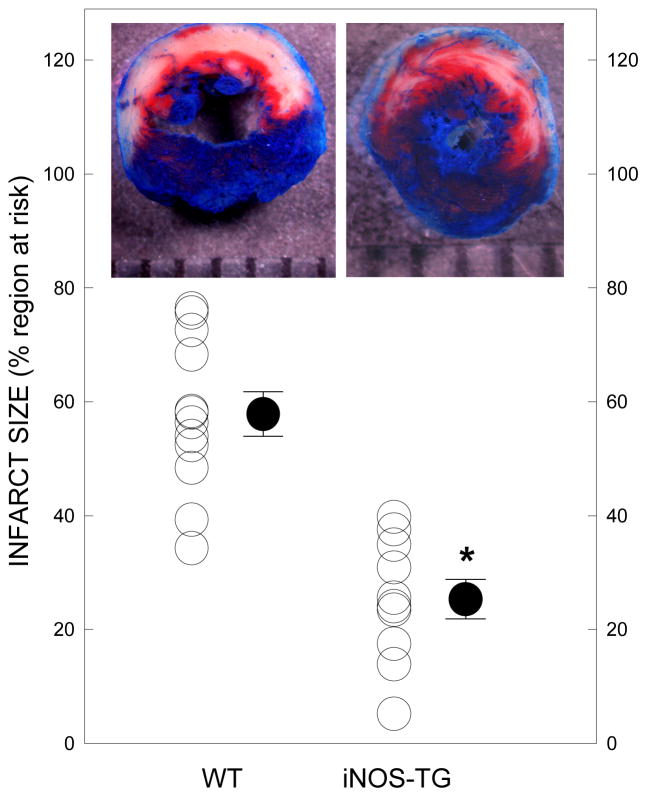
To examine the effects of chronic iNOS overexpression on ischemic tolerance, WT and iNOS-TG mice were subjected to 30 min of coronary occlusion followed by 24 h of reperfusion. The iNOS-TG mice and their WT littermates were not different with respect to the size of the risk region (35±3 mg versus 41±2 mg); however, as shown in Fig. 1, infarct size was significantly smaller in iNOS-TGhearts compared with WT hearts (25±3% versus 58±4% of the region at risk, P<0.05). These observations indicate that cardiomyocyte-specific iNOS expression produces a chronically-protected cardiac phenotype.
Figure 1. iNOS overexpression limits infarct size following ischemia-reperfusion in vivo.
WT and iNOS-TGhearts were subjected to a 30-min coronary occlusion followed by 24 h of reperfusion. Infarct size was measured by TTC staining and expressed as a percent of the risk region (*P<0.05 versus WT; n=10–12).
iNOS decreases ischemic injury in perfused hearts
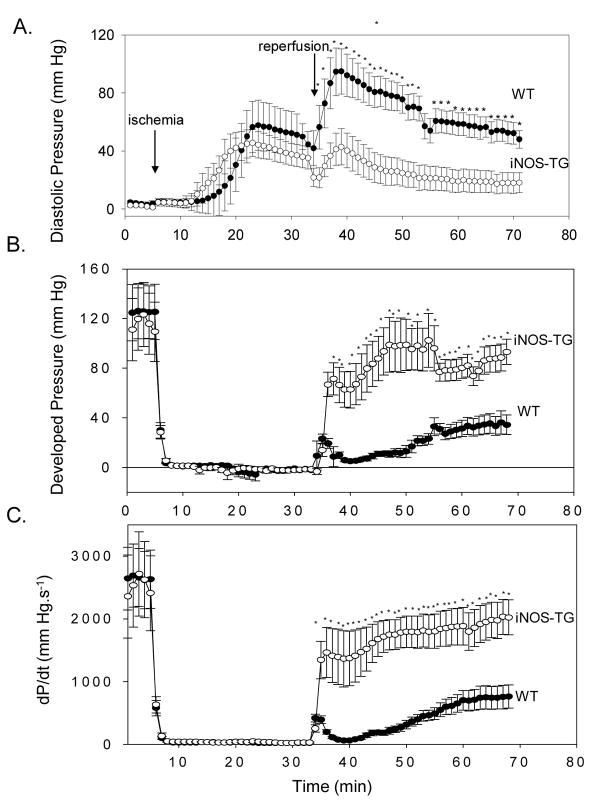
Ischemia and reperfusion cause extensive mitochondrial damage and induction of MPT has been suggested to be the critical event in the evolution of I/R injury15–17. To examine changes in MPT, we utilized the isolated perfused heart preparation, which in contrast to in vivo models, allows direct measurements of MPT. Perfused heart preparations from either mouse strain were stable, with <10 % loss of LV developed pressure per hour. During 30 min of ischemia, the hearts developed ischemic contracture, which was not significantly different between WT and iNOS-TG hearts (Fig. 2A). After reperfusion, however, LV developed pressure recovered to a much greater extent in iNOS-TG that in WT hearts (Fig. 2B). At 30 min of reperfusion, developed pressure was 75±11% of baseline in iNOS-TG hearts versus 17±3% in WT hearts (P<0.001). Similarly, LV +dP/dt was strikingly greater in iNOS-TG versus WT hearts, averaging 2004±298 mmHg/s versus 507±93 mmHg/s, respectively, at the end of reperfusion (P<0.001; Fig. 2C). In WT hearts, LV diastolic pressure rose markedly upon reperfusion, averaging 56±8 mmHg after 40 min; in contrast, in iNOS-TGhearts diastolic pressure was much lower (19±8 mmHg at 30 min; P<0.01; Fig. 2A).
Figure 2. Constitutive expression of iNOS improves functional recovery after ischemia-reperfusion ex vivo.
Hearts from WT or iNOS-TG mice were perfused in the Langendorff mode and subjected to 30 min of global ischemia followed by 40 min of reperfusion. Developed pressure was measured via a water-filled balloon connected to a pressure transducer inserted into the left ventricle. (A) LV diastolic pressure, (B) LV developed pressure, and (C) LV +dP/dt in WT (●) and iNOS -TG (○) mice. * P < 0.05 versus WT (n=7).
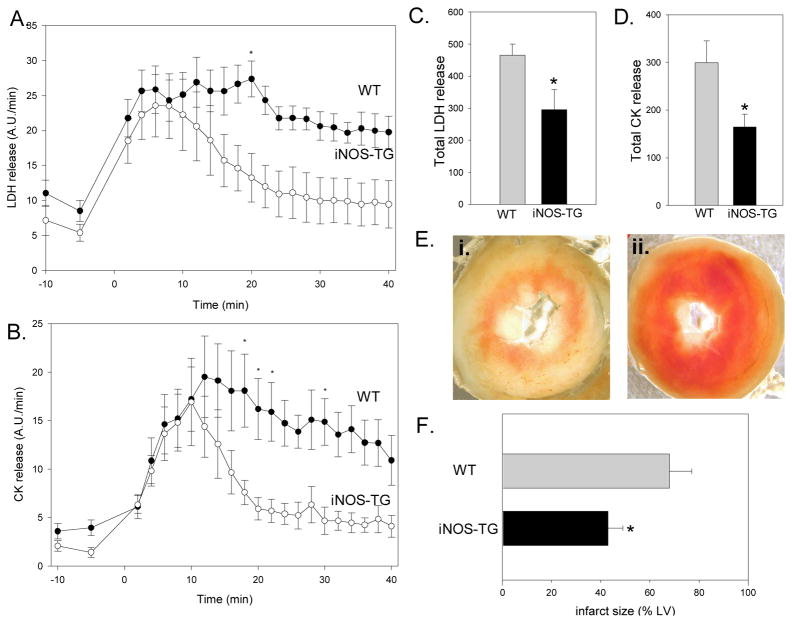
The greater functional recovery of iNOS-TG hearts was associated with reduction in tissue injury (Fig. 3A–F). Total LDH release during reperfusion was greater in WT than in iNOS-TG hearts (465±35 A.U. versus 296±63 A.U., respectively, P<0.05; Fig. 3A and C); CK release was also greater in WT (299±46 A.U.) than iNOS-TG (165±27 A.U.; P<0.005; Figs. 3B and D) hearts. Reduction in tissue injury was confirmed by tetrazolium-based measurements of infarct size, which was reduced from 68±9% of the LV in WT to 43±6% in iNOS-TG hearts (P<0.05; Fig. 3E and F). Taken together, these data corroborate the in vivo results by demonstrating that constitutive overexpression of iNOS protects the heart against I/R injury in the same preparation that we used to assess MPT.
Figure 3. Constitutive expression of iNOS decreases ischemia-reperfusion injury in isolated perfused hearts.
Hearts from WT and iNOS-TG mice were subjected to 30 min of ischemia followed by reperfusion and the coronary effluent was collected at the indicated times for measurement of LDH (A) or CK (B). Reperfusion was initiated at time 0. Measurements of total LDH and CK release during the entire 40 min of reperfusion are shown in panels C and D, respectively. (*P<0.05 vs WT, n=4–9). Panel E shows representative images of WT (i.) and transgenic (ii.) hearts after TTC staining. (F) Infarct size, delineated by TTC staining, is expressed as percent of the LV (*P<0.05 versus WT; n=7–12).
Constitutive expression of iNOS prevents MPT
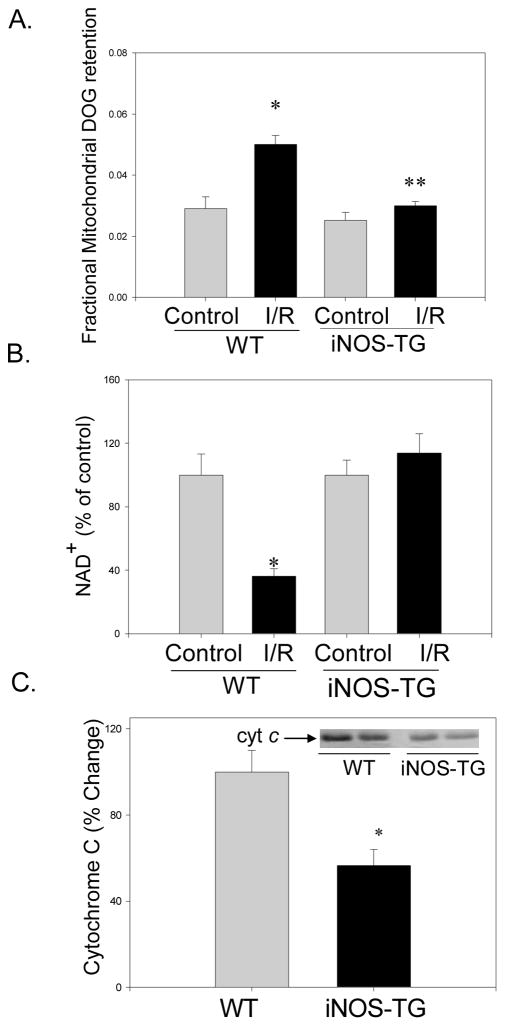
Changes in MPT were determined by measuring the mitochondrial retention of DOG, the loss of NAD+, and the release of cytochrome c into the cytoplasm. Perfusion with aerobic buffer for 60 min led to basal retention of radioactivity in the mitochondrial fraction. This was significantly increased (P<0.05) in mitochondria isolated from WT hearts subjected to 30 min of ischemia followed by 15 min of reperfusion, indicating induction of MPT (Fig. 4A). In contrast, mitochondria from iNOS-TG hearts subjected to I/R exhibited essentially no increase in radioactivity (Fig. 4A). These observations indicate that MPT is inhibited in iNOS-TG hearts. In addition to MPT, loss of NAD+ is another measure of MPT15. In WT hearts subjected to I/R, the NAD+ content was 36% of that in non-ischemic hearts. No decrease in NAD+ was observed in iNOS-TG hearts (Fig. 4B). Additionally, after I/R, cytoplasmic cytochrome c level was significantly less in iNOS-TG than WT hearts (Fig. 4C). Collectively, all three indices indicate a decrease in MPT in iNOS-TG hearts subjected to I/R.
Figure 4. Inhibition of mitochondrial permeability transition in iNOS-TG hearts.
(A) Isolated perfused WT or iNOS-TG hearts were loaded with 2-deoxy-[3H]-glucose (DOG) and mitochondrial entrapment of the label was measured to assess MPT. The radioactivity associated with the mitochondrial fraction was normalized to total radioactivity in the homogenate. Bars represent fractional radioactivity retained in the mitochondria. (*P<0.05 versus WT control; n=3–6, **P<0.001 versus WT I/R; n=4–6); (B) I/R-induced NAD+ depletion in WT and iNOS-TG hearts. Bars represent NAD+ levels in I/R hearts expressed as a percentage to the appropriate perfusion controls (*P<0.0005 versus WT; n=8); and (C) Cytochrome c was detected by Western blot using anti-cytochrome c antibodies in post-mitochondrial fractions prepared from WT and iNOS-TG hearts after I/R and normalized to total protein (*P<0.005 versus WT; n=5).
To determine whether MPT is also prevented in hearts preconditioned in vivo, we employed a pharmacologic model of PC26. For this, WT mice received 4, i.v. injections of 0.1 mg/kg DETA/NO; and 24 h later the hearts were excised and subjected to 30 min of ischemia followed by 30 min of reperfusion. Treatment with DETA/NO attenuated NAD+ depletion when compared with vehicle-treated hearts (Supplemental Fig. 6). Compared with vehicle-treated hearts, hearts preconditioned with DETA/NO also showed less oxidative stress, as measured by the accumulation of protein adducts of the lipid peroxidation product – 4-hydroxy-trans-2-nonenal (HNE; Supplemental Fig. 5). DETA/NO pretreatment did not prevent NAD+-depletion in hearts from iNOS-null mice (Supplemental Fig. 5), indicating that inhibition of MPT following pharmacologic PC is mediated by iNOS.
NO prevents permeability transition in isolated mitochondria
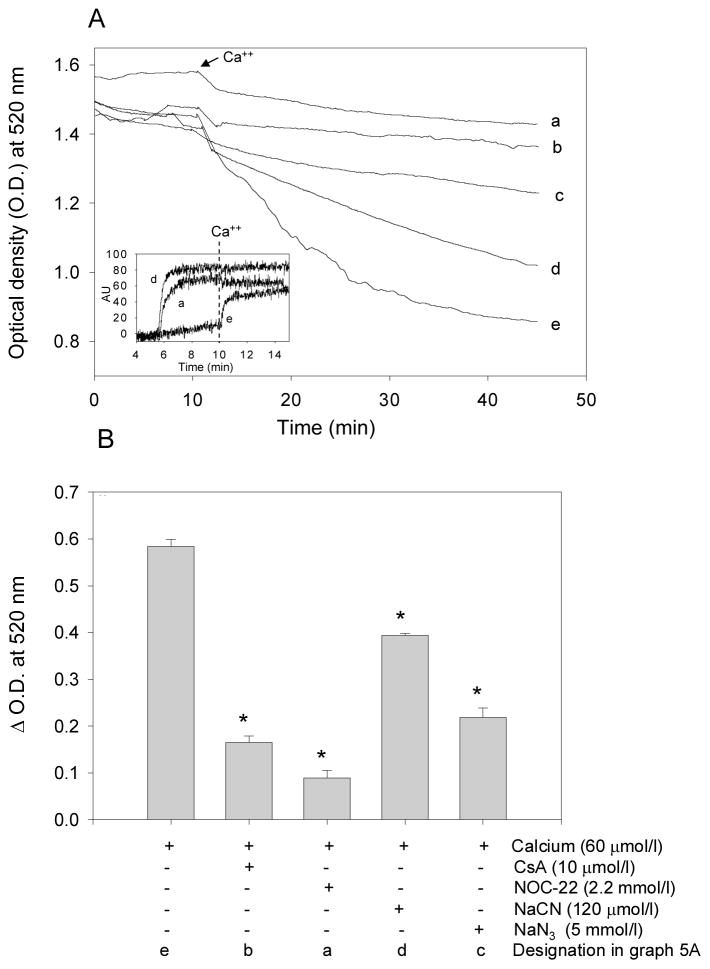
Having observed that increased iNOS expression is associated with a decrease in MPT during I/R, we examined whether NO directly prevents MPT. For this, isolated mitochondria were treated in suspension with Ca2+. As shown in Fig. 5, addition of Ca2+ led to an abrupt decrease in light absorbance (due to increased light scattering) indicating mitochondrial swelling. No change in absorbance was observed in the absence of Ca2+. The Ca2+-induced decrease in absorbance was inhibited by cyclosporin A, which prevents opening of the permeability pore, and NaCN, which inhibits cytochrome c oxidase (Fig. 5A&B). This decrease in absorbance was also accompanied by depolarization (loss of ΔΨ). Mitochondrial membrane potential was also abolished by cyanide, and Ca2+ did not cause additional depolarization or loss of absorbance in cyanide-poisoned mitochondria. Taken together, these data demonstrate that the high negative potential generated in well-energized (metabolically active) mitochondria favors Ca2+ uptake and that excessive accumulation of Ca2+ triggers MPT.
Figure 5. Nitric oxide inhibits mitochondrial permeability transition and promotes membrane depolarization.
Mitochondria were isolated from WT hearts and the opening of the permeability pore was measured by a decrease in absorbance at 520 nm. (A) After 10 min of equilibration, Ca2+ (60 μmoles/l) was added as indicated. Mitochondria were pre-incubated with no additive (e), with NOC-22 (a), cyclosporin A (CsA; b), azide (NaN3; c), or cyanide (NaCN; d) 5 min prior to the addition of calcium; (B) group data showing the change in optical density at 520 nm 35 min after the addition of calcium. Change of absorbance was normalized to the appropriate control for each individual experiment (#P<0.05 versus Ca2+ alone, *P<0.0005 versus Ca2+ alone; n=3–4/group). (inset) Membrane depolarization measurements in mitochondrial suspensions: Mitochondria were loaded with Rhodamine 123 (5 μmol/L) for 5 min and then allowed to incubate for 5 min in the absence (e) or presence of NaCN (d, 120 μmol/L) or NOC-22 (a, 2.2 mmol/L). Fluorescence intensity was monitored at excitation/emission wavelengths of 490/535 nm. Calcium (60 μmol/L) was added at t = 10 min and measurements were recorded for an additional 10 min. Increased fluorescence intensity indicates a decrease of ΔΨ (i.e., depolarization).
Pre-incubation with the NO-donor spermine NONOate (NOC-22) prevented Ca2+-induced loss of absorbance in mitochondrial suspension (Fig. 5). Similar to cyanide, NOC-22 induced depolarization and the mitochondria depolarized with NOC- 22 were insensitive to Ca2+. Using a t1/2 of 230 min, we estimate the flux of NO generated from the NOC-22 under the experimental conditions used to be ~10 nmol/min/mg protein, which is comparable to the estimated steady-state concentration of NO in cardiac myocytes (between 0.1 to 0.2 μmol/L6). No differences in Ca2+-induced MPT were observed, however, in mitochondria isolated from WT and iNOS-TG hearts (data not shown). These observations suggest that overexpression of iNOS does not alter the intrinsic sensitivity of the mitochondria to undergo permeability transition, however, physiological levels of NO inhibit permeability transition, in part by dissipating the membrane potential required for Ca2+ uptake.
NO prevents reperfusion-induced oxygen free radical generation
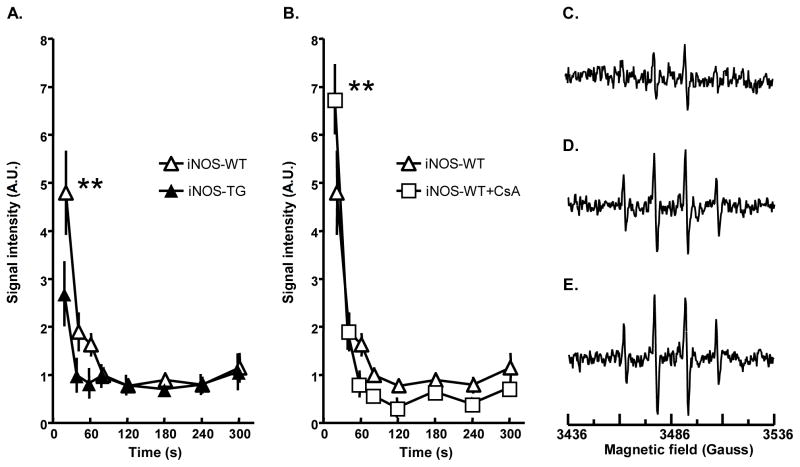
Reperfusion injury has been linked to the generation of oxygen-derived free radicals2;27. Hence, to further elucidate the cardioprotective mechanisms elicited by NO, we studied free radical generation in WT and iNOS-TG hearts. Oxygen radicals generated upon reperfusion were trapped with DMPO and quantified by EPR spectroscopy. As shown in Fig. 6A, reperfusion was associated with a burst of free radical production. The levels of spin-trapped radicals returned to baseline after 120 s of reperfusion. The amount of radicals trapped from reperfused hearts was significantly attenuated in iNOS-TG hearts versus WT hearts. In contrast, treatment with the MPT inhibitor, cyclosporin A, increased radical production (Fig. 6B). Although cyclosporin A was found to promote functional recovery of WT hearts subjected to 30 min of ischemia and 40 min of reperfusion (Supplemental Figure 6), and to decrease the release of cytochrome c in the cytosol, it did not affect mitochondrial retention of [3H]-DOG (Supplemental Figure 7). Furthermore, iNOS-mediated cardioprotection was not abolished by the MPT opener atractyloside and iNOS-TG hearts treated with atractyloside displayed better recovery of function and lower levels of LDH and CK release than did the WT hearts (Supplemental Figure 8). Collectively, these observations indicate that iNOS decreases reperfusion-induced oxygen radical production. NO maintains the mitochondrial pore in the closed state, so that its protection cannot be abolished by stabilizing the open state of the pore.
Figure 6. Generation of oxygen-derived free radicals in hearts subjected to ischemia-reperfusion.
(A) Graphs showing the time course of the EPR signals (mean ± SEM) after administration of 5,5′dimethyl-1-pyrroline-N-oxide (DMPO, 1 mole/L, 0.1 ml.min−1) during the first 5 min of reperfusion. The release of free radicals during the early period of reperfusion was significantly reduced in iNOS-TG mice (**, P < 0.001 versus WT), while (B) blockade of the mitochondrial transition pore by cyclosporin A increased the oxygen radical burst (**, P < 0.01 versus WT). (C–E): Representative EPR spectra illustrating free radical generation in the effluent of the heart in the first 20 s of reperfusion. EPR spectra from perfusates obtained from iNOS-TG (C), and WT (D) mouse hearts and WT hearts treated with cyclosporin A (CSA) (E).
DISCUSSION
The major finding of this study is that a cardiomyocyte-specific increase in iNOS leads to sustained cardioprotection without causing overt myocyte injury or dysfunction. Transgenic overexpression of iNOS in cardiac myocytes reduced infarct size, and decreased I/R-induced free radical production and MPT, indicating that overexpression of iNOS significantly enhances the ability of the heart to withstand oxidative and mitochondrial stress. Significantly, our observations demonstrate that manipulating a single myocyte gene can establish a chronically cardioprotected phenotype. This mode of cardioprotection differs in its time course from both early (which lasts 1–2 h) and late (48–72 h) PC, both of which offer only transient protection. The permanent cardioprotection as described here is due to the activity of iNOS, which is now widely recognized as an obligatory mediator of late PC1;4;28, and relates to favorable changes in two major determinants of I/R injury, i.e., free radical generation and MPT. These observations offer new insights into the mechanism of NO-mediated protection and have significant clinical implications for developing long-term prophylactic anti-ischemic interventions.
The results of this study show that a transgenic increase in iNOS is associated with a decrease in both - I/R injury in isolated perfused hearts and in infarct size following coronary occlusion in situ. These observations imply that chronic cardioprotection afforded by iNOS is independent of the experimental model used to assess its efficacy. The concordance between in situ and ex vivo models also indicates that iNOS-mediated protection is not related to systemic changes in blood-borne responses, favorable neutrophil-endothelium interactions or alterations in autonomic regulation, but can be attributed instead to changes in the response of cardiac myocytes to ischemia.
NO has been shown to play an obligatory role not only in the delayed cardioprotection elicited by ischemic PC, but also in that provided by physical exercise, NO donors, adenosine A1 receptor agonists, opioid δ1 receptor agonists and endotoxin derivatives1;4;28. Thus, generation of NO by iNOS appears to be a final common pathway that mediates the late phase of ischemic PC, pharmacologic PC and PC-induced by physical stimuli. Indeed, late PC could be viewed as a state of increased NO availability. However, to our knowledge, this is the first report that constitutive overexpression of iNOS in the heart is associated with sustained cardioprotection associated with a decrease in the production of oxygen-derived free radicals and alleviation of detrimental changes in the mitochondria. These observations reveal a new mechanism whereby iNOS imparts protection against I/R injury, i.e., protection of the mitochondria (without increasing mitochondrial biogenesis or inducing permanent changes in the mitochondrial structure and function) and advance our understanding of the cardiovascular function of this protein.
NO could protect the heart from ischemic injury by several mechanisms. Previous studies have shown that NO stimulates the opening of the mitochondrial KATP channel8 and inhibits apoptosis by nitrosylating caspases10. In addition, an increase in iNOS can enhance the expression of antioxidant proteins4. Our results suggest that the cardioprotection afforded by iNOS is not due to permanent changes in mitochondria because no difference in MPT was observed in mitochondria isolated from WT and iNOS-TG hearts. This observation is in agreement with results of other studies showing that mitochondria isolated from naive or preconditioned hearts show no difference in their ability to undergo MPT29–31. Thus, inhibition of MPT in iNOS-TG hearts, similar to that in hearts preconditioned by ischemia, does not appear to be due to a change in the intrinsic sensitivity of the mitochondria to undergo permeability transition per se. This view is consistent with our previous observation that NO must be generated during I/R, i.e., NO is not only a trigger but also a mediator of PC1;28. Based on these considerations, we propose that the cardioprotective networks stimulated by iNOS converge on the mitochondria, strengthening their defense against ischemic changes and preventing them from triggering cell death pathways. The protective effects of iNOS on the mitochondria may be facilitated by its localization. In iNOS-TG hearts significant levels of iNOS was associated with the mitochondria (Supplemental Fig. 9). Mitochondrial association of iNOS was also observed in WT hearts preconditioned by ischemia (Supplemental Fig. 10). No change in the distribution of eNOS or nNOS was observed. Although further experiments are required to understand the mechanism by which iNOS associates with the mitochondria and to fully assess the significance of these findings, these data suggest that mitochondrial protection by iNOS may be facilitated by the generation of NO near the mitochondria.
Although MPT induction has been proposed to be the key event that triggers myocyte cell death during reperfusion32;33, the mechanisms that trigger MPT during I/R are poorly understood. Increased generation of free radicals could be one trigger, which could increase mitochondrial calcium overload and trigger pore opening. Hence, our observation that cardiomyocyte-specific expression of iNOS prevents radical generation supports the notion that the protective effect of iNOS relate primarily to a decrease in free radical production, which in turn could prevent MPT and decrease I/R injury. The relationship between MPT and free radical generation is further clarified by the data obtained with cyclosporin A. Although cyclosporin A prevented cytochrome c release and decreased I/R injury (current study and15;34–36) in WT mice, it increased free radical generation. This observation is consistent with in vitro data showing that MPT decreases ROS generation by inhibiting complex I activity37. Hence, we propose that because cyclosporin A prevents MPT, it preserves complex I activity, ΔΨ and maintains the electron transport in a highly charged state, conditions which favor free radical generation. It follows then that the protective effects of iNOS (which are associated with a decrease in free radical generation) cannot be ascribed to cyclosporin A-like inhibition of MPT, but could be attributed instead to a decrease in the triggers of MPT, presumably oxygen-derived free radicals. That NO acts upstream of MPT is also supported by our observation and NO-mediated cardioprotection was not abolished by atractyloside, indicating that stabilization of the open-state of the pore does not abrogate NO-mediated cardioprotection; such a finding implies that NO-dependent cardioprotection relates to suppression of upstream triggers of pore opening rather than to the intrinsic ability of the pore to open. This is also consistent with the observation that mitochondria isolated from WT and iNOS-TG hearts were equally susceptible to MPT. It follows from these considerations that clinical interventions to directly inhibit MPT are likely to be less successful than those that increase iNOS in the heart (e.g., exercise, pharmacologic PC or iNOS gene therapy), because NO in addition to inhibiting MPT prevents free radical generation as well. Therefore, the dual protection provided by iNOS, which could be chronically induced in the heart, may be a therapeutically accessible pathway for establishing sustained resistance to myocardial I/R injury.
Supplementary Material
Acknowledgments
FUNDING SOURCES
This work was supported in part by NIH grants HL55477 and HL59378 (to A.B.), HL78825, HL55757, HL68088, HL76794, and HL70897 (to R.B.), HL65660 (to Y.T.X.), HL72410 (to B.D.), HL63744, HL65608 (to J.L.Z.), a VA merit award (to S.D.P.) and AHA predoctoral fellowships (to M.B.W. and B.G.H.).
Footnotes
DISCLOSURES
None
Reference List
- 1.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 2.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation. 2001;104:2981–2989. doi: 10.1161/hc4801.100038. [DOI] [PubMed] [Google Scholar]
- 3.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 4.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 5.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci U S A. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trochu JN, Bouhour JB, Kaley G, Hintze TH. Role of endothelium-derived nitric oxide in the regulation of cardiac oxygen metabolism: implications in health and disease. Circ Res. 2000;87:1108–1117. doi: 10.1161/01.res.87.12.1108. [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell VB, Chumley PH, Hogg N, Bloodsworth A, Darley-Usmar VM, Freeman BA. Nitric oxide inhibition of lipid peroxidation: kinetics of reaction with lipid peroxyl radicals and comparison with alpha-tocopherol. Biochemistry. 1997;36:15216–15223. doi: 10.1021/bi971891z. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki N, Sato T, Ohler A, O’Rourke B, Marban E. Activation of mitochondrial ATP-dependent potassium channels by nitric oxide. Circulation. 2000;101:439–445. doi: 10.1161/01.cir.101.4.439. [DOI] [PubMed] [Google Scholar]
- 9.Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 10.Rossig L, Fichtlscherer B, Breitschopf K, Haendeler J, Zeiher AM, Mulsch A, Dimmeler S. Nitric oxide inhibits caspase-3 by S-nitrosation in vivo. J Biol Chem. 1999;274:6823–6826. doi: 10.1074/jbc.274.11.6823. [DOI] [PubMed] [Google Scholar]
- 11.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 12.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 13.Jennings RB, Ganote CE. Structural changes in myocardium during acute ischemia. Circ Res. 1974;35 (Suppl 3):156–172. [PubMed] [Google Scholar]
- 14.Murphy E. Primary and secondary signaling pathways in early preconditioning that converge on the mitochondria to produce cardioprotection. Circ Res. 2004;94:7–16. doi: 10.1161/01.RES.0000108082.76667.F4. [DOI] [PubMed] [Google Scholar]
- 15.Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307 (Pt 1):93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 18.West MB, Rokosh G, Clark D, Liu S-Q, Guo Y, Bolli R, Bhatnagar A. Nitric oxide prevents mitochondrial permeability transition during ischemia-reperfusion:implications for the cardioprotective effects of late preconditioning. Circulation. 2004;110:III–236. [Google Scholar]
- 19.Heger J, Godecke A, Flogel U, Merx MW, Molojavyi A, Kuhn-Velten WN, Schrader J. Cardiac-specific overexpression of inducible nitric oxide synthase does not result in severe cardiac dysfunction. Circ Res. 2002;90:93–99. doi: 10.1161/hh0102.102757. [DOI] [PubMed] [Google Scholar]
- 20.Black RG, Jr, Guo Y, Ge ZD, Murphree SS, Prabhu SD, Jones WK, Bolli R, Auchampach JA. Gene dosage-dependent effects of cardiac-specific overexpression of the A3 adenosine receptor. Circ Res. 2002;91:165–172. doi: 10.1161/01.res.0000028007.91385.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol. 1998;275:H1375–H1387. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerr PM, Suleiman MS, Halestrap AP. Reversal of permeability transition during recovery of hearts from ischemia and its enhancement by pyruvate. Am J Physiol. 1999;276:H496–H502. doi: 10.1152/ajpheart.1999.276.2.H496. [DOI] [PubMed] [Google Scholar]
- 23.Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. Evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem. 1996;271:29223–29230. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- 24.Wang P, Chen H, Qin H, Sankarapandi S, Becher MW, Wong PC, Zweier JL. Overexpression of human copper, zinc-superoxide dismutase (SOD1) prevents postischemic injury. Proc Natl Acad Sci U S A. 1998;95:4556–4560. doi: 10.1073/pnas.95.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 26.Takano H, Tang XL, Qiu Y, Guo Y, French BA, Bolli R. Nitric oxide donors induce late preconditioning against myocardial stunning and infarction in conscious rabbits via an antioxidant-sensitive mechanism. Circ Res. 1998;83:73–84. doi: 10.1161/01.res.83.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 28.Bolli R, Dawn B, Tang XL, Qiu Y, Ping P, Xuan YT, Jones WK, Takano H, Guo Y, Zhang J. The nitric oxide hypothesis of late preconditioning. Basic Res Cardiol. 1998;93:325–338. doi: 10.1007/s003950050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Argaud L, Gateau-Roesch O, Chalabreysse L, Gomez L, Loufouat J, Thivolet-Bejui F, Robert D, Ovize M. Preconditioning delays Ca2+-induced mitochondrial permeability transition. Cardiovasc Res. 2004;61:115–122. doi: 10.1016/j.cardiores.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549:513–524. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khaliulin I, Schwalb H, Wang P, Houminer E, Grinberg L, Katzeff H, Borman JB, Powell SR. Preconditioning improves postischemic mitochondrial function and diminishes oxidation of mitochondrial proteins. Free Radic Biol Med. 2004;37:1–9. doi: 10.1016/j.freeradbiomed.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 33.Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 34.Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D, Ovize M. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol. 2005;38:367–374. doi: 10.1016/j.yjmcc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- 36.Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549:513–524. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batandier C, Leverve X, Fontaine E. Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J Biol Chem. 2004;279:17197–17204. doi: 10.1074/jbc.M310329200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.