Abstract
The eye lens is constantly subjected to oxidative stress from radiation and other sources. The lens has several mechanisms to protect its components from oxidative stress and to maintain its redox state, including enzymatic pathways and high concentrations of ascorbate and reduced glutathione. With aging, accumulation of oxidized lens components and decreased efficiency of repair mechanisms can contribute to the development of lens opacities or cataracts. Maintenance of transparency and homeostasis of the avascular lens depend on an extensive network of gap junctions. Communication through gap junction channels allows intercellular passage of molecules (up to 1 kDa) including antioxidants. Lens gap junctions and their constituent proteins, connexins (Cx43, Cx46, and Cx50), are also subject to the effects of oxidative stress. These observations suggest that oxidative stress-induced damage to connexins (and consequent altered intercellular communication) may contribute to cataract formation. Antioxid. Redox Signal. 11, 339–353.
The Eye Lens
The lens is a transparent and avascular organ whose main function is to transmit light and focus it on the retina. The lens is comprised of an anterior epithelial cell layer and of fiber cells that form the bulk of the organ. At the lens equator, epithelial cells differentiate into fiber cells, a process that involves cell elongation and loss of organelles. This differentiation process occurs throughout the lifespan of the organism. Most of the metabolic, synthetic, and active transport machinery in the lens is localized to surface (nucleated) cells (92). Fiber cell survival and the maintenance of transparency depend on the function of epithelial cells and on intercellular communication.
Gap Junctions and Intercellular Communication in the Lens
Lens intercellular communication is facilitated by an extensive network of gap junctions. Gap junctions are membrane specializations that contain clusters of intercellular channels that are permeable to ions and molecules up to 1 kDa. Lens fiber cells share ions and small metabolites through gap junction channels, and therefore behave as a functional syncytium (45, 91). Epithelial and fiber cells contain morphologically and physiologically distinct gap junctions (95, 123).
The lens appears to have an internal “circulation” in which flow of water and ions drives the movement of solutes throughout the organ (90). A model of this circulation has been developed based on surface currents recorded from lenses (90, 115, 133). In this model, current (i.e., flow of ions and associated water) enters the lens along the extracellular spaces between cells at the anterior and posterior poles, it crosses fiber cell membranes in the lens interior, and it flows back to the surface at the equator (through a cell-to-cell pathway). This model is consistent with the distribution of ion channels and pumps in the lens (29). The lens circulatory system provides a pathway for internal fiber cells to obtain essential nutrients, remove potentially toxic metabolites, and maintain resting potentials (43, 118). Therefore, it has been proposed that maintenance of lens intercellular communication is crucial for the support of fiber cells (44).
Gap junction channels are oligomeric assemblies of members of a family of related proteins called connexins (Cx) (14). Connexins contain four transmembrane domains with the amino and carboxyl termini on the cytoplasmic side. Channels formed by diverse connexins differ in physiological properties, including unitary conductance, permeability, gating, and regulation by different protein kinase-dependent pathways (reviewed in 51, 52, 138).
Three connexins have been identified in the lens with somewhat overlapping expression patterns (Fig. 1). Cx43 is expressed in lens epithelial cells (99), but its expression is turned off as epithelial cells in the equatorial region differentiate into fiber cells. Cx50 is also expressed in epithelial cells (24, 135, 158). Cx46 and Cx50 become abundantly expressed in the differentiating cells and are the two most abundant connexins in lens fiber cells (116, 170). Cx46 and Cx50 co-localize at gap junctional plaques and form mixed hexamers (60, 116). Recently, transcripts for another connexin, Cx23, have been detected in the zebrafish embryo lens (56) and in the mouse lens (121). Based on its pattern of expression and the lack of proper elongation of fiber cells in mice expressing a missense mutation of Cx23, Puk et al. (121) have proposed that Cx23 is involved in fiber cell differentiation. Lens cells from other mammalian species may also express Cx23, since it has been identified as an expressed sequence in mRNA from whole eyes or lenses (accession numbers for Oryctogalus cuniculus EST from eye: EB384661.1, EB384662.1; Bos taurus EST from lens: EG705869.1; Macaca mulatta EST from lens: EG703836). However, detection of Cx23 protein in lens cells, its abundance, and its contribution to lens gap junctional intercellular communication relative to the other lens connexins have not been reported. Therefore, Cx23 is not included in Fig. 1, nor further considered in this review.
FIG. 1.
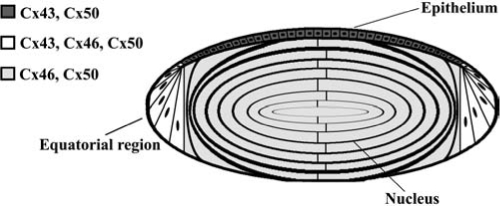
Diagram of the lens showing the distribution of connexin isoforms. Cells from the anterior epithelial layer express Cx43 and Cx50, cells from the equatorial region express Cx43, Cx46, and Cx50, and fiber cells (including those of the nucleus) contain Cx46 and Cx50.
The internal circulation between lens cells may change with aging, at least in humans. Sweeney and Truscott (157) and Moffat et al. (97) have shown restricted diffusion of GSH and water beyond the cortex/nucleus boundary in lenses from human subjects starting at middle age; while the restricted diffusion of GSH is observed in most lenses from subjects over the age of 30, the restriction to diffusion of water is observed much later (>65 years). These authors speculate that this barrier contributes to the development of age-related nuclear cataracts. The molecular basis for this restricted diffusion is unknown, but it is possible that connexin modifications in the aging lens lead to a decrease or loss of intercellular communication between cortical and nuclear fiber cells (preventing delivery of small molecules into the lens nucleus).
Cataracts
Clinical manifestation, classification, and etiology
Cataracts are a common pathological abnormality of the lens characterized by loss of lens transparency. They are the leading cause of blindness worldwide (129). Because of its high prevalence, this disease has substantial public health implications. In many countries, cataractous lenses that impair vision are surgically removed and replaced with prosthetic intraocular lenses. With increasing life expectancy, the corresponding increase in age-related cataracts will produce an even greater worldwide financial burden. Therefore, many efforts have been devoted to determine the factors that lead to cataract formation and to develop treatments to prevent their formation. Although the specific associated biochemical and histological changes may be diverse, a common biochemical change is the presence of high molecular weight insoluble protein aggregates.
Cataracts can be subdivided according to their anatomical location within the lens (e.g., cortical, nuclear, subcapsular), their appearance (e.g., total, pulverulent), and most commonly by a combination of these two parameters (e.g., nuclear pulverulent). They can also be subdivided according to their etiology (e.g., congenital, disease-related, or age-related). Many disease- and age-related cataracts may result from oxidative stress.
In this review, we will present an overview of the relationship between oxidative stress and cataract formation, the pathways involved in generating reactive oxygen species (ROS) in the lens, and pathways that ameliorate (protect and repair) the effects of oxidative stress upon lens components. Then, we will examine the effects of oxidative stress on lens gap junctions and how they may contribute to cataract formation. Finally, we will highlight the challenges for future research in this area.
Contribution of oxidative stress to cataract formation
The participation of oxidative stress in cataract formation was inferred after comparing the lipid composition, protein modifications, and concentrations of antioxidants between cataractous and clear lenses. Oxidative stress and hyperbaric oxygen therapy both cause similar biochemical changes and cataracts, further supporting the hypothesis (88, 113). Substantial evidence has accumulated to support the conclusion that ROS and resulting oxidative damage are factors contributing to the development of various types of cataracts (148, 155).
Oxidation of proteins, lipids, and DNA has been observed in cataractous lenses (6, 28, 64, 152, 180). Proteins from cataractous lenses lose sulfhydryl groups, contain oxidized methionines, become thiolated or crosslinked by nondisulfide bonds, form high molecular weight aggregates, and become insoluble (reviewed in 127, 161). All of these modifications may result from changes in the redox state of the lens. In fact, the normally high concentrations of reduced glutathione (GSH) are decreased in cataractous lenses (147), a decrease that may result from inactivation of glutathione reductase by disulfide bond formation and protein unfolding (174). Depletion of GSH in early postnatal mice causes cell damage to epithelial and fiber cells (70, 89), an effect that can be prevented by pretreatment with glutathione ester (89). Mixed protein–thiol and protein–protein disulfide bonds have been reported in lens proteins (23, 82, 83, 181). Because the formation of disulfide bonds precedes the characteristic morphological changes of cataracts, it has been proposed that this modification contributes to cataract formation following oxidative stress (82).
Oxidative stress may also have a role in selenite-induced and diabetic cataracts. In the selenite-induced cataract, several of the selenite-induced effects can be ameliorated by diethyldithiocarbamate which has antioxidant properties (57). In diabetic cataracts, hyperglycemia leads to an increase in superoxide in mitochondria (108, 134), and increased super-oxide dismutase (SOD) activity has been observed by some investigators (140), but not others (110).
Photooxidation may also contribute to age-related cataracts. Ultraviolet (UV) rays present in sunlight induce formation of ROS. In humans, the free UV filters (tryptophan derivatives) decrease with aging (16) whereas the levels of protein-bound UV filters which generate peroxides (mainly H2O2) upon UVA illumination (i.e., behaving as photosensitizers; 114) increase with age (96). Thus, the decrease in free UV filters and their binding to lens proteins cause the lens to be progressively more predisposed to UV damage and oxidation (16, 96, 114).
In summary, in response to oxidative stresses, lens proteins (including enzymes, crystallins, and other chaperones) may become modified, denatured, and aggregated, contributing to cataract formation.
Redox Systems in the Lens
ROS generation systems
Free radicals or ROS including the superoxide anion (·O2−), hydroxyl free radicals (·OH), and H2O2 may contribute to cataract formation. Among these, ·OH is the most reactive oxygen species. Because of its small size and neutral charge, H2O2 can cross the plasma membrane; therefore, its generation outside the lens may lead to oxidative stress in the lens and cataract formation. Whereas the normal concentration of H2O2 in the aqueous humor and lens is 25–30 μM, a three-fold increase has been found in lenses from several cataract patients (36, 149).
ROS can originate endogenously in different cellular compartments (e.g., mitochondria, peroxisomes, cytoplasm) as a result of normal metabolism through the enzymatic activities of lipoxygenases, NADPH oxidase and cytochrome P450, and mitochondrial electron transport, as a response to paracrine signals (e.g., inflammatory cytokines), or through the effects of exogenous factors (e.g., UV and ionizing radiation). Moreover, ROS may be generated by the interaction of metals with H2O2 through the Fenton reaction, especially in cataractous lenses that have high concentrations of redox-active iron (34). A summary of these pathways is presented in Fig. 2.
FIG. 2.
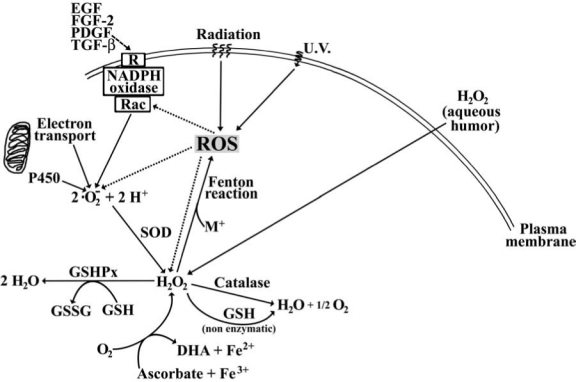
Pathways for generation of ROS in the lens. Normal electron transport and activity of cytochrome P450 (P450) can generate ·O2−· The NADPH oxidase complex, which assembles with participation of Rac GTPases and is associated with activation of plasma membrane receptors (R) by extracellular signals (e.g., growth factors), can also generate ·O2−· Growth factor receptors may also be activated by UV radiation-generated reactive oxygen species (ROS). H2O2 within the cell can be produced by the activity of superoxide dismutase (SOD) on ·O2− or it can originate by diffusion through the plasma membrane from the aqueous humor. H2O2 can also be generated by ascorbate and molecular oxygen in the presence of ferric ions. Reaction of H2O2 with metal ions (M+) can generate other ROS including ·OH or ·O2− through the Fenton reaction. H2O2 can be decomposed enzymatically by GSH-dependent peroxidases (GSHPx) or catalase, and nonenzymatically by reduced glutathione (GSH).
The NADPH oxidase system may participate in generating ROS in the lens. Immunoblotting and/or RT-PCR results have shown that different components of the NADPH oxidase complex (including p22phox, p40phox, p47phox, p67phox, gp91phox, Rac1, and Rac2) are expressed in the lens; the specific components expressed vary between human and mouse lenses (126). This complex has been most extensively characterized in phagocytes (reviewed in 169). An active phagocyte oxidase complex is formed upon stimulation by translocation of p40phox, p47phox, p67phox, and the GTPase Rac from the cytosol to the plasma membrane where they interact with the membrane-associated heterodimer of p22phox and gp91phox (the NADPH oxidase catalytic core). The active oxidase transfers electrons from NADPH to reduce oxygen and form superoxide. The p47phox subunit drives assembly, triggering the translocation of and interaction between the different subunits of the complex. Phosphorylation of the C-terminus of p47phox by protein kinase C (PKC), Akt, and/or mitogen-activated protein kinases disinhibits the autoinhibition of p47phox and drives assembly and activation.
ROS degradation/inactivation systems
Due to its external location and because its function is to transmit light to the retina, the lens is constantly exposed to light and radiation; therefore, it is one of the organs most prone to suffer oxidative stress produced in response to exogenous sources. However, the lens contains several nonenzymatic and enzymatic mechanisms to protect itself from oxidative stress and to repair oxidatively damaged cell components. The nonenzymatic mechanisms include the presence of high concentrations of ascorbate, reduced glutathione (GSH), crystallins (which act as protein chaperones), and free UV filters (e.g., tryptophan derivatives in the human lens and NAD(P)H in rabbit, guinea pig and frog lenses (2, 16, 125, 127).
ROS are degraded through the enzymatic activities of superoxide dismutase, catalase, and peroxidases (glutathione peroxidase, peroxiredoxins, and microperoxidases) (127, 132, 151). Superoxide dismutase catalyzes the conversion of ·O2− to H2O2. H2O2 can be degraded enzymatically by catalase, an enzyme that specifically uses H2O2 as a substrate, or by glutathione peroxidase (GSHPx-1), a selenoenzyme that reduces peroxides and requires GSH. Microperoxidases (which are heme peptide peroxidases derived from enzymatic cleavage of cytochrome c) also hydrolyze peroxides (151). H2O2 can be degraded nonenzymatically by reduced glutathione. Synthesis of the cofactor, GSH, involves the enzymes γ-glutamyl cysteine synthetase and glutathione synthase. In the biochemical reactions that require GSH, GSH is converted to its oxidized form, GSSG; GSSG is then converted back to its reduced form by GSSG reductase, an enzyme that requires NADPH (largely produced through the pentose phosphate pathway) as a cofactor (127). There likely is redundancy in the systems for degradation of peroxides since the lenses from animals lacking catalase or GSHPx-1 appear normal (53, 150). However, while lenses from catalase knockout mice do not exhibit increased sensitivity to oxidative stress (53), those from GSHPx-1-null mice are somewhat more sensitive to oxidative stress (150). Moreover, lenses from SOD-1 (CuZn-SOD)-null mice (containing twice the concentration of ·O2− of wild-type mice) appear normal, but they are more prone to develop photochemical cataracts (8). Lenses from peroxiredoxin 6-null mice have some histological abnormalities (i.e., abnormal cell localization in the bow region, some lens epithelial cells migrating to the region posterior to the equator, impaired loss of nuclei, and thickening of the capsule) and are more sensitive to oxidative stress (31).
Systems that protect/repair proteins from oxidative stress
Several protein modifications may result from oxidative stress including oxidation of methionines, oxidation of cysteines (with formation of protein–protein disulfide bonds and/or protein–thiol mixed disulfide bonds), and cross-linking of proteins through amino acids other than cysteine. These modifications can lead to formation of the high molecular weight insoluble aggregates that are common in cataractous lenses (17, 161, 162).
Repair of lens proteins after oxidative damage involves the participation of GSH-dependent thioltransferase, the NADPH-dependent thioredoxin/thioredoxin reductase system, and methionine sulfoxide reductases (as illustrated in Fig. 3). GSH-dependent thioltransferase catalyzes the dethiolation of protein–thiol mixed disulfides (124). Thioredoxin reduces protein–protein disulfide bonds in a reaction that leads to oxidation of thioredoxin. Oxidized thioredoxin is reduced by thioredoxin reductase, a homodimeric selenoenzyme that uses NADPH as a cofactor (177). Methionine sulfoxide reductases convert oxidized methionine (methionine sulfoxide) back to methionine in a reaction that involves thioredoxin reductase, the thioredoxin system, and NADPH (153). The expression of cystathionine-β-synthase in the lens has been reported (117). This enzyme is rate-limiting in a transsulfuration pathway that may contribute to the production of cysteine and glutathione (117). These enzymes work together to protect the lens from oxidative stress.
FIG. 3.
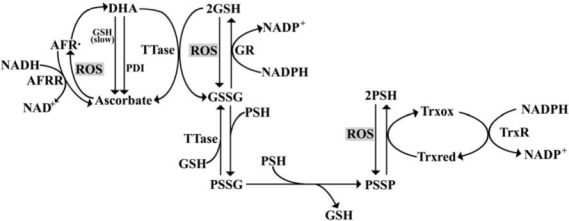
Redox pathways involved in oxidative stress in the eye lens. AFR·, ascorbate free radical; AFRR, NADH-dependent ascorbate free radical reductase; DHA, dehydroascorbate; GR, GSSG reductase; GSSG, oxidized glutathione; GSH, reduced glutathione; PDI, protein disulfide isomerase; PSH, sulfhydryl-containing protein; PSSG, glutathionated protein; PSSP, oxidized disulfide bond-containing protein; ROS, reactive oxygen species; TrxR, thioredoxin reductase; Trxox, oxidized thioredoxin; Trxred, reduced thioredoxin; TTase, thioltransferase.
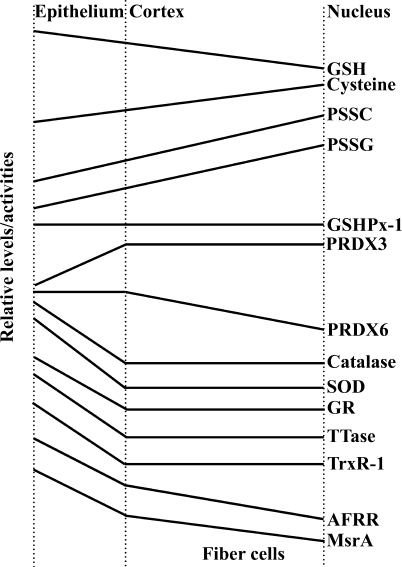
In the human lens, levels of GSH decrease from the cortical region towards the nucleus, whereas cysteine levels increase from the cortical region towards the nucleus (27). Since the nucleus and organelles are lost during fiber cell differentiation, it is not surprising that epithelial and differentiating fiber cells contain most of the redox systems of the lens. Indeed, many components of the redox system (including SOD, catalase, GSSG reductase, NADPH oxidase, peroxiredoxin 6, thioltransferase, thioredoxin reductase, ascorbate free radical reductase, methionine sulfoxide reductase) have much higher activities/levels in the epithelial/surface cells than in the rest of the lens (Fig. 4) (7, 15, 63, 65, 124, 126, 143, 153, 154, 177). Two enzymes that do not follow this pattern are GSHPx-1 (whose activity is comparable in the epithelium and in the rest of the mouse lens; 154) and peroxiredoxin 3 (a mitochondrial peroxidase with higher abundance in fiber cells than in epithelial cells; 72).
FIG. 4.
Regional distribution of components of the redox system within the lens. This graphical representation summarizes data from many studies regarding the relative levels/activities (shown in arbitrary units) of the different molecules/enzymes in the different regions of the lens. The levels/activities of most components of the redox system are higher in the epithelium. AFRR, NADH-dependent ascorbate free radical reductase; GR, GSSG reductase; GSH, reduced glutathione; GSHPx-1, glutathione-dependent peroxidase-1; MsrA, methionine sulfoxide reductase A; PRDX3, peroxiredoxin 3; PRDX6, peroxiredoxin 6; PSSC, protein-bound cysteine; PSSG, glutathionated protein; SOD, super-oxide dismutase; TrxR-1, thioredoxin reductase 1; TTase, thioltransferase.
Physiological Roles of ROS
ROS effects on enzymes
Oxidative stress can alter enzymatic activities directly by protein modification. For example, thiolation of an SH group at the active site of glycolytic enzymes (e.g., hexokinase or pyruvate kinase) leads to loss of activity, whereas thiolation of fructose 1,6-bisphosphatase, a gluconeogenic enzyme, leads to its activation (122). Oxidative stress may also affect enzymes indirectly (through regulation of gene expression). For example, exposure of porcine lenses to H2O2 results in induction of thioltransferase and thioredoxin/thioredoxin reductase (98). Similarly, exposure of cultured lens epithelial cells to low concentrations of H2O2 results in induction of peroxiredoxin 3 (72) and peroxiredoxin 6 (112). These compensatory changes may help the lens respond to oxidative stress and regain its normal redox state.
ROS effects on cell proliferation
ROS generation is a component of several signaling cascades involved in several processes including cell proliferation. In lens epithelial cells, several growth factors [epidermal growth factor (EGF), basic fibroblast growth factor (FGF-2), platelet-derived growth factor (PDGF), and transforming growth factor β (TGF-β)] increase ROS production; this effect can be blocked by NADPH oxidase inhibitors (126). Chen et al. (20) have suggested that G-protein coupled receptors and EGF receptors can modulate the mitogenic action of PDGF. This cellular signaling may also be affected by external influences, since UV radiation leads to ROS production and transient activation of the EGF receptor which induces translocation of Rac2 and NADPH oxidase activity (62).
Gap Junctions and Cataracts
As stated above, intercellular communication mediated by gap junction channels has been proposed to have a major role in the maintenance of lens transparency. This hypothesis has been substantiated by a number of genetic studies in mice. Targeted deletion of Cx46 or Cx50 results in the development of cataracts in homozygous mice (41, 171). The severities of the cataracts in both Cx46- and Cx50-null mice depend on the genetic background (35, 40). Furthermore, the cataract trait in several mutant mouse strains has been mapped to the lens connexin loci. Steele et al. (156) demonstrated that the No2 mouse, which develops congenital cataracts (especially in homozygotes), carries a missense mutation within the coding region of Cx50, resulting in a change of amino acid residue 47 from aspartate to alanine (Cx50D47A). Similarly, mice carrying a Cx50 mutation at amino acid residue 64 (changing from valine to alanine, Cx50V64A) exhibit dominantly-inherited cataracts (46). Another mouse with cataracts, Lop10, carries a missense mutation at amino acid residue 22 of Cx50 (Cx50G22R) (19).
Most importantly, mutations in lens connexins have been associated with human disease. Missense and frame-shift mutations of the Cx46 and Cx50 genes have been identified in members of families with inherited cataracts (usually dominant) of various different phenotypes. These mutants and their associated cataract phenotypes are summarized in Table 1.
Table 1.
Summary of Human Cx46 and Cx50 Mutants Associated with Cataract Formation
| |
Disease phenotype
|
||
|---|---|---|---|
| Mutant connexin | Cataract appearance | Other features | Reference |
| Cx46 | |||
| D3Y | Zonular pulverulent | 1 | |
| L11S | “Ant-egg” | 49 | |
| V28M | Variable | 25 | |
| F32L | Nuclear pulverulent | 59 | |
| R33L | Finely granular embryonal | 47 | |
| W45S | Nuclear | 86 | |
| P59L | Nuclear punctuate | 9 | |
| N63S | Zonular pulverulent | 87 | |
| R76G | Total | 25 | |
| R76H | Lamellar nuclear opacity surrounding pulverulent nuclear opacities. | Dominant inheritance with incomplete penetration | 18 |
| T87M | “Pearl box” | 48 | |
| P187L | Homogeneous zonular pulverulent | 128 | |
| N188T | Nuclear pulverulent | 74 | |
| fs380 | Zonular pulverulent | 87 | |
| Cx50 | |||
| R23T | Nuclear | 172 | |
| V44E | Total | Microcornea and variably associated with myopia | 26 |
| W45E | Jellyfish-like | Associated with microcornea | 165 |
| D47N | Nuclear pulverulent | 4 | |
| E48K | “Zonular nuclear” pulverulent | 10 | |
| V64G | Nuclear | 86 | |
| V79L | “Full moon” with Y-sutural opacities | 163 | |
| P88Q | Lamellar pulverulent | 5 | |
| Balloon-like with Y-sutural opacities | 164 | ||
| P88S | Zonular pulverulent | 142 | |
| P189L | Star-shaped nuclear opacity with a whitish central core | Associated with microcornea | 50 |
| R198Q | Not available | Microcornea and variably associated with myopia | 26 |
| fs203 | Total | Recessive inheritance; associated with nystagmus and amblyopia. | 120 |
| I247M | Zonular pulverulent | 119 | |
| S276F | Nuclear pulverulent | 175 | |
Gap Junctions and ROS
Because gap junction channels facilitate transfer of ions and molecules up to 1 kDa among coupled cells, they can allow sharing of molecules involved in maintaining a reduced environment in the lens including cysteine, ascorbate, free UV filters (e.g., tryptophan derivatives), and GSH. In fact, the permeability of Cx43 gap junction channels to GSH has been directly demonstrated (39). Thus, it is expected that gap junction function plays a pivotal role in maintaining the redox state of the lens. However, this function may be impaired by oxidative stress as demonstrated by the cellular uncoupling observed after treatment of cells with toxic compounds (e.g., CCl4 and paraquat) that lead to generation of free radicals (136, 137). Paradoxically, treatment of primary cultures of lens epithelial cells with 7-ketocholesterol, a cholesterol oxide that is increased in human cataracts, induces an increase in intercellular dye transfer; this effect is associated with an increase in the phosphorylated forms of Cx43, insolubility in 1% Triton X-100 and Cx43 at the plasma membrane (37).
Post-Translational Modification of Connexins
Like other proteins, connexins are subject to post-translational modification. Many of the connexins are phosphoproteins. A number of phosphorylated residues and the responsible kinases have been identified, and these phosphorylation events have been associated with various steps in the connexin life cycle (reviewed in 68; for Cx43, see 146). Connexins are also post-translationally modified by ubiquitinylation (66), S-nitrosylation (130), fatty acylation (80), hydroxylation (80), and γ-carboxyglutamylation (80). The functional role of many of these modifications remains unknown.
Cx43, Cx46, and Cx50 are phosphoproteins (61, 99, 158). Shearer et al. (141) have demonstrated acetylation of the second amino acid residue (glycine) and oxidation of methionines in the bovine orthologs of Cx46 and Cx50 (Cx44 and Cx49, respectively), and deamidation of asparagine121 in Cx49; however, the authors caution that both methionine oxidation and deamidation may occur during sample preparation (141).
Cx43 phosphorylation has been extensively studied in several different cell types. (The results may be extrapolated to Cx43 in lens epithelial cells.) Modification of Cx43 by phosphorylation decreases the electrophoretic mobility of the protein, resulting in an immunoblot pattern that contains three major immunoreactive Cx43 bands (designated NP, P1, and P2) (100). Depending on the cell type, the Cx43 band initially designated NP (for nonphosphorylated) may contain Cx43 phosphorylated at a site that does not alter its electrophoretic mobility (144). The P2 phosphoform has been correlated with localization of the protein to gap junctional plaques and insolubility in Triton X-100 (101). A P3 form with an electrophoretic mobility slower than P2 is observed in some cell lines during mitosis (67).
Several bands corresponding to different phosphorylated forms of Cx46 and Cx50 are detected in lens homogenates by immunoblotting. Phosphorylation of the lens fiber cell connexins has been implicated in regulation of intercellular communication, protein stability, and proteolytic cleavage (11–13, 21, 178). Ovine Cx49, the ortholog of Cx50, is phosphorylated by casein kinase I, and inhibition of casein kinase I increases intercellular communication between cultured lens cells (21). Phosphorylation may regulate stability of Cx56, the chicken ortholog of Cx46, since the phosphorylated forms of Cx56 with lower electrophoretic mobility show longer half-lives (11). Casein kinase II-mediated phosphorylation of Ser363 in Cx45.6, the chicken ortholog of Cx50, regulates its cleavage by a caspase-3-like protease (178).
Protein Kinase C
Protein kinase C (PKC) comprises a family of serine/threonine kinases that have central roles in cell signaling. The PKC isoenzymes have been grouped into three classes depending on the cofactors required for enzyme activation. Conventional PKC isoenzymes (α β, and γ) require diacylglycerol, Ca2+, and phosphatidylserine for activity; the novel PKC isoenzymes (δ, ɛ, θ, and η/L) do not require Ca2+ for activation; and the atypical PKC isoenzymes (ζ, ι/λ) require neither Ca2+ nor diacylglycerol (reviewed in 105). The PKC activators, diacylglycerol and phorbol esters (e.g., 12-O-tetradecanoyl phorbol ester, TPA), bind to cysteine-rich domains (C1) in conventional and novel PKCs. Because PKC activation may result from oxidative stress or lead to ROS generation, we will give special attention to the PKC-dependent effects on connexins and gap junction function.
Expression of protein kinase C in lens cells
The chicken lens expresses PKCα, γ, ɛ, and ι (13). Most of the PKC enzyme immunoreactivity detected with isoenzyme-specific antibodies localizes to the epithelium and annular pad. PKCγ and ɛ are present at lower levels in fiber cells where at least some PKCγ is localized at the plasma membrane (13). The specificity of expression of PKC isoenzymes is maintained in primary cultures of lens cells (13), where cells differentiate to form birefringent structures called lentoids that share similarities to differentiating fiber cells including expression of fiber cell connexins (11, 61, 93). Cultured ovine lens epithelial and lentoid cells have been reported to express PKCα; however, treatment with TPA does not induce translocation of PKCα to the plasma membrane of epithelial cells (159). The rabbit lens epithelial cell line, N/N1003A, expresses PKCα and γ (167). Saleh et al. (139) analyzed rat lenses and found expression of PKCγ in epithelium, the equatorial (bow) region, and the cortex; PKCα was not found in the cortex of these lenses, the only region analyzed for this isoenzyme. In cultured bovine lens epithelial cells, PKCα and γ were detected by immunoblotting (42).
Effects of PKC activation
In many cell types that express Cx43, treatment with TPA decreases intercellular communication. This change in gap junction function is associated with a relative increase in the P1 and P2 phosphoforms and a decrease in NP, a change associated with differential phosphorylation of Cx43 (reviewed in 138, 145). Serines at positions 368, 279/282, and 328 in Cx43 are phosphorylated after treatment with PKC activators (146). PKC-dependent phosphorylation of Ser368 in Cx43 leads to decreased intercellular communication due to a change in single channel behavior (69) and altered permeability (30); phosphorylation of Ser368 has also been implicated in trafficking/assembly of gap junctions (144).
As in these other systems, treatment of epithelial cells with phorbol esters (such as TPA) leads to a decrease in intercellular communication (81, 131, 159) associated with increased Cx43 phosphorylation (detectable by 32P incorporation) (131). Several mechanisms have been proposed for the TPA-induced decrease in intercellular communication. In cultured bovine epithelial cells, it has been suggested that the TPA-induced phosphorylation of Cx43 targets the protein (or converts the protein into a better substrate) for proteasomal degradation, because it is associated with an MG132-sensitive decrease in Cx43 levels (38). In cultures of ovine lens epithelial cells, TPA induced a gradual disappearance of Cx43 from gap junction plaques and a transient decrease in Cx43 levels which the authors interpreted as a transitory modification that affected its antigenicity (159). In these cultures, TPA also induces a decrease in levels of Cx49 mRNA and the disappearance of this connexin from gap junction plaques over a few hours (159). In contrast, activation of PKCα and γ in N/N1003A cells by treatment with TPA leads to a decrease in the number of gap junctional plaques without changes in Cx43 levels, suggesting redistribution of Cx43 (167). Similar results are obtained after treatment of N/N1003A cells with diacylglycerol (DAG), insulin-like growth factor 1 (IGF-1), or lens epithelium-derived growth factor (LEDGF), suggesting that IGF-1 and LEDGF may correspond to the endogenous agonists that trigger activation of PKC (78, 107). PKC involvement may also explain the decreased intercellular communication of lens epithelial cells elicited by extracellular ATP (84); this effect is sensitive to PKC and phospholipase C inhibitors and requires a serine at position 368 even though there is no requirement for a change in the phosphorylation state of this residue (85).
The effects of phorbol esters and PKC activation on fiber cell connexins have been studied in lentoid-containing cultures and lens organ cultures. TPA treatment of cultures from chicken lenses decreases dye coupling between lentoid cells (13). The TPA-induced decrease in dye coupling is associated with activation of PKCγ and a change in the immunoblot pattern of Cx56 (Fig. 5). The altered immunoblot pattern is associated with an increase in phosphorylation at Ser118, suggesting that phosphorylation leads to channel closure (12). Rat Cx46 hemichannels are also modulated by PKC phosphorylation; OAG reduces the amplitude of hemichannel currents and leads to their inactivation after prolonged incubation, an effect that can be inhibited by PKC inhibitors (106). PKCγ and Cx46 have been reported to co-localize in the equatorial and cortical regions of rat lenses, and 10% of the total PKCγ in lens cortex was co-precipitated with anti-Cx46 antisera (139).
FIG. 5.
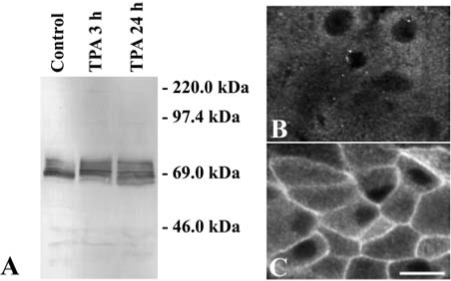
Effects of TPA on Cx56 and PKCγ in cultured chicken embryonic lens cells. (A) Immunoblot of Cx56 from homogenates of lentoid-containing cultures that were left untreated (Control) or that were treated with 200 nM TPA for 3 or 24 h. The migration positions of the molecular mass standards are indicated. (B, C). Photomicrographs show the distribution of anti-PKCγ immunoreactivity in lentoid cells left untreated (B) or treated with 200 nM TPA for 30 min (C). TPA treatment activated PKCγ (as demonstrated by its translocation to the plasma membrane) and produced a change in the immunoblot pattern of phosphorylated Cx56 forms. Bar, 12 μm in B and 15 μm in C.
Although we have shown that phorbol esters lead to uncoupling of lentoid cells derived from chicken lenses (13), the effects of these PKC activators on fiber cell communication have varied in different systems (and may result, at least in part, from species differences). For example, TenBroek et al. (159) found no effect of phorbol esters on dye coupling in lentoids from ovine lenses. Cx49, the ovine Cx50 ortholog, appears not to be a substrate for PKC (3), while Cx45.6, the chicken ortholog, is a good PKC substrate (61). Treatment of whole rat lenses with TPA leads to an increase in PKCγ activity associated with a decrease in anti-Cx50 immunoreactivity in gap junctions, an increase in anti-Cx50 immunoreactivity in the nonjunctional plasma membrane (hemichannels), and a decrease in Lucifer yellow transfer in the lens cortex measured in whole lenses. Co-precipitation of PKCγ with anti-caveolin-1 antibodies was also detected after TPA treatment, suggesting that PKCγ translocates to caveolin-1-containing membrane fractions, which also contain Cx46 and Cx50. These results suggest that TPA-induced activation of PKC leads to undocking and dispersion of Cx50 hemichannels (179).
Effects of Oxidative Stress on Lens Connexins
Oxidative stress may be linked to changes in connexins and gap junctions. We have observed that treatment of chicken lentoid-containing cultures with H2O2 leads to dose-and time-dependent changes in the immunoblot pattern of Cx56 (Fig. 6). These results suggest that H2O2 leads to differential phosphorylation of Cx56. The appearance of a cleaved form when cultures are treated with 500 μM H2O2 is associated with cell death, suggesting that strong oxidizing treatments lead to deleterious effects that surpass the restoring capacity of the cells. Treatment of N/N1003A cells with H2O2 leads to an increase in PKCγ activity, an effect that appears to result from direct oxidation of the enzyme with formation of disulfide bonds. Activation of PKCγ resulted in increased phosphorylation of Cx43 in Ser368 as detected by immunoblotting, a decrease in the number of gap junction plaques, and a decrease in dye coupling (77). Similar effects on lens fiber connexins were observed when rat lenses were treated with H2O2 (i.e., H2O2 induced activation of PKCγ and increased phosphorylation of Cx46 and Cx50) (75). In both N/N1003A cells and lenses, PKCγ co-precipitated with connexins using anti-caveolin-1 antibodies, leading the authors to conclude that activated PKCγ translocated to and phosphorylated connexins in lipid rafts. In the selenite-induced cataract, a decrease in the relative levels of the Cx46 phosphorylated form with the slowest electrophoretic mobility and an increase in the levels of the cleaved form of Cx50 in the lens cortex have been reported (33).
FIG. 6.
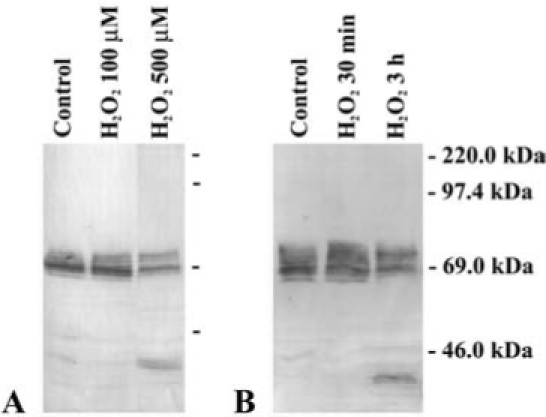
Effects of oxidative stress on Cx56 in cultured embryonic chicken lens cells. (A) Immunoblot of Cx56 from homogenates of lentoid-containing cultures that were left untreated (Control) or that were treated with 100 or 500 μM H2O2 for 3 h. (B) Immunoblot of Cx56 from homogenates of lentoid-containing cultures that were left untreated (Control) or that were treated with 500 μM H2O2 for 30 min or 3 h. The migration positions of the molecular mass standards are indicated. Only subtle changes in the immunoblot pattern of Cx56 bands were detected after treatment with 100 μM H2O2 for 3 h. A prominent degradation product of Cx56 was generated after treatment with 500 μM H2O2 for 3 h.
ROS and Apoptosis
Oxidative stress corresponds to an imbalance between the rate of ROS production and their rate of degradation, leading to accumulation of oxidized cell components. When this exceeds the cell's ability to repair these components, or the oxidizing stress is too high, apoptosis occurs. Apoptosis may play important roles in lens development and pathology (reviewed in 173, 176). Cellular stresses like H2O2 (76) or activators of the intrinsic pathway of apoptosis (160) can cause cell death of lens epithelial cells. Gap junction channels may propagate apoptosis signals through a bystander-like mechanism as proposed previously for other cell types (22, 32, 79, 94). However, other studies suggest a role for PKC-dependent closure of gap junction channels as a protective/survival mechanism, because the induced channel closure might prevent the intercellular transmission of apoptotic signals (77). Thus, published data suggest that PKC activation by H2O2 can either generate apoptotic signals (that may be propagated through gap junction channels) or lead to closure of those channels (blocking that propagation).
Involvement of Gap Junctions in Growth Factor Signaling
ROS may influence connexins and gap junction-mediated intercellular communication through their involvement in cellular signaling under normal physiological conditions. As noted above, stimulation of several growth factor receptors leads to ROS generation. Activation of various growth factor receptors leads to Cx43 phosphorylation and decreased intercellular communication in various non-lens cells (168). In chicken embryo lens cells, FGF treatment or ERK activation increases intercellular coupling (71). This difference may be due to the expression of additional connexins in lens cells (71).
Nitrogen Oxides in the Lens in Relation to Connexins and Cataract Formation
This review would not be complete without considering the possible role of nitrogen oxides in cataract formation. Nitric oxide (NO) is generated by nitric oxide synthases (NOS). NO can generate several different nitrogen oxide species including peroxynitrite which is formed after reaction with ·O2−. Peroxynitrite is a highly reactive species that can oxidize proteins and nonprotein sulfhydryl groups. Nitrogen oxides can modify proteins by nitration and nitrosation of their aromatic amino acid residues. For example, incubation of α-crystallin with the nitrite ion results in oxidative damage of the protein similar to the modifications found in cataracts (111).
Participation of inducible nitric oxide synthase (iNOS) and nitrite ion in cataractogenesis has been proposed in several models. In selenite-induced cataracts, an increase in nitrite and iNOS is observed within one day of treatment; the development of opacification can be inhibited by inhibitors of NOS and accelerated by the NOS substrate, arginine (57, 58). In the Shumiya cataract strain, appearance of opacities is preceded by increases in iNOS, Ca2+, and proteolysis; treatment with aminoguanidine, an inhibitor of iNOS, leads to a decrease in iNOS protein and Ca2+. The authors have suggested that the increase in Ca2+ leads to calpain-mediated proteolysis, decreased protein solubility, and opacification (54, 55). In the UPL rat, another hereditary cataractous strain, the appearance of opacities is associated with a decrease in ATP and GSH levels, a decrease in cytochrome c oxidase activity, and increased NO and Ca2+ levels, Na+/K+ ratio, and water-insoluble/water-soluble protein ratio. Treatment with NOS inhibitors delays the development (or decreases the severity) of cataracts (102, 103). The temporal course of these changes has led to the proposal that the increase in iNOS leads to excess production of NO which in turn leads to mitochondrial genome damage and cytochrome c oxidase dysfunction with a consequent decrease in ATP production, a decrease in Ca2+-ATPase function and an increase in lens Ca2+, which leads to opacification (103). In the SOD-1 (CuZn-SOD)-null mice, development of high glucose-induced cataracts can be delayed by NOS inhibitors and increased by NO donors (109). Nitric oxide donors also lead to decreases in ATP and GSH levels in the lens in organ culture (166). Treatment of the human lens epithelial cell line, SRA 01/04, with interferon-γ and LPS leads to iNOS expression, a decrease in ATP levels, and an increase in Ca2+-ATPase activity, effects that are attenuated by NOS inhibitors (73, 104).
Direct modification of connexins by nitrogen oxides has been reported (130). S-nitrosylation of Cx43 has been associated with opening of connexin hemichannels (measured by dye uptake) and an increase in the number of channels at the plasma membrane in cultured astrocytes (130); the dye uptake can be reduced by high intracellular concentrations of glutathione (130). A similar protective mechanism could operate in the Cx43-expressing nucleated cells of the lens as long as their normal high levels of glutathione are maintained.
Concluding Remarks
Because epithelial cells differentiate into fiber cells throughout the life span of the individual, somatic DNA mutations in epithelial cells acquired through oxidative stress may have detrimental consequences for lens homeostasis and transparency. Similarly, during aging, cells in the lens interior may accumulate modifications in enzymes and other components that are important for maintenance of a reduced environment and become more dependent on repair mechanisms (provided by the more externally located cells via intercellular communication). It is thus possible that protein or gene modifications in nucleated lens cells that result in decreased antioxidant activity would lead to cataract formation. Similarly, mutations that reduce intercellular communication would also lead to cataracts, because antioxidant molecules would not reach the interior of the lens. The effects of oxidative stress on lens proteins, lipids, and DNA may result in changes in intercellular communication because of changes in the lipid environment, activation of signaling pathways/enzymes that affect post-translational modification of connexins, direct oxidation of connexin molecules, or somatic mutations in genes, including those of connexins.
To elucidate the roles of altered gap junction function in oxidative stress-induced cataracts, it still remains to determine the temporal correlation between changes in connexin-mediated function and levels of antioxidants, ATP, and Ca2+, and the activities of enzymes involved in maintaining the redox state in an oxidative stress model of cataract. Biochemical determination of connexin protein modification can help elucidate whether direct oxidation of lens connexins occurs during cataract formation. Such analysis could clarify the relation between connexin modifications and changes in intercellular communication, and whether they precede the development of lens opacities. Care should be taken when performing these experiments, because proteins may be oxidized or some modifications may be lost (or acquired) during tissue dissection and sample preparation.
The real challenge to study the relationship between oxidative stress, connexin function, and cataracts lies in designing a good model to study this interrelationship. When considering this model, one has to take into account that the anterior of the lens is bathed by the aqueous humor, while the posterior of the lens is bathed by the vitreous. (Both humors contain signaling molecules that may affect how lens cells respond to a given stimulus.) To mimic the situation in vivo, an ideal in vitro model would consist of lenses correctly oriented in organ cultures in a container with two-chambers (one containing media mimicking the vitreous and the other the aqueous); this model would permit application of oxidative stresses to the lens from either chamber. It would even be better to develop techniques that would allow assessment of connexin function in vivo to study this interrelationship.
Taken together, there is strong evidence linking oxidative stress to cataract formation, oxidative stress to altered gap junction-mediated intercellular communication, and altered intercellular communication to cataracts. These results suggest that oxidative stress may lead to decreased connexin function and cataract formation, but the direct relationship between all three: oxidative stress, connexin function, and cataracts remains to be demonstrated.
Abbreviations
AFR·, ascorbate free radical; AFRR, NADH-dependent ascorbate free radical reductase; C1 domain, cysteine-rich region of protein kinase C isoenzymes; Cx, connexin; DAG, diacylglycerol; DHA, dehydroascorbate; EGF, epidermal growth factor; ERK, extracellular signal-regulated kinase; EST, expressed sequence tag; FGF-2, basic fibroblast growth factor; GR, oxidized glutathione reductase (GSSG reductase); GSSG, oxidized glutathione; GSH, reduced glutathione; GSHPx, glutathione-dependent peroxidase; IGF-I, insulin-like growth factor I; iNOS, inducible nitric oxide synthase; LEDGF, lens epithelium-derived growth factor; LPS, lipopolysaccharide; MsrA, methionine sulfoxide reductase A; NO, nitric oxide; ·O2−, superoxide anion; OAG, 1-oleoyl-2-acetyl-sn-glycerol; ·OH, hydroxyl free radical; P1, P2 and P3, different phosphorylated forms of Cx43; PDI, protein disulfide isomerase; PDGF, platelet-derived growth factor; PKC, protein kinase C; PRDX3, peroxiredoxin 3; PRDX6, peroxiredoxin 6; PSH, sulfhydryl-containing protein; PSSC, protein-bound cysteine; PSSG, gluthationated protein; PSSP, oxidized disulfide bond-containing protein; ROS, reactive oxygen species; SH, sulfhydryl; SOD, superoxide dismutase; TGF-β, transforming growth factor β; TPA, 12-O-tetrade-canoyl phorbol ester; TrxR, thioredoxin reductase; Trxox, oxidized thioredoxin; Trxred, reduced thioredoxin; TTase, thioltransferase.
Acknowledgments
The research in the author's laboratories is supported by NIH grant EY08368.
References
- 1. Addison PK. Berry V. Holden KR. Espinal D. Rivera B. Su H. Srivastava AK. Bhattacharya SS. A novel mutation in the connexin 46 gene (GJA3) causes autosomal dominant zonular pulverulent cataract in a Hispanic family. Mol Vis. 2006;12:791–795. [PubMed] [Google Scholar]
- 2. Andley UP. The lens epithelium: Focus on the expression and function of the α-crystallin chaperones. Int J Biochem Cell Biol. 2008;40:317–323. doi: 10.1016/j.biocel.2007.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arneson ML. Cheng H-L. Louis CF. Characterization of the ovine-lens plasma-membrane protein-kinase substrates. Eur J Biochem. 1995;234:670–679. doi: 10.1111/j.1432-1033.1995.670_b.x. [DOI] [PubMed] [Google Scholar]
- 4. Arora A. Minogue PJ. Liu X. Addison PK. Russel-Eggitt I. Webster AR. Hunt DM. Ebihara L. Beyer EC. Berthoud VM. Moore AT. A novel connexin50 mutation associated with congenital nuclear pulverulent cataracts. J Med Genet. 2008;45:155–160. doi: 10.1136/jmg.2007.051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arora A. Minogue PJ. Liu X. Reddy MA. Ainsworth JR. Bhattacharya SS. Webster AR. Hunt DM. Ebihara L. Moore AT. Beyer EC. Berthoud VM. A novel GJA8 mutation is associated with autosomal dominant lamellar pulverulent cataract: further evidence for gap junction dysfunction in human cataract. J Med Genet. 2006;43:e2. doi: 10.1136/jmg.2005.034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Babizhayev MA. Costa EB. Lipid peroxide and reactive oxygen species generating systems of the crystalline lens. Biochim Biophys Acta. 1994;1225:326–337. doi: 10.1016/0925-4439(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 7. Bando M. Obazawa H. Regional and subcellular distribution of ascorbate free radical reductase activity in the human lens. Tokai J Exp Clin Med. 1991;16:217–222. [PubMed] [Google Scholar]
- 8. Behndig A. Karlsson K. Reaume AG. Sentman ML. Marklund SL. In vitro photochemical cataract in mice lacking copper-zinc superoxide dismutase. Free Radic Biol Med. 2001;31:738–744. doi: 10.1016/s0891-5849(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 9. Bennett TM. Mackay DS. Knopf HL. Shiels A. A novel missense mutation in the gene for gap-junction protein α3 (GJA3) associated with autosomal dominant “nuclear punctate” cataracts linked to chromosome 13q. Mol Vis. 2004;10:376–382. [PubMed] [Google Scholar]
- 10. Berry V. Mackay D. Khaliq S. Francis PJ. Hameed A. Anwar K. Mehdi SQ. Newbold RJ. Ionides A. Shiels A. Moore T. Bhattacharya SS. Connexin 50 mutation in a family with congenital “zonular nuclear” pulverulent cataract of Pakistani origin. Hum Genet. 1999;105:168–170. doi: 10.1007/s004399900094. [DOI] [PubMed] [Google Scholar]
- 11. Berthoud VM. Bassnett S. Beyer EC. Cultured chicken embryo lens cells resemble differentiating fiber cells in vivo and contain two kinetic pools of connexin56. Exp Eye Res. 1999;68:475–484. doi: 10.1006/exer.1998.0635. [DOI] [PubMed] [Google Scholar]
- 12. Berthoud VM. Beyer EC. Kurata WE. Lau AF. Lampe PD. The gap junction protein connexin56 is phosphorylated in both the intracellular loop and the carboxy-terminal region. Eur J Biochem. 1997;244:89–97. doi: 10.1111/j.1432-1033.1997.00089.x. [DOI] [PubMed] [Google Scholar]
- 13. Berthoud VM. Westphale EM. Grigoryeva A. Beyer EC. PKC isoenzymes in the chicken lens and TPA-induced effects on intercellular communication. Invest Ophthalmol Vis Sci. 2000;41:850–858. [PubMed] [Google Scholar]
- 14.Beyer EC.Berthoud VM. The family of connexin genes. In: Harris A, editor; Locke D, editor. Connexin: A Guide. New York, NY: Humana Press; 2008. pp. 3–25. [Google Scholar]
- 15. Bhuyan KC. Bhuyan DK. Superoxide dismutase of the eye: relative functions of superoxide dismutase and catalase in protecting the ocular lens from oxidative damage. Biochim Biophys Acta. 1978;542:28–38. doi: 10.1016/0304-4165(78)90229-5. [DOI] [PubMed] [Google Scholar]
- 16. Bova LM. Sweeney MH. Jamie JF. Truscott RJW. Major changes in human ocular UV protection with age. Invest Ophthalmol Vis Sci. 2001;42:200–205. [PubMed] [Google Scholar]
- 17. Buckingham RH. The behaviour of reduced proteins from normal and cataractous lenses in highly dissociating media: cross-linked protein in cataractous lenses. Exp Eye Res. 1972;14:123–129. doi: 10.1016/0014-4835(72)90057-7. [DOI] [PubMed] [Google Scholar]
- 18. Burdon KP. Wirth MG. Mackey DA. Russell-Eggitt IM. Craig JE. Elder JE. Dickinson JL. Sale MM. A novel mutation in the Connexin 46 gene causes autosomal dominant congenital cataract with incomplete penetrance. J Med Genet. 2004;41:e106. doi: 10.1136/jmg.2004.018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang B. Wang X. Hawes NL. Ojakian R. Davisson MT. Lo WK. Gong XH. A Gja8 (Cx50) point mutation causes an alteration of α3 connexin (Cx46) in semi-dominant cataracts of Lop10 mice. Hum Mol Genet. 2002;11:507–513. doi: 10.1093/hmg/11.5.507. [DOI] [PubMed] [Google Scholar]
- 20. Chen KC. Zhou Y. Zhang W. Lou MF. Control of PDGF-induced reactive oxygen species (ROS) generation and signal transduction in human lens epithelial cells. Mol Vis. 2007;13:374–387. [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng HL. Louis CF. Functional effects of casein kinase I-catalyzed phosphorylation on lens cell-to-cell coupling. J Membr Biol. 2001;181:21–30. doi: 10.1007/s0023200100055. [DOI] [PubMed] [Google Scholar]
- 22. Colombo BM. Benedetti S. Ottolenghi S. Mora M. Pollo B. Poli G. Finocchiaro G. The “bystander effect”: association of U-87 cell death with ganciclovir-mediated apoptosis of nearby cells and lack of effect in athymic mice. Hum Gene Ther. 1995;6:763–772. doi: 10.1089/hum.1995.6.6-763. [DOI] [PubMed] [Google Scholar]
- 23. Cui XL. Lou MF. The effect and recovery of long-term H2O2 exposure on lens morphology and biochemistry. Exp Eye Res. 1993;57:157–167. doi: 10.1006/exer.1993.1111. [DOI] [PubMed] [Google Scholar]
- 24. Dahm R. Van Marle J. Prescott AR. Quinlan RA. Gap junctions containing α8-connexin (MP70) in the adult mammalian lens epithelium suggests a re-evaluation of its role in the lens. Exp Eye Res. 1999;69:45–56. doi: 10.1006/exer.1999.0670. [DOI] [PubMed] [Google Scholar]
- 25. Devi RR. Reena C. Vijayalakshmi P. Novel mutations in GJA3 associated with autosomal dominant congenital cataract in the Indian population. Mol Vis. 2005;11:846–852. [PubMed] [Google Scholar]
- 26. Devi RR. Vijayalakshmi P. Novel mutations in GJA8 associated with autosomal dominant congenital cataract and microcornea. Mol Vis. 2006;12:190–195. [PubMed] [Google Scholar]
- 27. Dickerson JE Jr. Lou MF. Free cysteine levels in normal human lenses. Exp Eye Res. 1997;65:451–454. doi: 10.1006/exer.1997.0343. [DOI] [PubMed] [Google Scholar]
- 28. Dische Z. Zil H. Studies on the oxidation of cysteine to cystine in lens proteins during cataract formation. Am J Ophthalmol. 1951;34:104–113. doi: 10.1016/0002-9394(51)90013-x. [DOI] [PubMed] [Google Scholar]
- 29. Donaldson P. Kistler J. Mathias RT. Molecular solutions to mammalian lens transparency. News Physiol Sci. 2001;16:118–123. doi: 10.1152/physiologyonline.2001.16.3.118. [DOI] [PubMed] [Google Scholar]
- 30. Ek-Vitorin JF. King TJ. Heyman NS. Lampe PD. Burt JM. Selectivity of connexin 43 channels is regulated through protein kinase C-dependent phosphorylation. Circ Res. 2006;98:1498–1505. doi: 10.1161/01.RES.0000227572.45891.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fatma N. Kubo E. Sharma P. Beier DR. Singh DP. Impaired homeostasis and phenotypic abnormalities in Prdx6−/− mice lens epithelial cells by reactive oxygen species: increased expression and activation of TGFβ. Cell Death Differ. 2005;12:734–750. doi: 10.1038/sj.cdd.4401597. [DOI] [PubMed] [Google Scholar]
- 32. Fick J. Barker FG. Dazin P. Westphale EM. Beyer EC. Israel MA. The extent of heterocellular communication mediated by gap junctions is predictive of bystander tumor cytotoxicity in vitro. Proc Natl Acad Sci USA. 1995;92:11071–11075. doi: 10.1073/pnas.92.24.11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fleschner CR. Connexin 46 and connexin 50 in selenite cataract. Ophthalmic Res. 2006;38:24–28. doi: 10.1159/000088527. [DOI] [PubMed] [Google Scholar]
- 34. Garner B. Roberg K. Qian M. Eaton JW. Truscott RJW. Distribution of ferritin and redox-active transition metals in normal and cataractous human lenses. Exp Eye Res. 2000;71:599–607. doi: 10.1006/exer.2000.0912. [DOI] [PubMed] [Google Scholar]
- 35. Gerido DA. Sellitto C. Li L. White TW. Genetic background influences cataractogenesis, but not lens growth deficiency, in Cx50-knockout mice. Invest Ophthalmol Vis Sci. 2003;44:2669–2674. doi: 10.1167/iovs.02-1311. [DOI] [PubMed] [Google Scholar]
- 36. Giblin FJ. McCready JP. Kodama T. Reddy VN. A direct correlation between the levels of ascorbic acid and H2O2 in aqueous humor. Exp Eye Res. 1984;38:87–93. doi: 10.1016/0014-4835(84)90142-8. [DOI] [PubMed] [Google Scholar]
- 37. Girão H. Catarino S. Pereira P. 7-Ketocholesterol modulates intercellular communication through gap-junction in bovine lens epithelial cells. Cell Commun Signal. 2004;2:2. doi: 10.1186/1478-811X-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Girão H. Pereira P. Phosphorylation of connexin 43 acts as a stimuli for proteasome-dependent degradation of the protein in lens epithelial cells. Mol Vis. 2003;9:24–30. [PubMed] [Google Scholar]
- 39. Goldberg GS. Lampe PD. Nicholson BJ. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol. 1999;1:457–459. doi: 10.1038/15693. [DOI] [PubMed] [Google Scholar]
- 40. Gong X. Agopian K. Kumar NM. Gilula NB. Genetic factors influence cataract formation in α3 connexin knockout mice. Dev Genet. 1999;24:27–32. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<27::AID-DVG4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 41. Gong X. Li E. Klier G. Huang Q. Wu Y. Lei H. Kumar NM. Horwitz J. Gilula NB. Disruption of α3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–843. doi: 10.1016/s0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- 42. Gonzalez K. Udovichenko I. Cunnick J. Takemoto DJ. Protein kinase C in galactosemic and tolrestat-treated lens epithelial cells. Curr Eye Res. 1993;12:373–377. doi: 10.3109/02713689308999462. [DOI] [PubMed] [Google Scholar]
- 43. Goodenough DA. Lens gap junctions: a structural hypothesis for nonregulated low-resistance intercellular pathways. Invest Ophthalmol Vis Sci. 1979;18:1104–1122. [PubMed] [Google Scholar]
- 44. Goodenough DA. The crystalline lens. A system networked by gap junctional intercellular communication. Semin Cell Biol. 1992;3:49–58. doi: 10.1016/s1043-4682(10)80007-8. [DOI] [PubMed] [Google Scholar]
- 45. Goodenough DA. Dick J. Lyons JE. Lens metabolic cooperation: a study of mouse lens transport and permeability visualized with freeze-substitution autoradiography and electron microscopy. J Cell Biol. 1980;86:576–589. doi: 10.1083/jcb.86.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Graw J. Loster J. Soewarto D. Fuchs H. Meyer B. Reis A. Wolf E. Balling R. De Angelis MH. Characterization of a mutation in the lens-specific MP70 encoding gene of the mouse leading to a dominant cataract. Exp Eye Res. 2001;73:867–876. doi: 10.1006/exer.2001.1096. [DOI] [PubMed] [Google Scholar]
- 47. Guleria K. Sperling K. Singh D. Varon R. Singh JR. Vanita V. A novel mutation in the connexin 46 (GJA3) gene associated with autosomal dominant congenital cataract in an Indian family. Mol Vis. 2007;13:1657–1665. [PubMed] [Google Scholar]
- 48. Guleria K. Vanita V. Singh D. Singh JR. A novel “pearl box” cataract associated with a mutation in the connexin 46 (GJA3) gene. Mol Vis. 2007;13:797–803. [PMC free article] [PubMed] [Google Scholar]
- 49. Hansen L. Yao W. Eiberg H. Funding M. Riise R. Kjaer KW. Hejtmancik JF. Rosenberg T. The congenital “ant-egg” cataract phenotype is caused by a missense mutation in connexin46. Mol Vis. 2006;12:1033–1039. [PubMed] [Google Scholar]
- 50. Hansen L. Yao W. Eiberg H. Kjaer KW. Baggesen K. Hejtmancik JF. Rosenberg T. Genetic heterogeneity in microcornea-cataract: five novel mutations in CRYAA, CRYGD, and GJA8. Invest Ophthalmol Vis Sci. 2007;48:3937–3944. doi: 10.1167/iovs.07-0013. [DOI] [PubMed] [Google Scholar]
- 51. Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 52. Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. 2007;94:120–143. doi: 10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ho YS. Xiong Y. Ma W. Spector A. Ho DS. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem. 2004;279:32804–32812. doi: 10.1074/jbc.M404800200. [DOI] [PubMed] [Google Scholar]
- 54. Inomata M. Hayashi M. Shumiya S. Kawashima S. Ito Y. Aminoguanidine-treatment results in the inhibition of lens opacification and calpain-mediated proteolysis in Shumiya cataract rats (SCR) J Biochem. 2000;128:771–776. doi: 10.1093/oxfordjournals.jbchem.a022814. [DOI] [PubMed] [Google Scholar]
- 55. Inomata M. Hayashi M. Shumiya S. Kawashima S. Ito Y. Involvement of inducible nitric oxide synthase in cataract formation in Shumiya cataract rat (SCR) Curr Eye Res. 2001;23:307–311. doi: 10.1076/ceyr.23.4.307.5455. [DOI] [PubMed] [Google Scholar]
- 56. Iovine MK. Gumpert AM. Falk MM. Mendelson TC. Cx23, a connexin with only four extracellular-loop cysteines, forms functional gap junction channels and hemi-channels. FEBS Lett. 2008;582:165–170. doi: 10.1016/j.febslet.2007.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ito Y. Cai H. Terao M. Tomohiro M. Preventive effect of diethyldithiocarbamate on selenite-induced opacity in cultured rat lenses. Ophthalmic Res. 2001;33:52–59. doi: 10.1159/000055642. [DOI] [PubMed] [Google Scholar]
- 58. Ito Y. Nabekura T. Takeda M. Nakao M. Terao M. Hori R. Tomohiro M. Nitric oxide participates in cataract development in selenite-treated rats. Curr Eye Res. 2001;22:215–220. doi: 10.1076/ceyr.22.3.215.5516. [DOI] [PubMed] [Google Scholar]
- 59. Jiang H. Jin Y. Bu L. Zhang W. Liu J. Cui B. Kong X. Hu L. A novel mutation in GJA3 (connexin46) for autosomal dominant congenital nuclear pulverulent cataract. Mol Vis. 2003;9:579–583. [PubMed] [Google Scholar]
- 60. Jiang JX. Goodenough DA. Heteromeric connexons in lens gap junction channels. Proc Natl Acad Sci USA. 1996;93:1287–1291. doi: 10.1073/pnas.93.3.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jiang JX. Goodenough DA. Phosphorylation of lens-fiber connexins in lens organ cultures. Eur J Biochem. 1998;255:37–44. doi: 10.1046/j.1432-1327.1998.2550037.x. [DOI] [PubMed] [Google Scholar]
- 62. Jiang Q. Zhou C. Healey S. Chu W. Kouttab N. Bi Z. Wan Y. UV radiation down-regulates Dsg-2 via Rac/NADPH oxidase-mediated generation of ROS in human lens epithelial cells. Int J Mol Med. 2006;18:381–387. [PubMed] [Google Scholar]
- 63. Kantorow M. Hawse JR. Cowell TL. Benhamed S. Pizarro GO. Reddy VN. Hejtmancik JF. Methionine sulfoxide reductase A is important for lens cell viability and resistance to oxidative stress. Proc Natl Acad Sci USA. 2004;101:9654–9659. doi: 10.1073/pnas.0403532101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kleiman NJ. Spector A. DNA single strand breaks in human lens epithelial cells from patients with cataract. Curr Eye Res. 1993;12:423–431. doi: 10.3109/02713689309024624. [DOI] [PubMed] [Google Scholar]
- 65. Kubo E. Miyazawa T. Fatma N. Akagi Y. Singh DP. Development- and age-associated expression pattern of peroxiredoxin 6, and its regulation in murine ocular lens. Mech Aging Dev. 2006;127:249–256. doi: 10.1016/j.mad.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 66. Laing JG. Beyer EC. The gap junction protein connexin43 is degraded via the ubiquitin proteasome pathway. J Biol Chem. 1995;270:26399–6403. doi: 10.1074/jbc.270.44.26399. [DOI] [PubMed] [Google Scholar]
- 67. Lampe PD. Kurata WE. Warn-Cramer BJ. Lau AF. Formation of a distinct connexin43 phosphoisoform in mitotic cells is dependent upon p34cdc2 kinase. J Cell Sci. 1998;111:833–841. doi: 10.1242/jcs.111.6.833. [DOI] [PubMed] [Google Scholar]
- 68. Lampe PD. Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lampe PD. TenBroek EM. Burt JM. Kurata WE. Johnson RG. Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol. 2000;149:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Laver NM. Robison WG Jr. Calvin HI. Fu SC. Early epithelial lesions in cataracts of GSH-depleted mouse pups. Exp Eye Res. 1993;57:493–498. doi: 10.1006/exer.1993.1151. [DOI] [PubMed] [Google Scholar]
- 71. Le AC. Musil LS. A novel role for FGF and extracellular signal-regulated kinase in gap junction-mediated intercellular communication in the lens. J Cell Biol. 2001;154:197–216. doi: 10.1083/jcb.200101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee W. Wells T. Kantorow M. Localization and H2O2-specific induction of PRDX3 in the eye lens. Mol Vis. 2007;13:1469–1474. [PubMed] [Google Scholar]
- 73. Li DX. Wang SL. Ito Y. Zhang JH. Wu CF. Diethyldithiocarbamate inhibits iNOS expression in human lens epithelial cells stimulated by IFN-gamma and LPS. Acta Pharmacol Sin. 2005;26:359–363. doi: 10.1111/j.1745-7254.2005.00041.x. [DOI] [PubMed] [Google Scholar]
- 74. Li Y. Wang J. Dong B. Man H. A novel connexin46 (GJA3) mutation in autosomal dominant congenital nuclear pulverulent cataract. Mol Vis. 2004;10:668–671. [PubMed] [Google Scholar]
- 75. Lin D. Lobell S. Jewell A. Takemoto DJ. Differential phosphorylation of connexin46 and connexin50 by H2O2 activation of protein kinase Cγ. Mol Vis. 2004;10:688–695. [PubMed] [Google Scholar]
- 76. Lin D. Shanks D. Prakash O. Takemoto DJ. Protein kinase C γ mutations in the C1B domain cause caspase-3-linked apoptosis in lens epithelial cells through gap junctions. Exp Eye Res. 2007;85:113–122. doi: 10.1016/j.exer.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lin D. Takemoto DJ. Oxidative activation of protein kinase C γ through the C1 domain. Effects on gap junctions. J Biol Chem. 2005;280:13682–13693. doi: 10.1074/jbc.M407762200. [DOI] [PubMed] [Google Scholar]
- 78. Lin D. Boyle DL. Takemoto DJ. IGF-I-induced phosphorylation of connexin 43 by PKC γ: regulation of gap junctions in rabbit lens epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:1160–1168. doi: 10.1167/iovs.02-0737. [DOI] [PubMed] [Google Scholar]
- 79. Lin JH. Weigel H. Cotrina ML. Liu S. Bueno E. Hansen AJ. Hansen TW. Goldman S. Nedergaard M. Gap-junction-mediated propagation and amplification of cell injury. Nat Neurosci. 1998;1:494–500. doi: 10.1038/2210. [DOI] [PubMed] [Google Scholar]
- 80. Locke D. Koreen IV. Harris AL. Isoelectric points and post-translational modifications of connexin26 and connexin32. FASEB J. 2006;20:1221–1223. doi: 10.1096/fj.05-5309fje. [DOI] [PubMed] [Google Scholar]
- 81. Long AC. Colitz CM. Bomser JA. Regulation of gap junction intercellular communication in primary canine lens epithelial cells: role of protein kinase C. Curr Eye Res. 2007;32:223–231. doi: 10.1080/02713680601186714. [DOI] [PubMed] [Google Scholar]
- 82. Lou MF. Dickerson JE Jr. Garadi R. The role of protein-thiol mixed disulfides in cataractogenesis. Exp Eye Res. 1990;50:819–826. doi: 10.1016/0014-4835(90)90133-f. [DOI] [PubMed] [Google Scholar]
- 83. Lou MF. McKellar R. Chyan O. Quantitation of lens protein mixed disulfides by ion-exchange chromatography. Exp Eye Res. 1986;42:607–616. doi: 10.1016/0014-4835(86)90050-3. [DOI] [PubMed] [Google Scholar]
- 84. Lurtz MM. Louis CF. Calmodulin and protein kinase C regulate gap junctional coupling in lens epithelial cells. Am J Physiol Cell Physiol. 2003;285:C1475–C1482. doi: 10.1152/ajpcell.00361.2002. [DOI] [PubMed] [Google Scholar]
- 85. Lurtz MM. Louis CF. Intracellular calcium regulation of connexin43. Am J Physiol Cell Physiol. 2007;293:C1806–C1813. doi: 10.1152/ajpcell.00630.2006. [DOI] [PubMed] [Google Scholar]
- 86. Ma Z. Zheng J. Yang F. Ji J. Li X. Tang X. Yuan X. Zhang X. Sun H. Two novel mutations of connexin genes in Chinese families with autosomal dominant congenital nuclear cataract. Br J Ophthalmol. 2005;89:1535–1537. doi: 10.1136/bjo.2005.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mackay D. Ionides A. Kibar Z. Rouleau G. Berry V. Moore A. Shiels A. Bhattacharya S. Connexin46 mutations in autosomal dominant congenital cataract. Am J Hum Genet. 1999;64:1357–1364. doi: 10.1086/302383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Marsili S. Salganik RI. Albright CD. Freel CD. Johnsen S. Peiffer RL. Costello MJ. Cataract formation in a strain of rats selected for high oxidative stress. Exp Eye Res. 2004;79:595–612. doi: 10.1016/j.exer.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 89. Martensson J. Steinherz R. Jain A. Meister A. Glutathione ester prevents buthionine sulfoximine-induced cataracts and lens epithelial cell damage. Proc Natl Acad Sci USA. 1989;86:8727–8731. doi: 10.1073/pnas.86.22.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mathias RT. Rae JL. Baldo GJ. Physiological properties of the normal lens. Physiol Rev. 1997;77:21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- 91.Mathias RT.Rae JL. Cell to cell communication in lens. In: Sperelakis N, editor; Cole WC, editor. Cell Interactions and Gap Junctions. Boca Raton, FL: CRC Press, Inc.; 1989. pp. 29–50. [Google Scholar]
- 92. Mathias RT. Rae JL. The lens: local transport and global transparency. Exp Eye Res. 2004;78:689–698. doi: 10.1016/j.exer.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 93. Menko AS. Klukas KA. Johnson RG. Chicken embryo lens cultures mimic differentiation in the lens. Dev Biol. 1984;103:129–141. doi: 10.1016/0012-1606(84)90014-9. [DOI] [PubMed] [Google Scholar]
- 94. Mesnil M. Piccoli C. Tiraby G. Willecke K. Yamasaki H. Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. Proc Natl Acad Sci USA. 1996;93:1831–1835. doi: 10.1073/pnas.93.5.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Miller TM. Goodenough DA. Evidence for two physiologically distinct gap junctions expressed by the chick lens epithelial cell. J Cell Biol. 1986;102:194–199. doi: 10.1083/jcb.102.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mizdrak J. Hains PG. Truscott RJW. Jamie JF. Davies MJ. Tryptophan-derived ultraviolet filter compounds covalently bound to lens proteins are photosensitizers of oxidative damage. Free Radic Biol Med. 2008;44:1108–1119. doi: 10.1016/j.freeradbiomed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 97. Moffat BA. Landman KA. Truscott RJW. Sweeney MH. Pope JM. Age-related changes in the kinetics of water transport in normal human lenses. Exp Eye Res. 1999;69:663–669. doi: 10.1006/exer.1999.0747. [DOI] [PubMed] [Google Scholar]
- 98. Moon S. Fernando MR. Lou MF. Induction of thioltransferase and thioredoxin/thioredoxin reductase systems in cultured porcine lenses under oxidative stress. Invest Ophthalmol Vis Sci. 2005;46:3783–3789. doi: 10.1167/iovs.05-0237. [DOI] [PubMed] [Google Scholar]
- 99. Musil LS. Beyer EC. Goodenough DA. Expression of the gap junction protein connexin43 in embryonic chick lens: molecular cloning, ultrastructural localization, and post-translational phosphorylation. J Membr Biol. 1990;116:163–175. doi: 10.1007/BF01868674. [DOI] [PubMed] [Google Scholar]
- 100. Musil LS. Cunningham BA. Edelman GM. Goodenough DA. Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines. J Cell Biol. 1990;111:2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Musil LS. Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Nabekura T. Koizumi Y. Nakao M. Tomohiro M. Inomata M. Ito Y. Delay of cataract development in hereditary cataract UPL rats by disulfiram and aminoguanidine. Exp Eye Res. 2003;76:169–174. doi: 10.1016/s0014-4835(02)00284-1. [DOI] [PubMed] [Google Scholar]
- 103. Nagai N. Ito Y. Adverse effects of excessive nitric oxide on cytochrome c oxidase in lenses of hereditary cataract UPL rats. Toxicology. 2007;242:7–15. doi: 10.1016/j.tox.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 104. Nagai N. Liu Y. Fukuhata T. Ito Y. Inhibitors of inducible nitric oxide synthase prevent damage to human lens epithelial cells induced by interferon-gamma and lipopolysaccharide. Biol Pharm Bull. 2006;29:2077–2081. doi: 10.1248/bpb.29.2077. [DOI] [PubMed] [Google Scholar]
- 105. Newton AC. Protein kinase C: Structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 106. Ngezahayo A. Zeilinger C. Todt I. Marten I. Kolb H-A. Inactivation of expressed and conducting rCx46 hemichannels by phosphorylation. Pflügers Arch. 1998;436:627–629. doi: 10.1007/s004240050681. [DOI] [PubMed] [Google Scholar]
- 107. Nguyen TA. Boyle DL. Wagner LM. Shinohara T. Takemoto DJ. LEDGF activation of PKC γ and gap junction disassembly in lens epithelial cells. Exp Eye Res. 2003;76:565–572. doi: 10.1016/s0014-4835(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 108. Nishikawa T. Edelstein D. Du XL. Yamagishi S. Matsumura T. Kaneda Y. Yorek MA. Beebe D. Oates PJ. Hammes HP. Giardino I. Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyper-glycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 109. Olofsson EM. Marklund SL. Behndig A. Glucose-induced cataract in CuZn-SOD null lenses: An effect of nitric oxide? Free Radic Biol Med. 2007;42:1098–1105. doi: 10.1016/j.freeradbiomed.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 110. Ozmen B. Ozmen D. Erkin E. Guner I. Habif S. Bayindir O. Lens superoxide dismutase and catalase activities in diabetic cataract. Clin Biochem. 2002;35:69–72. doi: 10.1016/s0009-9120(01)00284-3. [DOI] [PubMed] [Google Scholar]
- 111. Paik DC. Dillon J. The nitrite/alpha crystallin reaction: A possible mechanism in lens matrix damage. Exp Eye Res. 2000;70:73–80. doi: 10.1006/exer.1999.0761. [DOI] [PubMed] [Google Scholar]
- 112. Pak JH. Kim TI. Joon KM. Yong KJ. Choi HJ. Kim SA. Tchah H. Reduced expression of 1-cys peroxiredoxin in oxidative stress-induced cataracts. Exp Eye Res. 2006;82:899–906. doi: 10.1016/j.exer.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 113. Palmquist BM. Philipson B. Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol. 1984;68:113–117. doi: 10.1136/bjo.68.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Parker NR. Jamie JF. Davies MJ. Truscott RJW. Protein-bound kynurenine is a photosensitizer of oxidative damage. Free Radic Biol Med. 2004;37:1479–1489. doi: 10.1016/j.freeradbiomed.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 115. Parmelee JT. Measurement of steady currents around the frog lens. Exp Eye Res. 1986;42:433–441. doi: 10.1016/0014-4835(86)90003-5. [DOI] [PubMed] [Google Scholar]
- 116. Paul DL. Ebihara L. Takemoto LJ. Swenson KI. Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Persa C. Pierce A. Ma Z. Kabil O. Lou MF. The presence of a transsulfuration pathway in the lens: a new oxidative stress defense system. Exp Eye Res. 2004;79:875–886. doi: 10.1016/j.exer.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 118. Piatigorsky J. Intracellular ions, protein metabolism, and cataract formation. Curr Top Eye Res. 1980;3:1–39. [PubMed] [Google Scholar]
- 119. Polyakov AV. Shagina IA. Khlebnikova OV. Evgrafov OV. Mutation in the connexin 50 gene (GJA8) in a Russian family with zonular pulverulent cataract. Clin Genet. 2001;60:476–478. doi: 10.1034/j.1399-0004.2001.600614.x. [DOI] [PubMed] [Google Scholar]
- 120. Ponnam SP. Ramesha K. Tejwani S. Ramamurthy B. Kannabiran C. Mutation of the gap junction protein alpha 8 (GJA8) gene causes autosomal recessive cataract. J Med Genet. 2007;44:e85. doi: 10.1136/jmg.2007.050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Puk O. Loster J. Dalke C. Soewarto D. Fuchs H. Budde B. Nurnberg P. Wolf E. De Angelis MH. Graw J. Mutation in a novel connexin-like gene (Gjf1) in the mouse affects early lens development and causes a variable small-eye phenotype. Invest Ophthalmol Vis Sci. 2008;49:1525–1532. doi: 10.1167/iovs.07-1033. [DOI] [PubMed] [Google Scholar]
- 122. Qiao F. Xing K. Lou MF. Modulation of lens glycolytic pathway by thioltransferase. Exp Eye Res. 2000;70:745–753. doi: 10.1006/exer.2000.0836. [DOI] [PubMed] [Google Scholar]
- 123. Rae JL. Kuszak JR. The electrical coupling of epithelium and fibers in the frog lens. Exp Eye Res. 1983;36:317–326. doi: 10.1016/0014-4835(83)90114-8. [DOI] [PubMed] [Google Scholar]
- 124. Raghavachari N. Lou MF. Evidence for the presence of thioltransferase in the lens. Exp Eye Res. 1996;63:433–441. doi: 10.1006/exer.1996.0133. [DOI] [PubMed] [Google Scholar]
- 125. Rao CM. Zigler JS., Jr Levels of reduced pyridine nucleotides and lens photodamage. Photochem Photobiol. 1992;56:523–528. doi: 10.1111/j.1751-1097.1992.tb02196.x. [DOI] [PubMed] [Google Scholar]
- 126. Rao PV. Maddala R. John F. Zigler JS., Jr Expression of nonphagocytic NADPH oxidase system in the ocular lens. Mol Vis. 2004;10:112–121. [PubMed] [Google Scholar]
- 127. Reddy VN. Glutathione and its function in the lens-an overview. Exp Eye Res. 1990;50:771–778. doi: 10.1016/0014-4835(90)90127-g. [DOI] [PubMed] [Google Scholar]
- 128. Rees MI. Watts P. Fenton I. Clarke A. Snell RG. Owen MJ. Gray J. Further evidence of autosomal dominant congenital zonular pulverulent cataracts linked to 13q11 (CZP3) and a novel mutation in connexin 46 (GJA3) Hum Genet. 2000;106:206–209. doi: 10.1007/s004390051029. [DOI] [PubMed] [Google Scholar]
- 129. Resnikoff S. Pascolini D. Etya'ale D. Kocur I. Pararajasegaram R. Pokharel GP. Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 130. Retamal MA. Cortés CJ. Reuss L. Bennett MVL. Sáez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: Induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci USA. 2006;103:4475–4480. doi: 10.1073/pnas.0511118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Reynhout JK. Lampe PD. Johnson RG. An activator of protein kinase C inhibits gap junction communication between cultured bovine lens cells. Exp Cell Res. 1992;198:337–342. doi: 10.1016/0014-4827(92)90388-o. [DOI] [PubMed] [Google Scholar]
- 132. Rhee SG. Kang SW. Chang TS. Jeong W. Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001;52:35–41. doi: 10.1080/15216540252774748. [DOI] [PubMed] [Google Scholar]
- 133. Robinson KR. Patterson JW. Localization of steady currents in the lens. Curr Eye Res. 1982;2:843–847. doi: 10.3109/02713688209020020. [DOI] [PubMed] [Google Scholar]
- 134. Rolo AP. Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 135. Rong P. Wang X. Niesman I. Wu Y. Benedetti LE. Dunia I. Levy E. Gong X. Disruption of Gja8 (α8 connexin) in mice leads to microphthalmia associated with retardation of lens growth and lens fiber maturation. Development. 2002;129:167–174. doi: 10.1242/dev.129.1.167. [DOI] [PubMed] [Google Scholar]
- 136. Ruch RJ. Klaunig JE. Inhibition of mouse hepatocyte intercellular communication by paraquat-generated oxygen free radicals. Toxicol Appl Pharmacol. 1988;94:427–436. doi: 10.1016/0041-008x(88)90283-9. [DOI] [PubMed] [Google Scholar]
- 137. Sáez JC. Bennett MV. Spray DC. Carbon tetrachloride at hepatotoxic levels blocks reversibly gap junctions between rat hepatocytes. Science. 1987;236:967–969. doi: 10.1126/science.3576214. [DOI] [PubMed] [Google Scholar]
- 138. Sáez JC. Berthoud VM. Brañes MC. Martínez AD. Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 139. Saleh SM. Takemoto LJ. Zoukhri D. Takemoto DJ. PKC-γ phosphorylation of connexin 46 in the lens cortex. Mol Vis. 2001;7:240–246. [PubMed] [Google Scholar]
- 140. Scharf J. Dovrat A. Superoxide dismutase molecules in human cataractous lenses. Ophthalmic Res. 1986;18:332–337. doi: 10.1159/000265459. [DOI] [PubMed] [Google Scholar]
- 141. Shearer D. Ens W. Standing K. Valdimarsson G. Post-translational modifications in lens fiber connexins identified by off-line-HPLC MALDI-quadrupole time-of-flight mass spectrometry. Invest Ophthalmol Vis Sci. 2008;49:1553–1562. doi: 10.1167/iovs.07-1193. [DOI] [PubMed] [Google Scholar]
- 142. Shiels A. Mackay D. Ionides A. Berry V. Moore A. Bhattacharya S. A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am J Hum Genet. 1998;62:526–532. doi: 10.1086/301762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Singh AK. Shichi H. Peroxiredoxin in bovine ocular tissues: immunohistochemical localization and in situ hybridization. J Ocul Pharmacol Ther. 2001;17:279–286. doi: 10.1089/108076801750295308. [DOI] [PubMed] [Google Scholar]
- 144. Solan JL. Fry MD. TenBroek EM. Lampe PD. Connexin43 phosphorylation at S368 is acute during S and G2/M and in response to protein kinase C activation. J Cell Sci. 2003;116:2203–2211. doi: 10.1242/jcs.00428. [DOI] [PubMed] [Google Scholar]
- 145. Solan JL. Lampe PD. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim Biophys Acta. 2005;1711:154–163. doi: 10.1016/j.bbamem.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 146. Solan JL. Lampe PD. Key connexin 43 phosphorylation events regulate the gap junction life cycle. J Membr Biol. 2007;217:35–41. doi: 10.1007/s00232-007-9035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Spector A. Oxidation and cataract. Ciba Found Symp. 1984;106:48–64. doi: 10.1002/9780470720875.ch4. [DOI] [PubMed] [Google Scholar]
- 148. Spector A. Review: Oxidative stress and disease. J Ocul Pharmacol Ther. 2000;16:193–201. doi: 10.1089/jop.2000.16.193. [DOI] [PubMed] [Google Scholar]
- 149. Spector A. Garner WH. Hydrogen peroxide and human cataract. Exp Eye Res. 1981;33:673–681. doi: 10.1016/s0014-4835(81)80107-8. [DOI] [PubMed] [Google Scholar]
- 150. Spector A. Kuszak JR. Ma W. Wang RR. Ho Y. Yang Y. The effect of photochemical stress upon the lenses of normal and glutathione peroxidase-1 knockout mice. Exp Eye Res. 1998;67:457–471. doi: 10.1006/exer.1998.0548. [DOI] [PubMed] [Google Scholar]
- 151. Spector A. Ma W. Wang RR. Kleiman NJ. Microperoxidases catalytically degrade reactive oxygen species and may be anti-cataract agents. Exp Eye Res. 1997;65:457–470. doi: 10.1006/exer.1997.0336. [DOI] [PubMed] [Google Scholar]
- 152. Spector A. Roy D. Disulfide-linked high molecular weight protein associated with human cataract. Proc Natl Acad Sci USA. 1978;75:3244–3248. doi: 10.1073/pnas.75.7.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Spector A. Scotto R. Weissbach H. Brot N. Lens methionine sulfoxide reductase. Biochem Biophys Res Commun. 1982;108:429–434. doi: 10.1016/0006-291x(82)91884-8. [DOI] [PubMed] [Google Scholar]
- 154. Spector A. Yang Y. Ho YS. Magnenat JL. Wang RR. Ma W. Li WC. Variation in cellular glutathione peroxidase activity in lens epithelial cells, transgenics and knockouts does not significantly change the response to H2O2 stress. Exp Eye Res. 1996;62:521–540. doi: 10.1006/exer.1996.0063. [DOI] [PubMed] [Google Scholar]
- 155. Srivastava SK. Ansari NH. Bhatnagar A. Sugar induced cataractogenesis: A paradigm of oxidative tissue pathology? Lens Eye Toxic Res. 1990;7:161–171. [PubMed] [Google Scholar]
- 156. Steele EC Jr. Lyon MF. Favor J. Guillot PV. Boyd Y. Church RL. A mutation in the connexin 50 (Cx50) gene is a candidate for the No2 mouse cataract. Curr Eye Res. 1998;17:883–889. doi: 10.1076/ceyr.17.9.883.5144. [DOI] [PubMed] [Google Scholar]
- 157. Sweeney MH. Truscott RJW. An impediment to glutathione diffusion in older normal human lenses: a possible precondition for nuclear cataract. Exp Eye Res. 1998;67:587–595. doi: 10.1006/exer.1998.0549. [DOI] [PubMed] [Google Scholar]
- 158. TenBroek EM. Johnson R. Louis CF. Cell-to-cell communication in a differentiating ovine lens culture system. Invest Ophthalmol Vis Sci. 1994;35:215–228. [PubMed] [Google Scholar]
- 159. TenBroek EM. Louis CF. Johnson R. The differential effects of 12-O-tetradecanoylphorbol-13-acetate on the gap junctions and connexins of the developing mammalian lens. Dev Biol. 1997;191:88–102. doi: 10.1006/dbio.1997.8703. [DOI] [PubMed] [Google Scholar]
- 160. Theiss C. Mazur A. Meller K. Mannherz HG. Changes in gap junction organization and decreased coupling during induced apoptosis in lens epithelial and NIH-3T3 cells. Exp Cell Res. 2007;313:38–52. doi: 10.1016/j.yexcr.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 161. Truscott RJW. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 162. Truscott RJW. Augusteyn RC. The state of sulphydryl groups in normal and cataractous human lenses. Exp Eye Res. 1977;25:139–148. doi: 10.1016/0014-4835(77)90126-9. [DOI] [PubMed] [Google Scholar]
- 163. Vanita V. Hennies HC. Singh D. Nurnberg P. Sperling K. Singh JR. A novel mutation in GJA8 associated with autosomal dominant congenital cataract in a family of Indian origin. Mol Vis. 2006;12:1217–1222. [PubMed] [Google Scholar]
- 164. Vanita V. Singh JR. Singh D. Varon R. Sperling K. A mutation in GJA8 (p.P88Q) is associated with “balloon-like” cataract with Y-sutural opacities in a family of Indian origin. Mol Vis. 2008;14:1171–1175. [PMC free article] [PubMed] [Google Scholar]
- 165. Vanita V. Singh JR. Singh D. Varon R. Sperling K. A novel mutation in GJA8 associated with jellyfish-like cataract in a family of Indian origin. Mol Vis. 2008;14:323–326. [PMC free article] [PubMed] [Google Scholar]
- 166. Varma SD. Hegde KR. Susceptibility of the ocular lens to nitric oxide: implications in cataractogenesis. J Ocul Pharmacol Ther. 2007;23:188–195. doi: 10.1089/jop.2006.0124. [DOI] [PubMed] [Google Scholar]
- 167. Wagner LM. Saleh SM. Boyle DJ. Takemoto DJ. Effect of protein kinase Cγ on gap junction disassembly in lens epithelial cells and retinal cells in culture. Mol Vis. 2002;8:59–66. [PubMed] [Google Scholar]
- 168. Warn–Cramer BJ. Lau AF. Regulation of gap junctions by tyrosine protein kinases. Biochim Biophys Acta. 2004;1662:81–95. doi: 10.1016/j.bbamem.2003.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Werner E. GTPases and reactive oxygen species: Switches for killing and signaling. J Cell Sci. 2004;117:143–153. doi: 10.1242/jcs.00937. [DOI] [PubMed] [Google Scholar]
- 170. White TW. Bruzzone R. Goodenough DA. Paul DL. Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Mol Biol Cell. 1992;3:711–720. doi: 10.1091/mbc.3.7.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. White TW. Goodenough DA. Paul DL. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol. 1998;143:815–825. doi: 10.1083/jcb.143.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Willoughby CE. Arab S. Gandhi R. Zeinali S. Arab S. Luk D. Billingsley G. Munier FL. Heon E. A novel GJA8 mutation in an Iranian family with progressive autosomal dominant congenital nuclear cataract. J Med Genet. 2003;40:e124. doi: 10.1136/jmg.40.11.e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Wride MA. Minireview: Apoptosis as seen through a lens. Apoptosis. 2000;5:203–209. doi: 10.1023/a:1009653326511. [DOI] [PubMed] [Google Scholar]
- 174. Yan H. Harding JJ. Xing K. Lou MF. Revival of glutathione reductase in human cataractous and clear lens extracts by thioredoxin and thioredoxin reductase, in conjunction with α-crystallin or thioltransferase. Curr Eye Res. 2007;32:455–463. doi: 10.1080/02713680701257837. [DOI] [PubMed] [Google Scholar]
- 175. Yan M. Xiong C. Ye SQ. Chen Y. Ke M. Zheng F. Zhou X. A novel connexin 50 (GJA8) mutation in a Chinese family with a dominant congenital pulverulent nuclear cataract. Mol Vis. 2008;14:418–424. [PMC free article] [PubMed] [Google Scholar]
- 176. Yan Q. Liu JP. Li DW. Apoptosis in lens development and pathology. Differentiation. 2006;74:195–211. doi: 10.1111/j.1432-0436.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 177. Yegorova S. Liu A. Lou MF. Human lens thioredoxin: molecular cloning and functional characterization. Invest Ophthalmol Vis Sci. 2003;44:3263–3271. doi: 10.1167/iovs.02-1322. [DOI] [PubMed] [Google Scholar]
- 178. Yin X. Gu S. Jiang JX. The development-associated cleavage of lens connexin 45.6 by caspase-3-like protease is regulated by casein kinase II-mediated phosphorylation. J Biol Chem. 2001;276:34567–34572. doi: 10.1074/jbc.M106073200. [DOI] [PubMed] [Google Scholar]
- 179. Zampighi GA. Planells AM. Lin D. Takemoto D. Regulation of lens cell-to-cell communication by activation of PKCγ and disassembly of Cx50 channels. Invest Ophthalmol Vis Sci. 2005;46:3247–3255. doi: 10.1167/iovs.04-1504. [DOI] [PubMed] [Google Scholar]
- 180. Zigler JS Jr. Huang QL. Du XY. Oxidative modification of lens crystallins by H2O2 and chelated iron. Free Radic Biol Med. 1989;7:499–505. doi: 10.1016/0891-5849(89)90025-7. [DOI] [PubMed] [Google Scholar]
- 181. Zigman S. Paxhia T. McDaniel T. Lou MF. Yu NT. Effect of chronic near-ultraviolet radiation on the gray squirrel lens in vivo. Invest Ophthalmol Vis Sci. 1991;32:1723–1732. [PubMed] [Google Scholar]