Abstract
Pine, Martin J. (Roswell Park Memorial Institute, Buffalo, N.Y.). Metabolic control of intracellular proteolysis in growing and resting cells of Escherichia coli. J. Bacteriol. 92:847–850. 1966.—Protein breakdown was examined in Escherichia coli under varying conditions of growth and during nutritional deprivation. Optimal breakdown rates, estimated over short time periods of protein synthesis and decay, are invariant and unrepressible by any condition of growth or starvation. A more important aspect of metabolic control is manifest in the selection imposed by the physiological state of the cell for breakdown of limited populations of proteins. During the progress of growth, one-half to three-quarters of the protein susceptible in the resting state is progressively spared, and breakdown is continued in populations that are more selected and more frequently regenerated.
Full text
PDF
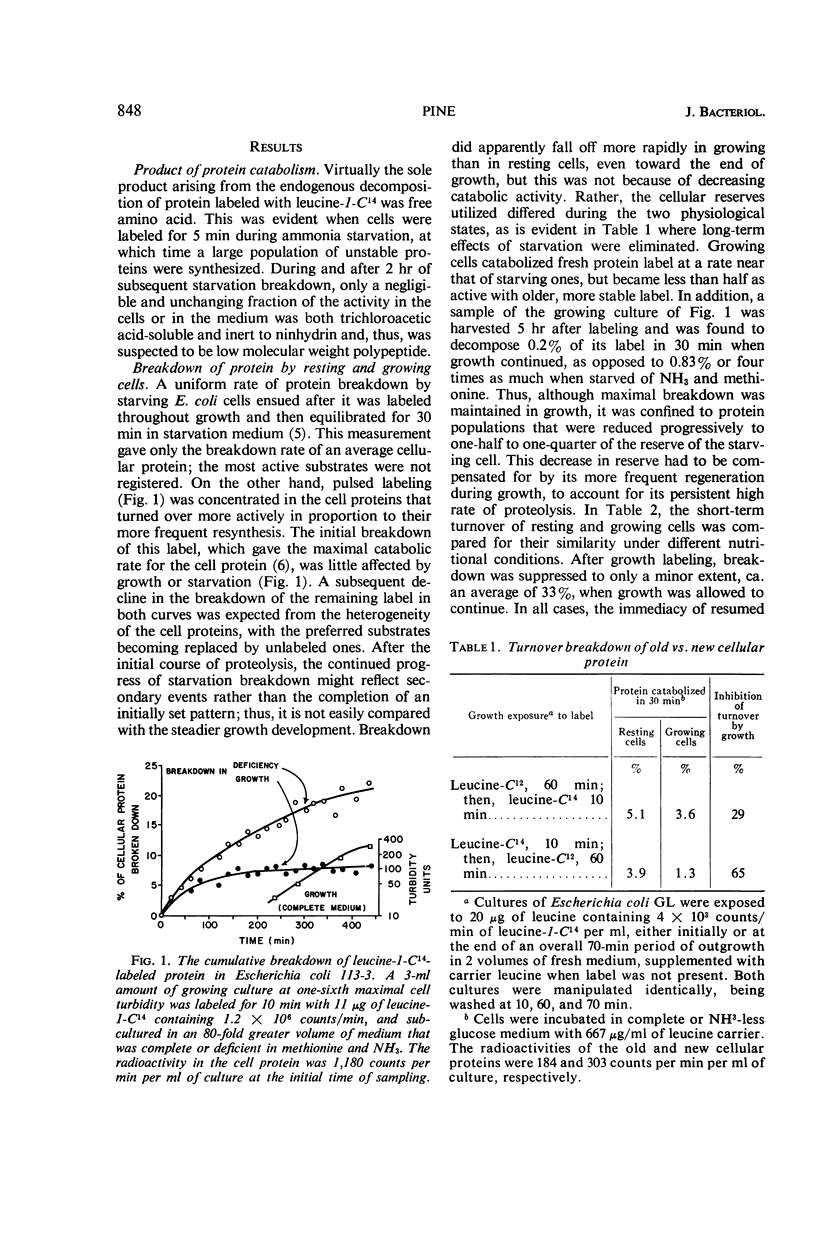
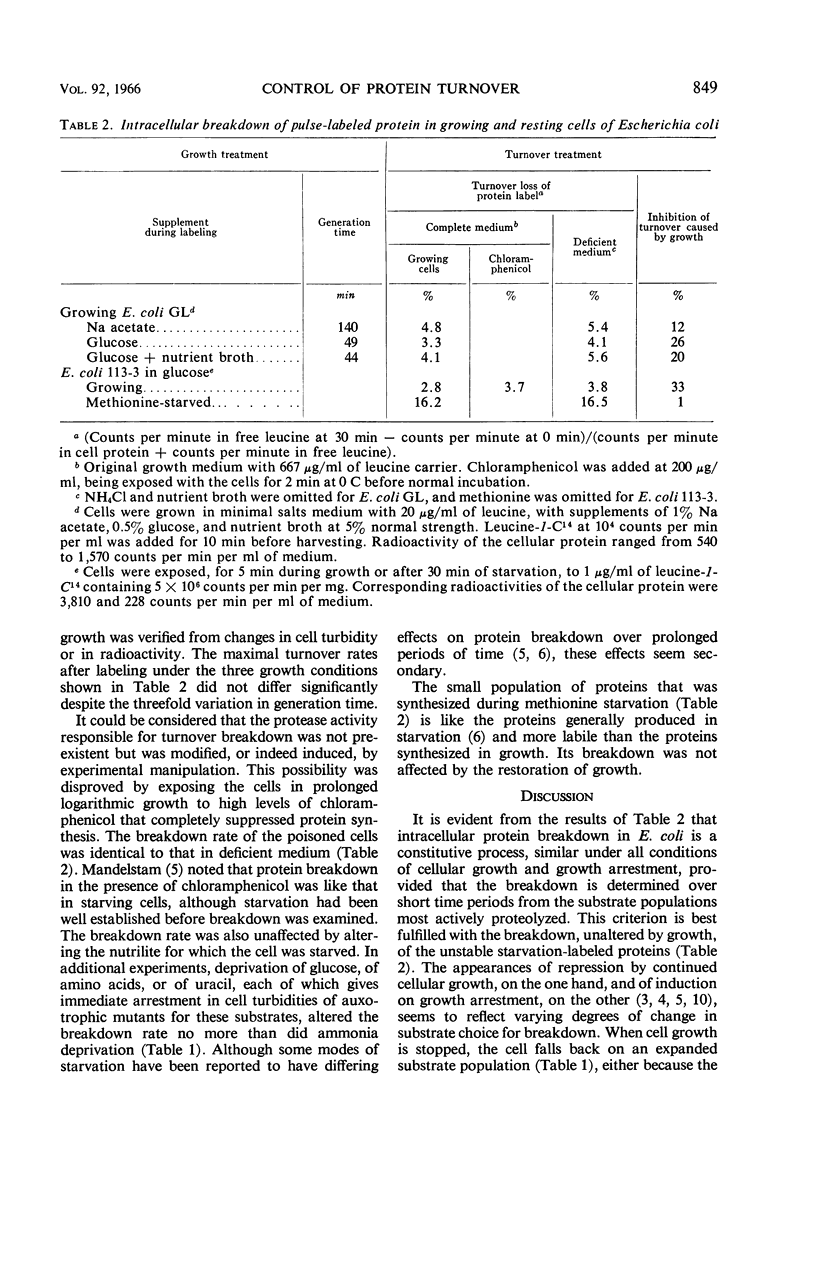

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EAGLE H., PIEZ K. A., FLEISCHMAN R., OYAMA V. I. Protein turnover in mammaliar cell cultures. J Biol Chem. 1959 Mar;234(3):592–597. [PubMed] [Google Scholar]
- HALVORSON H. Studies on protein and nucleic acid turnover in growing cultures of yeast. Biochim Biophys Acta. 1958 Feb;27(2):267–276. doi: 10.1016/0006-3002(58)90333-0. [DOI] [PubMed] [Google Scholar]
- KOCH A. L., LEVY H. R. Protein turnover in growing cultures of Escherichia coli. J Biol Chem. 1955 Dec;217(2):947–957. [PubMed] [Google Scholar]
- MANDELSTAM J. Turnover of protein in growing and non-growing populations of Escherichia coli. Biochem J. 1958 May;69(1):110–119. doi: 10.1042/bj0690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Heterogeneity of protein turnover in Escherichia coli. Biochim Biophys Acta. 1965 Jul 8;104(2):439–456. doi: 10.1016/0304-4165(65)90349-1. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Sweeney E. W., Berlin C. M. An analysis of the kinetics of rat liver tryptophan pyrrolase induction: the significance of both enzyme synthesis and degradation. Biochem Biophys Res Commun. 1964 Mar 26;15(3):214–219. doi: 10.1016/0006-291x(64)90148-2. [DOI] [PubMed] [Google Scholar]
- TRAYSER K. A., COLOWICK S. P. Properties of crystalline hexokinase from yeast. III. Studies on glucose-enzyme interaction. Arch Biochem Biophys. 1961 Jul;94:169–176. doi: 10.1016/0003-9861(61)90025-x. [DOI] [PubMed] [Google Scholar]
- URBA R. C. Protein breakdown in Bacillus cereus. Biochem J. 1959 Mar;71(3):513–518. doi: 10.1042/bj0710513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S. Protein degradation during diauxic growth of Escerichia coli. Biochem Biophys Res Commun. 1965 Sep 22;20(6):692–696. doi: 10.1016/0006-291x(65)90071-9. [DOI] [PubMed] [Google Scholar]


