Abstract
Physiological cell turnover plays an important role in maintaining normal tissue function and architecture. This is achieved by the dynamic balance of cellular regeneration and elimination, occurring periodically in tissues such as the uterus and mammary gland, or at constant rates in tissues such as the gastrointestinal tract and adipose tissue. Apoptosis has been identified as the prevalent mode of physiological cell loss in most tissues. Cell turnover is precisely regulated by the interplay of various endocrine and paracrine factors, which modulate tissue and cell-specific responses on proliferation and apoptosis, either directly, or by altering expression and function of key cell proliferative and/or death genes. Although recent studies have provided significant information on specific tissue systems, a clearly defined pathway that mediates cell turnover has not yet emerged for any tissue. Several similarities exist among the various tissues with regard to the intermediates that regulate tissue homeostasis, enabling a better understanding of the general mechanisms involved in the process. Here we review the mechanisms by which hormonal and cytokine factors mediate cell turnover in various tissues, emphasizing common themes and tissue-specific differences.
Keywords: Endocrine, Thymocytes, Breast, Uterus, Prostate, Adipocytes, Bone
Introduction
Tissue homeostasis is an important physiological phenomenon that ensures a dynamic balance between cell proliferation and cell death in the maintenance and regulation of normal tissue morphology and function (Hsueh et al. 1996; Uren and Vaux 1996). Tissue maintenance is a continuous process by which progenitor cells are recruited to differentiate into specific cell types competent in performing specialized functions, and unwanted cells are eliminated without affecting neighboring cells. The normal life span of a cell may vary between a few days and several years, depending on the cell type. The constant or cyclic pattern of cell turnover is apparent in tissues such as the thymus and uterus, but it is also vital in stable tissues such as bone, adipose tissue and nervous tissue (Gosden and Spears 1997; Prins and O’Rahilly 1997; Spelsberg et al. 1999; Ucker et al. 1994).
Cell death has been broadly classified into two categories, necrosis and apoptosis (Fawthrop et al. 1991; Trump et al. 1997; Wyllie et al. 1980). There is ongoing debate as to the mutually exclusive nature of the two categories, and it seems possible that there are many subcategories with blurred distinctions. Nevertheless, cell death clearly accompanied by an inflammatory response, resulting from cell swelling, loss of plasma membrane integrity and leakage of cellular contents into the extracellular space can certainly be classified as necrotic (Raffray and Cohen 1997; Wyllie et al. 1980). Such cell death is usually a pathological event occurring in response to cell/tissue injury or environmental insults. On the other hand cell death which occurs in seemingly random fashion in isolated individual cells within healthy tissues, and more extensively at times during ontogeny, without triggering an inflammatory response, can be termed apoptotic (Ellis et al. 1991; Kerr 1971; Schwartzman and Cidlowski 1993). Apoptosis is an energy-requiring process that often involves altered expression of key cell proliferation and death-inducing genes. Sometimes, however, it can be initiated by receptors that feed directly into cascades that activate constitutive proenzymes sufficient to kill. Apoptotic cell death is responsible for most of the developmental tissue modeling and has been documented in several cases of physiological cell loss (Gosden and Spears 1997; Kerr et al. 1972; Prins and O’Rahilly 1997; Spelsberg et al. 1999; Ucker et al. 1994). Examples are the classical clonal deletion of T and B cells during negative selection associated with an immune response and the hormonally regulated cyclic regression of the uterine endometrium (Gosden and Spears 1997; Owen and Jenkinson 1992; Thompson 1994).
The significance of physiological apoptosis and cell turnover is apparent from the diseases associated with loss of such homeostatic regulation (Hetts 1998; Rudin and Thompson 1997). A defect in the apoptotic machinery has been implicated in autoimmune lymphoproliferative disorders (Abbas 1996; Van Parijs et al. 1996) and in cancer (Fisher 1994). Excessive or abnormally occurring apoptosis has been associated with neurodegenerative disorders (Bredesen 1995) including Alzheimer’s disease, and in stroke and myocardial infarction (Colucci 1996; Olivetti et al. 1997). Understanding the physiological regulation of apoptosis can potentially lead to new avenues for the diagnosis and treatment of a variety of diseases.
Two important aspects of apoptosis should be borne in mind as one reads this review. First, it is a fact that many compounds and circumstances can provoke apoptosis. Furthermore, a particular agent may produce apoptosis in a given cell or tissue, but may not in another, or in a given cell at one time during its life history but not at another. Whether a cell responds to such an agent by dying must therefore depend on the specific array of interactive molecular regulatory systems active in that cell at that time. To understand how any specific hormone regulates apoptosis and cell turnover, it will be necessary to understand more fully the details of how these systems work, interact, and are arrayed in the cells and tissues of interest. For no hormone or cell system is this presently known in full. The reader will see, therefore, many apparent gaps, contradictory results, and paradoxes in the information below. Nevertheless, general patterns can often be discerned, and the direction in which further work is needed can be seen.
The second important thing to remember is that while these many initiatory pathways all eventually lead to cell death, debate continues on the question of whether they all eventually initiate the identical “final common pathway,” or whether there are multiple systems which can be activated to produce non-inflammatory cell death. This report is not a proper place to evaluate this question, and we will just refer to cells dying non-necrotically as apoptotic, without prejudice as to the specific systems that carry out the process. Certainly several major molecular players involved in regulation and execution of cell death have been identified: caspases and other proteases, the Bcl-2 family of proteins, mitochondrial elements, for instance. These are referred to when appropriate.
The initiators of physiological cell turnover and apoptosis may include cellular environment, growth factors, cytokines and hormones, acting in autocrine, paracrine or endocrine fashion. Here, we will focus on the influence of the endocrine system in maintaining tissue homeostasis in a healthy adult human being. Continuous tissue renewal by balancing cell division and cell deletion is mediated by circulating levels of specific hormones (Kiess and Gallaher 1998; Martimbeau and Tilly 1997; Tenniswood et al. 1992; Thompson 1994). Additionally, hormonal availability also determines the expression of growth factors and cytokines, which modulate cell turnover in target tissues. Endocrine gland functions and secretions are also subject to regulation via apoptosis of hormone-synthesizing cells, as is observed in the adrenal gland when deprived of adrenocorticotropic hormone. Table 1 lists some of the proliferative and apoptotic actions of individual hormones in hormone-responsive tissues.
Table 1.
Endocrine regulation of cell turnover in various tissues
| Tissue | Hormone | Response | References |
|---|---|---|---|
| Thymus | Corticosteroids | Induce thymocyte apoptosis, influence positive selection |
Dougherty 1952; Goya and Bolognani 1999 |
| Thymic peptides | Affect thymic selection | ||
| Testis | Gonadotropins | Promote spermatogenesis; prevent germ cell apoptosis |
Means et al. 1976; Tapanainen et al. 1993 |
| Androgens | Prevent germ cell apoptosis | ||
| Prostate | Androgens | Stimulate epithelial cell proliferation, prevent apoptosis |
Kyprianou and Isaacs 1988; Rennie et al. 1989 |
| Glucocorticoids | Prevent epithelial cell apoptosis | ||
| Mammary gland |
Progesterone plus estrogen |
Stimulate cell proliferation in resting breast, prevent apoptosis |
Ferguson and Anderson 1981a; Atwood et al. 1995 |
| Prolactin | Stimulate epithelial cell proliferation; postlactation withdrawal induces apoptosis |
||
| Glucocorticoids | Stimulate epithelial cell proliferation | ||
| Ovary | GnRH | Inhibit follicle development; induce atresia | Hsueh et al. 1994 |
| Gonadotropins | Promote follicular growth, prevent atresia | ||
| Estrogens | Promote follicular growth | ||
| Androgens | Induce follicular atresia | ||
| Inhibin | Inhibit follicular degeneration; promote atresia | ||
| Activin | Promote follicular atresia | ||
| Uterus | Estrogen | Promote epithelial cell proliferation; prevent apoptosis |
Pollard et al. 1987; Terada et al. 1989 |
| Progesterone | Induce epithelial cell differentiation, prevent apoptosis | ||
| Bone | Estrogen/androgens | Prevent osteoblastogenesis and osteoclastogenesis, promote osteoclast apoptosis |
Spelsberg et al. 1999; Canalis 1996; Nijweide et al. 1986 |
| Glucocorticoids | Promote osteoblast apoptosis | ||
| Parathyroid hormone | Induce osteoclastogenesis and bone resorption | ||
| Calcitonin | Block bone resorption; decrease osteoclastogenesis | ||
| Adipose tissue |
Leptin | Block preadipocyte differentiation, induce adipocyte apoptosis |
Prins and O’Rahilly 1997; Qian et al. 1998a; Geloen et al. 1989a; Devenport et al. 1989 |
| Insulin | Promote preadipocyte replication and differentiation, prevent adipocyte apoptosis |
||
| Corticosteroids | Proadipogenic at low concentrations, antiadipogenic at high concentrations, by inducing leptin |
||
| GI tract | Somatostatin | Promote epithelial and stem cell apoptosis | Lehy et al. 1979 |
Role of glucocorticoids in thymocyte selection
In the process of developing and maintaining a normal immune system, T cells undergo a rigorous selection process in the thymus. A complex balance of factors, both extracellular and intracellular, leads to proper selection of mature thymic lymphocytes for a normal immune system. This selection is dependent on the avidity of the T-cell receptor (TCR) for autologous antigen presented on major histocompatibility complex (MHC) molecules (Jameson et al. 1995; Sprent et al. 1988). Those T cells that recognize self-antigens/MHC molecules with high avidity are eliminated by activation-induced apoptosis. Those that do not express properly rearranged TCR or whose TCRs do not recognize antigenic peptide-MHC complexes at all undergo default apoptosis (death by neglect). Positive selection requires the rescue and survival of those T cells with moderate avidity for self-antigen/MHC molecules (Jameson et al. 1995). It is not clear how the same signaling pathway, mediated by TCR, can lead to both survival and apoptosis. The role of other regulators, including hormonal signals, in thymocyte deletion is being actively investigated (Iwata et al. 1996; Jameson et al. 1995; Sprent et al. 1988; Thompson 1999). Thymocyte selection and clonal deletion occurs primarily in the immature cortical double-positive (DP) CD4+, CD8+ population. Double-negative CD4−, CD8− or mature single-positive medullary and peripheral thymocytes are relatively resistant (Fowlkes et al. 1988; Jameson et al. 1995; Sprent et al. 1988).
Glucocorticoids (GCs) have long been known to have profound effects on the immune system, a classical response being the thymic involution that promptly follows administration of pharmacological amounts of GCs (Dougherty 1952; Ingle 1940). Several studies have demonstrated that immature DP thymocytes are preferentially susceptible to GC-evoked apoptosis (Ucker et al. 1994; Zilberman et al. 1996). In conditions of physiological stress, increased circulating glucocorticoids lead to thymic involution (Tarcic et al. 1998). Thus the massive apoptotic cell death in the thymus that is associated with T-cell selection appears to be under the control of corticosteroid hormones. GC-evoked apoptosis is mediated by its receptor, the GR, and can be blocked by the specific GR inhibitor, RU 38486 (Thompson 1999; Thompson et al. 1995). In tissue culture, lymphoid cells selected for GC resistance often show loss of, or mutant, dysfunctional GR. Thymocytes of transgenic mice with deliberately mutated GR show in vitro GC resistance (Kellendonk et al. 1999; Reichardt et al. 1998). Mice expressing thymus-specific antisense GR transcripts (GR-TKO mice) exhibited loss of double-negative thymocytes, indicating the importance of GC in double-negative thymocyte survival and maturation to the DP stage (King et al. 1995).
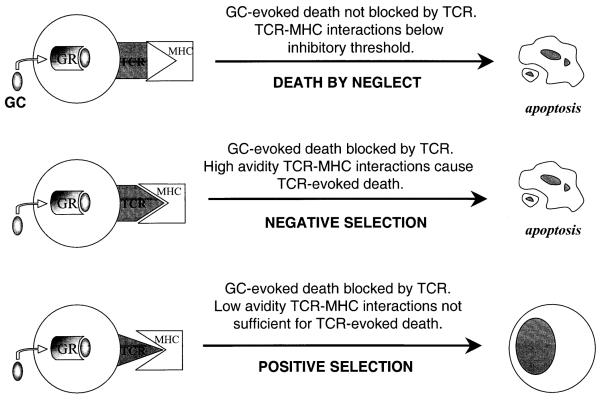
It has been proposed that GCs also are important mediators of death by neglect, which is associated with apoptotic deletion of thymocytes lacking functional TCR (Cohen and Duke 1984; Sprent et al. 1988; Vacchio et al. 1996). In support of this hypothesis, metyrapone, an inhibitor of GC synthesis, increased thymocyte recovery in fetal thymus organ cultures of thymi lacking functional MHC expression (King et al. 1995; Vacchio et al. 1996). In addition, GR-TKO mice are prone to thymomas derived from TCR minus cells, presumably owing to disabled apoptosis, and survival of thymocytes that normally would have been expunged via the death by neglect pathway (King et al. 1995; Vacchio et al. 1996). Positive selection refers to the survival of T cells selected due to their “intermediate” avidity for antigen/MHC molecules (Jameson et al. 1995). Thus the interaction of TCR with antigen-presenting MHC molecules provides a protective signal that excludes them from apoptosis, in essence rendering them corticosteroid resistant (Vacchio and Ashwell 1997; Vacchio et al. 1996). Negative selection involves thymocytes that have escaped GC-evoked apoptosis through the default pathway, but have such high TCR avidity for MHC antigen that it forces cell death (Sprent et al. 1988; von Boehmer and Kisielow 1990). The TCR avidity “threshold” that distinguishes between positive selection (low avidity) and negative selection (high avidity) is critical in maintaining normal T-cell selection. It has been suggested that this threshold is determined by GC levels and thus that inhibition of GC production lowers the threshold of avidity beyond which negative selection ensues (Iwata et al. 1991; Vacchio and Ashwell 1997; Vacchio et al. 1996). This is consistent with the hypothesis that GCs are required to antagonize TCR-mediated apoptosis to facilitate positive selection of low to moderately avid TCR. Indeed, GR-TKO mice have reduced thymic size owing primarily to loss of DP thymocytes that exhibit enhanced susceptibility to TCR-mediated apoptosis (King et al. 1995). Thus the balance between TCR and GC-mediated signaling determines the fate of a given thymocyte and the two antagonize each other’s apoptotic responses (Iwata et al. 1991; Wagner et al. 1996). Mutual antagonism between activation-induced and GC-induced apoptotic pathways has also been demonstrated in vitro in T-cell hybridomas (Zacharchuk et al. 1990). Further evidence for the critical interactions of the MHC-TCR system and GCs in the thymus comes from NOD mice (Atkinson and Leiter 1999), in which the normal MHC haplotype has been mutated. Thymocytes from these mice show GC resistance, and the distribution of thymocyte subtypes has been disrupted from failure of the normal selective turnover process. The effects of sex steroids and 1,25-dihydroxyvitamin D3 on this system highlight the complex interplay of hormones involved in the GC/MHC/TCR regulation of normal thymocyte apoptotic turnover (Casteels et al. 1998). While the full mechanisms of GC-mediated regulation of thymocyte apoptosis are still being investigated, a general model has developed (Fig. 1).
Fig. 1.
Glucocorticoid regulation of thymocyte selection: Glucocorticoids evoke thymocyte apoptosis unless TCR-MHC recognition antagonizes the apoptotic response. In the case of high-avidity TCR-MHC interactions, negative selection ensues, where thymocytes protected from glucocorticoid-evoked death undergo TCR-evoked apoptosis. When TCR-MHC interactions are moderate, glucocorticoid-evoked death is blocked; however, TCR does not evoke a death response, resulting in positive selection
The thymus itself is an endocrine organ and although the major source of circulating GCs is the adrenal gland, GCs are also produced locally in thymic epithelial cells, which are in direct contact with cortical DP immature thymocytes (Vacchio et al. 1994). In contrast to the adrenal gland, intrathymic corticosteroid production is maximal in the neonatal period, when thymocyte differentiation is at its peak (Vacchio et al. 1994). GC levels in the thymic environment are therefore sufficient to mediate a normal apoptotic signal in the neonate. In adults, adrenally derived steroids make up a significant fraction of the steroids contributing towards regulation of thymocyte numbers. The thymus also produces corticotropin-releasing factor (CRF) and adrenocorticotropic hormone (ACTH) (Aird et al. 1993; Batanero et al. 1992). It has been suggested that thymic CRF may have paracrine effects on immune function (Aird et al. 1993; Batanero et al. 1992). Thymosin, a peptide product of thymic stromal cells, abets thymocyte resistance to GCs and, along with a variety of thymic hormones, affects tissue turnover (Baumann et al. 1997; Goya and Bolognani 1999).
GC-evoked thymocyte apoptosis had been extensively studied for the elucidation of the molecular events preceding cell death (Distelhorst 1997; Thompson 1999). Studies of isolated thymocytes and leukemic lymphoblasts in culture undergoing GC-evoked T-cell death helped define the classical morphological features of apoptosis. Biochemically, these cells show altered expression of the oncogenes c-jun and c-myc, morphological changes, chromatin condensation, caspase and endonuclease activation, and DNA fragmentation (Cohen and Duke 1984; Martins and Aguas 1998; Thompson et al. 1999; Wyllie 1980). Regulation of thymocyte selection is influenced by other cellular factors and growth regulators, including intracellular levels of Ca2+, cAMP, and the various members of the protein kinase C family (Distelhorst 1997; Distelhorst and Dubyak 1998; McConkey et al. 1989, 1990). GC-evoked thymocyte apoptosis is associated with the elevation of cytosolic Ca2+ and the activation of nuclear Ca2+-dependent and -independent endonuclease(s), facilitating the accompanying DNA fragmentation (Cohen and Duke 1984; Distelhorst and Dubyak 1998; Wyllie 1980). Ca2+ may also exert its effects on thymocyte apoptosis via calmodulin-mediated activation of the Ca2+-dependent protein phosphatase, calcineurin (McConkey et al. 1992). Another second messenger, cAMP, promotes GC-mediated thymocyte apoptosis by as yet unidentified mechanisms (McConkey et al. 1993; Medh et al. 1998). Cyclic AMP alone can also evoke apoptosis of thymocyte cell lines in culture. Like GCs, cAMP antagonizes TCR-mediated negative selection (McConkey et al. 1990). The classic path of cAMP signaling is through activation of protein kinase A leading to modulation of gene transcription via phosphorylation of cAMP response element binding protein (CREB) (Montminy 1997). How this pathway interacts with the GC-mediated activation of GR-induced transcriptional responses is not well understood and is being intensely studied.
In normal mouse thymocytes, GC-evoked apoptosis is linked to the generation of ceramides via the sequential production or activation of phosphoinositide-specific phospholipase C (PI-PLC), diacyl glycerol (DAG), protein kinase C and acidic sphingomyelinase (aSMase) (Cifone et al. 1999). This process is dependent on GR, since RU 38486 can block Dex-induced aSMase production in correlation with inhibiting apoptosis. This pathway is also linked to activation of the JNK/SAPK cascade, as demonstrated in stress-induced apoptosis of leukemic U937 cells (Verheij et al. 1996). The aSMase-dependent apoptotic pathway is apparently distinct from the p53-dependent pathway, since p53 and aSMase knockout mice exhibit distinct tissue-specific sensitivity to radiation-induced apoptosis (Santana et al. 1996), although thymocytes of both strains of mice exhibit defects in stress-induced apoptosis when compared to their wild-type counterparts. Thymocytes from mice deficient in p53 exhibit normal sensitivity to GC-induced apoptosis; however, no reports are available on the susceptibility of aSMase-deficient thymocytes to GCs. Acid SMase activation appears to mediate downstream activation of caspases, since inhibition of intermediate steps in the pathway blocks caspase activation in parallel with prevention of apoptosis (Cifone et al. 1999).
Caspase activity can be detected in immature DP thymocytes that are subject to apoptotic selection, but not in double-negative or mature thymocyte populations (Jiang et al. 1999), implicating them as apoptotic effectors. In recent years, mice generated by targeted disruption of individual caspase genes have yielded important information on their role in normal thymocyte development and selection (Colussi and Kumar 1999). Mice deficient in caspase 1, 2, 3 or 9 exhibit normal development and distribution of thymocyte subpopulations (Colussi and Kumar 1999, and refs. therein), suggesting that either caspase activation is not essential for thymocyte selection, or that there is redundancy in their action. GC-evoked apoptosis of thymic T cells is dependent on caspase 9 but not on caspase 3 activation, since thymocytes from caspase 3 knockout mice are normal (Kuida et al. 1996), while those from caspase 9 knockout mice exhibit a delayed death response to Dex (Kuida et al. 1998; Hakem et al. 1998). Thymic cells of caspase 3 knockout mice are also susceptible to CD95- and TCR-mediated apoptosis. Corresponding peripheral thymocytes are resistant to both stimuli, suggesting a significant difference in the apoptotic pathways activated in cells from individual compartments (Colussi and Kumar 1999). Cell-specific differences in the recruitment of individual caspases have been reported in different subsets of isolated normal mouse T lymphocytes and T-cell lines when triggered to undergo apoptosis by the same stimulus (Sarin et al. 1996). In CEM cells, GC-evoked apoptosis is dependent on the activity of a member of the caspase 3 subfamily, but not on caspase 1 or its homologs, which are involved in Fas/Fas-L-mediated apoptosis (Geley et al. 1997, R.D. Medh, E.B. Thompson, unpublished observations). Thus, great diversity exists in the recruitment of individual caspases by various apoptotic pathways. Deciphering the significance of their selective induction will be instrumental in fully understanding the physiological basis of thymic selection.
Hormonal regulation of apoptosis in the testis and prostate
The prostate and testis involute upon deprivation of hormonal survival factors, e.g., upon castration or hypophysectomy (Kiess and Gallaher 1998; Tenniswood et al. 1992). A majority of reports describing involution of these tissues focus on apoptosis associated with non-physiological hormone ablation; however, in the intact animal there is some degree of spontaneous physiological cell turnover and apoptosis, which may also be hormonally regulated (Huckins 1978; Russell and Clermont 1976; Sinha Hikim and Swerdloff 1999).
Testis
Spermatogenesis occurs in the seminiferous tubules in the presence of testosterone secreted by the Leydig cells of the testis and gonadotropic hormones secreted by the anterior pituitary (Means et al. 1976; Steinberger 1971; Tapanainen et al. 1993). Beginning at puberty and continuing through life thereafter, the germinal epithelial cells continually proliferate and differentiate into spermatozoa in the presence of these hormones. During specific stages of spermatogenesis, however, there is significant loss of developing germinal cells, but not Leydig or Sertoli cells (Hsueh et al. 1996). It is estimated that only approximately 25% of spermatogonia actually develop into mature sperm (Blanco-Rodriguez and Martinez-Garcia 1996; Huckins 1978). In an early study, morphological features of physiological cell death during various stages of spermatogenesis in rats were similar to death associated with hypophysectomy, and included condensed chromosomes and nuclear distortion (Huckins 1978). The authors noted that in normal as well as hypophysectomized rats, the same types of germinal cells were undergoing death, but with a marked difference in frequency. Although these early studies report the death as necrotic, subsequent studies have determined that a majority of cell death is apoptotic (Blanco-Rodriguez and Martinez-Garcia 1996; Hsueh et al. 1996). The reason for spermatogonial degeneration is not well understood, but the ratio of spermatogonia to Sertoli cells may be a contributing factor, with survival of an optimal number of germ cells that can be sustained by Sertoli cells (Blanco-Rodriguez and Martinez-Garcia 1996; Huckins 1978). Loss of spermatogonia may also be a mechanism to eliminate cells with defective chromosomes (Blanco-Rodriguez and Martinez-Garcia 1996; Huckins 1978). Under conditions of gonadotropic hormone deprivation, such as after hypophysectomy, massive apoptosis reduces the pool of somatic Sertoli and Leydig cells as well as germ cells (Tapanainen et al. 1993). High expression of a gene known as SGP2 has been noted in such Sertoli cells (see “Prostate” below). Testicular involution can be prevented by gonadotropin replacement, supporting the hypothesis that follicle-stimulating hormone (FSH) and luteinizing hormone (LH) prevent cell death in addition to supporting testicular cell differentiation and proliferation (Hsueh et al. 1996; Tapanainen et al. 1993). Replenishment of testosterone by promoting Leydig cell function and survival also has a protective effect on germ cell survival (Tapanainen et al. 1993).
Germ cell apoptosis in the testis is regulated by the Fas-Fas ligand (FasL) system (Lee et al. 1997; Pentikainen et al. 1999). It has been proposed that Sertoli cells express FasL and cause apoptosis of Fas-expressing germ cells (Lee et al. 1997). Another study (Woolveridge et al. 1999) demonstrated colocalization of Fas and FasL in those spermatogonial cell types that undergo apoptosis following destruction of androgen-secreting Leydig cells by ethane dimethanesulfonate, although there was a decline in expression of Fas and FasL in testis by this treatment. Changes in expression were analyzed in a mixed cell population from testis by Western blotting; hence it is difficult to interpret how they relate to apoptosis of individual cells or types of cells. Interpretation of results from studies on Fas-deficient lpr (lymphoproliferation) mice is complicated by a leaky phenotype of these mice (Nagata and Suda 1995). Original studies had demonstrated that lpr mice undergo normal spermatogenesis and are fertile (Allen et al. 1990). More recently it has been demonstrated that although a majority of tissues in the lpr mouse lack Fas expression, testicular Fas expression is comparable to that in normal mice (Lee et al. 1997), explaining the maintenance of testicular homeostasis and fertility in lpr mice. Androgen deprivation caused an increase in Bax expression in the testicular cell population, implicating Bax in hormone-ablation triggered apoptosis. A marginal induction of Bcl-2 was also reported (Woolveridge et al. 1999); however, it may merely be a reflection of inadequacy of Western blotting in making quantitative interpretations. Transgenic mice expressing high levels of Bclxl or Bcl-2 fail to undergo the normal early wave of spermatogonial apoptosis, show highly abnormal spermatogenesis, and are sterile (Rodriguez et al. 1997). The protooncogene c-myc has also been implicated in spermatogonial apoptosis. Transgenic mice expressing the rat c-myc protooncogene were sterile due to defective spermatogonic differentiation and increased apoptosis (Suzuki et al. 1996). Thus, overlapping influences of multiple death/survival factors modulate spermatogonial development and maturation to maintain cellular homeostasis in the testis.
Prostate
Androgens stimulate cell proliferation and prevent apoptosis of prostate glandular epithelial cells. A low level of dynamic cell turnover occurs in the adult rat prostate such that a relatively constant number of cells is maintained (Isaacs 1984). Thus androgens function as survival factors, in the absence of which prostate epithelial cells undergo rapid apoptotic death, as is seen a few days after castration (English et al. 1989; Kyprianou and Isaacs 1988). This effect is far greater than can be explained by a mere inhibition of proliferation during the constant cellular turnover (Isaacs 1984). In addition, replacement of androgens to levels that are insufficient for a proliferative response is adequate to protect from castration-evoked cell death. Interestingly, after an initial exponential cell loss following castration, the fraction of cells that survived after 20 days could be induced by testosterone treatment to proliferate and reconstitute the gland. Subsequent successive hormone withdrawal and replacement resulted in similar repeated waves of apoptosis and residual cell survival, suggesting a key role for testosterone in prostate epithelial cell proliferation (Sandford et al. 1984).
Prostate involution following androgen withdrawal is a dynamic process involving derepression of genes normally repressed by circulating androgens, and the repression of normally expressed genes (Briehl and Miesfeld 1991; Guenette and Tenniswood 1994; Kiess and Gallaher 1998; Tenniswood et al. 1992). Some of these altered genes appear to be involved in prostatic cell turnover. Expression of the testosterone-repressed prostate marker 2 (TRPM-2) gene increased upon androgen withdrawal, in conjunction with the onset of cell death (Buttyan et al. 1989). Other instances of apoptosis are associated with overexpression of TRPM-2 homologs, including SGP-2 in Sertoli cells and clusterin in testis, suggesting an important role in apoptosis (Bettuzzi et al. 1989). Use of antiandrogens to block androgen action causes prostate cell apoptosis that is also associated with derepression of TRPM-2 expression (Leger et al. 1988). Treatment with high doses of corticosteroids protects from castration-induced prostate regression, and in parallel represses TRPM-2 expression (Rennie et al. 1989). While the physiological functions of TRPM-2 and its homologs are not clearly understood, this family of proteins have been implicated in spermatogenesis, regulation of lipid transport and modulation of immune function (Blaschuk et al. 1983; de Silva et al. 1990; Griswold et al. 1986).
Involution of the prostate is associated with destruction of the extracellular matrix, a process that is facilitated by synthesis and secretion of proteases (Tenniswood et al. 1992). Activity of plasminogen activators (PAs) has been shown to increase in association with prostate cell apoptosis (Rennie et al. 1984). Cortisol-mediated inhibition of PA activity by increasing PA inhibitor (PAI) expression has been suggested to play a role in its protective effect on prostate regression (Rennie et al. 1988). Another gene that is upregulated in temporal correlation with androgen depletion and apoptosis in prostate is TGFβ, and several studies suggest that TGFβ plays a role in inhibition of cell proliferation and activation of cell death (Ilio et al. 1995; Kyprianou and Isaacs 1989; Martikainen et al. 1990). TGFβ expression is maximal in rat ventral prostate 4 days after castration, approximately coinciding with the peaks of TRPM-2 expression and apoptosis (Kyprianou and Isaacs 1989). Postcastration androgen replacement reverts TGFβ levels to baseline (Isaacs 1984). In addition, TGFβ administration to non-castrated rats results in apoptosis of approximately 25% of prostatic glandular cells, even in the presence of physiological levels of androgens (Martikainen et al. 1990), implicating TGFβ as an intermediate in the apoptotic pathway. Derepression of TGFβ signaling has been implicated in castration-induced prostate tumor regression in vivo (Wikstrom et al. 1999) and suppression of tumorigenicity of prostate cancer cells in SCID mice (Guo and Kyprianou 1999). Despite the apoptogenic effect of TGFβ, its in vivo administration to castrated rats in conjunction with androgen replacement did not affect proliferative regrowth of prostatic glandular cells. Similarly, androgen-induced in vitro cell proliferation in organ culture was also not affected by TGFβ (Suzuki et al. 1996). In contrast, prostatic epithelial cell proliferation is inhibited in vitro in primary cultures (Rodriguez et al. 1997). In an attempt to reconcile the results, it has been hypothesized that stromal-derived growth factors, such as fibroblast growth factor (FGF) and epidermal growth factor (EGF), attenuate the inhibitory response of TGFβ on prostate cell proliferation in vivo and in organ cultures (Rodriguez et al. 1997; Suzuki et al. 1996). Overexpression of TGFβ in human prostate cancer LNCaP cells causes a concomitant upregulation of proapoptotic proteins Bax and caspase-1, and suppression of Bcl-2 (Guo and Kyprianou 1999). Other genes whose expression is increased during postcastration apoptosis of glandular epithelial cells include transglutaminase, poly (ADP-ribose) polymerase and hsp27 (Guenette and Tenniswood 1994). Several of these genes have been known to directly participate in the apoptotic process, in many diverse systems.
Apoptosis of prostatic epithelial cells is associated with characteristic chromatin condensation, endonuclease activation and increase in intracellular calcium (Kyprianou and Isaacs 1988). While apoptosis and cell turnover in the prostate may be limited in extent under physiological conditions, castration-induced apoptosis in this tissue provides several valuable hints for understanding the mechanism of hormonally regulated physiological apoptosis in other related systems (Tenniswood et al. 1992).
Hormonally regulated apoptosis in the mammary gland
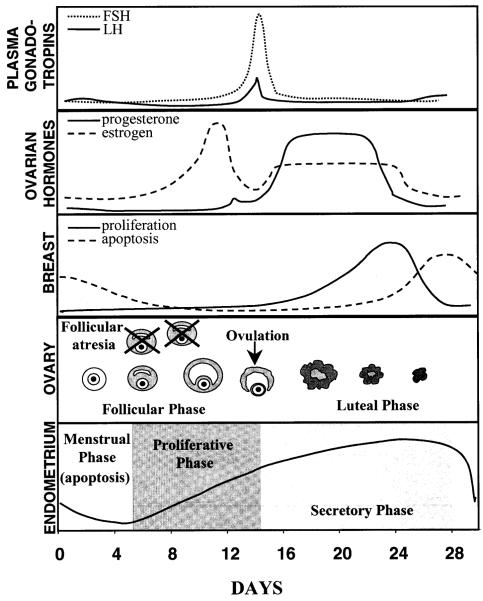
The mammary gland goes through various physiological stages, during initial development, puberty, pregnancy, maturation and weaning. Mammary epithelial cell proliferation and maintenance is regulated by the complex interactions of multiple hormones, including estrogens, progesterone, glucocorticoids, insulin and prolactin (Tenniswood et al. 1992). Unlike the prostate, regression of the mammary secretory epithelial cells following cessation of lactation represents a physiological process triggered by decreases in hormonal levels of pituitary prolactin, and placental estrogens, progesterone and somatotropin. In addition, a cyclic pattern of cell turnover has been observed in concordance with the ovarian cycle (Fig. 2) (Ferguson and Anderson 1981a, 1981b).
Fig. 2.
Correlation of hormonal secretions with proliferative and apoptotic events in human female reproductive tissues: Gonadotropins secreted by the anterior pituitary gland and ovarian hormones regulate cell turnover in the resting mammary gland, ovaries and the uterine endometrium (please refer to text for details)
Cell turnover in the non-lactating breast
Studies in human mammary tissue samples have suggested that, after development is complete, mammary epithelial cell proliferation and mitosis occur during the luteal phase of the ovarian cycle, coincident with the increased secretion of estrogen and progesterone from the ovaries (Ferguson and Anderson 1981a). Thus mitosis appears to be stimulated in the resting breast either by progesterone alone or by the synergistic effect of progesterone and estrogen. Estrogen alone does not seem to evoke proliferation in non-lactating mammary epithelial cells, since it does not occur in response to the surge of estrogen secretion during the preovulatory follicular phase. Mammary epithelial cell apoptosis peaks a few days after the proliferative peak, towards the end of the luteal phase (Ferguson and Anderson 1981a). This apoptotic peak coincides temporally with decreased secretion of estrogen and progesterone from the ovaries, as does endometrial apoptosis. A low level of apoptosis occurs in lobules throughout the breast (Ferguson and Anderson 1981a). Ultrastructural changes confirmed the apoptotic nature of this mammary epithelial cell death (Ferguson 1988; Ferguson and Anderson 1981b). Thus, normal non-lactating breast tissue undergoes dynamic and physiological cell turnover in a cyclic pattern in response to fluctuations in estrogen and progesterone secretions from the ovaries. This dynamic cellular replenishment plays an important role in maintenance of tissue architecture and function (Ferguson 1988).
Apoptosis in the postlactational mammary gland
During pregnancy the placental secretion of estrogen promotes proliferation and maturation of mammary ducts (Battersby and Anderson 1988). For this to occur, the hormonal background including progesterone, glucocorticoids, somatotropin, insulin and IGF-1 is important (Atwood et al. 1995; Plaut et al. 1993). Mammary gland differentiation is promoted by prolactin and antagonized by the counteracting effects of estrogens and progesterone. In whole organ cultures, combinations of insulin, aldosterone, hydrocortisone and prolactin have been shown to induce cell proliferation and differentiation into lobuloalveolar structures (Atwood et al. 1995). These hormones directly affect epithelial cell growth, and they alter expression of growth-promoting factors by surrounding myoepithelial cells. Epithelial cell proliferation also requires interactions with extracellular matrix components and with stromal cells (Barcellos-Hoff et al. 1989; Streuli and Bissell 1990). The marked tissue involution following weaning is due to apoptosis of the secretory epithelial cells of the breast (Strange et al. 1992; Walker et al. 1989). An important initiating event is the sharp decline in prolactin secretion. It has been proposed that, during lactation, mammary epithelial cells are terminally differentiated so as to require stimulation by lactogenic hormones for survival; lack of such a stimulation after weaning triggers apoptosis (Travers et al. 1996). Morphological features associated with mammary gland involution include chromatin condensation and formation of secretory vesicles containing lipid and proteins (Strange et al. 1992; Walker et al. 1989). Epithelial cell loss during weaning is accompanied by basement membrane degradation and activation of matrix metalloproteinase (Pullan et al. 1996). The synthesis and activation of PAs is also increased upon onset of weaning. PAs contribute towards extracellular matrix degradation via proteolytic cleavage of plasminogen to active plasmin, a potent serine protease (Pullan et al. 1996; Tenniswood et al. 1992). Other proteases implicated in destruction of the basement membrane during epithelial cell apoptosis include stromolysin, cathepsin D and collagenase (Basset et al. 1990; Lefebvre et al. 1992). These changes facilitate the extensive tissue remodeling associated with apoptosis of the postlactational breast. Similar activation of plasminogen activators and other proteases occurs in the prostate and other tissues where apoptosis is associated with basement membrane degradation. The expression of many other genes is altered during postlactational mammary gland involution. Expression of ornithine decarboxylase, a proliferative gene, is severely suppressed. At the same time, SGP-2/TRPM-2 is induced. As noted above, this gene has been implicated in the apoptotic death in the prostate and in vertebrate limb development (Guenette et al. 1994; Strange et al. 1992). Other genes induced in conjunction with mammary tissue regression are tissue transglutaminase and poly (ADP) ribose polymerase. Both are also implicated in prostate involution following castration and, as in the prostate, TGFβ expression is induced during mammary gland involution (Silberstein and Daniel 1987). By use plastic of implants, slowly released TGFβ has been shown to inhibit ductal growth in the mouse mammary gland (Silberstein et al. 1990). TGFβ expression is negatively regulated by estrogens and progesterone (Jeng and Jordan 1991; Knabbe et al. 1987). Thus several biochemical features of mammary cell apoptosis closely resemble those associated with apoptosis in the prostate (Tenniswood et al. 1992). These correlations enable identification of crucial proapoptotic genes such as TGFβ and TRPM-2, which may be effective in multiple tissues. However, there are significant differences. Induction of HSP27 expression is observed during prostate, but not mammary, epithelial cell apoptosis. Another significant difference between prostate and mammary tissue involution is the trapping of secretory vesicles in the mammary gland concomitant with other morphological features of apoptosis. In the prostate, secretory function ceases before manifestations of morphological features of apoptosis. These observations suggest tissue-specific variations in the cell death pathway, probably caused by selective expression of some genes.
Endocrine regulation of involution in the ovary and uterus
As in the prostate and testis, cells of the ovary and the uterus undergo death triggered by the loss of secretion of crucial hormonal survival factors. Unlike the male reproductive tissues, however, cyclic endocrine-dependent cell death in these female tissues is a normal physiological process closely linked to the cyclic fluctuations in hormonal secretions (Martimbeau and Tilly 1997). The phases of the female ovarian and menstrual cycles closely correlate with alterations in hormonal levels (Gosden and Spears 1997; Kiess and Gallaher 1998; Martimbeau and Tilly 1997) (Fig. 2).
Ovary
In the human ovary, only 0.1% of the approximately 400,000 ovarian follicles present at the onset of puberty undergo ovulation before the onset of menopause. The remaining 99.9% undergo degeneration (Hsueh et al. 1994, 1996; Tsafriri and Braw 1984). Follicular development in the ovaries is begun by 6–12 follicles but is completed only in one dominant follicle during each ovarian cycle. Ovulation of the dominant follicle requires a surge in LH and FSH secretion. The LH triggers granulosa cells of the dominant follicle to secrete progesterone and suppress estrogen secretion, initiating the luteal phase of the cycle. The remaining non-dominant follicles undergo atresia and degenerative death, mostly at the penultimate stage of follicle development (Amsterdam et al. 1998; Hsueh et al. 1994). A majority of atretic follicles exhibit DNA fragmentation and morphological features characteristic of apoptosis (Amsterdam et al. 1998; Hsueh et al. 1994). The remainder show more extreme forms of cell death, but this may only represent later stages, after the apoptotic event. The selective maturation of one follicle is regulated by tight control of hormonal and cytokine secretions. The dominant follicle outgrows the others because of its greater secretion of estrogen into its follicular fluid, providing a protective environment. Furthermore, an increase in FSH receptors on its granulosa cells accelerates growth by a feedforward mechanism (Hirshfield 1991; Hsueh et al. 1994). Increased estrogen from the dominant follicle depresses further FSH secretion from the anterior pituitary, contributing to atresia of non-dominant follicles (Hsueh et al. 1994). Blocking the preovulatory surge of gonadotropin secretion in rats promotes follicular atresia (Bill and Greenwald 1981), while exogenous gonadotropins have been shown to protect granulosa cells from degeneration (Chun et al. 1996). Increasing androgen relative to estrogen levels in the non-dominant follicles has also been shown to promote their apoptotic death (Billig et al. 1993; Carson et al. 1981). While estrogen has been shown to promote granulosa cell proliferation in rat ovaries (Richards 1980), androgens reverse the estrogen response and cause follicle degeneration as demonstrated in hypophysectomized rats (Hillier and Ross 1979) and in vitro culture models (Schomberg et al. 1976). Several reports indicate that gonadotropin-releasing hormone (GnRH) directly inhibits follicle differentiation and induces follicular atresia via specific receptors on granulosa and thecal cells (Billig et al. 1994; Hsueh and Jones 1981). Ovarian inhibin and activin modulate FSH secretion by the anterior pituitary, and have direct effects on follicular development and apoptosis (Ling et al. 1986; Vale et al. 1988). In turn gonadotropins induce inhibin production by granulosa cells in vitro (Bicsak et al. 1986). Injection of recombinant inhibin into the ovarian bursa of immature rats increases follicular development. In contrast, activin promotes follicular atresia (Woodruff et al. 1990). Thus, FSH, estrogens and inhibin act to proliferate and sustain cells, progesterone and LH mediate differentiation, while androgens, GnRH and activin promote apoptosis.
Intraovarian growth factors, including epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF), inhibit apoptosis by autocrine/paracrine mechanisms in in vitro models of preovulatory follicular growth (Luciano et al. 1994; Tilly et al. 1992). Insulin-like growth factor-1 (IGF-1) synergizes with FSH and LH in stimulating estrogen and progesterone production by granulosa cells, thereby functioning as a follicular survival factor (Chun et al. 1994). It has been postulated that FSH stimulates granulosa cells to secrete IGF-1, which in turn signals thecal cells to produce EGF and TGFα, thereby exerting an antiapoptotic response (Hsueh et al. 1994). In contrast, expression of TGFβ in hormone-deprived follicles may promote apoptosis (Martimbeau and Tilly 1997). Other pro- and antiapoptotic genes have been shown to affect follicular atresia. Notably, there are reports indicating that the bcl-2 gene family proteins play an important role in determining susceptibility of granulosa cells to apoptosis. Gonadotropins are known to promote the expression of the antiapoptotic members of the bcl-2 gene family (Johnson et al. 1996; Tilly et al. 1995). Mice deficient in the proapoptotic Bax protein exhibit enhanced follicular survival, and conversely Bax expression is induced in atretic granulosa cells (Martimbeau and Tilly 1997). Thus as in many systems of carefully regulated cell death, the balance of signals for and against initiation of cell death, superimposed on the levels of various intracellular Bcl-2 family proteins, determines the outcome.
Uterine endometrium
The cyclic pattern of estrogen and progesterone secretion from the ovaries regulates the cyclic phases of proliferation, secretion and menstruation in the uterine endometrium (Fig. 2) (Martin and Finn 1968; Sandow et al. 1979). Estrogen promotes epithelial cell proliferation in the uterine endometrium (Pollard et al. 1987). In the luteal phase of the ovarian cycle, progesterone secretion is predominant, promoting epithelial cell differentiation and initiation of the secretory phase of the endometrial cycle (Dallenbach-Hellweg 1988). During involution of the corpus luteum, when both estrogen and progesterone secretion are depressed, endometrial cells undergo rapid regression and epithelial cell death (Gosden and Spears 1997). Early studies on human and hamster uterine epithelium suggested that the endometrial involution occurs via apoptosis, with typical nuclear fragmentation, chromatin condensation and changes in cell shape (Hopwood and Levison 1976; Sandow et al. 1979). The stromal cells’ viability is not affected. More detailed studies repeatedly have confirmed the key role of the sex steroids in endometrial cell turnover. In the mouse uterus, 17β-estradiol treatment results in epithelial proliferation, while an inactive estrogen, estriol, causes epithelial cell loss via apoptosis, which can be prevented by progesterone and estrogen (Pollard et al. 1987). Progesterone has been reported to block 17β-estradiol-induced cell proliferation of epithelial cells in mouse uterine endometrium (Terada et al. 1989). In rabbit uterine epithelium, progesterone suppressed, while its antagonist RU 38486 induced, apoptosis (Rotello et al. 1992). In the mouse uterus, apoptotic cell death induced by estrogen withdrawal can be blocked by progesterone, estrogen or glucocorticoid hormones (Jo et al. 1993). These steroidal effects are mediated by altered expression of specific genes. TGFβ has been implicated as one of the gene products controlling the apoptotic process. In primary cultures of rabbit uterine epithelial cells, TGFβ inhibited cell proliferation and induced apoptosis, suggesting that it may also modulate endometrial regression in vivo (Rotello et al. 1991). Indeed, expression of TGFβ1 and TGFβ4 isoforms was more pronounced in the mid to late secretory and menstrual phases of the endometrial cycle, in comparison to the proliferative phase (Casslen et al. 1998; Tabibzadeh et al. 1998), implying progesterone dependence for their expression. Consistent with this implication, stimulation of proliferative phase endometrial explants with estrogen plus progesterone increased TGFβ1 expression (Casslen et al. 1998). Simply increasing the concentration of TGFβ may not be sufficient; an activation step may be required. It has been proposed that TGFβ1 is in its latent form until onset of the menstrual phase (Casslen et al. 1998), when activated TGFβ1 is known to induce apoptosis.
Several studies have also implicated the Bcl-2 family in regulation of cell viability in this tissue. Bcl-2 has been detected in glandular, stromal and myometrial cells of the human endometrium. In glandular cells Bcl-2 expression peaks in coincidence with the proliferative phase and shows only negligible expression in the secretory phase and during apoptosis (Gompel et al. 1994; Otsuki et al. 1994). This suggests that bcl-2 gene expression also may be under hormonal control, and may modulate apoptotic involution of the endometrium. Such regulation is somewhat unusual; Bcl-2 itself is often constitutive, while other family members show regulated expression. How the sex steroids regulate bcl-2 gene in one tissue and not another is not known. The progesterone and estrogen receptors also increased in abundance during the proliferative phase of the cycle in parallel with Bcl-2, consistent with a connection between hormonal activity and Bcl-2 expression (Otsuki et al. 1994). In rat uterine endometrium undergoing postimplantation decidualization, expression of Bcl-2 and its antagonistic protein, Bax, were regulated by estrogen and progesterone. Hormone injections in ovariectomized rats caused an induction of Bax and suppression of Bcl-2 expression (Akcali et al. 1996). These data suggest that Bcl-2 family proteins play a key role in mediating apoptosis of epithelial or stromal cells in the absence or presence, respectively, of blastocyst implantation.
Hormonal regulation of bone turnover
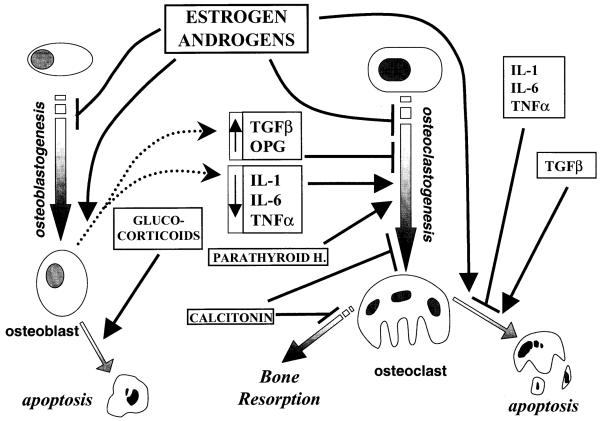
Bone remodeling is a process by which old bone is replaced by new, to regulate bone strength, shape, and repair. Remodeling is also important for the increase in bone mass associated with growth and is responsible for bone loss associated with old age (Vaughan 1981). In a normal adult the continual deposition and absorption of bone tissue occurs in discrete pockets called bone-remodeling units, where bone-forming osteoblasts and bone-resorbing osteoclasts are in dynamic equilibrium (Nijweide et al. 1986). These dual processes are tightly regulated by cytokines and hormones, especially estrogen and other sex steroids (Jilka 1998) (Fig. 3). Hormonal action on bone tissue is mediated via direct effects on osteoblast and osteoclast cells harboring specific hormone receptors, or via paracrine effects of hormonally regulated cytokine secretion by neighboring cells. For example, estrogen regulates bone turnover both by direct effects on osteoblast and osteoclast formation and function, and indirectly by regulating cytokine production (Spelsberg et al. 1999). Another relevant action of estrogen is to promote osteoclast apoptosis, thereby controlling the number of osteoclasts available for bone resorption (Kameda et al. 1995, 1997; Spelsberg et al. 1999). The reduction in circulating estrogens after menopause causes osteopenia and osteoporosis, due to excessive bone resorption not matched by an equivalent increase in new bone formation (Delmas et al. 1997; Robinson et al. 1997). In the mouse, ovariectomy stimulates osteoblastogenesis and osteoclastogenesis, while suppressing osteoclast apoptosis (Hughes et al. 1996; Jilka 1998; Jilka et al. 1992; Kimble et al. 1996). Although estrogen deficiency increases the differentiation of bone marrow progenitor cells into osteoclasts and osteoblasts, osteoclastogenesis predominates (Jilka et al. 1998a; Riggs et al. 1998). Since osteoblasts and stromal cells secrete cytokines necessary for osteoclastogenesis, even the increase in osteoblastogenesis contributes to osteoclast formation and overall bone resorption.
Fig. 3.
Hormonal regulation of bone resorption: Estrogens and androgens regulate osteoblastogenesis, osteoclastogenesis and osteoclast apoptosis. Osteoblast-derived cytokines, whose release is hormonally modulated, play a central role in osteoclastogenesis as well as osteoclast apoptosis. Parathyroid hormone and calcitonin have opposing effects on osteoclastogenesis. In addition, calcitonin blocks osteoclast bone-resorptive activity. Glucocorticoids promote osteoblast apoptosis
Estrogen-mediated regulation of osteoblast formation and activity
Osteoblastic cells are able to support osteoclastogenesis as is shown by the reduction in osteoclast cell number in mice that are genetically engineered to be defective in osteoblastogenesis (Jilka et al. 1996; Komori et al. 1997). Multiple reports have demonstrated the expression of ERs in osteoblasts, as well as ER upregulation during osteoblast differentiation (Bodine et al. 1998; Eriksen et al. 1988). In osteoblasts, stromal cells and macrophages, estrogen regulates the expression of certain cytokines, growth factors, transcription factors and bone matrix proteins. The production of osteoclastogenic cytokines IL-1, IL-6 and TNFα by osteoblasts is suppressed by estrogens; so during states of decreased estrogen availability synthesis is increased (Ishimi et al. 1990; Jilka 1998; Miyaura et al. 1995). These osteoclastogenic cytokines regulate one another; hence a small increase in certain ones has a stimulatory effect on others (Jilka 1998). Other factors from osteoblasts contribute to bone turnover as well. One factor is the TNF-related activation-induced cytokine (TRANCE/RANKL/OPGL). It is an inhibitory ligand for osteoprotegerin (OPG), a secreted protein which inhibits osteoclast formation and is implicated in the regulation and protection of bone mass (Fuller et al. 1998; Simonet et al. 1997). There are reports of estrogen-mediated upregulation of OPG synthesis and secretion by osteoblast cells (Hofbauer et al. 1998). Transgenic mice overexpressing OPG develop osteopetrosis, while OPG knockout mice have excessive osteoclast activity and are osteoporotic (Boyce et al. 1999). The synthesis of TGFβ, another osteoclastogenesis inhibitor, also is stimulated by estrogens in osteoblast cells (Finkelman et al. 1992). As in other tissues, TGFβ also induces osteoclast apoptosis, thus suppressing bone resorption. Thus, estrogens indirectly promote bone formation by stimulating osteoblastic production of proteins that promote bone formation/retention and repressing production of proteins that promote bone resorption.
Loss of osteoblasts occurs via apoptosis, which is mediated via the interplay between various hormonal and cytokine factors. The detailed map of these interactions is not yet available. IGF-I, IGF-II, insulin and bFGF have been shown to promote osteoblast proliferation in vitro (Hill et al. 1997), while serum deprivation or TNF administration each result in apoptosis (Jilka et al. 1998b). TGFβ and IL-6 are antiapoptotic for osteoblasts (Jilka et al. 1998b). This is especially interesting since TGFβ is generally proapoptotic in most tissues and also induces apoptosis of osteoclasts. The opposite actions of this vital cytokine on bone-forming and -resorbing cells demonstrate how precisely bone tissue turnover is regulated. Thus the net effect of TGFβ on the bone is growth promotion. Coordinate regulation of osteoblast apoptosis and formation has been proposed as a mechanism of regulating the pool of osteoblasts during bone healing (Landry et al. 1997).
Estrogen-mediated regulation of osteoclast differentiation and activity
As outlined above, osteoclastogenesis is modulated by a number of osteoblast- and stromal-cell-derived cytokines that promote osteoclast differentiation from hematopoietic progenitors (Martin and Ng 1994). Estrogen, in addition to regulating cytokine production, is thought to have some direct actions on osteoclast activity, presumably via the functional ER expressed in the cells (Kameda et al. 1997). ER-binding estrogen antagonists were able to reverse estrogen-mediated prevention of formation of bone resorption pits in vitro, suggesting that estrogen action was mediated via the ER (Shiokawa-Sawada et al. 1997). The recent development of an ER knockout mouse (ERKO) has highlighted the role of estrogens in bone tissue modeling. There are two isoforms of ER, α and β. ERαKO mice exhibit multiple effects on the skeletal system, with generally increased rates of bone resorption and remodeling in females (Couse and Korach 1999). However, male ERαKO mice exhibited a greater decrease in femoral bone density and mineral content than ERαKO females. Females showed a greater decrease in bone length and diameter. ERβ may modulate some of the actions of estrogen. Also, ERαKO mice exhibit elevated serum androgen levels, which may influence skeletal architecture and function.
Estrogen has been shown to inhibit osteoclast-induced bone resorption by enhancing expression of an IL-1 “decoy” receptor [IL-1R (type II)] that competes for ligand binding but does not properly transduce the signal. At the same time estrogen downregulates the IL-1-signaling receptor IL-1R1 (Sunyer et al. 1997). The decoy receptor blocks IL-1-mediated action on osteoclast bone resorption activity. Estrogen also has been shown to reduce osteoclast levels of the mRNAs for the bone-resorbing enzymes cathepsin K and carbonic anhydrase II (Kameda et al. 1997). By a direct action on them, estrogen also promotes osteoclast apoptosis, thereby reducing osteoclast numbers and subsequently decreasing bone resorption (Hughes and Boyce 1997; Hughes et al. 1996; Kameda et al. 1997; Lutton et al. 1996). In osteoblasts, in addition to suppressing IL-1, IL-6 and TNFα production, estrogen also induces production of TGFβ, which enhances estrogen-evoked osteoclast apoptosis in vivo (Hughes et al. 1996). In fact, TGFβ by itself can induce osteoclast apoptosis (Beaudreuil et al. 1995). Estrogens decrease osteoblastic production of IL-1, IL-6 and TNFα, all of which have antiapoptotic effects on osteoclasts (Hughes and Boyce 1997; Jilka 1998). All osteoclasts eventually die by apoptosis, but, by speeding up that process, estrogen and TGFβ shorten osteoclast life span and hence decrease their total numbers.
Thus, estrogen directly suppresses bone resorption by negatively affecting osteoblastogenesis and osteoclastogenesis and promoting osteoclast apoptosis. Additionally, estrogen induces osteoblastic secretion of cytokines that inhibit osteoclastogenesis (TGFβ and OPG), and suppresses production of osteoclastogenic cytokines (IL-1, IL-6 and TNFα).
Role of other hormones in bone remodeling
Loss of bone mass with advancing age occurs to varying extents in both men and women. In males, decline of gonadal androgen levels contributes to age-related loss of skeletal strength and bone mass (Stepan et al. 1989; Vanderschueren et al. 1992) due to increased bone remodeling and resorption. The increase in bone mass at puberty seen in males is a response to increased testicular androgen production (Spelsberg et al. 1999). Testosterone directly influences bone mass via the androgen receptors identified in osteoblasts and osteoclasts, and it can also be aromatized into estrogen for action via the ER (Bellido et al. 1995). Evidence for the direct action of androgens comes from use of the non-aromatizable analog, dehydrotestosterone, which can regulate cytokine expression to influence osteoclastogenesis and osteoclast apoptosis in a manner similar to estrogen (Bellido et al. 1995; Vanderschueren et al. 1992). Estrogen derived from testosterone also plays an important role in maintaining skeletal strength and integrity in males. In a young male estrogen deficiency associated with lack of aromatase activity resulted in decreased bone mass and skeletal dimensions (Morishima et al. 1997). Thus in males both estrogens and androgens are important physiological regulators of skeletal integrity.
Glucocorticoids induce a variety of responses in the skeletal system, the net effect being decreased bone formation and increased bone resorption; hence excessive amounts of GCs are known to induce osteoporosis (Canalis 1996; Reid 1997). Osteoblasts seem to be the major target of glucocorticoid action in the bone. GCs decrease osteoblast number and function by altering expression of several osteoblast-specific genes in the bone, including repression of osteocalcin (Meyer et al. 1997) and type I collagen (Delany et al. 1995a), and induction of collagenase (Delany et al. 1995b). GCs decrease IGF-I synthesis in osteoblasts, where IGF-I has been shown to have profound anabolic effects, inducing proliferation of preosteoblasts, increase in collagen I synthesis and decrease in collagenase. GCs inhibit TGFβ-mediated proliferative responses on osteoblasts. GCs have also been shown to promote osteoblast apoptosis in vivo (Weinstein et al. 1998). Aside from the direct effects of GCs on expression and function of bone-specific genes outlined above, GCs decrease intestinal Ca2+ absorption and increase its excretion, thus decreasing bone mineralization (Morris et al. 1990). GC-mediated bone resorption is postulated to be partially mediated by enhanced secretion or activity of parathyroid hormone (Hattersley et al. 1994), since parathyroidectomy relieves GC-induced bone resorption (Hattersley et al. 1994; Reid 1997). GCs decrease gonadotropin secretion, and hence androgen and estrogen levels (Lukert and Raisz 1990).
Parathyroid hormone plays an important role in bone remodeling. Hyperparathyroidism is associated with decreased bone formation and increased resorption of calcium and phosphate from bone (Nijweide et al. 1986; Vaughan 1981). Parathyroid hormone action is mediated via receptors on osteoblast cells, directly altering osteoblast-mediated cytokine production. Osteoblastic synthesis of osteoclastogenic factors such as IL-1, IL-6 and TNFα is increased (de la Mata et al. 1995; Grey et al. 1996; Silverberg and Bilezikian 1996), thereby promoting bone resorption. Parathyroid hormone is osteoclastogenic, the increase in osteoclast numbers contributing towards bone resorption. Calcitonin, from the thyroid gland, decreases the resorptive activity of osteoclasts and bone dissolution, and promotes calcium deposition in bone. Calcitonin also decreases the rate of osteoclastogenesis and osteoblastogenesis, thus reducing bone turnover (Nijweide et al. 1986; Selander et al. 1996; Vaughan 1981). Calcitonin modulates its actions by inactivation of osteoclasts by altering the cytoskeletal microfilament arrangement. Paradoxically, using an in vitro calvarial culture model system, one study demonstrated a reduction of osteoclast apoptosis in the presence of calcitonin and a significant increase in the presence of parathyroid hormone, suggesting that calcitonin-meditated suppression of bone resorption may be mediated by osteoclast inactivation rather than apoptosis (Selander et al. 1996).
Thus, multiple hormonal and cytokine factors contribute towards the regulation of bone turnover. Osteoblast and osteoclast apoptosis play an important role in bone loss, but osteoclast-mediated bone resorption is an additional mechanism of tissue turnover in the bone.
Hormonal control of adipose tissue turnover
The potential for tremendous changes in mass of adipose tissue is due to the ability to vary both the average adipose cell volume and the total cell number, both of which are regulated by endocrine and cytokine factors (Bjorntorp 1991). The volume of each adipocyte reflects the balance between lipogenesis and lipolysis (Arner 1988). Replication of preadipocytes and their differentiation into adipocytes (Van and Roncari 1978) is offset by ongoing apoptosis (Niesler et al. 1998; Prins et al. 1994b). A coordinate regulation of adipocyte volume and number has been suggested. In the event of energy intake exceeding usage, the resultant stored fat is first accommodated by an increase in volume of individual adipocytes until a threshold is reached, beyond which recruitment of preadipocytes to adipocytes further contributes to fat (Bjorntorp 1991). Hormonal regulation of the dynamic process of adipocyte turnover is mediated by signals originating from the hypothalamus, several traditional endocrine glands and the adipocytes themselves (Ailhaud et al. 1991; Prins and O’Rahilly 1997). Animal models of obesity, such as the obese (ob/ob) mouse, the diabetic (db/db) mouse, the fatty (fa/fa) rat, and others have provided insights into regulatory mechanisms that govern adipose tissue mass. We will focus here on endocrine mechanisms regulating adipocyte recruitment and deletion via apoptosis.
Leptin
Leptin, the product of the ob gene, is secreted by adipose cells, and acts on the hypothalamus and adipose tissue (Campfield et al. 1995; Rohner-Jeanrenaud and Jeanrenaud 1996). Leptin increases the basal metabolic rate and diminishes appetite, and therefore energy intake (Halaas et al. 1995; Pelleymounter et al. 1995; Qian et al. 1998a). Thus, leptin is the afferent signal from the adipose tissue to the hypothalamus, serving as a feedback regulator of adipose tissue mass. The ob/ob mouse does not produce functional leptin, and hence is obese. The hypothalamus expresses high levels of the leptin receptor, which is mutated in the db/db mouse and the fa/fa rat models of obesity. Through the leptin receptor, leptin inhibits secretion of neuropeptide Y (NPY) and increases production of the corticotropin-releasing factor (CRF), thereby modulating downstream effector pathways culminating in the utilization of body fat for energy production (York 1996). NPY is known to induce obesity by causing hyperphagia, stimulating insulin secretion and increasing the activity of lipogenic enzymes (Zarjevski et al. 1993). CRF has the opposite effect of inhibiting insulin secretion and food intake, preventing the development of obesity (Arase et al. 1989).
Leptin production by adipose cells is regulated by various endocrine factors, including insulin and corticosteroids (De Vos et al. 1995; Kolaczynski et al. 1996; Leroy et al. 1996). Both depletion of adipocyte lipid content via lipolysis and deletion of adipocyte cells via apoptosis contribute to leptin-induced reduction in fat content (Niesler et al. 1998; Prins et al. 1994a, 1994b). Adipocytes of mice administered intracerebroventricular leptin exhibit characteristic features of apoptosis, including internucleosomal DNA fragmentation, DNA strand breaks and a reduction in average adipocyte size (Qian et al. 1998a). In adipocytes, leptin regulates expression of various genes, including peroxisome proliferator-activated receptors (PPAR), uncoupling protein 2 (UCP-2) and TNFα (Qian et al. 1998b; Zhou et al. 1997). Adipose tissue predominantly expresses the PPARγ isoform, which is upregulated by leptin and suppressed during fasting. PPARγ plays a critical role in promoting adipocyte differentiation and lipid storage (Chawla and Lazar 1994; Chawla et al. 1994; Tugwood et al. 1992). Leptin induces PPARγ; thus the balance of actions maintains homeostatic control of adipose tissue mass (Qian et al. 1998a). Under certain circumstances PPARγ can be antiadipogenic. PPARγ phosphorylation inhibits adipogenesis (Hu et al. 1996) and heterodimers between PPARγ and the retinoid receptor RXR promote adipocyte apoptosis (Chawla and Lazar 1994). The role of other PPAR isoforms in adipose tissue turnover is being actively investigated. UCP-2 is also widely expressed in adipose tissue, where it is upregulated by both leptin and some PPAR ligands (Aubert et al. 1997; Zhou et al. 1997). UCP-2 overexpression in response to a fatty diet in A/J mice has been correlated with their resistance to obesity (Aubert et al. 1997). UCP-2 uncouples mitochondrial respiration from ATP (energy) generation, and thus mediates leptin-induced increase in energy expenditure. UCP-2 promotes free radical generation and by doing so may activate the apoptotic pathway in adipose tissue (Negre-Salvayre et al. 1997; Qian et al. 1998b).
The cytokine TNFα plays an important role in adipose tissue metabolism and apoptosis (Petruschke and Hauner 1993; Prins et al. 1998). After leptin administration, TNFα was observed to decrease in parallel with reduction in fat mass (Hotamisligil et al. 1995; Qian et al. 1998b). That TNFα might be a proximal cause of fat loss is supported by the observation that TNFα stimulates lipolysis by decreasing lipoprotein lipase activity, and increasing hormone-sensitive lipase activity (Argiles et al. 1997). In fact, in wasting syndromes, TNFα is greatly increased, causing the excessive loss of body fat (Argiles et al. 1997). Thus TNFα appears to function in an autocrine-negative feedback loop to tightly regulate the extent of fat accumulation (Argiles et al. 1997; Grunfeld et al. 1996; Hotamisligil et al. 1995; Prins and O’Rahilly 1997). Paradoxically, TNFα expression is markedly increased in adipocytes of obese individuals who exhibit insulin resistance (Kern et al. 1995), and may reflect obesity-associated TNFα resistance in parallel (Argiles et al. 1997). Analogous to insulin resistance, TNFα resistance is also associated with overproduction of the cytokine. Thus, the inability of TNFα to modulate lipolysis may be a key factor in the development of the obese phenotype. In addition to its lipolytic action on adipocytes, TNFα also blocks differentiation of human preadipocytes (Petruschke and Hauner 1993) and has been shown to induce preadipocyte and mature adipocyte apoptosis (Prins et al. 1997). This is consistent with the net effect of TNFα in both reducing adipocyte volume and number.
Insulin
Insulin is a lipogenic hormone (Denton et al. 1981; Geloen et al. 1989a), as is demonstrated by the loss of adipose tissue associated with insulin-deprived conditions such as untreated insulin-dependent diabetes mellitus. This loss of fat is due to a reduction of adipocyte number, in addition to lipolysis (Geloen et al. 1989b). An antiapoptotic action of insulin has been suggested in preserving adipocyte cell numbers (Prins and O’Rahilly 1997). Insulin also promotes preadipocyte replication and differentiation (Dixon-Shanies et al. 1975). Feedback regulation of adipose tissue mass occurs via insulin-mediated induction of leptin expression. Furthermore, long-term hyperinsulinemia sends antiadipogenic signals to the hypothalamus (Cusin et al. 1995; Dagogo-Jack et al. 1996). Insulin-mediated uptake of glucose in the intracellular compartment is mediated by specific transport proteins called glucose transporters. Among them, Glut 4 seems to have an important physiological role in regulating adipose tissue mass. Overexpression of Glut 4 in adipose tissue was associated with a huge increase in glucose uptake by adipocytes and a selective increase in adipocyte cell number rather than cell size (Shepherd et al. 1993). This suggests that glucose transport may affect adipocyte proliferation and differentiation. Thus insulin has a dual action on adipogenesis: a centrally regulated increase in lipogenesis and hence adipocyte cell volume, and a Glut-4-mediated increase in adipose cell recruitment and hence cell number.
Corticosteroids
Many studies suggest that adrenal glucocorticoids are necessary for development of obesity. The classical syndrome of corticosteroid excess (Cushing’s syndrome) is associated with a peculiar pattern of adipose tissue gain, predominantly in the visceral compartment (Rebuffe-Scrive et al. 1988). In contrast, Addison’s disease, characterized by diminished corticosteroid secretion, is associated with a loss of adipose tissue. In the brain, corticosteroid binding to both mineralocorticoid (type I) and glucocorticoid (type II) receptors plays an important role in regulation of food intake, and CNS true glucocorticoid receptor activity is required for fat accumulation (Santana et al. 1995). Contrasting results have been obtained in a transgenic mouse model in which antisense GR was selectively expressed in neural tissues, to reduce GR levels there (Richard et al. 1993). The transgenic mice developed obesity, implying that GCs may have an antiadipogenic effect in normal animals. However, residual GR expression, rather than complete lack of expression, in such a transgenic antisense model may be sufficient to mediate some of the actions of GC. Alternatively, it has been proposed that GR overexpression in peripheral tissues, rather than neural-specific antisense GR expression, mediates the obese response (York 1996). Further debate on the role of GR in regulation of adipose tissue mass arises from studies in normal rats. In lean rats, physiological concentrations of corticosteroids were found to promote fat accumulation, while pharmacological levels caused a decrease in adipose mass (De Vos et al. 1995; Devenport et al. 1989). Another study demonstrated that the dual action was contributed to by opposing responses from type I and type II steroid receptors, with aldosterone (a type I receptor ligand) causing an increase, and dexamethasone (a type II receptor ligand) causing a decrease in body weight gain and caloric intake. This is consistent with the saturation of type I receptors at low corticosteroid concentrations, mediating an anabolic response. Higher corticosteroid concentrations cause progressive occupation of the type II receptors, whose catabolic effects mask the modest anabolic effects mediated by the type I receptors. In the GR knockout animals, decreased CNS expression of these type II receptors could account for the obesity. Clearly, many discrepancies regarding the role of corticosteroids in adipose tissue regulation need reconciliation. All of the observations presented here suggest that alterations in normal GC signaling cause overlapping effects within the CNS and peripheral tissues, resulting in paradoxical effects on overall fat accumulation. It is clear that GCs generally have a permissive effect for fat accumulation. The precise mechanism of the adipogenic activity of physiological concentrations of corticosteroids is not fully understood, although it includes effects on preadipocyte differentiation and lipogenesis (Hauner et al. 1989; Ottosson et al. 1994).
In considering specific mechanisms, GCs positively regulate insulin secretion and are known to restore hypersecretion of insulin in adrenalectomized fa/fa rats (Stubbs and York 1991). GCs also synergize with insulin in upregulation of Glut 4, another adipogenic factor (Hauner et al. 1989). Pharmacological levels of GCs induce leptin production (De Vos et al. 1995; Devenport et al. 1989). Leptin stimulates production of CRF and decreases NPY synthesis (York 1996). Administration of CRF at doses that do not activate the pituitary-adrenal axis has been shown to reverse obesity, and decrease food intake and insulin production in fa/fa rats (Arase et al. 1989; Rohner-Jeanrenaud et al. 1989; York 1996), suggesting that CRF affects fat homeostasis by mechanisms independent of its stimulation of the synthesis of adrenal glucocorticoids. Transgenic mice overexpressing CRF and exhibiting elevated levels of hypothalamic-pituitary-adrenal activity develop obesity owing to increased corticosteroid production. Thus it is hypothetically possible that a deleterious loop could occur where an increase in GCs causes an increase in leptin production and a subsequent increase in CRF, which further enhances GC production.
Regulation of cell turnover in the gastrointestinal tract
It is clear that apoptosis of intestinal epithelial cells plays a fundamental role in maintenance of normal gut function, and that abnormalities of apoptosis contribute to some gastrointestinal diseases. The epithelium of the small intestine consists of numerous villi extending out into the lumen from pits or crypts. Stem cells close to the base of the crypt are the source of constant cell renewal, facilitating cellular turnover and replacement of dying cells (Potten 1998; Potten et al. 1997). The villi are lined by epithelial cells which form in the crypts, and as they mature progress upward along the villus. These cells undergo rapid turnover, and when eradicated are lost into the intestinal lumen (Jones and Gores 1997; Potten 1992). The average life span of an epithelial cell in this highly proliferative tissue is 3–5 days (Potten 1992). It has been suggested that, at the end of their migratory path, all elimination of epithelial cells at the luminal end of the villi occurs via apoptosis. The rapidity of the process has made it difficult to estimate whether apoptosis occurs only at the tips of the villi (Jones and Gores 1997; Pritchard and Watson 1996). However, there is strong evidence demonstrating apoptosis of stem cells within the crypts as well, where about 10% of the cells have been shown to undergo apoptosis (Potten 1998; Potten et al. 1997). Consistent with the observation of apoptosis in stem cells of the small intestinal crypts, they express Bax, which may contribute to the low incidence of cancers of the small intestine (Jones and Gores 1997). The proapoptotic protein Bak has been shown to colocalize with regions of epithelial cell apoptosis in human intestinal epithelium, and Bak induction correlates with apoptosis in human and rat intestinal cell lines (Moss et al. 1996).
Colonic epithelial cells also have a rapid rate of turnover, and there too the high proliferative index is balanced by an equally high rate of cell death, which is believed to occur along the luminal surface via apoptosis (Jones and Gores 1997). It has been demonstrated that epithelial cells in the colonic lumen express the proapoptotic Bax protein while those in the crypts are rich in Bcl-2 (Hockenbery et al. 1991; Krajewski et al. 1994). In addition, colonic stem cells express Bcl-2. Bcl-2 has been implicated as a contributor to high rates of colonic cancer (Jones and Gores 1997; Krajewski et al. 1994).
Physiological mediators of intestinal apoptosis
Most studies thus far have focussed on establishing that apoptosis is indeed the mechanism of cell death during normal gastrointestinal cell turnover. Very few studies have addressed the issue of the biological mediators of physiological apoptosis. Candidates are plentiful, among the various growth factors, cytokines and dietary factors that mediate gastrointestinal cell proliferation and apoptosis (Goodlad and Wright 1990). An inverse correlation has been established between intestinal epithelial cell apoptosis and glutathione (GSH) levels. Cells of the villus tips in monkey intestine with a high apoptotic index exhibited greatly reduced levels of GSH (Madesh et al. 1999). Luminal enterocyte cells contain oxidants and decreased mitochondrial-reducing activity. This combination would put them at risk for apoptosis, since mitochondrial-reducing capability is vital for preservation of mitochondrial function and inhibition of apoptotic events.
Hormones and paracrines secreted by the intestinal enteroendocrine cells and pancreatic cells also mediate epithelial and stem cell turnover. Overexpression of growth hormone, glucagon-like peptide-2 (GLP-2) or insulin-like growth factor promotes intestinal cell proliferation in mice (Tsai et al. 1997; Ulshen et al. 1993). In addition to stimulating epithelial cell proliferation and intestinal growth, GLP-2 increases villus height and prevents enterocyte apoptosis (Munroe et al. 1999; Tsai et al. 1997). The cytokine IL-6 has been shown to prevent apoptosis of intestinal enterocytes induced by hemorrhage by increasing expression of enterocyte Bcl-2 (Rollwagen et al. 1998). Enterocyte apoptosis associated with adaptation to partial gut resection can be prevented by EGF-EGF receptor complex (Helmrath et al. 1998). Rat small intestinal and human colonic cell lines were stimulated to proliferate in vitro in the presence of EGF-1 and insulin (Bjork et al. 1993). TGFβ has been shown to induce epithelial cell apoptosis directly and expression of TGFβ has been localized to the tip of the villus (Barnard et al. 1989). The proliferation of rat intestinal epithelial cells in culture is significantly inhibited upon expression of TGFβ. Resistant cells that continue to grow in the presence of TGFβ have suppressed TGFβ receptors and increased cyclooxygenase-2 (Cox-2) activity (Sheng et al. 1999). In mice colonic eicosanoids are well-known regulators of cell proliferation. Overexpression of Cox-2 with subsequent increase in prostaglandin E2 production has been reported in colon cancers, suggesting a role in inducing cell proliferation or inhibiting apoptosis (Sano et al. 1995). Cox-2 inhibitors have been shown to induce apoptosis in murine colon cancer cell lines (Erickson et al. 1999). Cox-2 overexpression is associated with an induction of Bcl-2 and suppression of cyclin D1 and of TGFβ receptors.
Some enteroendocrine hormones appear involved in controlling apoptosis, while others are not. Injections of gastrin did not significantly affect cell proliferation of small intestinal epithelial cells, but stimulated cell proliferation in the colon of rats (Fatemi et al. 1984). Somatostatin, on the other hand, had an inhibitory effect on DNA synthesis of gastrointestinal stem cells in vivo in rats (Lehy et al. 1979) and rat intestinal epithelial cells in vitro (Bjork et al. 1993). Somatostatin and its analogs have been shown to induce enterocyte apoptosis and impair intestinal regeneration after resection in rabbits (Thompson 1998, 1999). Apoptosis occurs in both crypt and villus compartments and is associated with decrease in crypt depth (Thompson 1998). Somatostatin has been used as an antitumor agent owing to its proapoptotic activity (Boros et al. 1998; Imam et al. 1997). Although these reports do not necessarily implicate a role for enteroendocrine hormones in physiological cell turnover and apoptosis, they provide a basis for future investigations.
Conclusions
In this review we have attempted to provide a consolidated overview of the hormonal regulation of physiological cell turnover and maintenance of tissue homeostasis. Part of this regulation occurs by control of apoptosis, an integral component of body physiology, not only during development, but also in preserving health in adults. Either too much or too little apoptosis has been implicated in several diseases. Apoptosis has a distinct and unique role to play in each tissue, and endocrine, paracrine and autocrine factors ensure tight regulation of the process. Some tissues such as the uterine endometrium and postlactational mammary gland undergo massive tissue involution via apoptosis. These processes are an integral part of normal reproductive physiology and are under rigorous endocrine regulation. In other tissues such as the gut, the process of apoptotic elimination of old cells and replacement with new ones is a constant event to preserve tissue function. Though subtle in appearance, such apoptosis is critically important for normal function. In the thymus, apoptosis is the basis of elimination of all but those thymocytes that are capable of normal function. Selection of thymocyte repertoire also involves intricate hormonal and TCR-mediated regulation. Apoptosis in the bone and adipose tissue is regulated by a complex set of endocrine and cytokine factors, and occurs in seemingly random cells. Bone and adipose cells are long lived and the extent of cell turnover generally determines the functional integrity and mass of the tissue.
In spite of the varied physiological roles of apoptosis in individual tissues, the general morphological and molecular features of dying cells are remarkably similar. The slight but distinctive differences in these features between tissues and cell types reflect differences in the pathways causing death, or in the makeup of cells responding to a common pathway. Intracellular apoptosis-inducing proteins such as the caspases, other proteases, TRPM-2 and Bax as well as antiapoptotic factors such as Bcl-2 seem to play an important role in multiple tissues during the commitment and execution phases of the apoptotic process. Hormonal factors, and cytokines such as TGFβ on the other hand, have highly tissue specific and sometimes opposite roles in different tissues, presumably due to their specific expressed network of genes. For example, estrogen has a proliferative effect in multiple tissues, including mammary gland, endometrium and bone. Glucocorticoids are proapoptotic for some thymic lymphocytes, yet protective to others and are generally antiapoptotic in the mammary gland and adipose tissue. The opportunity to answer the precise controls over cellular proliferation and apoptosis holds promise for future therapeutic approaches to combat various diseases.
References
- Abbas AK. Die and let live: eliminating dangerous lymphocytes. Cell. 1996;84:655–657. doi: 10.1016/s0092-8674(00)81042-9. [DOI] [PubMed] [Google Scholar]
- Ailhaud G, Amri E, Bardon S, Barcellini-Couget S, Bertrand B, Catalioto RM, Dani C, Doglio A, Forest C, Gaillard D. Growth and differentiation of regional adipose tissue: molecular and hormonal mechanisms. Int J Obes. 1991;15(Suppl 2):87–90. [PubMed] [Google Scholar]
- Aird F, Clevenger CV, Prystowsky MB, Redei E. Corticotropin-releasing factor mRNA in rat thymus and spleen. Proc Natl Acad Sci U S A. 1993;90:7104–7108. doi: 10.1073/pnas.90.15.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akcali KC, Khan SA, Moulton BC. Effect of decidualization on the expression of bax and bcl-2 in the rat uterine endometrium. Endocrinology. 1996;137:3123–3131. doi: 10.1210/endo.137.7.8770938. [DOI] [PubMed] [Google Scholar]
- Allen RD, Marshall JD, Roths JB, Sidman CL. Differences defined by bone marrow transplantation suggest that lpr and gld are mutations of genes encoding an interacting pair of molecules. J Exp Med. 1990;172:1367–1375. doi: 10.1084/jem.172.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A, Dantes A, Hosokawa K, Schere-Levy CP, Kotsuji F, Aharoni D. Steroid regulation during apoptosis of ovarian follicular cells. Steroids. 1998;63:314–318. doi: 10.1016/s0039-128x(98)00016-6. [DOI] [PubMed] [Google Scholar]
- Arase K, Shargill NS, Bray GA. Effects of corticotropin releasing factor on genetically obese (fatty) rats. Physiol Behav. 1989;45:565–570. doi: 10.1016/0031-9384(89)90074-7. [DOI] [PubMed] [Google Scholar]
- Argiles JM, Lopez-Soriano J, Busquets S, Lopez-Soriano FJ. Journey from cachexia to obesity by TNF. FASEB J. 1997;11:743–751. doi: 10.1096/fasebj.11.10.9271359. [DOI] [PubMed] [Google Scholar]
- Arner P. Control of lipolysis and its relevance to development of obesity in man. Diabetes Metab Rev. 1988;4:507–515. [PubMed] [Google Scholar]
- Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: as good as it gets? Nat Med. 1999;5:601–604. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Ikeda M, Vonderhaar BK. Involution of mouse mammary glands in whole organ culture: a model for studying programmed cell death. Biochem Biophys Res Comm. 1995;207:860–867. doi: 10.1006/bbrc.1995.1265. [DOI] [PubMed] [Google Scholar]
- Aubert J, Champigny O, Saint-Marc P, Negrel R, Collins S, Ricquier D, Ailhaud G. Up-regulation of UCP-2 gene expression by PPAR agonists in preadipose and adipose cells. Biochem Biophys Res Comm. 1997;238:606–611. doi: 10.1006/bbrc.1997.7348. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard JA, Beauchamp RD, Coffey RJ, Moses HL. Regulation of intestinal epithelial cell growth by transforming growth factor type beta. Proc Natl Acad Sci USA. 1989;86:1578–1582. doi: 10.1073/pnas.86.5.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset P, Bellocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, Podhajcer OL, Chenard MP, Rio MC, Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990;348:699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- Batanero E, de Leeuw FE, Jansen GH, van Wichen DF, Huber J, Schuurman HJ. The neural and neuro-endocrine component of the human thymus. II. Hormone immunoreactivity. Brain Behav Immun. 1992;6:249–264. doi: 10.1016/0889-1591(92)90047-r. [DOI] [PubMed] [Google Scholar]
- Battersby S, Anderson TJ. Proliferative and secretory activity in the pregnant and lactating human breast. Virchows Archiv A Pathol Anat Histopathol. 1988;413:189–196. doi: 10.1007/BF00718610. [DOI] [PubMed] [Google Scholar]
- Baumann CA, Badamchian M, Goldstein AL. Thymosin alpha 1 antagonizes dexamethasone and CD3-induced apoptosis of CD4+ CD8+ thymocytes through the activation of cAMP and protein kinase C dependent second messenger pathways. Mech Ageing Dev. 1997;94:85–101. doi: 10.1016/s0047-6374(96)01860-x. [DOI] [PubMed] [Google Scholar]
- Beaudreuil J, Mbalaviele G, Cohen-Solal M, Morieux C, De Vernejoul MC, Orcel P. Short-term local injections of transforming growth factor-beta 1 decrease ovariectomy-stimulated osteoclastic resorption in vivo in rats. J Bone Miner Res. 1995;10:971–977. doi: 10.1002/jbmr.5650100619. [DOI] [PubMed] [Google Scholar]
- Bellido T, Jilka RL, Boyce BF, Girasole G, Broxmeyer H, Dalrymple SA, Murray R, Manolagas SC. Regulation of interleukin-6, osteoclastogenesis, and bone mass by androgens. The role of the androgen receptor. J Clin Invest. 1995;95:2886–2895. doi: 10.1172/JCI117995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettuzzi S, Hiipakka RA, Gilna P, Liao ST. Identification of an androgen-repressed mRNA in rat ventral prostate as coding for sulphated glycoprotein 2 by cDNA cloning and sequence analysis. Biochem J. 1989;257:293–296. doi: 10.1042/bj2570293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicsak TA, Tucker EM, Cappel S, Vaughan J, Rivier J, Vale W, Hsueh AJ. Hormonal regulation of granulosa cell inhibin biosynthesis. Endocrinology. 1986;119:2711–2719. doi: 10.1210/endo-119-6-2711. [DOI] [PubMed] [Google Scholar]
- Bill CHD, Greenwald GS. Acute gonadotropin deprivation. I. A model for the study of follicular atresia. Biol Reprod. 1981;24:913–921. doi: 10.1095/biolreprod24.4.913. [DOI] [PubMed] [Google Scholar]
- Billig H, Furuta I, Hsueh AJ. Estrogens inhibit and androgens enhance ovarian granulosa cell apoptosis. Endocrinology. 1993;133:2204–2212. doi: 10.1210/endo.133.5.8404672. [DOI] [PubMed] [Google Scholar]
- Billig H, Furuta I, Hsueh AJ. Gonadotropin-releasing hormone directly induces apoptotic cell death in the rat ovary: biochemical and in situ detection of deoxyribonucleic acid fragmentation in granulosa cells. Endocrinology. 1994;134:245–252. doi: 10.1210/endo.134.1.8275940. [DOI] [PubMed] [Google Scholar]
- Bjork J, Nilsson J, Hultcrantz R, Johansson C. Growth-regulatory effects of sensory neuropeptides, epidermal growth factor, insulin, and somatostatin on the non-transformed intestinal epithelial cell line IEC-6 and the colon cancer cell line HT 29. Scand J Gastroenterol. 1993;28:879–884. doi: 10.3109/00365529309103129. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. Adipose tissue distribution and function. Int J Obes. 1991;15(Suppl 2):67–81. [PubMed] [Google Scholar]
- Blanco-Rodriguez J, Martinez-Garcia C. Spontaneous germ cell death in the testis of the adult rat takes the form of apoptosis: re-evaluation of cell types that exhibit the ability to die during spermatogenesis. Cell Prolif. 1996;29:13–31. doi: 10.1111/j.1365-2184.1996.tb00091.x. [DOI] [PubMed] [Google Scholar]
- Blaschuk O, Burdzy K, Fritz IB. Purification and characterization of a cell-aggregating factor (clusterin), the major glycoprotein in ram rete testis fluid. J Biol Chem. 1983;258:7714–7720. [PubMed] [Google Scholar]
- Bodine PV, Henderson RA, Green J, Aronow M, Owen T, Stein GS, Lian JB, Komm BS. Estrogen receptor-alpha is developmentally regulated during osteoblast differentiation and contributes to selective responsiveness of gene expression. Endocrinology. 1998;139:2048–2057. doi: 10.1210/endo.139.4.5897. [DOI] [PubMed] [Google Scholar]
- Boros LG, Brandes JL, Yusuf FI, Cascante M, Williams RD, Schirmer WJ. Inhibition of the oxidative and nonoxidative pentose phosphate pathways by somatostatin: a possible mechanism of antitumor action. Med Hypotheses. 1998;50:501–506. doi: 10.1016/s0306-9877(98)90271-7. [DOI] [PubMed] [Google Scholar]
- Boyce BF, Hughes DE, Wright KR, Xing L, Dai A. Recent advances in bone biology provide insight into the pathogenesis of bone diseases. Lab Invest. 1999;79:83–94. [PubMed] [Google Scholar]
- Bredesen DE. Neural apoptosis. Ann Neurol. 1995;38:839–851. doi: 10.1002/ana.410380604. [DOI] [PubMed] [Google Scholar]
- Briehl MM, Miesfeld RL. Isolation and characterization of transcripts induced by androgen withdrawal and apoptotic cell death in the rat ventral prostate. Mol Endocrinol. 1991;5:1381–1388. doi: 10.1210/mend-5-10-1381. [DOI] [PubMed] [Google Scholar]
- Buttyan R, Olsson CA, Pintar J, Chang C, Bandyk M, Ng PY, Sawczuk IS. Induction of the TRPM-2 gene in cells undergoing programmed death. Mol Cell Biol. 1989;9:3473–3481. doi: 10.1128/mcb.9.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Canalis E. Clinical review 83: mechanisms of glucocorticoid action in bone: implications to glucocorticoid-induced osteoporosis. J Clin Endocrinol Metabol. 1996;81:3441–3447. doi: 10.1210/jcem.81.10.8855781. [DOI] [PubMed] [Google Scholar]
- Carson RS, Findlay JK, Clarke IJ, Burger HG. Estradiol, testosterone, and androstenedione in ovine follicular fluid during growth and atresia of ovarian follicles. Biol Reprod. 1981;24:105–113. doi: 10.1095/biolreprod24.1.105. [DOI] [PubMed] [Google Scholar]
- Casslen B, Sandberg T, Gustavsson B, Willen R, Nilbert M. Transforming growth factor beta1 in the human endometrium. Cyclic variation, increased expression by estradiol and progesterone, and regulation of plasminogen activators and plasminogen activator inhibitor-1. Biol Reprod. 1998;58:1343–1350. doi: 10.1095/biolreprod58.6.1343. [DOI] [PubMed] [Google Scholar]
- Casteels KM, Gysemans CA, Waer M, Bouillon R, Laureys JM, Depovere J, Mathieu C. Sex difference in resistance to dexamethasone-induced apoptosis in NOD mice: treatment with 1,25(OH)2D3 restores defect. Diabetes. 1998;47:1033–1037. doi: 10.2337/diabetes.47.7.1033. [DOI] [PubMed] [Google Scholar]
- Chawla A, Lazar MA. Peroxisome proliferator and retinoid signaling pathways co-regulate preadipocyte phenotype and survival. Proc Natl Acad Sci U S A. 1994;91:1786–1790. doi: 10.1073/pnas.91.5.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Schwarz EJ, Dimaculangan DD, Lazar MA. Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology. 1994;135:798–800. doi: 10.1210/endo.135.2.8033830. [DOI] [PubMed] [Google Scholar]
- Chun SY, Billig H, Tilly JL, Furuta I, Tsafriri A, Hsueh AJ. Gonadotropin suppression of apoptosis in cultured preovulatory follicles: mediatory role of endogenous insulin-like growth factor I. Endocrinology. 1994;135:1845–1853. doi: 10.1210/endo.135.5.7525255. [DOI] [PubMed] [Google Scholar]
- Chun SY, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJ. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinology. 1996;137:1447–1456. doi: 10.1210/endo.137.4.8625923. [DOI] [PubMed] [Google Scholar]
- Cifone MG, Migliorati G, Parroni R, Marchetti C, Millimaggi D, Santoni A, Riccardi C. Dexamethasone-induced thymocyte apoptosis: apoptotic signal involves the sequential activation of phosphoinositide-specific phospholipase C, acidic sphingomyelinase, and caspases. Blood. 1999;93:2282–2896. [PubMed] [Google Scholar]
- Cohen JJ, Duke RC. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984;132:38–42. [PubMed] [Google Scholar]
- Colucci WS. Apoptosis in the heart. N Engl J Med. 1996;335:1224–1226. doi: 10.1056/NEJM199610173351610. [DOI] [PubMed] [Google Scholar]
- Colussi PA, Kumar S. Targeted disruption of caspase genes in mice: what they tell us about the functions of individual caspases in apoptosis. Immunol Cell Biol. 1999;77:58–63. doi: 10.1046/j.1440-1711.1999.00788.x. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Cusin I, Sainsbury A, Doyle P, Rohner-Jeanrenaud F, Jeanrenaud B. The ob gene and insulin. A relationship leading to clues to the understanding of obesity. Diabetes. 1995;44:1467–1470. doi: 10.2337/diab.44.12.1467. [DOI] [PubMed] [Google Scholar]
- Dagogo-Jack S, Fanelli C, Paramore D, Brothers J, Landt M. Plasma leptin and insulin relationships in obese and nonobese humans. Diabetes. 1996;45:695–698. doi: 10.2337/diab.45.5.695. [DOI] [PubMed] [Google Scholar]
- Dallenbach-Hellweg G. The endometrium in natural and artificial luteal phases. Hum Reprod. 1988;3:165–168. doi: 10.1093/oxfordjournals.humrep.a136668. [DOI] [PubMed] [Google Scholar]
- de la Mata J, Uy HL, Guise TA, Story B, Boyce BF, Mundy GR, Roodman GD. Interleukin-6 enhances hypercalcemia and bone resorption mediated by parathyroid hormone-related protein in vivo. J Clin Invest. 1995;95:2846–2852. doi: 10.1172/JCI117990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva HV, Harmony JA, Stuart WD, Gil CM, Robbins J. Apolipoprotein J: structure and tissue distribution. Biochemistry. 1990;29:5380–5389. doi: 10.1021/bi00474a025. [DOI] [PubMed] [Google Scholar]
- De Vos P, Saladin R, Auwerx J, Staels B. Induction of ob gene expression by corticosteroids is accompanied by body weight loss and reduced food intake. J Biol Chem. 1995;270:15958–15961. doi: 10.1074/jbc.270.27.15958. [DOI] [PubMed] [Google Scholar]
- Delany AM, Gabbitas BY, Canalis E. Cortisol downregulates osteoblast alpha 1 (I) procollagen mRNA by transcriptional and posttranscriptional mechanisms. J Cell Biochem. 1995a;57:488–594. doi: 10.1002/jcb.240570314. [DOI] [PubMed] [Google Scholar]
- Delany AM, Jeffrey JJ, Rydziel S, Canalis E. Cortisol increases interstitial collagenase expression in osteoblasts by post-transcriptional mechanisms. J Biol Chem. 1995b;270:26607–26612. doi: 10.1074/jbc.270.44.26607. [DOI] [PubMed] [Google Scholar]
- Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, Draper M, Christiansen C. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641–1647. doi: 10.1056/NEJM199712043372301. [DOI] [PubMed] [Google Scholar]
- Denton RM, Brownsey RW, Belsham GJ. A partial view of the mechanism of insulin action. Diabetologia. 1981;21:347–362. doi: 10.1007/BF00252681. [DOI] [PubMed] [Google Scholar]
- Devenport L, Knehans A, Sundstrom A, Thomas T. Corticosterone’s dual metabolic actions. Life Sci. 1989;45:1389–1396. doi: 10.1016/0024-3205(89)90026-x. [DOI] [PubMed] [Google Scholar]
- Distelhorst CW. Glucocorticoid-induced apoptosis. Adv Pharmacol. 1997;41:247–270. doi: 10.1016/s1054-3589(08)61061-3. [DOI] [PubMed] [Google Scholar]
- Distelhorst CW, Dubyak G. Role of calcium in glucocorticosteroid-induced apoptosis of thymocytes and lymphoma cells: resurrection of old theories by new findings. Blood. 1998;91:731–734. [PubMed] [Google Scholar]
- Dixon-Shanies D, Rudick J, Knittle JL. Observations on the growth and metabolic functions of cultured cells derived from human adipose tissue. Proc Soc Exp Biol Med. 1975;149:541–545. doi: 10.3181/00379727-149-38846. [DOI] [PubMed] [Google Scholar]
- Dougherty T. Effect of hormone on lymphatic tissue. Physiol Rev. 1952;32:379–401. doi: 10.1152/physrev.1952.32.4.379. [DOI] [PubMed] [Google Scholar]
- Ellis RE, Yuan JY, Horvitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- English HF, Kyprianou N, Isaacs JT. Relationship between DNA fragmentation and apoptosis in the programmed cell death in the rat prostate following castration. Prostate. 1989;15:233–250. doi: 10.1002/pros.2990150304. [DOI] [PubMed] [Google Scholar]
- Erickson BA, Longo WE, Panesar N, Mazuski JE, Kaminski DL. The effect of selective cyclooxygenase inhibitors on intestinal epithelial cell mitogenesis. J Surg Res. 1999;81:101–107. doi: 10.1006/jsre.1998.5511. [DOI] [PubMed] [Google Scholar]
- Eriksen EF, Colvard DS, Berg NJ, Graham ML, Mann KG, Spelsberg TC, Riggs BL. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988;241:84–86. doi: 10.1126/science.3388021. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Cullan GE, Sharp JG. Evaluation of the effects of pentagastrin, gastrin and pancreatic glucagon on cell proliferation in the rat gastrointestinal tract. Cell Tissue Kinetics. 1984;17:119–133. doi: 10.1111/j.1365-2184.1984.tb00574.x. [DOI] [PubMed] [Google Scholar]
- Fawthrop DJ, Boobis AR, Davies DS. Mechanisms of cell death. Arch Toxicol. 1991;65:437–444. doi: 10.1007/BF01977355. [DOI] [PubMed] [Google Scholar]
- Ferguson DJ. An ultrastructural study of mitosis and cytokinesis in normal ‘resting’ human breast. Cell Tissue Res. 1988;252:581–587. doi: 10.1007/BF00216645. [DOI] [PubMed] [Google Scholar]
- Ferguson DJ, Anderson TJ. Morphological evaluation of cell turnover in relation to the menstrual cycle in the “resting” human breast. Br J Cancer. 1981a;44:177–181. doi: 10.1038/bjc.1981.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DJ, Anderson TJ. Ultrastructural observations on cell death by apoptosis in the “resting” human breast. Virchows Archiv A Pathol Anat Histol. 1981b;393:193–203. doi: 10.1007/BF00431076. [DOI] [PubMed] [Google Scholar]
- Finkelman RD, Bell NH, Strong DD, Demers LM, Baylink DJ. Ovariectomy selectively reduces the concentration of transforming growth factor beta in rat bone: implications for estrogen deficiency-associated bone loss. Proc Natl Acad Sci U S A. 1992;89:12190–12193. doi: 10.1073/pnas.89.24.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- Fowlkes BJ, Schwartz RH, Pardoll DM. Deletion of self-reactive thymocytes occurs at a CD4+8+ precursor stage. Nature. 1988;334:620–623. doi: 10.1038/334620a0. [DOI] [PubMed] [Google Scholar]
- Fuller K, Wong B, Fox S, Choi Y, Chambers TJ. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med. 1998;188:997–1001. doi: 10.1084/jem.188.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geley S, Hartmann BL, Kapelari K, Egle A, Villunger A, Heidacher D, Greil R, Auer B, Kofler R. The interleukin 1beta-converting enzyme inhibitor CrmA prevents Apo1/Fas- but not glucocorticoid-induced poly(ADP-ribose) polymerase cleavage and apoptosis in lymphoblastic leukemia cells. FEBS Lett. 1997;402:36–40. doi: 10.1016/s0014-5793(96)01496-2. [DOI] [PubMed] [Google Scholar]
- Geloen A, Collet AJ, Guay G, Bukowiecki LJ. Insulin stimulates in vivo cell proliferation in white adipose tissue. Am J Physiol. 1989a;256:C190–C196. doi: 10.1152/ajpcell.1989.256.1.C190. [DOI] [PubMed] [Google Scholar]
- Geloen A, Roy PE, Bukowiecki LJ. Regression of white adipose tissue in diabetic rats. Am J Physiol. 1989b;257:E547–E553. doi: 10.1152/ajpendo.1989.257.4.E547. [DOI] [PubMed] [Google Scholar]
- Gompel A, Sabourin JC, Martin A, Yaneva H, Audouin J, Decroix Y, Poitout P. Bcl-2 expression in normal endometrium during the menstrual cycle. Am J Pathol. 1994;144:1195–1202. [PMC free article] [PubMed] [Google Scholar]
- Goodlad RA, Wright NA. Growth control factors in the gastrointestinal tract. Baillieres Clin Gastroenterol. 1990;4:97–118. doi: 10.1016/0950-3528(90)90041-e. [DOI] [PubMed] [Google Scholar]
- Gosden R, Spears N. Programmed cell death in the reproductive system. Br Med Bull. 1997;53:644–661. doi: 10.1093/oxfordjournals.bmb.a011636. [DOI] [PubMed] [Google Scholar]
- Goya RG, Bolognani F. Homeostasis, thymic hormones and aging. Gerontology. 1999;45:174–178. doi: 10.1159/000022082. [DOI] [PubMed] [Google Scholar]
- Grey A, Mitnick MA, Shapses S, Ellison A, Gundberg C, Insogna K. Circulating levels of interleukin-6 and tumor necrosis factor-alpha are elevated in primary hyperparathyroidism and correlate with markers of bone resorption – a clinical research center study. J Clin Endocrinol Metabol. 1996;81:3450–3454. doi: 10.1210/jcem.81.10.8855783. [DOI] [PubMed] [Google Scholar]
- Griswold MD, Roberts K, Bishop P. Purification and characterization of a sulfated glycoprotein secreted by Sertoli cells. Biochemistry. 1986;25:7265–7270. doi: 10.1021/bi00371a003. [DOI] [PubMed] [Google Scholar]
- Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenette RS, Tenniswood M. The role of growth factors in the suppression of active cell death in the prostate: an hypothesis. Biochem Cell Biol. 1994;72:553–559. doi: 10.1139/o94-074. [DOI] [PubMed] [Google Scholar]
- Guenette RS, Corbeil HB, Leger J, Wong K, Mezl V, Mooibroek M, Tenniswood M. Induction of gene expression during involution of the lactating mammary gland of the rat. J Mol Endocrinol. 1994;12:47–60. doi: 10.1677/jme.0.0120047. [DOI] [PubMed] [Google Scholar]
- Guo Y, Kyprianou N. Restoration of transforming growth factor beta signaling pathway in human prostate cancer cells suppresses tumorigenicity via induction of caspase-1-mediated apoptosis. Cancer Res. 1999;59:1366–1371. [PubMed] [Google Scholar]
- Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, Elia A, de la Pompa JL, Kagi D, Khoo W, Potter J, Yoshida R, Kaufman SA, Lowe SW, Penninger JM, Mak TW. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Hattersley AT, Meeran K, Burrin J, Hill P, Shiner R, Ibbertson HK. The effect of long- and short-term corticosteroids on plasma calcitonin and parathyroid hormone levels. Calcif Tissue Int. 1994;54:198–202. doi: 10.1007/BF00301678. [DOI] [PubMed] [Google Scholar]
- Hauner H, Entenmann G, Wabitsch M, Gaillard D, Ailhaud G, Negrel R, Pfeiffer EF. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J Clin Invest. 1989;84:1663–1670. doi: 10.1172/JCI114345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmrath MA, Shin CE, Erwin CR, Warner BW. The EGF:EGF-receptor axis modulates enterocyte apoptosis during intestinal adaptation. J Surg Res. 1998;77:17–22. doi: 10.1006/jsre.1998.5362. [DOI] [PubMed] [Google Scholar]
- Hetts SW. To die or not to die: an overview of apoptosis and its role in disease. JAMA. 1998;279:300–337. doi: 10.1001/jama.279.4.300. [DOI] [PubMed] [Google Scholar]
- Hill PA, Tumber A, Meikle MC. Multiple extracellular signals promote osteoblast survival and apoptosis. Endocrinology. 1997;138:3849–3858. doi: 10.1210/endo.138.9.5370. [DOI] [PubMed] [Google Scholar]
- Hillier SG, Ross GT. Effects of exogenous testosterone on ovarian weight, follicular morphology and intraovarian progesterone concentration in estrogen-primed hypophysectomized immature female rats. Biol Reprod. 1979;20:261–268. doi: 10.1095/biolreprod20.2.261. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Hockenbery DM, Zutter M, Hickey W, Nahm M, Korsmeyer SJ. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991;88:6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer L, Khosla S, Dunstan C, Lacey D, Spelsberg T, Riggs B. Estrogen stimulates gene expression and protein production of osteoprotegerin by human osteoblastic cells. Bone. 1998;23:S172. doi: 10.1210/endo.140.9.7131. [DOI] [PubMed] [Google Scholar]
- Hopwood D, Levison DA. Atrophy and apoptosis in the cyclical human endometrium. J Pathol. 1976;119:159–166. doi: 10.1002/path.1711190305. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh AJ, Jones PB. Extrapituitary actions of gonadotropin-releasing hormone. Endocr Rev. 1981;2:437–461. doi: 10.1210/edrv-2-4-437. [DOI] [PubMed] [Google Scholar]
- Hsueh AJ, Billig H, Tsafriri A. Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev. 1994;15:707–724. doi: 10.1210/edrv-15-6-707. [DOI] [PubMed] [Google Scholar]
- Hsueh AJ, Eisenhauer K, Chun SY, Hsu SY, Billig H. Gonadal cell apoptosis. Recent Prog Horm Res. 1996;51:433–456. [PubMed] [Google Scholar]
- Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- Huckins C. The morphology and kinetics of spermatogonial degeneration in normal adult rats: an analysis using a simplified classification of the germinal epithelium. Anat Rec. 1978;190:905–926. doi: 10.1002/ar.1091900410. [DOI] [PubMed] [Google Scholar]
- Hughes DE, Boyce BF. Apoptosis in bone physiology and disease. Mol Pathol. 1997;50:132–137. doi: 10.1136/mp.50.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med. 1996;2:1132–1136. doi: 10.1038/nm1096-1132. [DOI] [PubMed] [Google Scholar]
- Ilio KY, Sensibar JA, Lee C. Effect of TGF-beta 1, TGF-alpha, and EGF on cell proliferation and cell death in rat ventral prostatic epithelial cells in culture. J Androl. 1995;16:482–490. [PubMed] [Google Scholar]
- Imam H, Eriksson B, Lukinius A, Janson ET, Lindgren PG, Wilander E, Oberg K. Induction of apoptosis in neuro-endocrine tumors of the digestive system during treatment with somatostatin analogs. Acta Oncol. 1997;36:607–614. doi: 10.3109/02841869709001323. [DOI] [PubMed] [Google Scholar]
- Ingle D. Effect of 2 steroid components on weight of thymus of adrenalectomized rats. Proc Natl Acad Sci USA. 1940;44:174–175. [Google Scholar]
- Isaacs JT. Antagonistic effect of androgen on prostatic cell death. Prostate. 1984;5:545–557. doi: 10.1002/pros.2990050510. [DOI] [PubMed] [Google Scholar]
- Ishimi Y, Miyaura C, Jin CH, Akatsu T, Abe E, Nakamura Y, Yamaguchi A, Yoshiki S, Matsuda T, Hirano T. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145:3297–3303. [PubMed] [Google Scholar]
- Iwata M, Hanaoka S, Sato K. Rescue of thymocytes and T cell hybridomas from glucocorticoid-induced apoptosis by stimulation via the T cell receptor/CD3 complex: a possible in vitro model for positive selection of the T cell repertoire. Eur J Immunol. 1991;21:643–648. doi: 10.1002/eji.1830210316. [DOI] [PubMed] [Google Scholar]
- Iwata M, Ohoka Y, Kuwata T, Asada A. Regulation of T cell apoptosis via T cell receptors and steroid receptors. Stem Cells. 1996;14:632–641. doi: 10.1002/stem.140632. [DOI] [PubMed] [Google Scholar]
- Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- Jeng MH, Jordan VC. Growth stimulation and differential regulation of transforming growth factor-beta 1 (TGF beta 1), TGF beta 2, and TGF beta 3 messenger RNA levels by norethindrone in MCF-7 human breast cancer cells. Mol Endocrinol. 1991;5:1120–1128. doi: 10.1210/mend-5-8-1120. [DOI] [PubMed] [Google Scholar]
- Jiang D, Zheng L, Lenardo MJ. Caspases in T-cell receptor-induced thymocyte apoptosis. Cell Death Differ. 1999;6:402–411. doi: 10.1038/sj.cdd.4400513. [DOI] [PubMed] [Google Scholar]
- Jilka RL. Cytokines, bone remodeling, and estrogen deficiency: a 1998 update. Bone. 1998;23:75–81. doi: 10.1016/s8756-3282(98)00077-5. [DOI] [PubMed] [Google Scholar]
- Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, Boyce B, Broxmeyer H, Manolagas SC. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257:88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Takahashi K, Parfitt AM, Manolagas SC. Linkage of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence. J Clin Invest. 1996;97:1732–1740. doi: 10.1172/JCI118600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, Takahashi K, Munshi M, Williams DC, Roberson PK, Manolagas SC. Loss of estrogen upregulates osteoblastogenesis in the murine bone marrow. Evidence for autonomy from factors released during bone resorption. J Clin Invest. 1998a;101:1942–1950. doi: 10.1172/JCI1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998b;13:793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- Jo T, Terada N, Saji F, Tanizawa O. Inhibitory effects of estrogen, progesterone, androgen and glucocorticoid on death of neonatal mouse uterine epithelial cells induced to proliferate by estrogen. J Steroid Biochem Mol Biol. 1993;46:25–32. doi: 10.1016/0960-0760(93)90205-b. [DOI] [PubMed] [Google Scholar]
- Johnson AL, Bridgham JT, Witty JP, Tilly JL. Susceptibility of avian ovarian granulosa cells to apoptosis is dependent upon stage of follicle development and is related to endogenous levels of bcl-xlong gene expression. Endocrinology. 1996;137:2059–2066. doi: 10.1210/endo.137.5.8612548. [DOI] [PubMed] [Google Scholar]
- Jones BA, Gores GJ. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. Am J Physiol. 1997;273:G1174–G1188. doi: 10.1152/ajpgi.1997.273.6.G1174. [DOI] [PubMed] [Google Scholar]
- Kameda T, Ishikawa H, Tsutsui T. Detection and characterization of apoptosis in osteoclasts in vitro. Biochem Biophys Res Comm. 1995;207:753–760. doi: 10.1006/bbrc.1995.1251. [DOI] [PubMed] [Google Scholar]
- Kameda T, Mano H, Yuasa T, Mori Y, Miyazawa K, Shiokawa M, Nakamaru Y, Hiroi E, Hiura K, Kameda A, Yang NN, Hakeda Y, Kumegawa M. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med. 1997;186:489–495. doi: 10.1084/jem.186.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C, Tronche F, Reichardt HM, Schutz G. Mutagenesis of the glucocorticoid receptor in mice. J Steroid Biochem Mol Biol. 1999;69:253–259. doi: 10.1016/s0960-0760(99)00041-2. [DOI] [PubMed] [Google Scholar]
- Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF. Shrinkage necrosis: a distinct mode of cellular death. J Pathol. 1971;105:13–20. doi: 10.1002/path.1711050103. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiess W, Gallaher B. Hormonal control of programmed cell death/apoptosis. Eur J Endocrinol. 1998;138:482–491. doi: 10.1530/eje.0.1380482. [DOI] [PubMed] [Google Scholar]
- Kimble RB, Srivastava S, Ross FP, Matayoshi A, Pacifici R. Estrogen deficiency increases the ability of stromal cells to support murine osteoclastogenesis via an interleukin-1 and tumor necrosis factor-mediated stimulation of macrophage colony-stimulating factor production. J Biol Chem. 1996;271:28890–28897. doi: 10.1074/jbc.271.46.28890. [DOI] [PubMed] [Google Scholar]
- King LB, Vacchio MS, Dixon K, Hunziker R, Margulies DH, Ashwell JD. A targeted glucocorticoid receptor antisense transgene increases thymocyte apoptosis and alters thymocyte development. Immunity. 1995;3:647–656. doi: 10.1016/1074-7613(95)90135-3. [DOI] [PubMed] [Google Scholar]
- Knabbe C, Lippman ME, Wakefield LM, Flanders KC, Kasid A, Derynck R, Dickson RB. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987;48:417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Kolaczynski JW, Nyce MR, Considine RV, Boden G, Nolan JJ, Henry R, Mudaliar SR, Olefsky J, Caro JF. Acute and chronic effects of insulin on leptin production in humans: Studies in vivo and in vitro. Diabetes. 1996;45:699–701. doi: 10.2337/diab.45.5.699. [DOI] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang HG, Reed JC. Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol. 1994;145:1323–1336. [PMC free article] [PubMed] [Google Scholar]
- Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- Kyprianou N, Isaacs JT. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology. 1988;122:552–562. doi: 10.1210/endo-122-2-552. [DOI] [PubMed] [Google Scholar]
- Kyprianou N, Isaacs JT. Expression of transforming growth factor-beta in the rat ventral prostate during castration-induced programmed cell death. Mol Endocrinol. 1989;3:1515–1522. doi: 10.1210/mend-3-10-1515. [DOI] [PubMed] [Google Scholar]
- Landry P, Sadasivan K, Marino A, Albright J. Apoptosis is coordinately regulated with osteoblast formation during bone healing. Tissue Cell. 1997;29:413–419. doi: 10.1016/s0040-8166(97)80027-4. [DOI] [PubMed] [Google Scholar]
- Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology. 1997;138:2081–2088. doi: 10.1210/endo.138.5.5110. [DOI] [PubMed] [Google Scholar]
- Lefebvre O, Wolf C, Limacher JM, Hutin P, Wendling C, LeMeur M, Basset P, Rio MC. The breast cancer-associated stromelysin-3 gene is expressed during mouse mammary gland apoptosis. J Cell Biol. 1992;119:997–1002. doi: 10.1083/jcb.119.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger JG, Le Guellec R, Tenniswood MP. Treatment with antiandrogens induces an androgen-repressed gene in the rat ventral prostate. Prostate. 1988;13:131–142. doi: 10.1002/pros.2990130205. [DOI] [PubMed] [Google Scholar]
- Lehy T, Dubrasquet M, Bonfils S. Effect of somatostatin on normal and gastric-stimulated cell proliferation in the gastric and intestinal mucosae of the rat. Digestion. 1979;19:99–109. doi: 10.1159/000198330. [DOI] [PubMed] [Google Scholar]
- Leroy P, Dessolin S, Villageois P, Moon BC, Friedman JM, Ailhaud G, Dani C. Expression of ob gene in adipose cells. Regulation by insulin. J Biol Chem. 1996;271:2365–2368. doi: 10.1074/jbc.271.5.2365. [DOI] [PubMed] [Google Scholar]
- Ling N, Ying SY, Ueno N, Shimasaki S, Esch F, Hotta M, Guillemin R. A homodimer of the beta-subunits of inhibin A stimulates the secretion of pituitary follicle stimulating hormone. Biochem Biophys Res Comm. 1986;138:1129–1137. doi: 10.1016/s0006-291x(86)80400-4. [DOI] [PubMed] [Google Scholar]
- Luciano AM, Pappalardo A, Ray C, Peluso JJ. Epidermal growth factor inhibits large granulosa cell apoptosis by stimulating progesterone synthesis and regulating the distribution of intracellular free calcium. Biol Reprod. 1994;51:646–654. doi: 10.1095/biolreprod51.4.646. [DOI] [PubMed] [Google Scholar]
- Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis: pathogenesis and management. Ann Intern Med. 1990;112:352–364. doi: 10.7326/0003-4819-112-5-352. [DOI] [PubMed] [Google Scholar]
- Lutton JD, Moonga BS, Dempster DW. Osteoclast demise in the rat: physiological versus degenerative cell death. Exp Physiol. 1996;81:251–260. doi: 10.1113/expphysiol.1996.sp003929. [DOI] [PubMed] [Google Scholar]
- Madesh M, Benard O, Balasubramanian KA. Apoptotic process in the monkey small intestinal epithelium: 2. Possible role of oxidative stress. Free Rad Biol Med. 1999;26:431–438. doi: 10.1016/s0891-5849(98)00218-4. [DOI] [PubMed] [Google Scholar]
- Martikainen P, Kyprianou N, Isaacs JT. Effect of transforming growth factor-beta 1 on proliferation and death of rat prostatic cells. Endocrinology. 1990;127:2963–2968. doi: 10.1210/endo-127-6-2963. [DOI] [PubMed] [Google Scholar]
- Martimbeau S, Tilly JL. Physiological cell death in endocrine-dependent tissues: an ovarian perspective. Clin Endocrinol. 1997;46:241–254. doi: 10.1046/j.1365-2265.1997.00157.x. [DOI] [PubMed] [Google Scholar]
- Martin L, Finn CA. Hormonal regulation of cell division in epithelial and connective tissues of the mouse uterus. J Endocrinol. 1968;41:363–371. doi: 10.1677/joe.0.0410363. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Ng KW. Mechanisms by which cells of the osteoblast lineage control osteoclast formation and activity. J Cell Biochem. 1994;56:357–366. doi: 10.1002/jcb.240560312. [DOI] [PubMed] [Google Scholar]
- Martins TC, Aguas AP. Involvement of c-myc in the resistance of non-obese diabetic mice to glucocorticoid-induced apoptosis. Immunology. 1998;95:377–382. doi: 10.1046/j.1365-2567.1998.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey DJ, Nicotera P, Hartzell P, Bellomo G, Wyllie AH, Orrenius S. Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys. 1989;269:365–370. doi: 10.1016/0003-9861(89)90119-7. [DOI] [PubMed] [Google Scholar]
- McConkey DJ, Orrenius S, Jondal M. Agents that elevate cAMP stimulate DNA fragmentation in thymocytes. J Immunol. 1990;145:1227–1230. [PubMed] [Google Scholar]
- McConkey DJ, Jondal M, Orrenius S. Cellular signaling in thymocyte apoptosis. Semin Immunol. 1992;4:371–377. [PubMed] [Google Scholar]
- McConkey DJ, Orrenius S, Okret S, Jondal M. Cyclic AMP potentiates glucocorticoid-induced endogenous endonuclease activation in thymocytes. FASEB J. 1993;7:580–585. doi: 10.1096/fasebj.7.6.8386120. [DOI] [PubMed] [Google Scholar]
- Means AR, Fakunding JL, Huckins C, Tindall DJ, Vitale R. Follicle-stimulating hormone, the Sertoli cell, and spermatogenesis. Recent Prog Horm Res. 1976;32:477–527. doi: 10.1016/b978-0-12-571132-6.50027-0. [DOI] [PubMed] [Google Scholar]
- Medh RD, Saeed MF, Johnson BH, Thompson EB. Resistance of human leukemic CEM-C1 cells is overcome by synergism between glucocorticoid and protein kinase A pathways: correlation with c-Myc suppression. Cancer Res. 1998;58:3684–3693. [PubMed] [Google Scholar]
- Meyer T, Carlstedt-Duke J, Starr DB. A weak TATA box is a prerequisite for glucocorticoid-dependent repression of the osteocalcin gene. J Biol Chem. 1997;272:30709–30714. doi: 10.1074/jbc.272.49.30709. [DOI] [PubMed] [Google Scholar]
- Miyaura C, Kusano K, Masuzawa T, Chaki O, Onoe Y, Aoyagi M, Sasaki T, Tamura T, Koishihara Y, Ohsugi Y. Endogenous bone-resorbing factors in estrogen deficiency: cooperative effects of IL-1 and IL-6. J Bone Miner Res. 1995;10:1365–1373. doi: 10.1002/jbmr.5650100914. [DOI] [PubMed] [Google Scholar]
- Montminy M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- Morishima A, Grumbach M, Bilezikian J. Estrogen markedly increases bone mass in an estrogen deficient young man with aromatase deficiency. J Bone Miner Res. 1997;12:S126. [Google Scholar]
- Morris HA, Need AG, O’Loughlin PD, Horowitz M, Bridges A, Nordin BE. Malabsorption of calcium in corticosteroid-induced osteoporosis. Calcif Tissue Int. 1990;46:305–308. doi: 10.1007/BF02563820. [DOI] [PubMed] [Google Scholar]
- Moss SF, Agarwal B, Arber N, Guan RJ, Krajewska M, Krajewski S, Reed JC, Holt PR. Increased intestinal Bak expression results in apoptosis. Biochem Biophys Res Comm. 1996;223:199–203. doi: 10.1006/bbrc.1996.0869. [DOI] [PubMed] [Google Scholar]
- Munroe DG, Gupta AK, Kooshesh F, Vyas TB, Rizkalla G, Wang H, Demchyshyn L, Yang ZJ, Kamboj RK, Chen H, McCallum K, Sumner-Smith M, Drucker DJ, Crivici A. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc Natl Acad Sci U S A. 1999;96:1569–1573. doi: 10.1073/pnas.96.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- Negre-Salvayre A, Hirtz C, Carrera G, Cazenave R, Troly M, Salvayre R, Penicaud L, Casteilla L. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 1997;11:809–815. [PubMed] [Google Scholar]
- Niesler CU, Siddle K, Prins JB. Human preadipocytes display a depot-specific susceptibility to apoptosis. Diabetes. 1998;47:1365–1368. doi: 10.2337/diab.47.8.1365. [DOI] [PubMed] [Google Scholar]
- Nijweide PJ, Burger EH, Feyen JH. Cells of bone: proliferation, differentiation, and hormonal regulation. Physiol Rev. 1986;66:855–886. doi: 10.1152/physrev.1986.66.4.855. [DOI] [PubMed] [Google Scholar]
- Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- Otsuki Y, Misaki O, Sugimoto O, Ito Y, Tsujimoto Y, Akao Y. Cyclic bcl-2 gene expression in human uterine endometrium during menstrual cycle. Lancet. 1994;344:28–29. doi: 10.1016/s0140-6736(94)91051-0. [DOI] [PubMed] [Google Scholar]
- Ottosson M, Vikman-Adolfsson K, Enerback S, Olivecrona G, Bjorntorp P. The effects of cortisol on the regulation of lipoprotein lipase activity in human adipose tissue. J Clin Endocrinol Metabol. 1994;79:820–825. doi: 10.1210/jcem.79.3.8077367. [DOI] [PubMed] [Google Scholar]
- Owen JJ, Jenkinson EJ. Apoptosis and T-cell repertoire selection in the thymus. Ann N Y Acad Sci. 1992;663:305–310. doi: 10.1111/j.1749-6632.1992.tb38673.x. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Pentikainen V, Erkkila K, Dunkel L. Fas regulates germ cell apoptosis in the human testis in vitro. Am J Physiol. 1999;276:E310–E316. doi: 10.1152/ajpendo.1999.276.2.E310. [DOI] [PubMed] [Google Scholar]
- Petruschke T, Hauner H. Tumor necrosis factor-alpha prevents the differentiation of human adipocyte precursor cells and causes delipidation of newly developed fat cells. J Clin Endocrinol Metabol. 1993;76:742–747. doi: 10.1210/jcem.76.3.8445033. [DOI] [PubMed] [Google Scholar]
- Plaut K, Ikeda M, Vonderhaar BK. Role of growth hormone and insulin-like growth factor-I in mammary development. Endocrinology. 1993;133:1843–1848. doi: 10.1210/endo.133.4.8404627. [DOI] [PubMed] [Google Scholar]
- Pollard JW, Pacey J, Cheng SV, Jordan EG. Estrogens and cell death in murine uterine luminal epithelium. Cell Tissue Res. 1987;249:533–540. doi: 10.1007/BF00217324. [DOI] [PubMed] [Google Scholar]
- Potten CS. The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Can Metastasis Rev. 1992;11:179–195. doi: 10.1007/BF00048063. [DOI] [PubMed] [Google Scholar]
- Potten CS. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond Biol. 1998;353:821–830. doi: 10.1098/rstb.1998.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Wilson JW, Booth C. Regulation and significance of apoptosis in the stem cells of the gastrointestinal epithelium. Stem Cells. 1997;15:82–93. doi: 10.1002/stem.150082. [DOI] [PubMed] [Google Scholar]
- Prins JB, O’Rahilly S. Regulation of adipose cell number in man. Clin Sci. 1997;92:3–11. doi: 10.1042/cs0920003. [DOI] [PubMed] [Google Scholar]
- Prins JB, Walker NI, Winterford CM, Cameron DP. Apoptosis of human adipocytes in vitro. Biochem Biophys Res Comm. 1994a;201:500–507. doi: 10.1006/bbrc.1994.1730. [DOI] [PubMed] [Google Scholar]
- Prins JB, Walker NI, Winterford CM, Cameron DP. Human adipocyte apoptosis occurs in malignancy. Biochem Biophys Res Comm. 1994b;205:625–630. doi: 10.1006/bbrc.1994.2711. [DOI] [PubMed] [Google Scholar]
- Prins JB, Niesler CU, Winterford CM, Bright NA, Siddle K, O’Rahilly S, Walker NI, Cameron DP. Tumor necrosis factor-alpha induces apoptosis of human adipose cells. Diabetes. 1997;46:1939–1944. doi: 10.2337/diab.46.12.1939. [DOI] [PubMed] [Google Scholar]
- Prins J, Ledgerwood E, Ameloot P, Vandenabeele P, Faraco P, Bright N, O’Rahilly S, Bradley J. Tumour necrosis factor induced autophagy and mitochondrial morphological abnormalities are mediated by TNFR-I and/or TNFR-II and do not invariably lead to cell death. Biochem Soc Trans. 1998;26:S314. doi: 10.1042/bst026s314. [DOI] [PubMed] [Google Scholar]
- Pritchard DM, Watson AJ. Apoptosis and gastrointestinal pharmacology. Pharmacol Ther. 1996;72:149–169. doi: 10.1016/s0163-7258(96)00102-7. [DOI] [PubMed] [Google Scholar]
- Pullan S, Wilson J, Metcalfe A, Edwards GM, Goberdhan N, Tilly J, Hickman JA, Dive C, Streuli CH. Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J Cell Sci. 1996;109:631–642. doi: 10.1242/jcs.109.3.631. [DOI] [PubMed] [Google Scholar]
- Qian H, Azain MJ, Compton MM, Hartzell DL, Hausman GJ, Baile CA. Brain administration of leptin causes deletion of adipocytes by apoptosis. Endocrinology. 1998a;139:791–794. doi: 10.1210/endo.139.2.5908. [DOI] [PubMed] [Google Scholar]
- Qian H, Hausman GJ, Compton MM, Azain MJ, Hartzell DL, Baile CA. Leptin regulation of peroxisome proliferator-activated receptor-gamma, tumor necrosis factor, and uncoupling protein-2 expression in adipose tissues. Biochem Biophys Res Comm. 1998b;246:660–667. doi: 10.1006/bbrc.1998.8680. [DOI] [PubMed] [Google Scholar]
- Raffray M, Cohen GM. Apoptosis and necrosis in toxicology – a continuum or distinct modes of cell death. Pharmacol Ther. 1997;75:153–177. doi: 10.1016/s0163-7258(97)00037-5. [DOI] [PubMed] [Google Scholar]
- Rebuffe-Scrive M, Krotkiewski M, Elfverson J, Bjorntorp P. Muscle and adipose tissue morphology and metabolism in Cushing’s syndrome. J Clin Endocrinol Metab. 1988;67:1122–1128. doi: 10.1210/jcem-67-6-1122. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schutz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Reid IR. Glucocorticoid osteoporosis – mechanisms and management. Eur J Endocrinol. 1997;137:209–217. doi: 10.1530/eje.0.1370209. [DOI] [PubMed] [Google Scholar]
- Rennie PS, Bouffard R, Bruchovsky N, Cheng H. Increased activity of plasminogen activators during involution of the rat ventral prostate. Biochem J. 1984;221:171–178. doi: 10.1042/bj2210171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie PS, Bowden JF, Bruchovsky N, Cheng H. The relationship between inhibition of plasminogen-activator activity and prostatic involution. Biochem J. 1988;252:759–764. doi: 10.1042/bj2520759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie PS, Bowden JF, Freeman SN, Bruchovsky N, Cheng H, Lubahn DB, Wilson EM, French FS, Main L. Cortisol alters gene expression during involution of the rat ventral prostate. Mol Endocrinol. 1989;3:703–708. doi: 10.1210/mend-3-4-703. [DOI] [PubMed] [Google Scholar]
- Richard D, Chapdelaine S, Deshaies Y, Pepin MC, Barden N. Energy balance and lipid metabolism in transgenic mice bearing an antisense GCR gene construct. Am J Physiol. 1993;265:R146–R150. doi: 10.1152/ajpregu.1993.265.1.R146. [DOI] [PubMed] [Google Scholar]
- Richards JS. Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol Rev. 1980;60:51–89. doi: 10.1152/physrev.1980.60.1.51. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton LJ., III A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13:763–773. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- Robinson JA, Harris SA, Riggs BL, Spelsberg TC. Estrogen regulation of human osteoblastic cell proliferation and differentiation. Endocrinology. 1997;138:2919–2927. doi: 10.1210/endo.138.7.5277. [DOI] [PubMed] [Google Scholar]
- Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P. An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J. 1997;16:2262–2270. doi: 10.1093/emboj/16.9.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohner-Jeanrenaud F, Jeanrenaud B. Obesity, leptin, and the brain. N Engl J Med. 1996;334:324–325. doi: 10.1056/NEJM199602013340511. [DOI] [PubMed] [Google Scholar]
- Rohner-Jeanrenaud F, Walker CD, Greco-Perotto R, Jeanrenaud B. Central corticotropin-releasing factor administration prevents the excessive body weight gain of genetically obese (fa/fa) rats. Endocrinology. 1989;124:733–739. doi: 10.1210/endo-124-2-733. [DOI] [PubMed] [Google Scholar]
- Rollwagen FM, Yu ZY, Li YY, Pacheco ND. IL-6 rescues enterocytes from hemorrhage induced apoptosis in vivo and in vitro by a bcl-2 mediated mechanism. Clin Immunol Immunopathol. 1998;89:205–213. doi: 10.1006/clin.1998.4600. [DOI] [PubMed] [Google Scholar]
- Rotello RJ, Lieberman RC, Purchio AF, Gerschenson LE. Coordinated regulation of apoptosis and cell proliferation by transforming growth factor beta 1 in cultured uterine epithelial cells. Proc Natl Acad Sci U S A. 1991;88:3412–3415. doi: 10.1073/pnas.88.8.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotello RJ, Lieberman RC, Lepoff RB, Gerschenson LE. Characterization of uterine epithelium apoptotic cell death kinetics and regulation by progesterone and RU 486. Am J Pathol. 1992;140:449–456. [PMC free article] [PubMed] [Google Scholar]
- Rudin CM, Thompson CB. Apoptosis and disease: regulation and clinical relevance of programmed cell death. Annu Rev Med. 1997;48:267–281. doi: 10.1146/annurev.med.48.1.267. [DOI] [PubMed] [Google Scholar]
- Russell LD, Clermont Y. Degeneration of germ cells in normal, hypophysectomized and hormone treated hypophysectomized rats. Anat Res. 1976;187:347–366. doi: 10.1002/ar.1091870307. [DOI] [PubMed] [Google Scholar]
- Sandford NL, Searle JW, Kerr JF. Successive waves of apoptosis in the rat prostate after repeated withdrawal of testosterone stimulation. Pathology. 1984;16:406–410. doi: 10.3109/00313028409084731. [DOI] [PubMed] [Google Scholar]
- Sandow BA, West NB, Norman RL, Brenner RM. Hormonal control of apoptosis in hamster uterine luminal epithelium. Am J Anat. 1979;156:15–35. doi: 10.1002/aja.1001560103. [DOI] [PubMed] [Google Scholar]
- Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- Santana P, Akana SF, Hanson ES, Strack AM, Sebastian RJ, Dallman MF. Aldosterone and dexamethasone both stimulate energy acquisition whereas only the glucocorticoid alters energy storage. Endocrinology. 1995;136:2214–2222. doi: 10.1210/endo.136.5.7720670. [DOI] [PubMed] [Google Scholar]
- Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman EH, Fuks Z, Kolesnick R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- Sarin A, Wu ML, Henkart PA. Different interleukin-1 beta converting enzyme (ICE) family protease requirements for the apoptotic death of T lymphocytes triggered by diverse stimuli. J Exp Med. 1996;184:2445–2450. doi: 10.1084/jem.184.6.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomberg DW, Stouffer RL, Tyrey L. Modulation of progestin secretion in ovarian cells by 17beta-hydroxy-5alpha-androstan-3-one (dihydrotestosterone): a direct demonstration in monolayer culture. Biochem Biophys Res Comm. 1976;68:77–81. doi: 10.1016/0006-291x(76)90012-7. [DOI] [PubMed] [Google Scholar]
- Schwartzman RA, Cidlowski JA. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev. 1993;14:133–151. doi: 10.1210/edrv-14-2-133. [DOI] [PubMed] [Google Scholar]
- Selander KS, Harkonen PL, Valve E, Monkkonen J, Hannuniemi R, Vaananen HK. Calcitonin promotes osteoclast survival in vitro. Mol Cell Endocrinol. 1996;122:119–129. doi: 10.1016/0303-7207(96)03870-1. [DOI] [PubMed] [Google Scholar]
- Sheng H, Shao J, O’Mahony CA, Lamps L, Albo D, Isakson PC, Berger DH, DuBois RN, Beauchamp RD. Transformation of intestinal epithelial cells by chronic TGF-beta1 treatment results in downregulation of the type II TGF-beta receptor and induction of cyclooxygenase-2. Oncogene. 1999;18:855–867. doi: 10.1038/sj.onc.1202397. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Gnudi L, Tozzo E, Yang H, Leach F, Kahn BB. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem. 1993;268:22243–22246. [PubMed] [Google Scholar]
- Shiokawa-Sawada M, Mano H, Hanada K, Kakudo S, Kameda T, Miyazawa K, Nakamaru Y, Yuasa T, Mori Y, Kumegawa M, Hakeda Y. Down-regulation of gap junctional intercellular communication between osteoblastic MC3T3-E1 cells by basic fibroblast growth factor and a phorbol ester (12-O-tetradecanoylphorbol-13-acetate) J Bone Miner Res. 1997;12:1165–1173. doi: 10.1359/jbmr.1997.12.8.1165. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Daniel CW. Reversible inhibition of mammary gland growth by transforming growth factor-beta. Science. 1987;237:291–293. doi: 10.1126/science.3474783. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Strickland P, Coleman S, Daniel CW. Epithelium-dependent extracellular matrix synthesis in transforming growth factor-beta 1-growth-inhibited mouse mammary gland. J Cell Biol. 1990;110:2209–2219. doi: 10.1083/jcb.110.6.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg SJ, Bilezikian JP. Cytokines in primary hyperparathyroidism – factors that matter. J Clin Endocrinol Metabol. 1996;8:3448–3449. doi: 10.1210/jcem.81.10.8855782. [DOI] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Sinha Hikim AP, Swerdloff RS. Hormonal and genetic control of germ cell apoptosis in the testis. Rev Reproduction. 1999;4:38–47. doi: 10.1530/ror.0.0040038. [DOI] [PubMed] [Google Scholar]
- Spelsberg TC, Subramaniam M, Riggs BL, Khosla S. The actions and interactions of sex steroids and growth factors/cytokines on the skeleton. Mol Endocrinol. 1999;13:819–828. doi: 10.1210/mend.13.6.0299. [DOI] [PubMed] [Google Scholar]
- Sprent J, Lo D, Gao EK, Ron Y. T cell selection in the thymus. Immunol Rev. 1988;101:173–190. doi: 10.1111/j.1600-065x.1988.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Steinberger E. Hormonal control of mammalian spermatogenesis. Physiol Rev. 1971;51:1–22. doi: 10.1152/physrev.1971.51.1.1. [DOI] [PubMed] [Google Scholar]
- Stepan JJ, Lachman M, Zverina J, Pacovsky V, Baylink DJ. Castrated men exhibit bone loss: effect of calcitonin treatment on biochemical indices of bone remodeling. J Clin Endocrinol Metabol. 1989;69:523–527. doi: 10.1210/jcem-69-3-523. [DOI] [PubMed] [Google Scholar]
- Strange R, Li F, Saurer S, Burkhardt A, Friis RR. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development. 1992;115:49–58. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Bissell MJ. Expression of extracellular matrix components is regulated by substratum. J Cell Biol. 1990;110:1405–1415. doi: 10.1083/jcb.110.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs M, York DA. Central glucocorticoid regulation of parasympathetic drive to pancreatic B-cells in the obese fa/fa rat. Int J Obes. 1991;15:547–553. [PubMed] [Google Scholar]
- Sunyer T, Lewis J, Osdoby P. Estrogen decreases the steady state levels of the IL-1 signaling receptor (Type I) while increasing those of the IL-1 decoy receptor (Type II) mRNAs in human osteoclast-like cells. J Bone Miner Res. 1997;12:S135. [Google Scholar]
- Suzuki M, Abe K, Yoshinaga K, Obinata M, Furusawa M. Specific arrest of spermatogenesis caused by apoptotic cell death in transgenic mice. Genes to Cells. 1996;1:1077–1086. doi: 10.1046/j.1365-2443.1996.d01-228.x. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S, Lessey B, Satyaswaroop PG. Temporal and site-specific expression of transforming growth factor-beta4 in human endometrium. Mol Hum Reproduct. 1998;4:595–602. doi: 10.1093/molehr/4.6.595. [DOI] [PubMed] [Google Scholar]
- Tapanainen JS, Tilly JL, Vihko KK, Hsueh AJ. Hormonal control of apoptotic cell death in the testis: gonadotropins and androgens as testicular cell survival factors. Mol Endocrinol. 1993;7:643–650. doi: 10.1210/mend.7.5.8316250. [DOI] [PubMed] [Google Scholar]
- Tarcic N, Ovadia H, Weiss DW, Weidenfeld J. Restraint stress-induced thymic involution and cell apoptosis are dependent on endogenous glucocorticoids. J Neuroimmunol. 1998;82:40–46. doi: 10.1016/S0165-5728(97)00186-0. [DOI] [PubMed] [Google Scholar]
- Tenniswood MP, Guenette RS, Lakins J, Mooibroek M, Wong P, Welsh JE. Active cell death in hormone-dependent tissues. Cancer Metastasis Rev. 1992;11:197–220. doi: 10.1007/BF00048064. [DOI] [PubMed] [Google Scholar]
- Terada N, Yamamoto R, Takada T, Miyake T, Terakawa N, Wakimoto H, Taniguchi H, Li W, Kitamura Y, Matsumoto K. Inhibitory effect of progesterone on cell death of mouse uterine epithelium. J Steroid Biochem. 1989;33:1091–1096. doi: 10.1016/0022-4731(89)90414-7. [DOI] [PubMed] [Google Scholar]
- Thompson EB. Apoptosis and steroid hormones. Mol Endocrinol. 1994;8:665–673. doi: 10.1210/mend.8.6.7935482. [DOI] [PubMed] [Google Scholar]
- Thompson EB. Mechanisms of T-cell apoptosis by glucocorticoids. Trends Endocrinol Metabol. 1999;10:353–358. doi: 10.1016/s1043-2760(99)00187-3. [DOI] [PubMed] [Google Scholar]
- Thompson EB, Thulasi R, Saeed MF, Johnson BH. Glucocorticoid antagonist RU 486 reverses agonist-induced apoptosis and c-myc repression in human leukemic CEM-C7 cells. Ann N Y Acad Sci. 1995;761:261–275. doi: 10.1111/j.1749-6632.1995.tb31383.x. [DOI] [PubMed] [Google Scholar]
- Thompson EB, Medh RD, Zhou F, Ayala-Torres S, Ansari N, Zhang W, Johnson BH. Glucocorticoids, oxysterols, and cAMP with glucocorticoids each cause apoptosis of CEM cells and suppress c-myc. J Steroid Biochem Mol Biol. 1999;69:453–461. doi: 10.1016/s0960-0760(99)00063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JS. Somatostatin analogue predisposes enterocytes to apoptosis. J Gastrointest Surg. 1998;2:167–173. doi: 10.1016/s1091-255x(98)80009-7. [DOI] [PubMed] [Google Scholar]
- Thompson JS. Epidermal growth factor inhibits somatostatin-induced apoptosis. J Surg Res. 1999;81:95–100. doi: 10.1006/jsre.1998.5468. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Billig H, Kowalski KI, Hsueh AJ. Epidermal growth factor and basic fibroblast growth factor suppress the spontaneous onset of apoptosis in cultured rat ovarian granulosa cells and follicles by a tyrosine kinase-dependent mechanism. Mol Endocrinol. 1992;6:1942–1950. doi: 10.1210/mend.6.11.1480180. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Tilly KI, Kenton ML, Johnson AL. Expression of members of the bcl-2 gene family in the immature rat ovary: equine chorionic gonadotropin-mediated inhibition of granulosa cell apoptosis is associated with decreased bax and constitutive bcl-2 and bcl-xlong messenger ribonucleic acid levels. Endocrinology. 1995;136:232–241. doi: 10.1210/endo.136.1.7828536. [DOI] [PubMed] [Google Scholar]
- Travers MT, Barber MC, Tonner E, Quarrie L, Wilde CJ, Flint DJ. The role of prolactin and growth hormone in the regulation of casein gene expression and mammary cell survival: relationships to milk synthesis and secretion. Endocrinology. 1996;137:1530–1539. doi: 10.1210/endo.137.5.8612482. [DOI] [PubMed] [Google Scholar]
- Trump BF, Berezesky IK, Chang SH, Phelps PC. The pathways of cell death: oncosis, apoptosis, and necrosis. Toxicol Pathol. 1997;25:82–88. doi: 10.1177/019262339702500116. [DOI] [PubMed] [Google Scholar]
- Tsafriri A, Braw RH. Experimental approaches to atresia in mammals. Oxf Rev Reprod Biol. 1984;6:226–265. [PubMed] [Google Scholar]
- Tsai CH, Hill M, Asa SL, Brubaker PL, Drucker DJ. Intestinal growth-promoting properties of glucagon-like peptide-2 in mice. Am J Physiol. 1997;273:E77–E84. doi: 10.1152/ajpendo.1997.273.1.E77. [DOI] [PubMed] [Google Scholar]
- Tugwood JD, Issemann I, Anderson RG, Bundell KR, McPheat WL, Green S. The mouse peroxisome proliferator activated receptor recognizes a response element in the 5′ flanking sequence of the rat acyl CoA oxidase gene. EMBO J. 1992;11:433–439. doi: 10.1002/j.1460-2075.1992.tb05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucker DS, Hebshi LD, Blomquist JF, Torbett BE. Physiological T-cell death: susceptibility is modulated by activation, aging, and transformation, but the mechanism is constant. Immunol Rev. 1994;142:273–299. doi: 10.1111/j.1600-065x.1994.tb00893.x. [DOI] [PubMed] [Google Scholar]
- Ulshen MH, Dowling RH, Fuller CR, Zimmermann EM, Lund PK. Enhanced growth of small bowel in transgenic mice overexpressing bovine growth hormone. Gastroenterology. 1993;104:973–980. doi: 10.1016/0016-5085(93)90263-c. [DOI] [PubMed] [Google Scholar]
- Uren AG, Vaux DL. Molecular and clinical aspects of apoptosis. Pharmacol Ther. 1996;72:37–50. doi: 10.1016/s0163-7258(96)00098-8. [DOI] [PubMed] [Google Scholar]
- Vacchio MS, Ashwell JD. Thymus-derived glucocorticoids regulate antigen-specific positive selection. J Exp Med. 1997;185:2033–2038. doi: 10.1084/jem.185.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacchio MS, Papadopoulos V, Ashwell JD. Steroid production in the thymus: implications for thymocyte selection. J Exp Med. 1994;179:1835–1846. doi: 10.1084/jem.179.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacchio MS, King LB, Ashwell JD. Regulation of thymocyte development by glucocorticoids. Behring Inst Mitt. 1996:24–31. [PubMed] [Google Scholar]
- Vale W, Rivier C, Hsueh A, Campen C, Meunier H, Bicsak T, Vaughan J, Corrigan A, Bardin W, Sawchenko P. Chemical and biological characterization of the inhibin family of protein hormones. Recent Prog Horm Res. 1988;44:1–34. doi: 10.1016/b978-0-12-571144-9.50005-3. [DOI] [PubMed] [Google Scholar]
- Van Parijs L, Ibraghimov A, Abbas AK. The roles of costimulation and Fas in T cell apoptosis and peripheral tolerance. Immunity. 1996;4:321–328. doi: 10.1016/s1074-7613(00)80440-9. [DOI] [PubMed] [Google Scholar]
- Van RL, Roncari DA. Complete differentiation of adipocyte precursors. A culture system for studying the cellular nature of adipose tissue. Cell Tissue Res. 1978;195:317–329. doi: 10.1007/BF00236728. [DOI] [PubMed] [Google Scholar]
- Vanderschueren D, Van Herck E, Suiker AM, Visser WJ, Schot LP, Bouillon R. Bone and mineral metabolism in aged male rats: short and long term effects of androgen deficiency. Endocrinology. 1992;130:2906–2916. doi: 10.1210/endo.130.5.1572302. [DOI] [PubMed] [Google Scholar]
- Vaughan J. The physiology of bone. Clarendon Press; Oxford: 1981. [Google Scholar]
- Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, Haimovitz-Friedman A, Fuks Z, Kolesnick RN. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- von Boehmer H, Kisielow P. Self-nonself discrimination by T cells. Science. 1990;248:1369–1373. doi: 10.1126/science.1972594. [DOI] [PubMed] [Google Scholar]
- Wagner DH, Jr, Hagman J, Linsley PS, Hodsdon W, Freed JH, Newell MK. Rescue of thymocytes from glucocorticoid-induced cell death mediated by CD28/CTLA-4 costimulatory interactions with B7-1/B7-2. J Exp Med. 1996;184:1631–1638. doi: 10.1084/jem.184.5.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker NI, Bennett RE, Kerr JF. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am J Anat. 1989;185:19–32. doi: 10.1002/aja.1001850104. [DOI] [PubMed] [Google Scholar]
- Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom P, Westin P, Stattin P, Damber JE, Bergh A. Early castration-induced upregulation of transforming growth factor beta1 and its receptors is associated with tumor cell apoptosis and a major decline in serum prostate-specific antigen in prostate cancer patients. Prostate. 1999;38:268–277. doi: 10.1002/(sici)1097-0045(19990301)38:4<268::aid-pros2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Lyon RJ, Hansen SE, Rice GC, Mather JP. Inhibin and activin locally regulate rat ovarian folliculogenesis. Endocrinology. 1990;127:3196–3205. doi: 10.1210/endo-127-6-3196. [DOI] [PubMed] [Google Scholar]
- Woolveridge I, de Boer-Brouwer M, Taylor MF, Teerds KJ, Wu FC, Morris ID. Apoptosis in the rat spermatogenic epithelium following androgen withdrawal: changes in apoptosis-related genes. Biol Reprod. 1999;60:461–470. doi: 10.1095/biolreprod60.2.461. [DOI] [PubMed] [Google Scholar]
- Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- York DA. Lessons from animal models of obesity. Endocrinol Metabol Clin N Am. 1996;25:781–800. doi: 10.1016/s0889-8529(05)70354-6. [DOI] [PubMed] [Google Scholar]
- Zacharchuk CM, Mercep M, Chakraborti PK, Simons SS, Jr, Ashwell JD. Programmed T lymphocyte death. Cell activation- and steroid-induced pathways are mutually antagonistic. J Immunol. 1990;145:4037–4045. [PubMed] [Google Scholar]
- Zarjevski N, Cusin I, Vettor R, Rohner-Jeanrenaud F, Jeanrenaud B. Chronic intracerebroventricular neuropeptide-Y administration to normal rats mimics hormonal and metabolic changes of obesity. Endocrinology. 1993;133:1753–1758. doi: 10.1210/endo.133.4.8404618. [DOI] [PubMed] [Google Scholar]
- Zhou YT, Shimabukuro M, Koyama K, Lee Y, Wang MY, Trieu F, Newgard CB, Unger RH. Induction by leptin of uncoupling protein-2 and enzymes of fatty acid oxidation. Proc Natl Acad Sci U S A. 1997;94:6386–6390. doi: 10.1073/pnas.94.12.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman Y, Yefenof E, Oron E, Dorogin A, Guy R. T cell receptor-independent apoptosis of thymocyte clones induced by a thymic epithelial cell line is mediated by steroids. Cell Immunol. 1996;170:78–84. doi: 10.1006/cimm.1996.0136. [DOI] [PubMed] [Google Scholar]