Abstract
We report the design and synthesis of an orthogonally protected peptide nucleic acid (PNA) building block, Fmoc-PNA-U'-(Dde)-OH and its use in the construction of PNA FRET probes. This building block allows for the post-synthetic attachment of reporter groups to the amino group attached to the 5-position of U following selective deprotection of the Dde group. We illustrate the use of this building block for the synthesis of a series of FAM Cy5 donor acceptor pairs and their ability to detect a target DNA sequence.
Keywords: peptide nucleic acid, fluorescein, Cy5, FRET, orthogonally protected, labeling
The ability to directly detect and image gene expression inside living cells and organisms would greatly aid biological and biomedical research. One approach to image gene expression is to use antisense probes targeted to the mRNA of interest. Because endogenous mRNA transcripts are of very low copy number (1-10,000 copies/cell) it is critical that the signal from an antisense probe is greater than the background signal from unbound agent in the cell. To accomplish this, various types of fluorogenic antisense probes have been developed that are designed to become fluorescent only upon binding to the target mRNA, most notably molecular beacons and binary FRET1 probes.
Molecular beacons consist of a single antisense agent that bears a fluorescent donor on one end, and a quencher on the other (1, 2). In the absence of the target, the molecular beacon folds in such a way to bring the donor in close proximity to the quencher which quenches the fluorescence of the donor. In the presence of a target, the beacon unfolds to form a duplex, thereby distancing the quencher from the donor which results in a strong fluorescence signal. In principle molecular beacons should show high specificity and signal to noise but in practice, non-specific interactions with other biomolecules in a cell can open the beacon and activate fluorescence, as can cleavage of the backbone or linkers, which diminish the signal to noise.
Binary FRET probes consist of a pair of antisense agents one bearing a donor fluorophore, and the other, an acceptor fluorophore. When present in low concentrations, energy transfer from the donor to the acceptor can only take place efficiently in the presence of a complementary template sequence which brings the two components together. As a result, binary FRET probes cannot be activated by non-specific binding or degradation as can molecular beacons, and should possess much higher specificity and signal to noise. The first report of using binary FRET probes to detect a nucleic acid sequence in vitro came in 1988 (3), but it wasn't until 2000 that detection of an mRNA by a binary FRET probe in living cells was reported. Tsuji and coworkers showed that a pair of FRET probes could detect about 10,000 copies of c-fos mRNA produced from an over-expression vector by fluorescence microscopy (4). Later they showed that they could detect about 900 copies by using time-resolved fluorescence imaging (5). They have also been able to detect about 10,000 copies of IL-2 mRNA by flow cytometry (6). To improve signal to noise, Bao and coworkers have developed dual FRET molecular beacons, in which the donor and acceptor probes are made into molecular beacons, which only become fluorescent when bound to the target mRNA. More recently, Kool and coworkers demonstrated ability to detect mRNA in vivo by flow cytometry with a new class of quenched autoligating FRET probes (7).
Though the initial results in detecting mRNA in living cells by the binary FRET probes are promising, obtaining adequate signal to noise has been a problem. The FRET probes used in the previous cellular studies were all constructed from DNA, which can be degraded in vivo and thereby reduce the signal. Also, the RNA of DNA-RNA hybrids can be cleaved by RNAseH which would further reduce the signal. Another disadvantage of DNA is that it is negatively charged, making it difficult to penetrate regions of folded RNA that would have high negative electrostatic potential. Peptide nucleic acids (PNAs) are a synthetic mimic of DNA with an electrically neutral amide backbone that recognize DNA or mRNA in a sequence specific manner by Watson-Crick base pairing rules (8). PNA has become a particularly attractive nucleic acid analog for the development of therapeutics and diagnostics because of its ability to form more stable duplexes with RNA than DNA, its stability to proteases and nucleases, and its ability to invade regions of secondary structure in RNA, and not activate RNAse H (9, 10).
PNA has recently been used for the construction of binary FRET probes in which the donor and acceptor fluorophores were attached to the ends of two PNAs (11). To maximize the FRET signal, however, the two PNAs had to bind to sites separated by about 7 bases. In designing FRET probes for folded mRNA in vivo, it would be easier if one only had to target a single accessible site, rather than two accessible sites that are within 5-10 nt of each other, which would be much rarer. When targeting a single site, however, one of the fluorophores would have to be attached to an internal position of the PNA to maximize the FRET signal. More recently, PNAs internally labeled at C's with donor and acceptor fluorophores were used to create ligatible FRET probes (12).
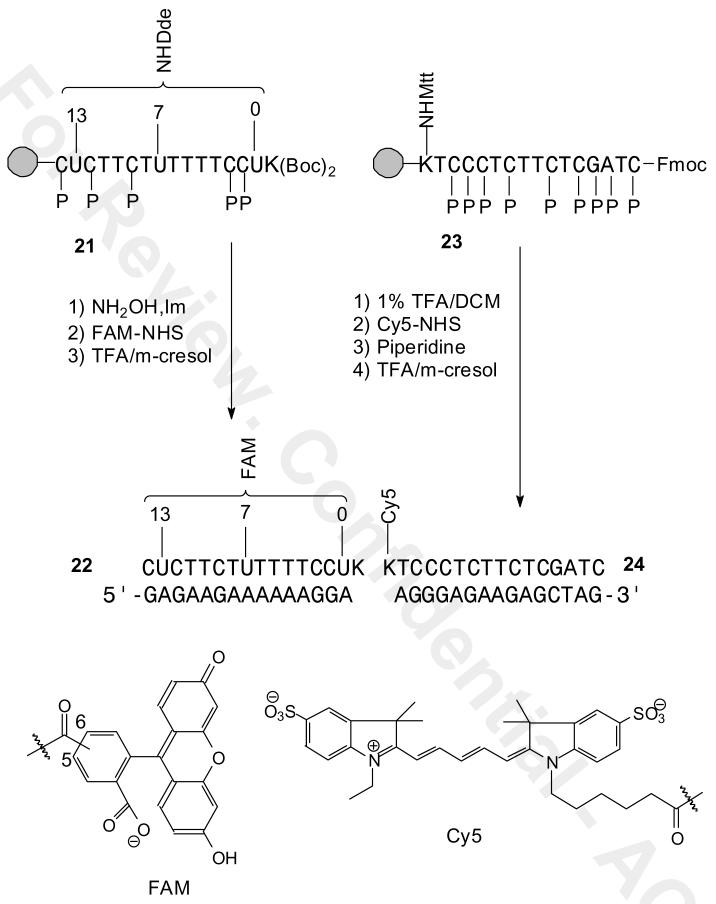
Unlike endogenous genes, trans-genes can be genetically engineered to produce a high number of transcripts, and to have any desired sequence. To take advantage of these features for the development of binary PNA FRET probes, we have undertaken the design and synthesis of mRNA cassettes that can be appended to a transgene to tag the mRNA with a repeating antisense site. Such a multimeric antisense tag would allow for the simultaneous binding of multiple binary FRET probes and could thereby greatly amplify the signal from such probes. To obtain an optimal signal output from a binary FRET probe requires that the distance between donor and acceptor can be varied, and the optimal placement of the donor and acceptor may ultimately depend on the number of cassettes and the conformation of the mRNA in vivo. Our initial design of an antisense cassette sequence is one that is devoid of secondary structure, and is purine rich. The sequence contains numerous A's and A-tracts, making it possible to easily vary the position of donor or acceptor fluorophores in a pair of adjacent PNAs through the use of a derivatizable T (Figure 1). Runs of purines in the mRNA sequence would also make it possible to further increase the affinity of a probe by triplex clamp formation. The purine rich nature of the antisense cassette is also optimal for the complementary PNA sequence, as empirical rules dictate that optimal PNAs have <60% purine content, and no more than four purines or three guanines in a row (http://www.appliedbiosystems.com/support/seqguide.cfm)(13).
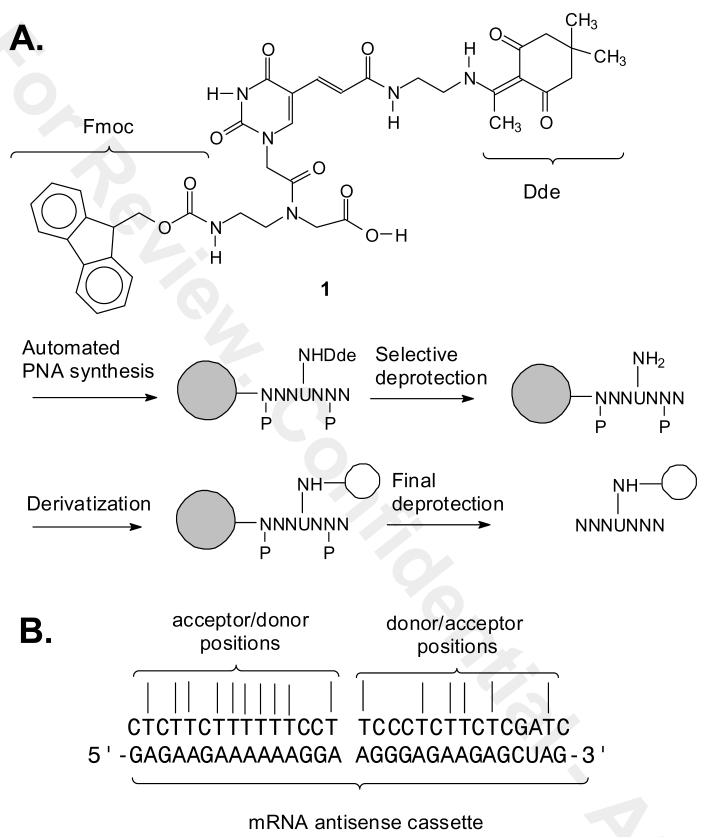
Figure 1.
A) Structure of the orthogonally protected Fmoc-PNA-U′-(Dde)-OH building block and its use for selectively derivatizing T's of PNAs. B) Antisense cassette sequence and the possible complementary binary FRET PNA probes that could be prepared.
PNAs have been derivatized with fluorophores directly through the amino group at the amino terminus, or at both amino or carboxy termini via addition of amino acids such as lysine or cysteine. Internally labeled PNAs have been prepared by Fmoc or Boc synthesis via derivatization of alkylamino derivative of the amino groups of A, C and G (14) or at C or T by Boc synthesis through an aminoalkyl-modified backbone (15). For detecting our antisense cassette, however, we required an Fmoc PNA building block for derivatizing at T. We have previously developed a building block for introducing C5-thiomethyl uracil (U) into PNA by Fmoc synthesis which can be post-synthetically derivatized via maleimide-linked reagents, though we found that a large excess of the maleimide is required to obtain a good yield of product (16). For our purposes, we decided to develop a new Fmoc building block for introducing a donor or acceptor fluorophore to a T through an amine linker. Furthermore we wished that the linker have a well defined orientation so that it might also be used to better report on the relative orientation between donor and acceptor groups. To this end we designed two routes to a C5-derivative of U, compound 1, containing a Dde-protected alkylamino group which we show can be selectively deprotected and derivatized prior to complete deprotection of the PNA (Figure 1). We chose the Dde group because it has been previously shown to be compatible with Fmoc PNA synthesis and orthogonal to the other protecting groups (17). We also demonstrate the application of our building block to the synthesis of a binary FRET probe for the detection of the DNA sequence corresponding to our antisense mRNA cassette.
EXPERIMENTAL
General Procedures
All reactions were performed under an argon or nitrogen atmosphere unless otherwise specified. All commercially available materials were used without further purification unless otherwise noted. Anhydrous solvents for reactions, such as DMF, DCM, and THF were either used as obtained from Sigma-Aldrich or distilled from an appropriate drying agent, or dried with 4 Å molecular sieves. Analytical thin-layer chromatography (TLC) was performed using either Sigma-Aldrich's pre-coated silica gel plastic sheets 60 F254 (layer thickness of 250 μm) or general purpose pre-coated silica gel glass sheets 60 F254 (250 μm) for compounds that are difficult to resolve on the plastic TLC plates. Molecules were visualized using UV light unless otherwise stated. Flash column chromatography was carried out on E. Merck (Bodman Industries, Aston, PA) silica gel 60 (40-63 μm) using the stated solvent system. 1H NMR and 13C NMR spectra were obtained on a Varian Mercury-300, Varian Oxford-300 and VarianUnityPlus-300 (all 300 MHz for 1H NMR and 75 MHz for 13C NMR spectrometers). The chemical shifts are expressed in parts per million (ppm) using deuterated NMR solvents in reference to TMS at 0 ppm.
Synthesis of tert-butyl 2-(5-iodo-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl) acetate (3)
To a suspension of iodouracil 2 (11.9 g, 50 mmol) and anhydrous K2CO3 (7.6 g, 55 mmol) in anhydrous DMF (175 mL) at 0 °C, tert-butylbromoacetate (9.75 g, 50 mmol) in anhydrous DMF (25 mL) was slowly added via syringe under a nitrogen atmosphere. After addition, the resulting reaction mixture was warmed to rt and vigorously stirred for 24 h. The by-product precipitate of KBr salt was filtered under vacuum and washed with 10 mL of DMF. The solvent was evaporated in vacuo, and diluted with EtOAc (200 mL) and washed with water (3 × 100 mL). The aqueous phase was back extracted with EtOAc (3 × 100 mL). The organic layers were combined, dried over Na2SO4 and evaporated to dryness in vacuo to yield the crude product. The product was then precipitated by adding cold water and collected in a sintered glass filter and dried in vacuum overnight to give compound 3 as a white solid, 16.5 g, 94 % yield. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 11.8 (s, 1 H), 8.2 (s, 1 H), 4.4 (s, 2 H), 1.4 (s, 9 H); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 177.5, 166.9, 161.0, 150.5, 150.1, 81.9, 67.9, 49.0, 27.6; ESI-MS (positive ion mode) calcd for C10H14IN2O4 [M + H]+ 352.99; found 353.00.
Synthesis of (E)-3-(1-(2-tert-butoxy-2-oxoethyl)-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)acrylic acid (4)
To a clean, dry 250 mL round bottom flask was added tert-butyl-2-(5-iodo-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl) acetate (3) (5.28 g, 15 mmol), and N-tetrabutylammonium bromide (5.80 g, 18 mmol) and dissolved in anhydrous DMF (70 mL). Then, acrylic acid (2.16 g, 30 mmol) in dry DMF (10 mL) was slowly added to the reaction mixture via a stainless steel syringe under nitrogen. A mixture of DMF/water/DIEA (10:10:10 mL) was added via syringe, and the reaction was evacuated (freed of any oxygen gas) and kept with stirring at rt under nitrogen. The catalyst, Pd(OAc)2 (3% mol, 1.01 g, 4.5 mmol) was added under nitrogen. The flask was again evacuated and kept under nitrogen atmosphere. The reaction mixture was then heated to 80-90 °C in an oil bath for 16 h. The reaction mixture turned black for the first few hours and then palladium particles precipitated while the solution turned yellow. The black particles were filtered under vacuum, and the DMF was evaporated in vacuo at an elevated temperature. The reaction mixture was then washed with water (25 mL) and diethyl ether (5 × 50 mL). The organics were combined, dried over Na2SO4, and evaporated to give the crude product as yellow oil. The product (4) was then precipitated by adding DCM/Et2O mixture and few drops of hexane in an ice bath. The product was collected by vacuum filtration in a sintered glass funnel and dried in vacuum overnight to give compound 4 as a yellow powder, 2.4 g, 54% yield. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 12.3 (br, 1 H), 11.8 (s, 1 H), 8.3 (s, 1 H), 7.2 (d, J = 16 Hz, 1 H), 6.8 (d, J = 16 Hz, 1 H), 4.4 (s, 2 H), 1.4 (s, 9 H); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 168.5, 167.4, 162.9, 150.4, 149.3, 137.3, 118.5, 108.4, 82.8, 50.4, 28.3; LR ESI-MS (positive mode) calcd for C13H17IN2O6 [M + H]+ 297.11; found 297.11.
Synthesis of (E)-2,5-dioxopyrrolidin-1-yl-3-(1-(2-tert-butoxy-2-oxoethyl)-2, 4-dioxo1,2,3,4-tetrahydropyrimidin-5-yl)acrylate (5)
To (E)-3-(1-(2-tert-butoxy-2-oxoethyl)-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)acrylic acid (4) (0.59 g, 2.0 mmol) and NHS (0.46 g, 4 mmol) and DIEA (1.1 mL, 0.83 g, 6.4 mmol) in anhydrous DMF (20 mL) at 0 °C was added EDC·HCl (0.81 g, 4.2 mmol) in dry DMF (10 mL) dropwise via syringe under nitrogen. The reaction mixture was then warmed to rt and stirred for 12 h. The reaction was judged over by TLC, and the solvent was evaporated in vacuo. The crude product was purified by flash chromatography on a silica gel by with 5% MeOH in DCM. The solvents were evaporated, and the product was dried in vacuum overnight to give 0.59 g 5 as a white solid in 75% yield. The product was immediately used for the next step owing to its instability. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 8.1 (s, 1 H), 7.6 (d, J = 16 Hz, 1 H), 7.2 (d, J = 16 Hz, 1 H), 4.5 (s, 2 H), 2.8 (s, 4 H), 1.5 (s, 9 H); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 177.5, 170.3, 166.4, 162.6, 161.9, 151.6, 149.4, 142.9, 109.2, 107.1, 82.2, 50.0, 27.5, 25.4.
Synthesis of the TFA salt of 2-(1-(2-aminoethylamino)ethylidene)-5,5-dimethyl-1,3-cyclohexanedione (6)
Tert-butyl 2-(1-(4,4-dimethyl-2,6-dioxocyclohexylidene)ethylamino) ethylcarbamate, (18) (0.84 g, 2.6 mmol) was suspended in dry DCM (11.25 mL) and cooled to 0 °C. TFA (3.75 mL, 25 %) was added dropwise via a syringe under atmospheric pressure. The reaction was allowed to occur at 0 °C for 30 minutes and then warmed to rt, and stirred for another 30 minutes. After 1 h, the reaction was judged completed by TLC analysis in 5% MeOH/DCM (Rf = 0). The solvent was evaporated in vacuo, and the residual TFA was removed by repeatedly evaporating from chloroform in small portions to give the product 6 as a yellow oil in quantitative yield (0.58 g). The product was immediately used for the next step without further purification, owing to its high instability. 1H NMR (300 MHz, CDCl3) δ (ppm): 3.9 (t, J = 16 Hz, 2 H), 3.3 (t, J = 16 Hz, 2 H), 2.7 (s, 3 H), 2.5 (s, 4 H), 1.1 (s, 6 H).
Synthesis of (E)-tert-butyl 2-(5-(3-(2-(1-(4,4-dimethyl-2,6-dioxocyclohexylidene)ethylamino)ethylamino)-3-oxoprop-1-enyl)-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetate (7)
The basic form of compound 6 (0.17 g, 0.77 mmol) was freshly prepared by addition of DIEA until about pH 7-7.5 as judged by pH paper, and dissolved in dry DMF (5 mL). The solution was then slowly added via syringe under nitrogen to a reaction flask containing the NHS ester 5 (0.250 g, 0.64 mmol) in dry DMF (5 mL) at 0 °C and then warmed to rt and stirred for 4-6 h under nitrogen. The solvent was evaporated in vacuo, and the crude reaction mixture was dissolved in 70 mL of DCM and was extracted with 1 M aq. HCl (3 × 30 mL). The aqueous phase was back extracted with DCM and the organic layers were combined and concentrated in vacuo to give the crude product, which was chromatographed on silica gel with 5% MeOH in DCM to give 0.27 g compound 7 as a pure white solid in 85% yield. TLC in 5% MeOH/DCM, Rf = 0.25. 1H NMR (300 MHz, CD3OD) δ (ppm): 7.9 (s, 1 H), 7.2 (d, J = 16 Hz, 1 H), 7.1 (d, J = 16 Hz, 1 H), 4.5 (s, 2 H), 3.7 (t, J = 6 Hz, 2 H), 3.5 (t, J = 6 Hz, 2 H), 2.6 (s, 3 H), 2.4 (s, 4 H), 1.5 (s, 9 H) 1.0 (s, 6 H); 13C NMR (75 MHz, CD3OD) δ (ppm): 200.0, 176.1, 169.7, 168.4, 164.0, 151.8, 149.3, 134.5, 122.0, 110.7, 109.0, 84.1, 53.5, 51.0,49.9, 43.5, 39.9, 31.0, 28.4, 18.4; LR ESI-MS (positive ion mode) calcd for C25H35N4O7 [M + H]+ 503.25; found 503.24.
Synthesis of (E)-2-(5-(3-(2-(1-(4,4-dimethyl-2,6-dioxocyclohexylidene)ethyl-amino)ethylamino)-3-oxoprop-1-enyl)-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl) acetic acid (8)
Compound 7 (2.5 g, 5.0 mmol) was dissolved in CH2Cl2 (15 mL), and TFA (15 mL) was slowly added dropwise via syringe at 0 °C. The reaction mixture was then warmed to rt after addition of the reagents and stirred for 4 h. The reaction was monitored by TLC (5% MeOH in DCM) until the disappearance of the starting material and appearance of a new spot near the base line. After the reaction was complete, the solvent was evaporated in vacuo, and the residual TFA was co-evaporated several times with chloroform, and the resulting solid was triturated with methanol. The solid product was dried in vacuo and isolated by filtering through a sintered glass funnel under vacuum to afford 2.2 g of compound 8 as a white solid (quantitative yield). 1H NMR (300 MHz, DMSO-d6) δ (ppm): 13.2 (t, J = 6 Hz, 1 H), 11.7 (s, 1 H), 8.4 (t, J = 6 Hz, 1 H), 8.1 (s, 1 H), 7.1 (d, J = 16 Hz, 1 H), 7.0 (d, J = 16 Hz, 1 H), 4.5 (s, 2 H), 3.6 (m, 2 H), 3.4 (m, 2 H), 2.5 (s, 3 H), 2.3 (s, 4 H), 0.9 (s, 6 H); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 196.4, 173.2, 169.3, 166.1, 162.3, 149.8, 148.4, 132.1, 120.9, 108.2, 52.4, 49.0, 42.0, 40.3, 38.7, 38.4, 29.8, 27.9, 17.3; LR ESI-MS (positive ion mode): calcd for C21H27N4O7 [M + H]+ 447.19; found 447.18.
Synthesis of (E)-tert-butyl 2-(N-(2-(((9H-fluoren-9-yl)methoxy)carbonylami no)ethyl)-2-(5-(3-(2-(1-(4,4-dimethyl-2,6-dioxocyclohexylidene)ethylamino)ethylamino)-3-oxoprop-1-enyl)-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido) acetate (10)
To a solution of the carboxylic acid 8 (2.2 g, 5.1 mmol) and amine 9 (19 5315) (1 equiv, 2.2 g, 5.1 mmol) in dry DMF (30 mL) was added redistilled DIEA (2.1 mL, 1.5 g, 11.7 mmol) via syringe under nitrogen, and the temperature was lowered to 0 °C. In a separate round bottom flask, HATU (1.2 equiv, 2.3 g, 6.1 mmol) was dissolved in anhydrous DMF (10 mL) and transferred to the reaction mixture via syringe under a nitrogen, at 0 °C. The reaction was kept at 0 °C for 1 h and then warmed to rt and stirred for 8 h. The reaction was monitored by TLC (5% MeOH in DCM). The reaction mixture was worked up by pouring into 100 mL of DCM and washed with water (8 × 50 mL). The aqueous and DCM layers did not separate completely and both the clear organic layer and emulsion layer were collected, dried over Na2SO4 and evaporated in vacuo. The crude product was purified by flash chromatography with 5% MeOH in DCM to give 3.9 g of compound 10 as a white solid in 93% yield. TLC: 5% MeOH/DCM (Rf = 0.20). 1H NMR (300 MHz, acetone-d6) δ (ppm): 13.5 (t, J = 6 Hz, 1 H), 10.4 (s, 1 H), 7.9 (d, J = 16 Hz, 2 H), 7.8 (s, 1 H), 7.7 (d, J = 16 Hz, 2 H), 7.4 (t, J = 16 Hz, 2 H), 7.3 (t, J = 16 Hz, 2 H), 7.2 (d, J = 16 Hz, 1 H), 7.1 (d, J = 16 Hz, 1 H), 4.8 (s, 2 H), 4.4 - 4.2 (m, 3 H), 4.0 (s, 2 H), 3.7 - 3.3 (m, 8 H), 2.6 (s, 3 H), 2.3 (s, 4 H), 1.5 (s, 9 H), 1.0 (s, 6 H); 13C NMR (75 MHz, acetone-d6) δ (ppm): 205.7, 162.8, 162.3, 161.1, 158.9, 148.6, 144.5, 141.5, 132.9, 127.9, 127.4, 125.5, 121.4, 120.2, 109.3, 109.2, 80.3, 66.5, 66.3, 49.2, 48.7, 47.4, 42.6, 39.2, 39.0, 27.8, 27.6, 17.2; LR MS-ESI (positive ion mode) calcd for C44H532N6O10 [M + H]+ 825.38; found 825.36.
Synthesis of (E)-2-(N-(2-(((9H-fluoren-9-yl)methoxy)carbonylamino)ethyl)-2 -(5-(3-(2-(1-(4,4-dimethyl-2,6-dioxocyclohexylidene)ethylamino)ethylamino)-3-oxoprop-1-enyl)-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)acetic acid; Fmoc-U' (Dde)-OH (1)
TFA (7 mL) was slowly added via syringe at 0 °C to a solution of compound 10 (0.825 g, 1.0 mmol) in DCM (7 mL). The reaction mixture was then warmed to rt and stirred for 4-5 h. The reaction was monitored by TLC (DCM/MeOH; 5:1) and judged complete in 4-5 h after which the solvents were evaporated in vacuo and the residual TFA co-evaporated with CCl4 several times, and then evaporated from methanol to give 0.77 g of Fmoc-U'(Dde)-OH (1) as a white solid in quantitative yield. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 13.3 (t, J = 6 Hz, 1 H), 11.7 (s, 1 H), 8.4 (t, J = 6 Hz, 1 H), 8.0 (s, 1 H), 7.9 (d, J = 16 Hz, 2 H), 7.7 (d, J = 16 Hz, 2 H), 7.4 (t, J = 16 Hz, 2 H), 7.3 (t, J = 16 Hz, 2 H), 7.1 (d, J = 16 Hz, 1 H), 7.0 (d, J = 16 Hz, 1 H), 4.8 (s, 2 H), 4.3-4.2 (m, 3 H), 4.0 (s, 2 H), 3.6-3.2 (m, 8 H), 2.5 (s, 3 H), 2.3 (s, 4 H), 0.9 (s, 6 H); 13CNMR (75 MHz, DMSO-d6) δ (ppm): 196.3, 173.0, 170.3, 166.1, 162.3, 149.7, 148.6, 143.8, 140.7, 132.1, 127.5, 127.0, 125.1, 120.6, 120.0, 108.0, 65.4, 52.3, 48.2, 47.7, 46.7, 41.9, 38.3, 29.7, 27.8, 17.2; LR ESI-MS (positive ion mode) calcd for for C40H45N6O10 [M + H] 769.32; found 769.28.
Synthesis of (E)-tert-butyl 2-(3-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl) acrylamido)ethylcarbamate (13)
To the (E)-3-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5 -yl)acrylic acid (11) (20) (0.55 g, 3.0 mmol) and N-hydroxysuccinimide, NHS, (0.38 g, 3.3 mmol) was added anhydrous DMF (10 mL) under nitrogen. Then, mono-Boc ethylenediamine (12) (0.72 g, 4.5 mmol) (Alfa Aesar) and diisopropylamine (0.85 g, 1.2 mL, 6.6 mmol) in DMF (5 mL) were added dropwise via syringe to the reaction mixture. The reaction mixture was cooled to 0 °C with stirring under nitrogen. Then, EDC·HCl (0.632 g, 3.3 mmol) dissolved in anhydrous DMF (10 mL) was slowly added to the reaction mixture at 0 °C. After addition, the reaction mixture was allowed to warm to rt, and stirred for overnight. The DMF was evaporated at elevated temperature (100-110 °C) in vacuo. The crude product was purified by flash chromatography by using 5% MeOH in DCM to afford 0.83 g of compound 13 as an off white solid in 85% yield. Analytically pure compound was obtained by recrystallization from ethanol/ether. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 11.3 (s, 1 H), 8.1 (t, J = 7 Hz, 1 H), 7.9 (s, 1 H), 7.1 (d, J = 16 Hz, 1 H), 7.0 (d, J = 16 Hz, 1 H), 6.8 (t, J = 6 Hz, 1 H), 3.2 (m, 2 H), 3.0 (m, 2 H), 1.4 (s, 9 H); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 165.9, 162.8, 155.5, 150.2, 144.7, 132.4, 120.5, 107.8, 77.5, 40.2, 38.5, 28.1; ESI-MS (positive ion mode) calcd for C14H21N4O5 [M + H]+ 325.1512; found 325.1512.
Synthesis of (E)-N-(2-aminoethyl)-3-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin- 5-yl)acrylamide (14)
Compound 13 (0.70 g, 2.2 mmol) was dissolved in DCM (12 mL), and TFA (4 mL) was slowly added via syringe at 0 °C. The reaction mixture was warmed to rt and stirred for 2 h. The reaction was monitored by TLC in 10% MeOH /DCM until the disappearance of the starting material and appearance of a new spot (product) near the base line. When the reaction was complete, the solvent was evaporated in vacuo, and the remaining solvent was evaporated with small portions of carbon tetrachloride. The product was precipitated by adding a small amount of methanol to give a brownish white solid (0.49 g, 99%). 1H NMR (300 MHz, DMSO-d6) δ (ppm): 9.2 (br, 3 H), 8.3 (t, J = 6 Hz, 1 H), 7.9 (s, 1 H), 7.2-7.1 (d, J = 16 Hz, 1 H), 7.0-6.9 (d, J = 16 Hz, 1 H), 3.4-3.3 (m, 2 H), 2.9 (t, J = 6 Hz, 1 H); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 166.5, 162.8, 150.3, 145.0, 132.9, 120.0, 107.6, 38.7, 36.7; LR ESI-MS (positive ion mode) calcd for C9H13N4O3 [M + H] 225.10; found 225.09.
Synthesis of (E)-N-(2-(1-(4,4-dimethyl-2,6-dioxocyclohexylidene)ethylamino)-ethyl)-3-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)acrylamide (16)
Compound 14 (0.45 g, 2.2 mmol) was freshly prepared as described above and dissolved in dry DMF, and DIEA was slowly added at 0 °C until the pH of the solution was slightly basic. Dde-OH (15) (available from Aldrich) (0.48 g, 2.6 mmol) in DMF was then slowly added via syringe. The reaction mixture was refluxed under nitrogen for 24 h. The solvent was evaporated at elevated temperature and then co-evaporated with EtOH to almost complete dryness. The product was then precipitated out of ethanol in an ice bath to give 0.72 g of compound 16 as a pale brown solid in 84% yield. TLC: in 5% MeOH/DCM (Rf = 0.25). 1H NMR (300 MHz, DMSO-d6) δ (ppm): 13.2 (t, J = 6 Hz, 1 H), 11.3 (s, 2 H), 8.3 (t, J = 6 Hz, 1 H), 7.9 (s, 1 H), 7.1 (d, J = 16, 1 H), 7.0 (d, J = 16, 1 H), 3.6 (m, 2 H) 3.4 (m, 2 H), 2.5 (s, 3 H), 2.3 (s, 4 H), 0.9 (s, 6 H); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 196.3, 173.0, 166.3, 162.8, 150.2, 145.0, 132.9, 119.9, 107.6, 107.0, 52.2, 41.9, 40.2, 38.6, 38.3, 29.6, 27.8, 17.2; ESI-MS (positive ion mode) calcd for C19H25N4O3 [M + H] 389.1825; found 389.1822.
Synthesis of (E)-tert-butyl 2-(5-(3-(2-(1-(4,4-dimethyl-2,6-dioxocyclohexylidene)ethylamino)ethylamino)-3-oxoprop-1-enyl)-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetate (7) from 16
A solution of compound 16 (0.6 g, 1.5 mmol) and anhydrous K2CO3 (0.24 g, 1.7 mmol) were dissolved in anhydrous DMF (8 mL). A solution of t-butylbromoacetate (0.30 g, 1.5 mmol) in anhydrous DMF (4 mL) was slowly added via syringe at 0 °C. The reaction mixture was warmed up to rt and stirred under nitrogen overnight. The precipitate of KBr was filtered and washed several times with methanol (3 × 10 ml). The filtrates were combined, evaporated in vacuo at an elevated temperature, and co-evaporated with EtOH to almost complete dryness. The crude product was dissolved in 100 mL DCM and extracted with water (5 × 30 mL), 1 M HCl (2 × 20 mL), and finally with brine (1 × 20 mL). The aqueous phases were back extracted with DCM (1 × 100 mL), and the organics were combined, dried over Na2SO4, and evaporated in vacuo. The product was purified by flash chromatography by using 5% MeOH in DCM to give 0.69 g of compound 7 as a crystalline white solid in 92% yield with identical spectra as described for 7 prepared from 5.
General PNA Synthesis, Purification and Characterization Procedures
Anhydrous diethyl ether, DIEA, TFA, meta-cresol, hydrazine, hydroxylamine hydrochloride were purchased from Sigma (St. Louis, MO). High purity anhydrous DMF and NMP solvents for the synthesis and dilution of the PNA monomers were used as obtained from either Applied-Biosystems (Foster City, CA) or Sigma-Aldrich (St.- Louis, MO), HATU was purchased either from Applied Biosystems or GenScript Corp. (Piscataway, NJ). The Fmoc PNA monomers A, C, G, T, and reagents for peptide nucleic acid (PNA) synt hesis were from Applied Biosystems or ASM-Research Chemicals (Hannover, Germany). ε-Boc-α-Boc-Lys and ε-Mtt-α-Fmoc-Lys were from EMD Chemicals (Gibbstown, NJ). Cy5-NHS was from Amersham Biosciences Inc. (Piscataway, NJ). FAM-NHS was from Molecular Probes (Eugene, Oregon). Ultrafree-MC 0.2 μM filtered microcentrifuge tubes were used for cleavage of the PNA from the resin (Millipore Inc., Bedford, MA). Labeling of PNAs with fluorophore groups were carried out in anhydrous solvents unless otherwise specified. The synthesis of the PNAs was carried out by solid-phase Fmoc PNA synthesis on Fmoc-XAL-PEG-PS resin on an Expedite 8909 DNA/PNA synthesizer (Applied Biosystems) on a 2 μmol scale. DNA (ODNs) templates for fluorescence solution studies were purchased from Integrated DNA technologies Inc (Coralville, IA). Crude PNA FRET donor and acceptor probes were analyzed and purified by reversed phase high-performance liquid chromatography (RP-HPLC) on a Beckman instrument equipped with a UV array detector. PNAs were eluted with a 65 min, 1 ml/min, 0-70% linear gradient of acetonitrile in 0.1 % aqueous TFA on Varian Microsorb-MV column (C-18, 5 μm, 300 Å pore size, 4.6 × 250 mm). The PNAs were characterized by UV-vis on a Bausch and Lomb Spectronic1001 spectrophotometer or on a Varian Cary 100 Bio UV-Visible spectrophotometer and by fluorescence spectroscopy on a Varian Cary Eclipse fluorescence spectrophotometer in phosphate buffered saline (PBS, pH 7.0, 100 mM). MALDI-TOF mass spectrometry was carried out on a PerSeptive Voyager MALDI-TOF instrument using a sinapinic acid matrix (10 mg in 1 mL of 0.1% TFA in 2:1 H2O-CH3CN, mixed 1:1 with PNA). For PNAs with fluorescent tags, we were unable to obtain good MALDI spectra and instead relied on UV to identify the fractions containing the desired PNA by the ratio of the PNA and fluorophore absorbance at 260 nm to the fluorophore absorbance maximum in PBS. Estimated molar extinction coefficients at 260 nm were obtained from available data for PNA (21) and the fluorophores (http://www.basic.northwestern.edu/biotools/OligoCalcModifications.html) and also used to determine PNA concentration.
Incorporation of Fmoc-U'(Dde)-OH into PNA and selective Dde deprotection
PNAs were synthesized using Fmoc protected monomers and Fmoc-XAL-PEG-PS universal support on an 8909 Expedite PNA synthesizer using Bhoc-protected Fmoc PNA monomers and the PNA building block, Fmoc-U'-(Dde)-OH 1. The resin was divided into three micro-centrifuge reaction tubes. One tube was treated twice with 2% hydrazine in DMF for 30 min to remove the Dde group (22) (1 mL/2 μmol of the protected PNA resin). Another tube was treated with 1 mL of NH2OH·HCl/imidazole deprotection cocktail mixture (1 volume of DMF plus 5 volumes of 1.25 g of NH2OH•HCl and 0.918 g of imidazole in 5 mL of NMP) (17) for 3 h to remove the Dde group. The third reaction tube was not treated. Following completion of the reactions, the Fmoc was removed with 1 mL 20% piperidine/DMF for 20 min, and repeated. Then the resin was washed 5 × 1 mL of 1:1 DCM:DMF. The resins were then treated with 200 μL of TFA/mcresol (4:1) for 90 min at rt to remove the Bhoc protecting groups and cleave the PNA from the resin. The solution was removed and the remaining resin treated a second time. The solutions were combined and the PNA was precipitated by treatment with cold anhydrous diethyl ether (1 mL). The PNAs were then recovered as white pellets by spinning them down in a centrifuge at 2000 rpm (3 × 5 min) and removing the ether. The crude PNAs were purified by reversed phase HPLC as described in the experimental section by monitoring the UV absorbance at 260 nm. The desired fractions were collected, concentrated to dryness on a centrifugal evaporator overnight. The PNAs were re-dissolved in pure water and analyzed and characterized by UV, and further characterized by MALDI-TOF mass spectrometry using sinapinic acid matrix. PNA 18: MALDI (M+H)+Calcd 4327.7, found 4326.9; PNA 20 (M+H)+ Calcd 4163.6, via hydrazine deprotection: found 4161.7; via hydroxylamine/imidazole deprotection 4162.7.
Solid Phase Synthesis of the PNA-FAM 22 Donor Probes
The donor probes were synthesized on the Expedite 8909 PNA synthesizer using Fmoc protected monomers and the universal support Fmoc-XAL-PEG-PS in which one of the monomers was the PNA building block, Fmoc-U′-(Dde)-OH, and with ε-Boc-α-Boc-Lys at the N-terminus. The resin was treated with 1 mL of the deprotection mixture (NH2OH·HCl/imidazole in NMP/DMF) for 3 h, washed thoroughly with DCM/DMF (1:1) mixture (5 × 1 mL). The resin was then swelled with DMF (250 μL) and DIEA (11 μL) for 5 min and a 10 fold molar excess of FAM-NHS (10 equiv, 10 mg) was dissolved in a separate tube with 100 μL of anhydrous DMF, vortexed for few seconds to completely dissolve the activated FAM-NHS and immediately added to the resin all at once and stirred for 4-6 h at rt. The resin was washed with DCM/DMF (1:1) mixture (5 × 1 mL) to remove excess reagents and thoroughly dried by purging with nitrogen gas for several minutes. The PNAs were then cleaved from the resin and deprotected with 200 μL of TFA/m-cresol (4:1) cocktail mixture as described above. The crude PNA-FAM conjugates 22 were then recovered as yellow colored pellets by precipitation from cold anhydrous diethyl ether (3 × 1 mL). The crude PNAs were dissolved in 500 μL of water containing 0.1% TFA filtered using an Xpertek syringe filters (13 mm, 0.45 μm) before HPLC injection. The crude PNA-FAM donors 22 were purified by reversed phase HPLC with conditions described in the general procedure section. The HPLC fractions containing the PNAs were collected, concentrated to dryness on a centrifugal evaporator overnight and re-dissolved in freshly distilled deionized water, and characterized by UV in pH 7 PBS buffer. Ratio of 500/267 nm absorbances for PNA 22 expected: 0.60 based on an estimated absorbance of 70,000 M-1•cm-1 for FAM at 500 nm and 116,200 M-1•cm-1 at 260 nm (119,000 M-1•cm-1 - 20% hypochromicity for the PNA and 20,000 M-1•cm-1 for FAM); found: 0.52 for PNA 22-0, 0.51 for 22-7, and 0.61 for 22-13.
Solid phase Synthesis of the PNA-Cy5 FRET Acceptor Probe 24 via ε-Mtt-α-Fmoc-Lys
The PNA was synthesized on the Expedite 8909 PNA synthesizer as described above and ε-Mtt-α-Fmoc-Lys was added to the C-terminus. After the PNA synthesis was completed, the Mtt group was selectively deprotected by treating the resin with 1% TFA in DCM (5 × 1 mL) for 5 min (23, 24). After 3 cycles of the reaction, the yellow color of the deprotection mixture (an indication of trityl group deprotection) started to fade away, and finally cleared indicating the completion of the reaction. The resin was then thoroughly washed with DCM/DMF (1:1) mixture (5 × 1 mL) to remove the deprotected Mtt group and excess reagents. Then, it was swelled with DMF (250 μL) and DIEA (11 μL) for 5 min and Cy5-NHS ester (1 mg) in 100 μL of anhydrous DMF was immediately added to the resin and stirred for 4 h. The excess reagents were washed away with 5 × 1 mL DCM/DMF (1:1). Fmoc was removed with two treatments of 1 mL of 20% piperidine/DMF for 20 min and then washed 5 × 1 mL of DCM/DMF (1:1). The PNA was cleaved from the resin with 200 μL of TFA/m-cresol (4:1) for 90 min followed by filtration to remove the crude PNA. The cleavage step was repeated one more time and the filtrates were combined in one tube. The PNA-Cy5 FRET acceptor probe 24 was recovered by precipitation with cold anhydrous diethyl ether (1 mL). The crude PNA-Cy5 conjugate was purified by reversed phase HPLC and characterized by UV in pH 7 PBS buffer. Ratio of the absorbances at 650 and 260 nm for PNA 24 expected: 2.3, based on an estimated absorbance of 250,000 M-1•cm-1 for Cy5 at 650 nm, and 108,600 M-1•cm-1 at 260 nm (123,200 M-1•cm-1 for the PNA20% hypochromicity and 10,000 M-1•cm-1 for Cy5): found 2.0.
Fluorescence resonance energy transfer (FRET) studies
The acceptor probe (PNA Cy5 24) was added to the FRET donor probe (PNA-FAM 22) with or without DNA template and allowed to equilibrate at the concentration and time indicated in the figure legend. The emission spectrum was then recorded from 450 to 800 nm with excitation at 490 nm with 5 nm excitation and emission slit windows. The emission maximum for the PNA-Cy5 24 probe was at 667 nm.
RESULTS AND DISCUSSION
Building block synthesis
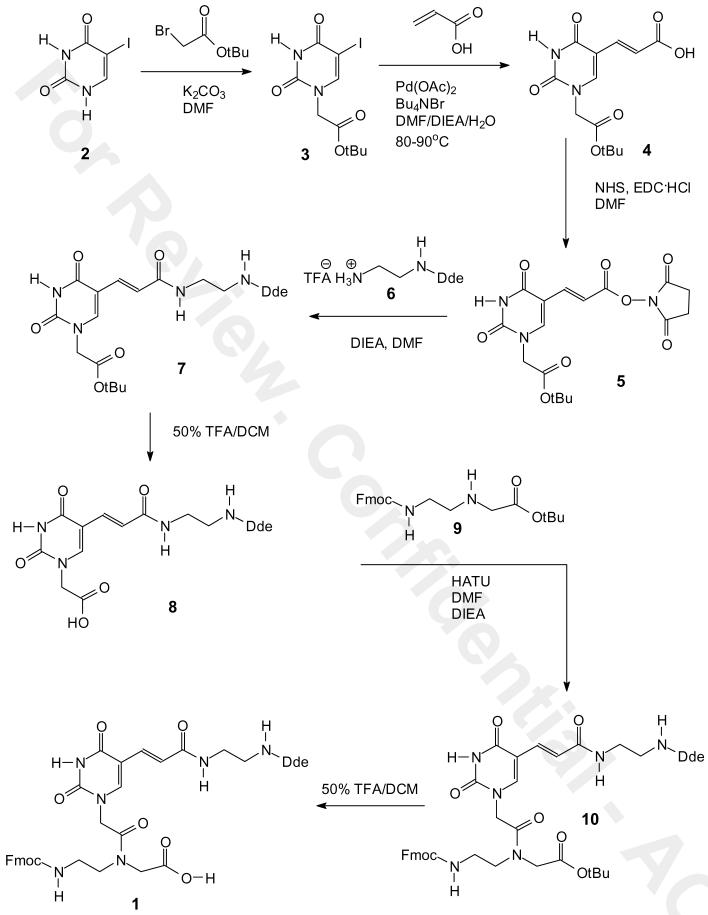
Two synthetic routes were developed to the building block. In route I (Scheme 1), commercially available iodouracil 2 was alkylated at N1 with tert-butyl bromoacetate in the presence of potassium carbonate in anhydrous DMF under nitrogen at room temperature for 24 h to give the tert-butyl ester 3 in 94% yield. The iodo group of 3 was replaced with an acryloyl group via Heck reaction with acrylic acid to give the carboxylic acid 4 in 54% yield. The carboxylic acid 4 was converted into an NHS ester 5 with N-hydroxysuccinamide and EDC in anhydrous DMF overnight in 75 % yield. The NHS ester was coupled with the TFA salt of mono-Dde protected ethylenediamine 6 (18) to give compound 7 in about 85 % yield.
Scheme 1.
Synthesis of Fmoc-U'-(Dde)-OH 1 from iodouracil via intermediate 7.
Compound 7 was then converted to the Fmoc building block 1 in three additional steps. In the first step, compound 7 was treated with 50% TFA/DCM from 0 oC to rt for 4 h to give the carboxylic acid 8 in a quantitative yield without disturbing the Dde group. The carboxylic acid 8 was then coupled with tert-butyl-N-Fmoc-aminoethyl-glycinate 9 (19) by activation with HATU in the presence of DIEA in anhydrous DMF to give compound 10 in 93% yield. Finally, deprotection of the tert-butyl ester group of 10 with 50% TFA in dichloromethane afforded the desired PNA building block 1 in quantitative yield and in 30% overall yield from iodouracil.
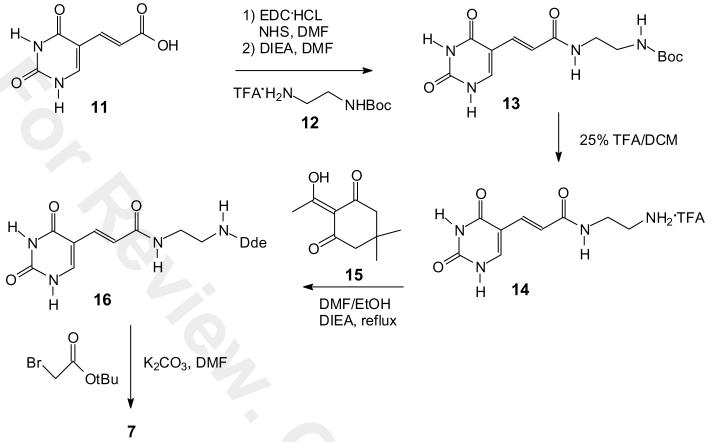
We also investigated a second route to 7 that, though two steps longer, may be preferable for large scale synthesis due to the ease of synthesis and purification of the intermediates and the better overall yield (Scheme 2). Acrylic acid 11 was first prepared in two steps in 85 % yield from commercially available 5′-hydroxymethyluracil (20). We initially planned to couple 11 with mono-Dde protected ethylenediamine 6 as we had done in the first route, but due to the instability of 6 we decided to couple the more stable mono-Boc protected ethylenediamine 12 and then convert the Boc group to a Dde group. Thus, acrylic acid 11 was then converted to the NHS ester in 87% yield by first forming the NHS ester with EDC and then adding commercially available mono-Boc ethylenediamine 12 in the presence of DIEA in dry DMF. The tert-butyl protecting group was then deprotected with 25% TFA/DCM for 3 h to give the TFA salt 14 in quantitative yield. The TFA salt was refluxed with Dde-OH 15 in the presence of DIEA in anhydrous DMF to give compound 16 in 84 % yield. Compound 16 was alkylated with tert-butyl bromoacetate in the presence of potassium carbonate in anhydrous DMF under nitrogen at rt for 12 h to afford compound 7 in 90% yield. Compound 7 was converted to the PNA building block 1 as previously described in 43 % overall yield for nine steps from hydroxymethyluracil.
Scheme 2.
Synthesis of intermediate 7 from uracil acrylic acid 11.
Incorporation of Dde-U into PNA
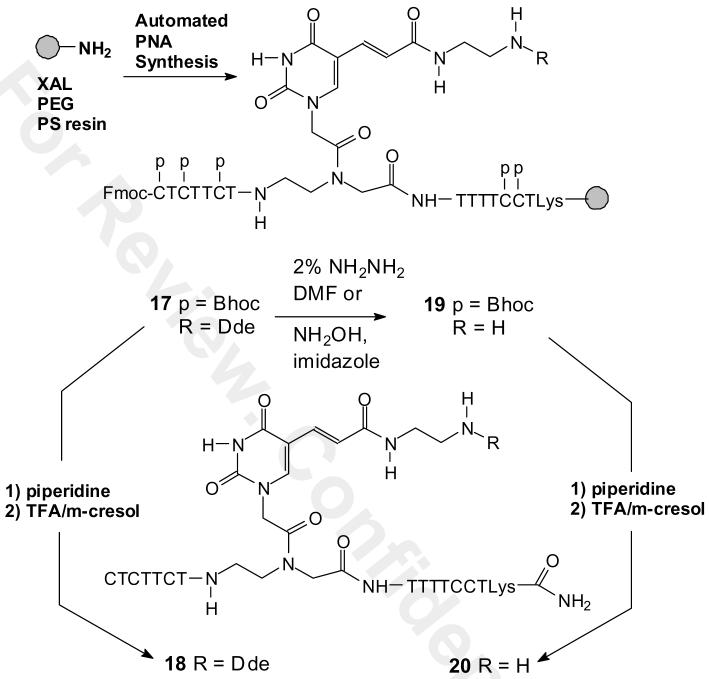
To demonstrate that the Dde-U building block 1 was compatible with automated PNA synthesis and that the Dde group can be selectively removed, we synthesized CTCTTCTU(Dde)TTTTCCTK, 18 and CTCTTCTU(NH2)TTTTCCTK, 20 according to Scheme 3. First Dde-U was incorporated into PNA by solid phase automated Fmoc PNA synthesis with standard Bhoc monomers on a XALPEG-PS resin to give the solid phase intermediate 17, followed by treatment with TFA/m-cresol to give the Dde-protected PNA 18 as confirmed by HPLC and MALDI analysis. The ability of the Dde group to survive strongly acidic deprotection conditions demonstrates its orthogonality to acid labile amine protecting groups such as Mtt that would therefore allow selective derivatization of other sites on the PNA.
Scheme 3.
Synthesis of the PNA-U'-(Dde) 18 and PNA-U'-(NH2) 20.
We then examined 2% hydrazine/DMF (22) and NH2OH/imidazole (17) as methods for removing the Dde group from the solid supported PNA 19 to give 20 (Scheme 3). Following either treatment the PNA was first treated with piperidine to remove the Fmoc group, and then cleaved from the support and completely deprotected with TFA/m-cresol. HPLC and MALDI analysis of the major product from both deprotection methods showed the same peak corresponding to the Dde deprotected PNA 20. In practice, the hydroxylamine reagent is preferable, as it is compatible with the Fmoc group, which undergoes a significant amount of deprotection with hydrazine (17).
Synthesis of FAM/Cy5 FRET probes
We selected a FAM/Cy5 FRET system to maximize detection sensitivity. Cy5 has a red-shifted absorption maxima which minimizes background signal due to direct excitation of Cy5 upon irradiation of fluorescein. Because the Cy5 emission is also red shifted, there is less background signal due to fluorescein emission at the Cy5 emission maximum. In spite of its red-shifted absorption spectrum, Cy5 still has sufficient overlap between its absorption spectrum and that of fluorescein to give a good FRET signal (7, 25). To determine the optimal positioning of the donor relative to the acceptor for maximum FRET, we synthesized PNAs with the fluorescein attached to three different sites (PNA-FAM 22-0, 22-7, and 22-13), and Cy5 to a single site on the ε-amino end of a carboxy terminal lysine group on the acceptor PNA-Cy5 24 (Scheme 4).
Scheme 4.
Synthesis of the PNA FRET donor and acceptor probes 22 and 24.
The FRET donor PNA-FAMs were prepared by removing the Dde group from the corresponding PNAs 21-0, 21-7, 21-13 with NH2OH/imidazole, followed by coupling with the NHS ester of 5/6-carboxyfluorescein (FAM) in the presence of DIEA for 2 h. To help drive the coupling to completion the support was additionally treated with 100 μl of HATU (0.2 M) in DMF an additional 2 h at room temperature. The support was then washed several times with DCM/DMF, cleaved from the support and deprotected with TFA/m-cresol to afford the crude PNA as a colored pellet. The PNAs were then purified by reverse phase HPLC in 0.1% aqueous TFA. When dissolved at pH 7, the fluorescein adopts the open fluorescent form as shown in Scheme 4. The FRET acceptor PNA-Cy5 24 was prepared by selectively deprotecting a carboxy terminal ε-Mtt-α-Fmoc-Lys PNA 23 with 1% TFA/DCM and coupling with the NHS ester of Cy5. The PNA was then treated with piperidine to remove the Fmoc group and then cleaved from the column and completely deprotected with TFA/m-cresol to give PNA-Cy5 24.
FRET experiments
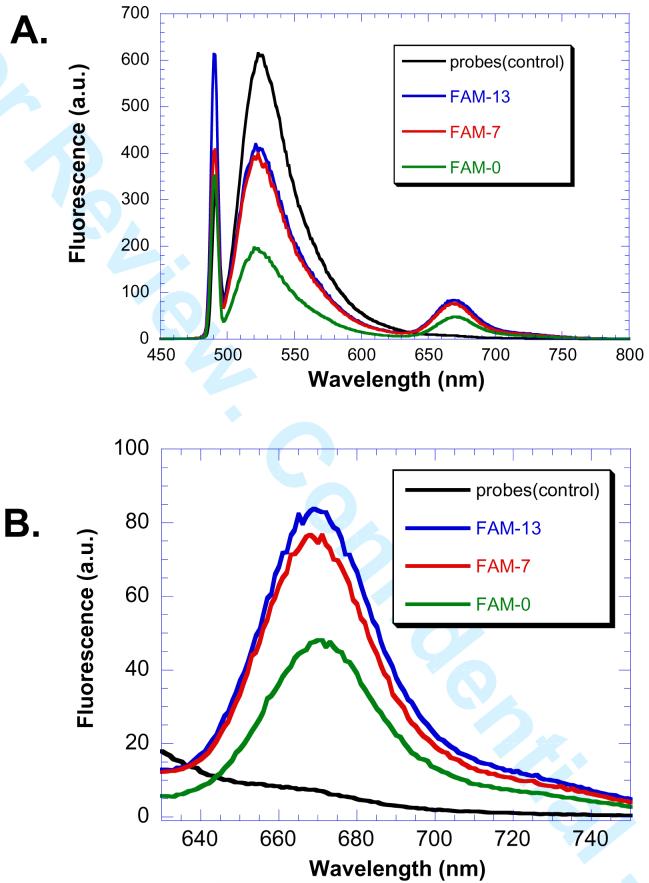
The donor and acceptor PNAs were then examined for their ability to detect a complementary DNA sequence by FRET. Fluorescence spectra were obtained with excitation at 491 nm in the presence of 2 μM PNA-FAM and 2 μM DNA and 4 μM PNA-Cy5. Fluorescence emission from fluorescein appears at 525 nm and that from Cy5 at 667 nm (Figure 2). The results clearly show a decrease in fluorescein emission and an increase in Cy5 emission for all the donor acceptor pairs. Maximal FRET was observed for fluorescein donors in positions 7 and 13. The similar FRET efficiencies for such two different spacings can be explained by similar donor acceptor distances resulting from the helical nature of the PNA•DNA duplex (26). Given the helical repeat of a PNA•DNA duplex as between 13 Å (27) and 15.6 Å (28), the fluorescein at position 7 will be out of phase with the Cy5 while the fluorescein at position 13 will be in phase with the Cy5. The donor with fluorescein in position 0 showed more quenching, but less Cy5 emisssion indicating other quenching pathways were operating, presumably due to the formation of fluorescein-Cy5 complexes (29).
Figure 2.
DNA templated FRET system. PNA-FAM donor 22-0, 7 or 13 (2 μM), and PNA-Cy5 24 (4 μM) were incubated with template DNA (2 μM) or without (control), in 100 mM PBS buffer pH 7.4 at 37 °C for 3 h. A) The emission spectrum was then recorded from 450-800 nm with excitation was at 491 nm with 5 nm emission and excitation slit windows. B) Expansion of the 630-750 nm region of the FRET signal.
Conclusion
In conclusion, we have developed a new Fmoc-PNA building block 1 containing an orthogonally Dde protected alkyl amino group linked to C5 of uracil. We demonstrated that this building block could be incorporated into PNAs site-specifically and then selectively deprotected and derivatized with donor and acceptor fluorophores. Several fluorescein/Cy5 binary PNA probes were shown to detect the DNA sequence corresponding to the antisense mRNA cassette, but the optimal positioning for detecting a single or multimeric mRNA cassette remains to be determined. Further modification of the PNAs will also be required to render them membrane permeable before they can be used to detect single and multiple mRNA cassettes in living cells.
Supplementary Material
Acknowledgments
We thank Dan Gu and Dian Su for acquiring the MALDI data. This work was supported by the National Institutes of Health as a Program of Excellence in Nanotechnology (1U01 HL080729-01) grant and by the Washington University NIH Mass Spectrometry Resource (Grant No. P41 RR000954) and Washington University NMR facility (Grant No. RR1571501).
Footnotes
Supporting Information Available. Proton and 13C NMR spectra of the intermediates and final product. This information is available free of charge via the Internet at http://pubs.acs.org.
Abbreviations. Boc: tert-butyloxycarbonyl; Bhoc: benzhydryloxycarbonyl; Cy5: cyanine-5; Dde: 1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl; DCM: dichloromethane; DIEA: diisopropylethylamine; DMF: N,N-dimethylformamide; EDC: N,N-dimethylpropylethlydicarbodimide; ESI: electrospray ionization; EtOAc: ethylacetate; FAB: fast atom bombardment; FAM: 5-(and-6)-carboxyfluorescein; FRET: fluorescence energy transfer; Fmoc: 9-fluorenylmethoxycarbonyl; HATU: O-(7-azabenzotriazole-1-yl)-N,N,N'N'-tetramethyluronium hexafluorophosphate; MeOH: methanol; Mtt: methyltrityl; NHS: N-hydroxysuccinamide; NMP, N-methyl-2-pyrrolidinone; PNA: peptide nucleic acid; TFA: trifluoroacetic acid; THF: tetrahydrofuran; TMS: tetramethylsilane.
References
- (1).Santangelo P, Nitin N, Bao G. Nanostructured probes for RNA detection in living cells. Ann Biomed Eng. 2006;34:39–50. doi: 10.1007/s10439-005-9003-6. [DOI] [PubMed] [Google Scholar]
- (2).Fang X, Mi Y, Li JJ, Beck T, Schuster S, Tan W. Molecular beacons: fluorogenic probes for living cell study. Cell Biochem. Biophys. 2002;37:71–81. doi: 10.1385/CBB:37:2:071. [DOI] [PubMed] [Google Scholar]
- (3).Cardullo RA, Agrawal S, Flores C, Zamecnik PC, Wolf DE. Detection of nucleic acid hybridization by nonradiative fluorescence resonance energy transfer. Proc Natl Acad Sci U S A. 1988;85:8790–4. doi: 10.1073/pnas.85.23.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Tsuji A, Koshimoto H, Sato Y, Hirano M, Sei-Iida Y, Kondo S, Ishibashi K. Direct observation of specific messenger RNA in a single living cell under a fluorescence microscope. Biophys J. 2000;78:3260–74. doi: 10.1016/S0006-3495(00)76862-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Tsuji A, Sato Y, Hirano M, Suga T, Koshimoto H, Taguchi T, Ohsuka S. Development of a time-resolved fluorometric method for observing hybridization in living cells using fluorescence resonance energy transfer. Biophys J. 2001;81:501–15. doi: 10.1016/S0006-3495(01)75717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Ishibashi K, Tsuji A. IL-2 or IL-4 mRNA as a potential flow cytometric marker molecule for selective collection of living T helper 1 or T helper 2 lymphocytes. Anal Chem. 2003;75:2715–23. doi: 10.1021/ac0206824. [DOI] [PubMed] [Google Scholar]
- (7).Abe H, Kool ET. Flow cytometric detection of specific RNAs in native human cells with quenched autoligating FRET probes. Proc. Natl. Acad. Sci. USA. 2006;103:263–8. doi: 10.1073/pnas.0509938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Nielsen PE, Egholm M, Berg RH, Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991;254:1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- (9).Nielsen PE. PNA Technology. Mol Biotechnol. 2004;26:233–48. doi: 10.1385/MB:26:3:233. [DOI] [PubMed] [Google Scholar]
- (10).Lundin KE, Good L, Stromberg R, Graslund A, Smith CI. Biological activity and biotechnological aspects of peptide nucleic acid. Adv Genet. 2006;56:1–51. doi: 10.1016/S0065-2660(06)56001-8. [DOI] [PubMed] [Google Scholar]
- (11).Robertson KL, Yu L, Armitage BA, Lopez AJ, Peteanu LA. Fluorescent PNA probes as hybridization labels for biological RNA. Biochemistry. 2006;45:6066–74. doi: 10.1021/bi052050s. [DOI] [PubMed] [Google Scholar]
- (12).Dose C, Seitz O. Single nucleotide specific detection of DNA by native chemical ligation of fluorescence labeled PNA-probes. Bioorg Med Chem. 2008;16:65–77. doi: 10.1016/j.bmc.2007.04.059. [DOI] [PubMed] [Google Scholar]
- (13).Xi C, Balberg M, Boppart SA, Raskin L. Use of DNA and peptide nucleic acid molecular beacons for detection and quantification of rRNA in solution and in whole cells. Appl Environ Microbiol. 2003;69:5673–8. doi: 10.1128/AEM.69.9.5673-5678.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Seitz O, Kohler O. Convergent strategies for the attachment of fluorescing reporter groups to peptide nucleic acids in solution and on solid phase. Chemistry. 2001;7:3911–25. doi: 10.1002/1521-3765(20010917)7:18<3911::aid-chem3911>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- (15).Englund EA, Appella DH. Synthesis of gamma-substituted peptide nucleic acids: a new place to attach fluorophores without affecting DNA binding. Org Lett. 2005;7:3465–7. doi: 10.1021/ol051143z. [DOI] [PubMed] [Google Scholar]
- (16).Cai J, Li X, Taylor JS. Improved Nucleic Acid Triggered Probe Activation through the Use of a 5-Thiomethyluracil Peptide Nucleic Acid Building Block. Org. Lett. 2005;7:751–4. doi: 10.1021/ol0478382. [DOI] [PubMed] [Google Scholar]
- (17).Diaz-Mochon JJ, Bialy L, Bradley M. Full orthogonality between Dde and Fmoc: the direct synthesis of PNA--peptide conjugates. Org Lett. 2004;6:1127–9. doi: 10.1021/ol049905y. [DOI] [PubMed] [Google Scholar]
- (18).Zhang Z, Fan E. Solid-phase and solution-phase syntheses of oligomeric guanidines bearing peptide side chains. J Org Chem. 2005;70:8801–10. doi: 10.1021/jo051226t. [DOI] [PubMed] [Google Scholar]
- (19).Thomson SA, Josey JA, Cadilla R, Gaul MD, Hassman CF, Luzzio MJ, Pipe AJ, Reed KL, Ricca DJ, Wiethe RW, Noble SA. Fmoc mediated synthesis of peptide nucleic acids. Tetrahedron. 1995;51:6179–94. [Google Scholar]
- (20).Ressner EC, Fraher P, Edelman MS, Mertes MP. Synthesis of 5-substituted uracil derivatives. Journal of Medicinal Chemistry. 1976;19:194–6. doi: 10.1021/jm00223a042. [DOI] [PubMed] [Google Scholar]
- (21).Nielsen PE. Peptide Nucleic Acids: Protocols and Applications. Taylor & Francis; 2004. [Google Scholar]
- (22).Bycroft BW, Chan WC, Chharbra SR, Hone ND. A novel lysine-protecting procedure for continuous flow solid phase synthesis of branched peptides. J. Chem. Soc., Chem. Commun. 1993:778–9. [Google Scholar]
- (23).Bourel L, Carion O, Gras-Masse H, Melnyk O. The deprotection of Lys(Mtt) revisited. J Pept Sci. 2000;6:264–70. doi: 10.1002/1099-1387(200006)6:6<264::AID-PSC248>3.3.CO;2-1. [DOI] [PubMed] [Google Scholar]
- (24).Thiam K, Loing E, Verwaerde C, Auriault C, Gras-Masse H. IFN-gamma-derived lipopeptides: influence of lipid modification on the conformation and the ability to induce MHC class II expression on murine and human cells. J Med Chem. 1999;42:3732–6. doi: 10.1021/jm991025f. [DOI] [PubMed] [Google Scholar]
- (25).Marti AA, Li X, Jockusch S, Stevens N, Li Z, Raveendra B, Kalachikov S, Morozova I, Russo JJ, Akins DL, Ju J, Turro NJ. Design and characterization of two-dye and three-dye binary fluorescent probes for mRNA detection. Tetrahedron. 2007;63:3591–3600. doi: 10.1016/j.tet.2006.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Clegg RM, Murchie AI, Zechel A, Lilley DM. Observing the helical geometry of double-stranded DNA in solution by fluorescence resonance energy transfer. Proc Natl Acad Sci U S A. 1993;90:2994–8. doi: 10.1073/pnas.90.7.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Eriksson M, Nielsen PE. Solution structure of a peptide nucleic acid-DNA duplex. Nat Struct Biol. 1996;3:410–3. doi: 10.1038/nsb0596-410. [DOI] [PubMed] [Google Scholar]
- (28).Lukeman PS, Mittal AC, Seeman NC. Two dimensional PNA/DNA arrays: estimating the helicity of unusual nucleic acid polymers. Chem Commun (Camb) 2004:1694–5. doi: 10.1039/b401103a. [DOI] [PubMed] [Google Scholar]
- (29).Dietrich A, Buschmann V, Muller C, Sauer M. Fluorescence resonance energy transfer (FRET) and competing processes in donor-acceptor substituted DNA strands: a comparative study of ensemble and single-molecule data. J Biotechnol. 2002;82:211–31. doi: 10.1016/s1389-0352(01)00039-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.