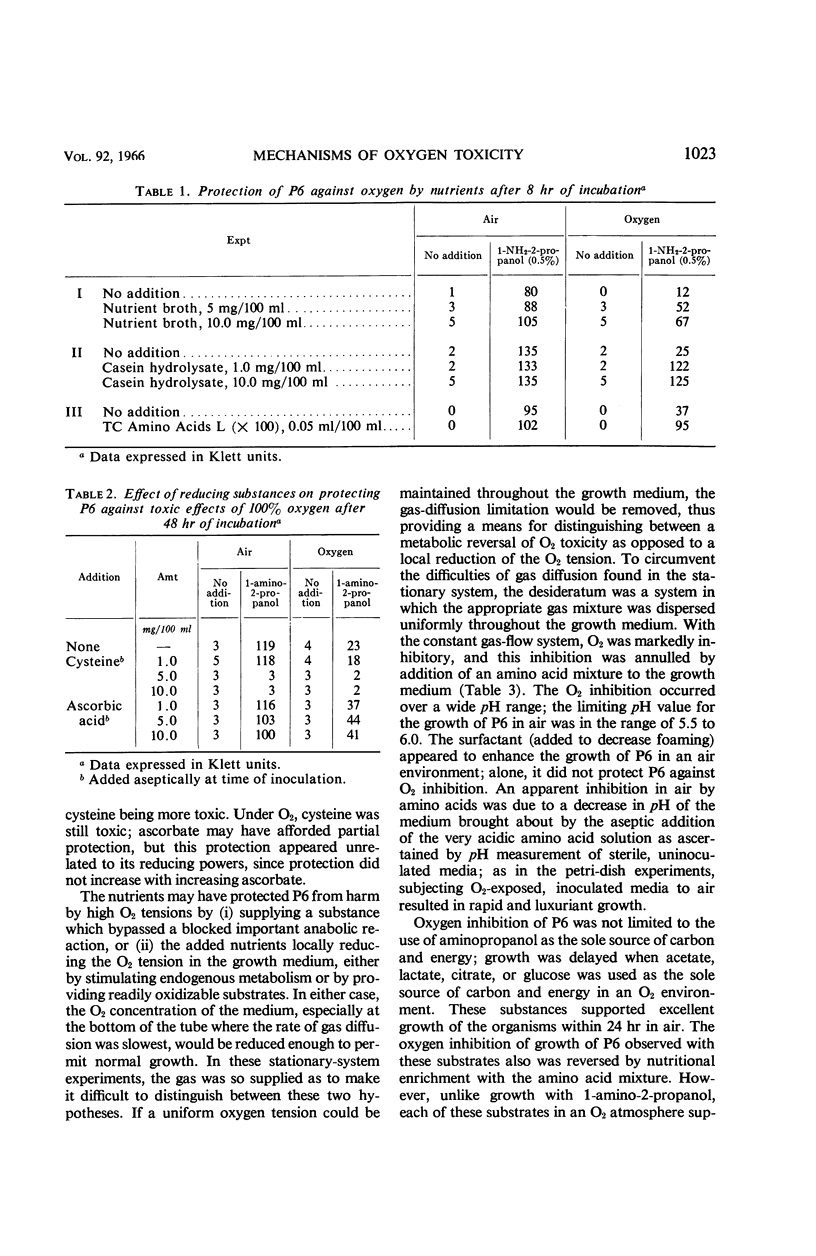
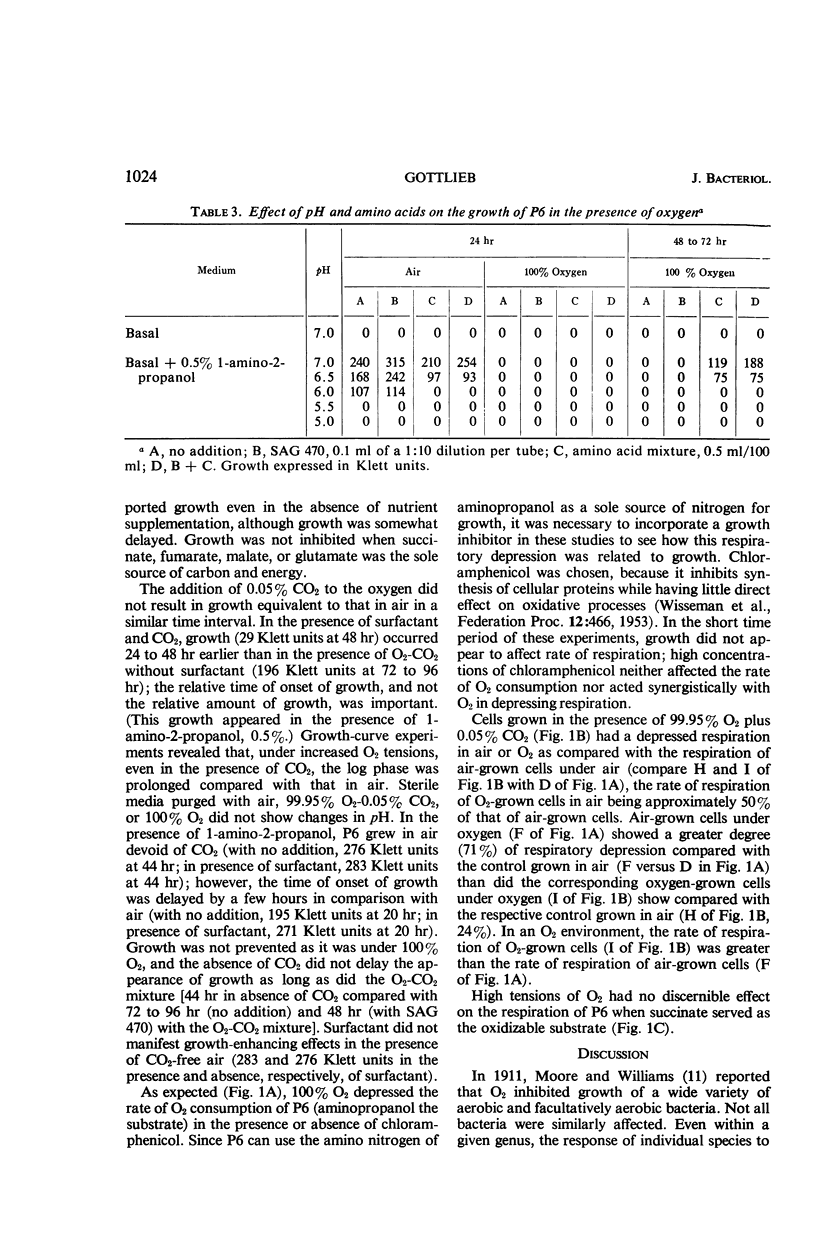
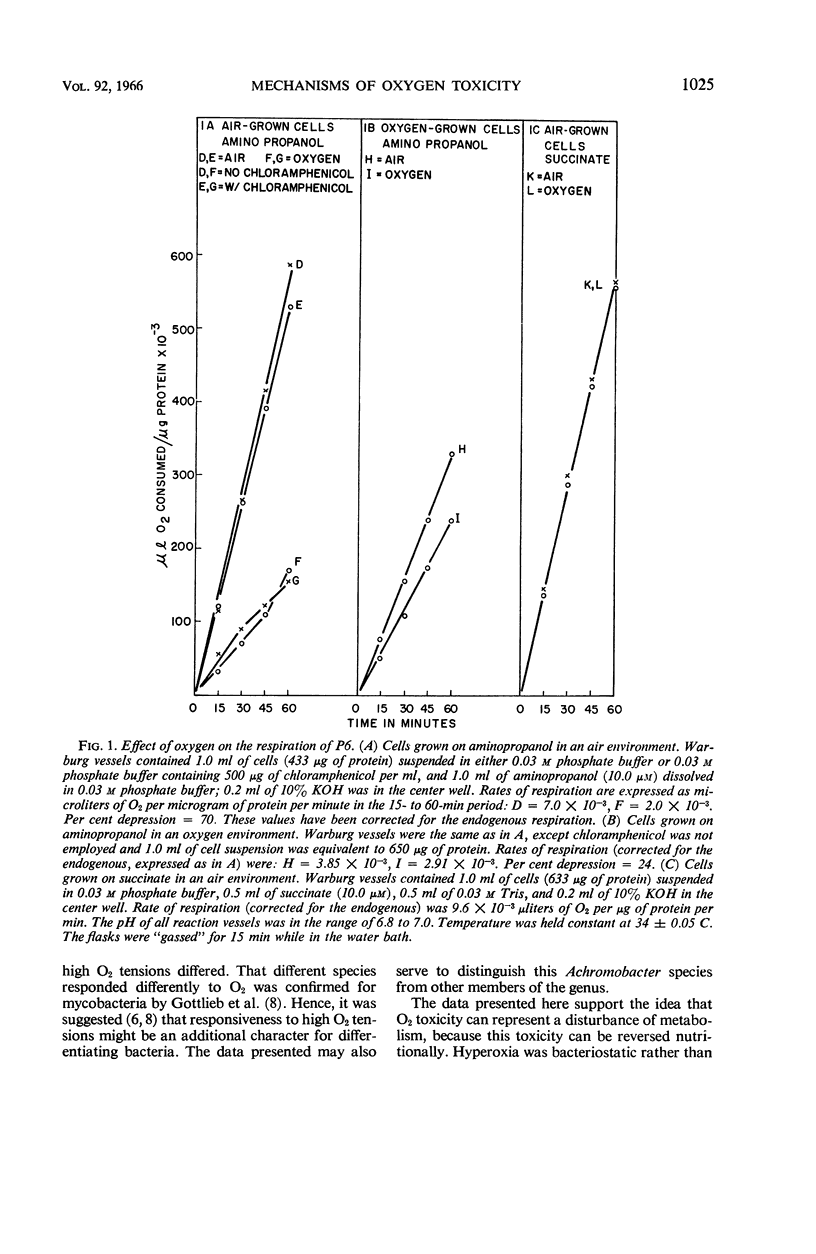
Abstract
Gottlieb, Sheldon F. (Union Carbide Corp., Tonawanda, N.Y.). Bacterial nutritional approach to mechanisms of oxygen toxicity. J. Bacteriol. 92:1021–1027. 1966.—Inhibition by oxygen of growth of the bacterium Achromobacter P6 was reversed by amino acid supplements. The reversal of oxygen-induced growth inhibition was not due to the presence of reducing substances in the growth medium. Oxygen primarily exerts a bacteriostatic effect. The oxygen inhibition of growth occurred over a wide pH range. Oxygen inhibition of growth was observed when 1-amino-2-propanol, acetate, lactate, citrate, or glucose was used as the sole source of carbon and energy. No inhibition of growth was obtained when succinate, fumarate, malate, or glutamate was used as the source of carbon and energy. Oxygen markedly depressed the respiration of P6 when 1-amino-2-propanol was the substrate. There was no depression of respiration under oxygen with succinate as substrate. P6 grown in the presence of high oxygen tensions had a higher rate of respiration under oxygen than similar air-grown cells. Chloramphenicol did not affect the rate of oxygen consumption or cause a further depression of the respiratory rate in the presence of oxygen. It is suggested that microbes may serve as a model system for studying the cellular and subcellular mechanisms of oxygen toxicity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKER J., MAPSON L. W. Studies in the respiratory and carbohydrate metabolism of plant tissues. VII. Experimental studies with potato tubers of an inhibition of the respiration and of a block in the tricarboxylic acid cycle induced by oxygen poisoning. Proc R Soc Lond B Biol Sci. 1955 May 17;143(913):523–549. doi: 10.1098/rspb.1955.0027. [DOI] [PubMed] [Google Scholar]
- Chance B., Jamieson D., Coles H. Energy-linked pyridine nucleotide reduction: inhibitory effects of hyperbaric oxygen in vitro and in vivo. Nature. 1965 Apr 17;206(981):257–263. doi: 10.1038/206257a0. [DOI] [PubMed] [Google Scholar]
- GERSHENOVICH Z. S., KRICHEVSKAIA A. A. [The protective role of arginine in oxygen poisoning]. Biokhimiia. 1960 Sep-Oct;25:790–795. [PubMed] [Google Scholar]
- GOTTLIEB S. F., MANDEL M. Utilization of 1-amino-2-propanol by a soil bacterium. Can J Microbiol. 1959 Aug;5:363–368. doi: 10.1139/m59-045. [DOI] [PubMed] [Google Scholar]
- GOTTLIEB S. F., ROSE N. R., MAURIZI J., LANPHIER E. H. OXYGEN INHIBITION OF GROWTH OF MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1964 Apr;87:838–843. doi: 10.1128/jb.87.4.838-843.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Z., Reinhold L. Rapid Changes in Permeability of Cell Membranes to Water Brought About by Carbon Dioxide & Oxygen. Plant Physiol. 1962 Jul;37(4):481–486. doi: 10.1104/pp.37.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S. F. Hyperbaric oxygenation. Adv Clin Chem. 1965;8:69–139. doi: 10.1016/s0065-2423(08)60413-8. [DOI] [PubMed] [Google Scholar]
- JAMIESON D., VAN DEN BRENK H. A. Pulmonary damage due to high pressure oxygen breathing in rats. 2. Changes in dehydrogenase activity of rat lung. Aust J Exp Biol Med Sci. 1962 Feb;40:51–56. doi: 10.1038/icb.1962.7. [DOI] [PubMed] [Google Scholar]
- Moore B., Williams R. S. The Growth of Various Species of Bacteria and Other Micro-Organisms in Atmospheres Enriched with Oxygen. Biochem J. 1911;5(4):181–187. doi: 10.1042/bj0050181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUART B., GERSCHMAN R., STANNARD J. N. Effect of high oxygen tension on potassium retentivity and colony formation of baker's yeast. J Gen Physiol. 1962 Jul;45:1019–1030. doi: 10.1085/jgp.45.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders A. P., Hall I. H., Woodhall B. Succinate: protective agent against hyperbaric oxygen toxicity. Science. 1965 Dec 31;150(3705):1830–1831. doi: 10.1126/science.150.3705.1830. [DOI] [PubMed] [Google Scholar]
- Thomas J. J., Neptune E. M., Sudduth H. C. Toxic effects of oxygen at high pressure on the metabolism of d-glucose by dispersions of rat brain. Biochem J. 1963 Jul;88(1):31–45. doi: 10.1042/bj0880031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD J. D., WATSON W. J. GAMMA-AMINOBUTYRIC ACID LEVELS IN THE BRAIN OF RATS EXPOSED TO OXYGEN AT HIGH PRESSURES. Can J Biochem Physiol. 1963 Sep;41:1907–1913. [PubMed] [Google Scholar]


