Abstract
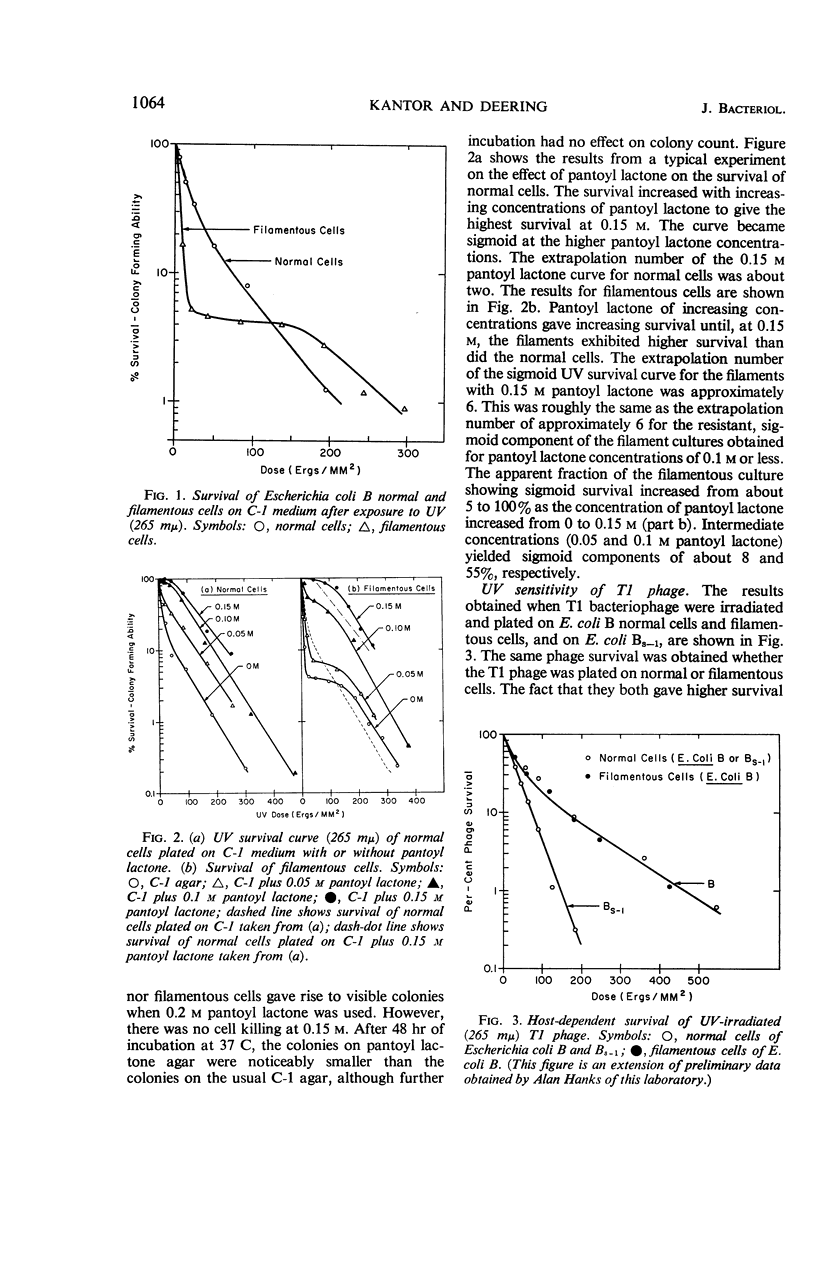
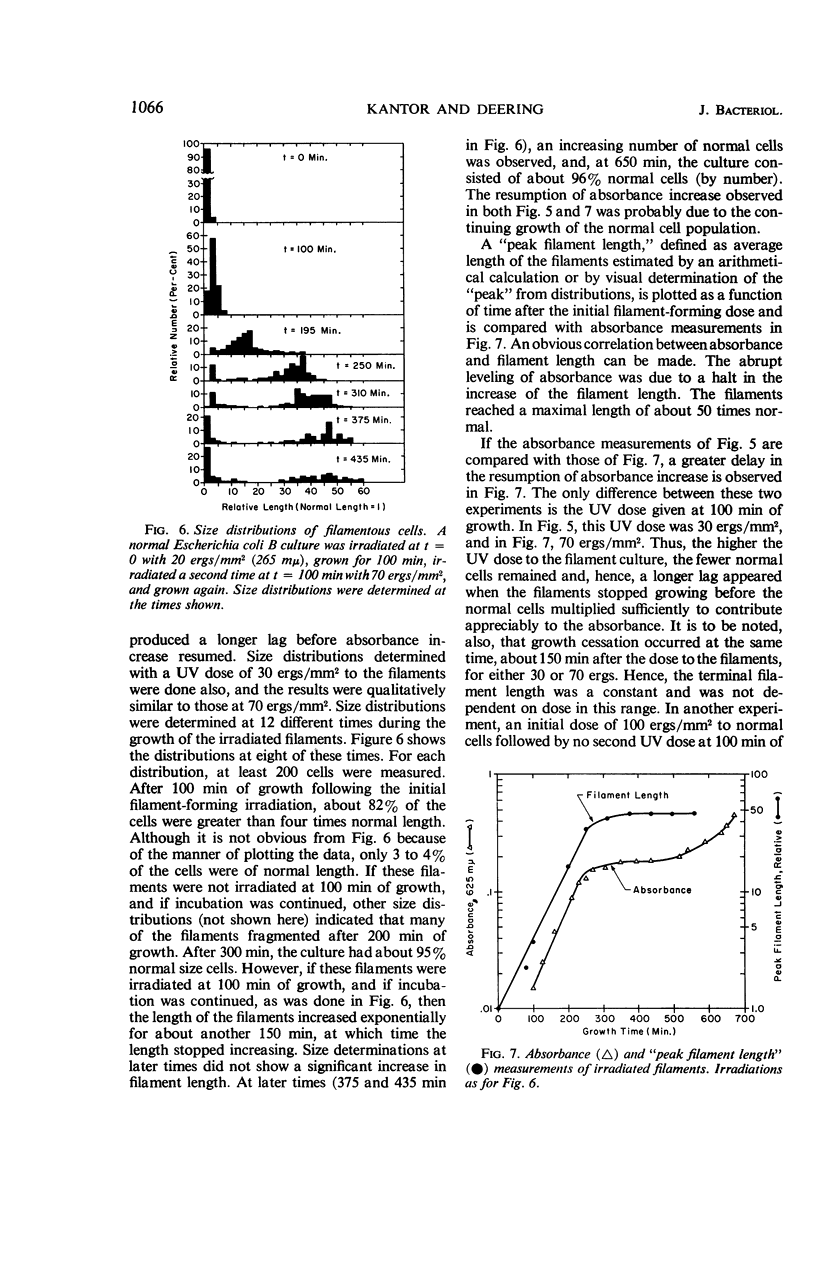
Kantor, George J. (The Pennsylvania State University, University Park), and R. A. Deering. Ultraviolet radiation studies of filamentous Escherichia coli B. J. Bacteriol. 92:1062–1069. 1966.—Small ultraviolet (UV) doses cause Escherichia coli B to grow into long filamentous single cells. A large fraction of these filaments can recover their division ability and can form colonies under appropriate conditions. Preformed filaments can be irradiated with UV, and their ability to still produce colonies can be compared with that of irradiated normal cells. In this regard, filaments are more sensitive to UV than normal cells. Filaments can still host-cell reactivate UV-irradiated T1 phage and can regain their own deoxyribonucleic acid (DNA) synthetic ability after it has been blocked by UV. This indicates that these filaments still retain mechanisms for repairing UV-damaged DNA. Pantoyl lactone, an agent that stimulates cell-division recovery in UV-irradiated E. coli B, causes increased UV resistance for both normal and filamentous cells, with the filaments becoming more resistant than normal cells. In the absence of pantoyl lactone, irradiated filaments grow to a length of about 50 times normal and then stop growing. These long filaments cannot subsequently divide and give colonies. We conclude that the UV dose given to the preformed filaments causes an additional division lag beyond that of unirradiated filaments, and that some critical length is reached after which division recovery and colony formation is impossible. Irradiated normal cells recover before reaching this critical length.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLER H. I., HARDIGREE A. A. POSTIRRADIATION GROWTH, DIVISION, AND RECOVERY IN BACTERIA. Radiat Res. 1965 May;25:92–102. [PubMed] [Google Scholar]
- Adler H. I., Fisher W. D., Hardigree A. A., Stapleton G. E. Repair of radiation-induced damage to the cell division mechanism of Escherichia coli. J Bacteriol. 1966 Feb;91(2):737–742. doi: 10.1128/jb.91.2.737-742.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEERING R. A., CHRISTIAN E. Action of T2 bacteriophage on filamentous Escherichia coli. Virology. 1958 Aug;6(1):150–156. doi: 10.1016/0042-6822(58)90066-7. [DOI] [PubMed] [Google Scholar]
- DEERING R. A. Radiation sensitivity of filamentous Escherichia coli. Biochim Biophys Acta. 1959 Jan;31(1):11–19. doi: 10.1016/0006-3002(59)90433-0. [DOI] [PubMed] [Google Scholar]
- DEERING R. A. Studies on division inhibition and filament formation of Escherichia coli by ultraviolet light. J Bacteriol. 1958 Aug;76(2):123–130. doi: 10.1128/jb.76.2.123-130.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELKIND M. M., BEAM C. A. Variation of the biological effectiveness of x-rays and alpha-particles on haploid Saccharomyces cerevisiae. Radiat Res. 1955 Sep;3(1):88–104. [PubMed] [Google Scholar]
- GRULA E. A., GRULA M. M. Cell division in a species of Erwinia III. Reversal of inhibition of cell division caused by D-amino acids, penicillin, and ultraviolet light. J Bacteriol. 1962 May;83:981–988. doi: 10.1128/jb.83.5.981-988.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAWALT P., SETLOW R. Effect of monochromatic ultraviolet light on macromolecular synthesis in Escherichia coli. Biochim Biophys Acta. 1960 Jul 1;41:283–294. doi: 10.1016/0006-3002(60)90011-1. [DOI] [PubMed] [Google Scholar]
- HILL R. F. A radiation-sensitive mutant of Escherichia coli. Biochim Biophys Acta. 1958 Dec;30(3):636–637. doi: 10.1016/0006-3002(58)90112-4. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOROWITZ H. J. Absorption effects in volume irradiation of microorganisms. Science. 1950 Mar 3;111(2879):229–229. doi: 10.1126/science.111.2879.229-a. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B. PHYSICAL CHANGES AND MUTAGENESIS. J Cell Physiol. 1964 Oct;64:SUPPL 1–1:68. [PubMed] [Google Scholar]
- Swenson P. A., Setlow R. B. Effects of ultraviolet radiation on macromolecular synthesis in Escherichia coli. J Mol Biol. 1966 Jan;15(1):201–219. doi: 10.1016/s0022-2836(66)80221-8. [DOI] [PubMed] [Google Scholar]
- VAN DE PUTTE P., WESTENBROEK C., ROERSCH A. THE RELATIONSHIP BETWEEN GENE-CONTROLLED RADIATION RESISTANCE AND FILAMENT FORMATION IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Oct 15;76:247–256. doi: 10.1016/0006-3002(63)90037-4. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Genetics of Resistance to Radiation in ESCHERICHIA COLI. Genetics. 1947 May;32(3):221–248. doi: 10.1093/genetics/32.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]


