Abstract
Intestinal cell kinase (ICK), originally cloned from the intestine and expressed in the intestinal crypt epithelium, is a highly conserved serine/threonine protein kinase that is similar to mitogen-activated protein kinases (MAPKs) in the catalytic domain and requires dual phosphorylation within a MAPK-like TDY motif for full activation. Despite these similarities to MAPKs, the biological functions of ICK remain unknown. In this study, we report that suppression of ICK expression in cultured intestinal epithelial cells by short hairpin RNA (shRNA) interference significantly impaired cellular proliferation and induced features of gene expression characteristic of colonic or enterocytic differentiation. Downregulation of ICK altered expression of cell cycle regulators (cyclin D1, c-Myc, and p21Cip1/WAF1) of G1-S transition, consistent with the G1 cell cycle delay induced by ICK shRNA. ICK deficiency also led to a significant decrease in the expression and/or activity of p70 ribosomal protein S6 kinase (S6K1) and eukaryotic initiation factor 4E (eIF4E), concomitant with reduced expression of their upstream regulators, the mammalian target of rapamycin (mTOR) and the regulatory associated protein of mTOR (Raptor). Furthermore, ICK interacts with the mTOR/Raptor complex in vivo and phosphorylates Raptor in vitro. These results suggest that disrupting ICK function may downregulate protein translation of specific downstream targets of eIF4E and S6K1 such as cyclin D1 and c-Myc through the mTOR/Raptor signaling pathway. Taken together, our findings demonstrate an important role for ICK in proliferation and differentiation of intestinal epithelial cells.
Keywords: intestinal epithelial cell proliferation and differentiation; mammalian target of rapamycin, regulatory associated protein of mTOR, caudal-type homeobox protein 2 (Cdx2)
intestinal cell kinase/MAK-related kinase (ICK/MRK) (1, 15, 48) encodes a conserved Ser/Thr kinase in the CMGC group of the protein kineome, clustering with MAK (male germ cell-associated kinase) (35) and MOK (MAPK/MAK/MRK overlapping kinase) (36). ICK, MAK, and MOK have similarities in the catalytic domain to both cyclin-dependent protein kinases (CDKs) and mitogen-activated protein kinases (MAPKs) (15, 37). The TDY motif of ICK and MAK and the TEY motif of MOK in their activation T-loops align with the TXY motif of classic MAPKs (15). ICK requires an intact and doubly phosphorylated TDY motif for maximum activity (15). PP5 (protein phosphatase 5) and CCRK (cell cycle-related kinase) are putative regulators for Thr-157 phosphorylation in the TDY motif (14). Unlike classic MAPKs, the ICK kinase activity was not acutely stimulated by serum or EGF under the same condition in which the ERK kinase activity was upregulated (48). In addition, ICK has a long COOH-terminal noncatalytic domain with postulated functions in protein-protein and protein-DNA interactions (15). These differences in domain structure and response to growth factors suggest that ICK may operate through a signaling pathway distinct from that of classic MAPKs.
ICK mRNA is expressed in many tissues and is highly abundant in intestine and lung (1, 48). In the small intestine, ICK mRNA expression appears to be restricted to the proliferative compartment of the crypt where the stem and progenitor cells reside (48). The specific localization of ICK mRNA in the crypt suggests an important function for ICK in some aspect of stem cell or progenitor cell activity. Various signaling cascades including Wnt, Hedgehog, BMP, and Notch have been implicated in the control of stem cell activity, proliferation, lineage commitment, terminal differentiation, and cell survival during normal development and tissue regeneration of the gastrointestinal epithelium (8, 41). Dysregulation of the signaling pathways that control these biological processes can lead to intestinal neoplasia (4, 41, 42). Recently, a role for the mammalian target of rapamycin (mTOR) signaling pathway during the development and morphogenesis of the intestinal epithelium has also emerged (30, 32).
mTOR plays a central role in cell growth and proliferation by integrating nutrient, hormonal, and energy signal inputs to control protein translation (16, 45). mTOR forms two structurally and functionally distinct multiprotein complexes, mTORC1 and mTORC2. mTOR when associated with its regulatory protein Raptor in complex 1 (28) is rapamycin sensitive and regulates protein translation by modulating the activity of two important translational regulators, the eukaryotic initiation factor 4E (eIF4E) and the ribosomal protein S6 kinases (S6K1 and S6K2). eIF4E is the mRNA cap-binding protein and a key component of the eIF4F translation initiation complex. Assembly of the eIF4F complex is rate limiting for translation initiation and is largely dependent on eIF4E availability. The impact of eIF4E availability on protein translation is selective and disproportional (17). Most cellular mRNAs containing short 5′-untranslated regions (UTRs) are insensitive to the alteration in eIF4E availability. Only “weak” mRNAs containing lengthy and highly structured 5′-UTRs such as c-myc, cyclin D1, VEGF, survivin, and autocrine growth factors are sensitive to the availability of eIF4E. Elevated eIF4E function can also enhance nucleocytoplasmic transport of mRNAs encoding growth-promoting proteins such as cyclin D1 (44). S6K1 has been postulated to modulate protein translation through multiple targets including 40S ribosomal protein S6 (24), eukaryotic initiation factor 4B (eIF4B) (16), and eukaryotic elongation factor 2 (eEF2) kinase (51).
mTORC1 controls cell growth and proliferation and contributes to efficient G1 cell cycle progression through its downstream effectors eIF4E and S6K1 (11, 12). However, the study of mTORC1 functions in mammalian organogenesis and morphogenesis is hindered because both mTOR and Raptor knockout mice die at an early embryonic stage (20). Nevertheless, a role for TORC1 signaling in the regulation of intestinal morphogenesis and development was revealed from studies in two eukaryotic model organisms. Disruption of TOR in Caenorhabditis elegans caused intestinal atrophy associated with inhibition of mRNA translation (30). During zebrafish development, although rapamycin induced only a mild overall developmental delay, the digestive tract development was arrested at the primitive gut tube stage (32), suggesting that the TOR signaling events are essential for epithelial growth, morphogenesis, and differentiation in the vertebrate intestine. Gene knockdown demonstrated that this defect in gut development is mediated specifically by the rapamycin-sensitive zTOR/Raptor complex, suggesting that the mTOR/Raptor complex may play a similar role in mammalian gastrointestinal development.
In this study we sought to investigate the biological functions of ICK during intestinal epithelial cell proliferation and differentiation by knocking down ICK expression in colorectal carcinoma and intestinal epithelial cell lines using lentiviral shRNA. Here we report that knockdown of ICK expression in replicating intestinal epithelial cells induced growth retardation, G1 cell cycle delay, and some features of gene expression characteristic of colonic or enterocytic differentiation. We also report that the expression and/or activity of several key regulatory components of G1 cell cycle progression and of major regulators of protein translation and cell growth in the mTORC1 pathway were significantly altered. Finally, we provide biochemical evidence showing that ICK may directly interact with the mTOR/Raptor complex and that Raptor may be a potential downstream substrate of ICK.
MATERIALS AND METHODS
Cell culture.
COLO 205, Caco-2, and HEK293T cells were obtained from American Type Culture Collection. RIE-1 rat intestinal epithelial cell line was obtained from Dr. Ken Brown, Cambridge, UK. Cells were maintained at a 37°C and 5% CO2 incubator. HEK293T and RIE cells were cultured in Dulbecco's modified Eagle's medium with high glucose supplemented with 10% fetal bovine serum. COLO205 cells were cultured in RPMI 1640 medium with 2 mM l-glutamine supplemented with 1.5 g/l sodium bicarbonate, 1.0 mM sodium pyruvate, 4.5 g/l glucose, 10 mM HEPES, and 10% fetal bovine serum. Caco-2 cells were cultured in Eagle's minimal essential medium with 2 mM l-glutamine and Earle's BSS supplemented with 1.5 g/l sodium bicarbonate, 1.0 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 10% fetal bovine serum.
Silencing ICK by lentiviral shRNAs.
The MISSION TRC ICK shRNA Target Set was obtained from Sigma. Human ICK shRNA-1 containing a hairpin insert sequence (5′-CCAGTGAAATTGACACAATAT-3′) and human ICK shRNA-2 containing a hairpin insert sequence (5′-CCTACCATCAAGCCATTGTTT-3′) are most effective at suppressing human ICK expression at protein levels (Fig. 1). Rat ICK shRNA containing a hairpin insert sequence (5′-CCAGTGAAATTGACACGATTT-3′) was constructed within the lentivirus plasmid vector pLKO.1-Puro. The Non-Target shRNA Control Vector (Sigma) contains a hairpin insert sequence (5′-CAACAAGATGAAGAGCACCAA-3′) that contains four base pair mismatches to any known human or mouse genes. This nontargeting shRNA vector is used as a negative control to monitor off-target effects in that it will activate RNA-induced silencing complex and the RNAi pathway but does not target any human or mouse genes.
Fig. 1.
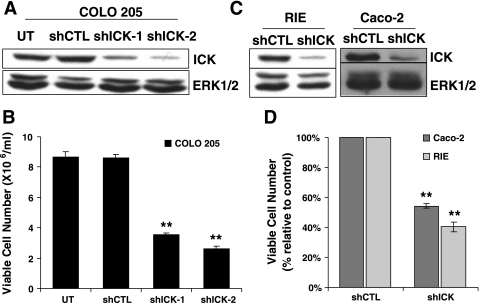
Knockdown of intestinal cell kinase (ICK) impairs proliferation of intestinal epithelial cells. A and B: COLO 205 cells were infected with lentiviral human ICK short hairpin (sh) RNA-1, ICK shRNA-2, or the control shRNA. The protein levels of ICK were assessed by Western blotting (A). Infected COLO 205 cells, including cells floating in culture medium and cells removed from culture dishes by trypsinization, were stained with Trypan blue and counted with a hemocytometer. Total cell numbers are shown in B; means ± SD, n = 3, **P < 0.01. C and D: RIE and Caco-2 cells were infected with lentiviral rat ICK shRNA and human ICK shRNA-1, respectively, or the control shRNA. The protein levels of ICK were assessed by Western blotting (C). D: 4 days after infection, the viable cell number was determined in RIE and Caco-2 cells expressing ICK shRNA and expressed relative to the control shRNA (means ± SD, n = 3, **P < 0.01).
Lentivector particles were generated in HEK293T cells by using a calcium-phosphate protocol as described in Ref. 14. Exponentially growing cells at ∼50–60% confluence were infected with either ICK shRNA or the control shRNA overnight (12–14 h) before change of medium. Twenty-four hours after infection, cells were plated at ∼6–8 × 105 cells/ml in 10-cm dishes and grown for 3–4 days in the presence of 5 μg/ml puromycin. Infected cells were harvested either for determination of cell number and cell cycle distribution or for mRNA and protein extraction.
Determination of viable cell number.
Infected COLO 205 or RIE cells were grown for 3–4 days before harvest. Unattached cells floating in culture medium and attached cells removed from tissue culture plates by trypsin-EDTA were pooled and counted by using a hemocytometer based on Trypan blue exclusion staining. Infected Caco-2 cells were cultured at preconfluence for 3–4 days. Because Caco-2 cells cluster, it is difficult to determine the total viable cell number by counting via a hemocytometer as described above. Therefore, we determined the Caco-2 cell number from cell culture infected with ICK shRNA relative to those infected with the control shRNA by counting the number of cell nuclei stained with 4,6-diamidino-2-phenylindole (DAPI). Forty optical fields under ×20 lens were randomly selected per coverslip for counting and triplicate coverslips per treatment were counted. The mean cell count of ICK shRNA treated cell culture was plotted as the percentage of the mean cell count of the control shRNA-treated cell culture.
Flow cytometry.
Attached and unattached COLO 205 or RIE cells were pooled as described above, washed in PBS, and fixed in a 1% paraformaldehyde solution. Fixed cells were permeabilized in 70% ethanol and stained with 80 μg/ml of propidium iodide (Sigma) in the presence of 100 μg/ml of ribonuclease (Roche). DNA content was determined by use of a FACSCalibur flow cytometer (Becton Dickinson Biosciences). Data were analyzed by use of Mod Fit Software (Verity Software House).
Quantitative real-time PCR.
Total RNA was isolated from COLO 205 and Caco-2 cells expressing either ICK shRNA or the control shRNA using RNeasy Protection Mini Kit (Qiagen). The first-strand cDNA was synthesized by reverse transcription from isolated RNA and used for PCR using Taqman PCR master mix in triplicates (Applied Biosystems). Quantity of target mRNA was measured by real-time PCR using an ABI PRISM SDS7000 detection system (Applied Biosystems). Taqman primer sets were obtained from Applied Biosystems: ICK (Hs00248170), CCND1 (Hs00277039), MYC (Hs01570247), CDX2 (Hs00230919), sucrase isomaltase (SI) (Hs00356112). The 18S RNA was used as internal reference for normalization.
Cell extract preparation and Western blot.
Cells were harvested in ice-cold PBS and lysed in lysis buffer [50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40 (NP-40), 2 mM EGTA, and 2 mM DTT supplemented with complete protease inhibitors (Roche), 1 mM Na3VO4, 1 μM microcystin LR, and 5 mM β-glycerophosphate]. Cell lysate was cleared by centrifugation. Protein concentration of the supernatant was determined by Bradford assay (Bio-Rad). Equal volume of cell extract and 2× sodium dodecyl sulfate (SDS) sample buffer (0.5 M Tris, pH 6.8, 10% SDS, 10% glycerol, 0.1% bromphenol blue) were mixed, heated for 5 min, and stored frozen at −20°C. Equal amounts of total protein from cells expressing either ICK shRNA or the control shRNA were loaded onto a SDS gel and transferred to a PVDF membrane for Western blotting.
Western blotting was done essentially as described in Ref. 15. A rabbit polyclonal ICK antibody was generated against the ICK peptide, (C)EQKNGEIKPKSRR (residues 388-400 of mouse ICK), purified on a peptide affinity column (Sulfolink from Pierce) and stored frozen in 50% glycerol. Antibodies against E-cadherin, cyclin D1, CDK4, p21Waf1/Cip1, p27Kip1, p15INK4B, Cdx2, mTOR, Raptor, eIF4E-binding proteins 1 and 2 (4EBP1 and 4EBP2), phospho-Cdk2 (Thr160), and phospho-p70 S6 kinase (Thr389) were from Cell Signaling Technology. Mouse monoclonal eIF4E and Cdk2 antibodies were from BD Transduction Laboratories. Mouse polyclonal PC-LKC antibody was from Novus Biologicals. Liver-intestine cadherin (LI-CDH) (H-167) rabbit polyclonal antibody and c-MYC (9E10) mouse monoclonal antibody were from Santa Cruz Biotechnology. Rabbit anti-ACTIVE MAPK (pTEpY) polyclonal antibody was from Promega.
GST pull-down assay.
Glutathione S-transferase (GST; 2 μg) or GST-ICK (10 μg) plasmid was cotransfected with AU1-mTOR (4 μg) and/or Flag-Raptor (2 μg) plasmids into HEK293T cells. Forty-eight hours after transfection, cells were harvested in ice-cold PBS and lysed in lysis buffer [50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 2 mM EGTA, complete mix of protease inhibitors (Roche), 1 mM Na3VO4, 1 μM microcystin LR, and 5 mM β-glycerophosphate]. The cell lysate was cleared by centrifugation. A portion of the cell lysate was used for Western blotting as a control for protein signal input. The rest of the cell lysate was incubated with glutathione-Sepharose beads (Amersham Biosciences) for 2 h at 4°C to absorb GST fusion proteins. The beads were washed extensively with lysis buffer followed by PBS buffer. The beads were boiled in SDS sample buffer for 5 min to elute binding proteins.
In vitro kinase assay.
Flag-tagged human Raptor or mTOR plasmid was transfected into HEK293T cells. Forty-eight hours after transfection, cells were harvested and lysed. To pull down Flag-Raptor or Flag-mTOR, the cell lysate was cleared by centrifugation and subsequently incubated with the anti-Flag (2–3 μg/ml) monoclonal antibody (Sigma) plus protein A-Sepharose beads (Pierce) at 4°C for 3–4 h. The beads were extensively washed in lysis buffer followed by kinase buffer (50 mM HEPES, pH 7.5, and 10 mM MgCl2 supplemented with 5 mM DTT, complete protease inhibitors [Roche], 1 mM Na3VO4, 1 μM microcystin LR and 5 mM β-glycerophosphate). The beads samples were incubated with 5 μCi [γ-32P]ATP (7,000 Ci/mmol), 100 μM ATP, and 1 to 2 μg of affinity-purified His-ICK(1-296) (14) in 50 μl kinase buffer at 30°C for 15 min with gentle agitation. The reaction was terminated by addition of 50 μl of 2× SDS sample buffer. The reaction sample was heated at 95°C for 5 min and separated on a 10% SDS gel. The gel was dried and exposed for autoradiography.
RESULTS
Suppression of ICK expression impairs intestinal epithelial cell proliferation.
A previous study indicates that ICK mRNA is restricted to the intestinal crypt, which is the proliferative compartment of the intestine where stem cells, progenitor cells, and the rapidly replicating transit-amplifying cells reside (48). Therefore, we examined the potential biological effects of ICK on proliferation by suppressing ICK expression in a highly proliferative colonic epithelial cell line, COLO 205, using lentiviral shRNA. Two different ICK shRNAs effectively decreased ICK protein level in COLO 205 cells compared with the control shRNA (Fig. 1A). COLO 205 cells were infected with the ICK shRNA, and the viable cell number was determined 4 days after infection when the protein level of ICK was markedly reduced. No significant difference in the total viable cell number was detected when comparing cell cultures that were not infected with those infected with the control shRNA. However, there was a significant decrease (by ∼60–70%) in the total viable cell number of cell cultures infected with either ICK shRNA-1 or ICK shRNA-2 compared with those infected with the control shRNA or uninfected (Fig. 1B).
We also examined the potential effect of ICK on cell proliferation in RIE-1 rat intestinal epithelial cell line and Caco-2 colon carcinoma cell line. ICK shRNA was able to effectively knockdown ICK protein level in both RIE-1 and Caco-2 cells compared with the control shRNA (Fig. 1C). There was a 50–60% reduction in the total viable cell number of replicating preconfluent Caco-2 cells and RIE cells infected with ICK shRNA compared with those infected with the control shRNA (Fig. 1D).
Silencing ICK triggers cell cycle delay at G1.
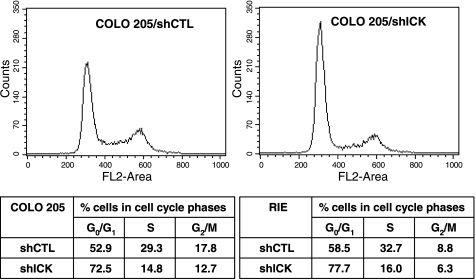
One possible reason for the significant decrease in viable cell numbers induced by ICK shRNA is the inhibition of cell cycle progression. Three days after ICK shRNA infection, cell cycle progression of exponentially growing COLO205 cells was assessed by flow cytometry. A significant increase (by ∼20%) in the proportion of cells in the G1 phase was observed (Fig. 2). Similarly, a ∼20% increase in the proportion of cells in the G1 phase was induced by ICK shRNA in replicating RIE-1 cells (Fig. 2). These results indicate that knockdown of ICK caused a significant delay of the cell cycle during G1. COLO 205 cultures infected with ICK shRNA lacked the typical sub-G0/G1 peak recognized in flow cytometry scans as dead cells and/or cell debris. Furthermore, cell nuclei appeared normal as revealed by DAPI staining and the activated form of caspase 3, the primary mediator of apoptosis, was not detected (data not shown). Thus programmed cell death is unlikely to be the major cause of the reduction in viable cell numbers induced by ICK shRNA.
Fig. 2.
ICK deficiency triggers a significant cell cycle delay at G1. After lentiviral shRNA infection, COLO 205 or RIE cells, including cells floating in culture medium, were harvested and stained with propidium iodide. The DNA content was determined by flow cytometry. The relative distribution of cells in the various phases of the cell cycle is shown.
Downregulation of ICK alters the expression of several cell cycle regulators during G1-S transition.
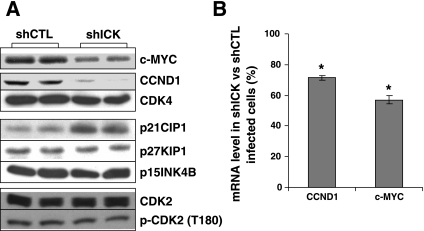
To explore the molecular mechanism underlying the prolonged G1 phase induced by the knockdown of ICK, we analyzed the expression of several cell cycle regulator genes, particularly those that are involved in G1-S transition. The levels of cyclin D1 and c-Myc, positive regulators of G1-S transition, were downregulated whereas that of p21Cip1, a Cdk inhibitor at G1, was upregulated in cells expressing ICK shRNA compared with cells expressing the control shRNA (Fig. 3A). In contrast, the levels of Cdk4, Cdk2, phosphorylated Cdk2, and Cdk inhibitors p27Kip1 and p15Ink4b were not altered significantly following the knockdown of ICK (Fig. 3A), suggesting that the G1 phase delay induced by ICK shRNA is a specific cell cycle event that is primarily dependent on the availability of cyclin D1 and/or c-Myc.
Fig. 3.
ICK deficiency alters gene expression of cell cycle regulators at G1. After lentiviral shRNA infection, COLO 205 cells were harvested as described in Fig. 2 and extracted for total cellular protein and mRNA. A: protein levels of cell cycle regulators in cells expressing ICK shRNA and the control shRNA were assessed by Western blotting. B: mRNA levels of cyclin D1 (CCND1) and c-Myc (c-myc) were determined by quantitative RT-PCR; means ± SD, n = 3, *P < 0.05.
Attenuating ICK function downregulates key regulators of protein translation and perturbs the mTORC1 pathway.
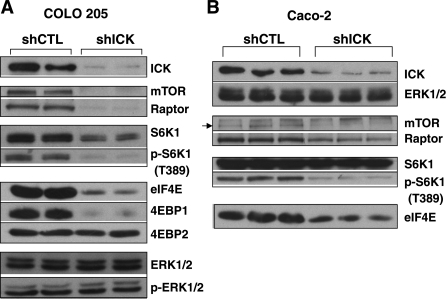
The expression of cyclin D1 and c-myc can be regulated at both the transcriptional and translational levels. Although ICK shRNA induced significant downregulation of cyclin D1 and c-Myc proteins (Fig. 3A), only a modest decrease in the levels of cyclin D1 and c-myc mRNAs was observed in the ICK-deficient COLO 205 cells (Fig. 3B). Given that the mRNAs of cyclin D1 and c-myc have lengthy, G+C rich, highly structured 5′-UTRs, they are sensitive to the availability of eIF4E (7). Therefore, we asked whether the decrease in cyclin D1 and c-Myc proteins might be the consequence of the reduced expression and/or activity of eIF4E. Indeed, a significant decrease in the protein level of eIF4E was observed in COLO 205 and Caco-2 cells treated with ICK shRNA compared with those treated with the control shRNA (Fig. 4). The activity of eIF4E can be inhibited by the binding of 4EBP1 and 4EBP2, direct downstream targets of mTORC1. The levels of 4EBP1 and 4EBP2 were determined in COLO 205 cells infected with ICK shRNA or the control shRNA. The 4EBP1 level was markedly reduced in ICK shRNA treated COLO 205 cells, whereas the 4EBP2 level was not affected (Fig. 4A).
Fig. 4.
Disrupting ICK function in intestinal epithelial cells impairs the expression and/or activity of key regulatory components of protein translation in the mTORC1 signaling pathway. After lentiviral shRNA infection, cells were harvested and extracted for total cellular protein. The protein levels of key components of the mTORC1 pathway and the classic MAPK pathway were assessed by Western blotting in COLO 205 cells (A) or Caco-2 cells (B) expressing either ICK shRNA or the control shRNA. mTOR, mammalian target of rapamycin; Raptor, regulatory associated protein of mTOR; S6K1, ribosomal protein S6 kinase (S6K1); eIF4E, eukaryotic initiation factor 4E; 4EBP1, eIF4E-binding protein 1.
In addition to eIF4E, p70 S6 kinase (S6K1) is another key regulator of cyclin D1 translation (29). S6K1 activation is initiated by mTOR/Raptor-mediated phosphorylation of Thr-389 (28). We found a decrease in the level of S6K1 protein in COLO205 cells expressing ICK shRNA compared with those expressing the control shRNA (Fig. 4A). Furthermore, the activity of S6K1, as assessed by phosphorylation of Thr-389, was markedly reduced, concomitant with a significant decrease in mTOR and Raptor proteins. In contrast to COLO205 cells, Caco-2 cells expressing ICK shRNA yielded a similar amount of S6K1 protein as cells expressing the control shRNA (Fig. 4B). However, a significant decrease in the activity of S6K1, as assessed by phosphorylation of Thr-389, was detected in Caco-2 cells expressing ICK shRNA. The reduced phosphorylation of S6K1 at Thr-389 was again associated with the reduced expression of mTOR/Raptor proteins (Fig. 4B). In contrast to S6K1, the level and activity of ERK1 and ERK2 were not affected by the deficiency of ICK (Fig. 4A).
ICK physically associates with the mTOR/Raptor complex and phosphorylates Raptor in vitro.
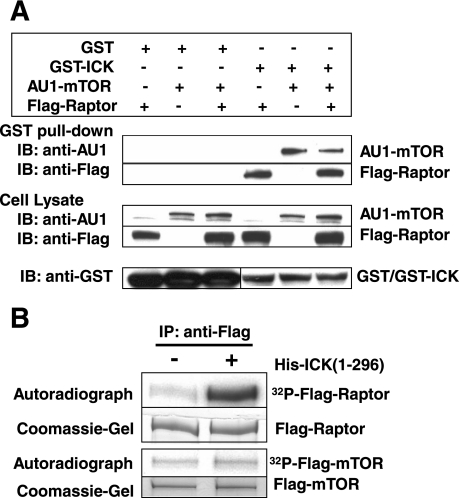
Given that suppression of ICK expression significantly downregulates the mTOR/Raptor signaling pathway, we tested whether ICK interacts with the mTOR/Raptor complex in vivo and whether mTOR and/or Raptor are putative substrates for ICK. To determine whether ICK has affinity for binding mTOR and/or Raptor, we examined the association of recombinant mTOR and Raptor with GST-ICK vs. control GST after purification from cell lysates using glutathione beads (Fig. 5A). Although GST was expressed at a much higher level than GST-ICK, no recombinant mTOR or Raptor was detectable in washed beads with bound GST. In contrast, a portion of recombinant mTOR and Raptor was bound by GST-ICK. We conclude that ICK has a selective affinity for binding mTOR and Raptor.
Fig. 5.
ICK interacts with mTOR/Raptor in vivo and phosphorylates Raptor in vitro. A: glutathione S-transferase (GST)-ICK or GST was coexpressed with Flag-Raptor and/or AU1-mTOR in HEK293T cells. GST fusion proteins were pulled down from cell lysate and analyzed for association with Flag-Raptor or AU1-mTOR by Western blotting against the anti-Flag and anti-AU1 antibodies, respectively. B: Flag-mTOR or Flag-Raptor was expressed in HEK293T cells and isolated by anti-Flag immunoprecipitation prior to the in vitro kinase assay with His-ICK(1-296).
To determine whether mTOR and/or Raptor are substrates for ICK, we tested the ability of active His-ICK(1-296) (14) to phosphorylate Flag-mTOR or Flag-Raptor immunoprecipitated from cells. Whereas the phosphorylation level of Flag-mTOR was unchanged, the phosphorylation level of Flag-Raptor was significantly increased after ICK and ATP/Mg treatment (Fig. 5B). This result implicates Raptor as a putative substrate for ICK.
ICK deficiency in COLO 205 cells induces elevated expression of genes associated with colonic epithelial polarization and differentiation.
To determine whether ICK also plays a role in regulating intestinal epithelial cell differentiation, we examined whether the knockdown of ICK expression induces changes in gene expression that are hallmarks of intestinal epithelial cell differentiation.
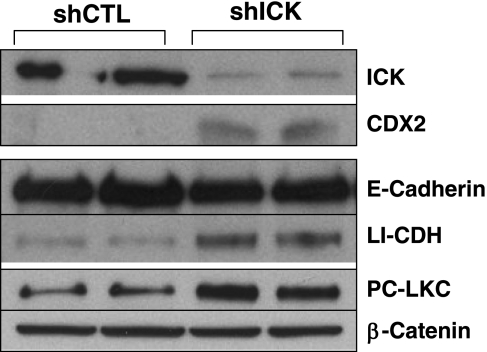
COLO205 cells are a highly proliferative and poorly differentiated colorectal carcinoma cell line. However, these cells can be induced to express morphological features of colonic epithelial differentiation by the overexpression of the homeodomain transcriptional factors Cdx1 or Cdx2 (27). Although some evidence suggests functional redundancy between Cdx1 and Cdx2 (10), Cdx2, in particular, has been regarded as an intestine-specific homeobox gene and a master regulator of the intestinal differentiation program during morphogenesis and development of intestinal epithelium (5, 13, 47). The major morphological feature of differentiated COLO205 cells induced by Cdx1 or Cdx2 is the compaction of cells that is driven by the upregulated interaction between E-cadherin and β- and p120-catenin (10, 27). A significant increase in Cdx2 protein was observed in the ICK-deficient COLO205 cells (Fig. 6). However, no significant clustering of COLO205 cells that expressed ICK shRNA was observed (data not shown). The expression of two well-established downstream target genes of Cdx2, sucrase isomaltase (SI) (38) and liver-intestin cadherin (LI-CDH) (21), was also examined in COLO 205 cells infected with ICK shRNA or the control shRNA. The mRNA of SI was not detectable by quantitative RT-PCR in COLO 205 cells (data not shown), consistent with a previous report (27). However, significant upregulation of LI-CDH protein (Fig. 6) and mRNA (data not shown) was detected by Western blotting and quantitative RT-PCR, respectively. Furthermore, significant upregulation of the cell adhesion protein protocadherin LKC (Liver, Kidney, Colon) (PC-LKC) was seen in ICK-deficient COLO 205 cells (Fig. 6). PC-LKC is specifically enriched in liver, kidney, and colon and functions as a molecular switch for contact inhibition of epithelial cells (40). In contrast, the protein level of E-cadherin was not altered following the knockdown of ICK, suggesting the ICK regulates only a subset of differentiation and polarization-related genes.
Fig. 6.
ICK deficiency in COLO 205 cells induces features of gene expression characteristic of colonic epithelial polarization and differentiation. After lentiviral shRNA infection, COLO 205 cells were harvested and extracted for total cellular protein. The protein levels of ICK, Cdx2, liver-intestine cadherin (LI-CDH), protocadherin LKC (PC-LKC), and E-cadherin were analyzed by Western blotting. Anti-β-catenin signal indicates equal loading of total proteins.
ICK deficiency in Caco-2 cells induces gene expression characteristic of enterocytic differentiation.
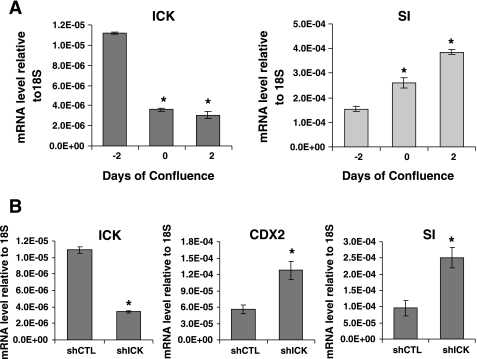
Caco-2 cells undergo spontaneous differentiation along the absorptive enterocytic cell lineage with time in culture, including cellular polarization and formation of tight junctions and microvilli, thus recapitulating some of the phenotypic changes that intestinal epithelial cells undergo during their migration along the crypt-villus axis. At the biochemical and molecular level, the expression of brush border hydrolases such as alkaline phosphatase and SI are increased in differentiated postconfluent Caco-2 cells compared with their expression in replicating subconfluent Caco-2 cells (9, 22). The level of ICK mRNA was significantly decreased when Caco-2 cells attained confluence in culture and underwent a spontaneous differentiation, as demonstrated by a steady increase in the mRNA level of SI (Fig. 7A). Furthermore, suppression of ICK expression by shRNA in preconfluent replicating Caco-2 cells resulted in the elevated expression of SI (∼2.5-fold) and Cdx2 (∼2-fold) (Fig. 7B) compared with control cells expressing ICK.
Fig. 7.
ICK deficiency in Caco-2 cells induces a significant increase in the mRNA level of sucrase isomaltase (SI), a marker for enterocytic differentiation. A: mRNA levels of ICK and SI were determined in Caco-2 cultures at different stages of confluence by quantitative RT-PCR; means ± SD, n = 3, *P < 0.05. B: mRNA levels of SI and Cdx2 in preconfluent replicating Caco-2 cells infected with either ICK shRNA or the control shRNA were determined by quantitative RT-PCR; means ± SD, n = 3, *P < 0.05.
DISCUSSION
In this study, we demonstrate that ICK plays a key role in the regulation of cell proliferation and G1 cell cycle progression. Our findings suggest that downregulation of ICK may modulate the transition of intestinal epithelial cells from proliferation to differentiation. Consistent with this conclusion, we provide evidence that knockdown of ICK altered gene expression of several key regulators of G1-S transition. Specifically, cyclin D1 and c-Myc were downregulated and p21Cip/WAF1 was upregulated, consistent with the G1 cell cycle delay induced by ICK shRNA. ICK deficiency also resulted in the downregulation of eIF4E and S6K1 expression and/or activity, in agreement with the reduced protein translation of specific downstream target genes of eIF4E and S6K1 such as cyclin D1 and c-Myc.
Evidence from our study supports a positive role for ICK in the proliferation and G1 cell cycle progression of intestinal epithelial cells in vitro, consistent with the restricted expression of ICK mRNA in the proliferative compartment of the small intestine in vivo. Although very little is known about the biological functions of these MAPK-related family members ICK/MAK/MOK in the intestine, the importance of the classic MAPK signaling pathways during intestinal epithelial cell proliferation and differentiation has been documented (2, 23, 49). By small molecule inhibition or gene knockout, it has been demonstrated that ERK supports proliferation whereas p38 MAPK and JNK promote differentiation of intestinal epithelial cells. Localization of MAP kinases along the crypt-villus axis of the small intestine is directly correlated with their biological functions. Because of the lack of reliable ICK antibodies for immunohistochemistry, the distribution of ICK protein and activity along the crypt-villus axis within the small intestine are not yet known. Further studies will be required to determine whether reduced ICK expression and/or activity will change the pattern of proliferation and differentiation of intestinal epithelial cells along the crypt-villus axis in vivo.
Although ICK is similar to both CDK2 and ERK2 in the catalytic domain and can be regulated within a MAPK-like TDY motif, knockdown of ICK in intestinal epithelial cells has little effect on the expression or activation of CDK2 and ERK2, suggesting a minimal functional overlap between ICK, ERK2, and CDK2 signaling cascades. This result is consistent with our observation that disrupting ICK signaling alone in intestinal epithelial cells only resulted in partial inhibition of growth and cell cycle. It is likely that other signaling cascades, such as the MAPK pathway, are still able to drive cell cycle progression to a significant extent in the absence of ICK depending on the physiological context of the cell.
The expression and/or activity of several key regulatory components of the protein translational machinery and the mTORC1 signaling pathway were significantly altered in response to ICK deficiency. This observation is consistent with reported gene microarray data indicating that protein translation is one of the major biological processes that are significantly downregulated during differentiation of Caco-2 intestinal epithelial cells in vitro and during maturation of intestinal epithelial cells along the crypt-villus axis in vivo (33, 34).
Our study provides further support for the role of mTORC1 pathway in the control of cell cycle and proliferation of intestinal epithelial cells during mammalian gastrointestinal morphogenesis and development. Reduced ICK expression in COLO 205 and Caco-2 cells significantly decreased mTOR and Raptor proteins, which led to reduced phosphorylation and activity of their downstream effectors such as S6K1. In addition, knockdown of ICK expression resulted in reduced expression of eIF4E protein in both COLO 205 and Caco-2 cells and reduced expression of S6K1 protein in COLO 205 cells but not in Caco-2 cells. We also observed a differential expression pattern of 4EBP1 and 4EBP2 in ICK-deficient COLO 205 cells, consistent with a previous report that the expression of 4EBP1 and 4EBP2 was differentially regulated during myeloid cell differentiation that is accompanied by a decrease in cell proliferation (18, 19). Taken together, these results suggest that disrupting ICK function may lead to downregulation of eIF4E and S6K1 through different mechanisms that are probably cell context dependent. The molecular basis for these signaling mechanisms through which ICK regulates protein translation awaits further investigation.
A recent study in rat intestinal epithelial and human colon cancer cell lines showed that suppression of Cdx2 stimulated the mTORC1 pathway, causing translational deregulation and G1-S acceleration (3). This result is consistent with our finding that upregulation of Cdx2 expression correlates with downregulation of mTORC1 activity following the knockdown of ICK. The molecular basis for the inverse relationship between Cdx2 expression and mTORC1 activity is unknown. Although it is possible that other signaling cascades may also be involved in conferring the phenotype induced by ICK shRNA, downregulation of the mTOR/Raptor signaling pathway appears to be a major factor involved in regulating cell cycle and cell growth of intestinal epithelial cells in response to ICK deficiency. Thus a direct or indirect interaction between ICK and mTORC1 signaling events may be critical for the morphogenesis and homeostasis of the intestinal epithelium. Indeed, our biochemical data suggest that not only is ICK capable of interacting with the mTOR/Raptor complex in vivo but it may also phosphorylate Raptor as a direct substrate to regulate the activity and/or stability of mTORC1. Further studies are required to elucidate the biological functions of the ICK phosphorylation site(s) of Raptor.
We observed a significant decrease in eIF4E and c-Myc proteins in ICK-deficient intestinal epithelial cells. It has been well documented that the intracellular expression level of c-Myc correlates strictly with cell proliferation and has profound effects on cell cycle progression (39). The decreased expression of c-Myc in the ICK-deficient COLO 205 cells coincides with the increased expression of p21Cip1/WAF1, which is consistent with prior reports showing that c-Myc represses the transcription of p21Cip1/WAF1 (6, 50). However, our data do not exclude the possibility that the increase in the protein level of p21Cip1/WAF1 may also be the consequence of reduced proteosomal degradation induced by ICK shRNA. The core promoter of eIF4E gene contains a pair of consensus c-Myc binding sites (26). The notion that eIF4E is a transcriptional target of c-Myc is well supported by data showing a strict correlation between eIF4E and c-Myc levels following growth induction (43). Given that c-Myc is also a selective translational target of eIF4E, it is conceivable that a positive feedback loop may exist to reciprocally regulate the expression of c-Myc and eIF4E in ICK-deficient intestinal epithelial cells.
In contrast to ICK, expression of its closely related kinase MAK is more restricted (35). Interestingly, MAK is highly expressed in testis where ICK expression is barely detectable (1, 35, 48). MAK, originally identified from and highly expressed in testicular germ cells, was speculated to have an important function in spermatogenesis (25, 35). However, the MAK null mouse is viable and fertile (46), suggesting the existence of functional redundancy and/or feedback compensation for the lack of MAK in testis. MAK was also identified as an androgen-inducible coactivator of androgen receptor in prostate cancer cells. Similar to the role of ICK in intestinal epithelial cells, MAK is also required for prostate epithelial cell replication (31, 52). However, unlike MAK whose expression and function may be tissue restricted, the roles of ICK in proliferation and differentiation may extend well beyond the intestinal epithelium given its much wider tissue distribution.
GRANTS
This work was supported by NIH DK064751 (to S. M. Cohn), American Cancer Society Institutional Research Grant (to Z. Fu), and NIH GM62890 (to T. W. Sturgill). This work was also supported by a Pilot Feasibility Award and a New Investigator Award (to Z. Fu) and the Molecular Biology/Gene Expression and Immunology Cell Isolation Cores of the UVA Digestive Health Research Center (NIH P30 DKO67629).
ACKNOWLEDGMENTS
We are especially grateful to Darlene Bruce for excellent technical assistance. We thank William Ross [University of Virginia (UVA) Digestive Health Research Center] for advice on flow cytometry, Lifu Wang and Thurl Harris (Pharmacology, UVA) for mTOR and Raptor constructs, and Michael J. Weber (UVA Cancer Center) for advice on this project.
REFERENCES
- 1.Abe S, Yagi T, Ishiyama S, Hiroe M, Marumo F, Ikawa Y. Molecular cloning of a novel serine/threonine kinase, MRK, possibly involved in cardiac development. Oncogene 11: 2187–2195, 1995 [PubMed] [Google Scholar]
- 2.Aliaga JC, Deschenes C, Beaulieu JF, Calvo EL, Rivard N. Requirement of the MAP kinase cascade for cell cycle progression and differentiation of human intestinal cells. Am J Physiol Gastrointest Liver Physiol 277: G631–G641, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Aoki K, Tamai Y, Horiike S, Oshima M, Taketo MM. Colonic polyposis caused by mTOR-mediated chromosomal instability in Apc+/ Delta716 Cdx2+/− compound mutant mice. Nat Genet 35: 323–330, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature 432: 324–331, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Beck F. The role of Cdx genes in the mammalian gut. Gut 53: 1394–1396, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claassen GF, Hann SR. A role for transcriptional repression of p21CIP1 by c-Myc in overcoming transforming growth factor beta-induced cell-cycle arrest. Proc Natl Acad Sci USA 97: 9498–9503, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL. eIF4E is a central node of an RNA regulon that governs cellular proliferation. J Cell Biol 175: 415–426, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Santa Barbara P, van den Brink GR, Roberts DJ. Development and differentiation of the intestinal epithelium. Cell Mol Life Sci 60: 1322–1332, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engle MJ, Goetz GS, Alpers DH. Caco-2 cells express a combination of colonocyte and enterocyte phenotypes. J Cell Physiol 174: 362–369, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Ezaki T, Guo RJ, Li H, Reynolds AB, Lynch JP. The homeodomain transcription factors Cdx1 and Cdx2 induce E-cadherin adhesion activity by reducing beta- and p120-catenin tyrosine phosphorylation. Am J Physiol Gastrointest Liver Physiol 293: G54–G65, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23: 3151–3171, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol 24: 200–216, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flores MV, Hall CJ, Davidson AJ, Singh PP, Mahagaonkar AA, Zon LI, Crosier KE, Crosier PS. Intestinal differentiation in zebrafish requires Cdx1b, a functional equivalent of mammalian Cdx2. Gastroenterology 135: 1665–1675, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Fu Z, Larson KA, Chitta RK, Parker SA, Turk BE, Lawrence MW, Kaldis P, Galaktionov K, Cohn SM, Shabanowitz J, Hunt DF, Sturgill TW. Identification of yin-yang regulators and a phosphorylation consensus for male germ cell-associated kinase (MAK)-related kinase. Mol Cell Biol 26: 8639–8654, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Z, Schroeder MJ, Shabanowitz J, Kaldis P, Togawa K, Rustgi AK, Hunt DF, Sturgill TW. Activation of a nuclear Cdc2-related kinase within a mitogen-activated protein kinase-like TDY motif by autophosphorylation and cyclin-dependent protein kinase-activating kinase. Mol Cell Biol 25: 6047–6064, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev 15: 807–826, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Graff JR, Konicek BW, Carter JH, Marcusson EG. Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res 68: 631–634, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Grolleau A, Sonenberg N, Wietzerbin J, Beretta L. Differential regulation of 4E-BP1 and 4E-BP2, two repressors of translation initiation, during human myeloid cell differentiation. J Immunol 162: 3491–3497, 1999 [PubMed] [Google Scholar]
- 19.Grolleau A, Wietzerbin J, Beretta L. Defect in the regulation of 4E-BP1 and 2, two repressors of translation initiation, in the retinoid acid resistant cell lines, NB4-R1 and NB4-R2. Leukemia 14: 1909–1914, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mlST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell 11: 859–871, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Hinoi T, Lucas PC, Kuick R, Hanash S, Cho KR, Fearon ER. CDX2 regulates liver intestine-cadherin expression in normal and malignant colon epithelium and intestinal metaplasia. Gastroenterology 123: 1565–1577, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Hirata M, Bamba T, Hosoda S. The human colon cancer cell line CaCo-2 produces secretory components during enterocytic differentiation. Gastroenterol Jpn 28: 528–534, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Houde M, Laprise P, Jean D, Blais M, Asselin C, Rivard N. Intestinal epithelial cell differentiation involves activation of p38 mitogen-activated protein kinase that regulates the homeobox transcription factor CDX2. J Biol Chem 276: 21885–21894, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. EMBO J 16: 3693–3704, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jinno A, Tanaka K, Matsushime H, Haneji T, Shibuya M. Testis-specific mak protein kinase is expressed specifically in the meiotic phase in spermatogenesis and is associated with a 210-kilodalton cellular phosphoprotein. Mol Cell Biol 13: 4146–4156, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones RM, Branda J, Johnston KA, Polymenis M, Gadd M, Rustgi A, Callanan L, Schmidt EV. An essential E box in the promoter of the gene encoding the mRNA cap-binding protein (eukaryotic initiation factor 4E) is a target for activation by c-myc. Mol Cell Biol 16: 4754–4764, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keller MS, Ezaki T, Guo RJ, Lynch JP. Cdx1 or Cdx2 expression activates E-cadherin-mediated cell-cell adhesion and compaction in human COLO 205 cells. Am J Physiol Gastrointest Liver Physiol 287: G104–G114, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with Raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110: 163–175, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Koziczak M, Hynes NE. Cooperation between fibroblast growth factor receptor-4 and ErbB2 in regulation of cyclin D1 translation. J Biol Chem 279: 50004–50011, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Long X, Spycher C, Han ZS, Rose AM, Muller F, Avruch J. TOR deficiency in C. elegans causes developmental arrest and intestinal atrophy by inhibition of mRNA translation. Curr Biol 12: 1448–1461, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Ma AH, Xia L, Desai SJ, Boucher DL, Guan Y, Shih HM, Shi XB, deVere White RW, Chen HW, Tepper CG, Kung HJ. Male germ cell-associated kinase, a male-specific kinase regulated by androgen, is a coactivator of androgen receptor in prostate cancer cells. Cancer Res 66: 8439–8447, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Makky K, Tekiela J, Mayer AN. Target of rapamycin (TOR) signaling controls epithelial morphogenesis in the vertebrate intestine. Dev Biol 303: 501–513, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariadason JM, Arango D, Corner GA, Aranes MJ, Hotchkiss KA, Yang W, Augenlicht LH. A gene expression profile that defines colon cell maturation in vitro. Cancer Res 62: 4791–4804, 2002 [PubMed] [Google Scholar]
- 34.Mariadason JM, Nicholas C, L'Italien KE, Zhuang M, Smartt HJ, Heerdt BG, Yang W, Corner GA, Wilson AJ, Klampfer L, Arango D, Augenlicht LH. Gene expression profiling of intestinal epithelial cell maturation along the crypt-villus axis. Gastroenterology 128: 1081–1088, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Matsushime H, Jinno A, Takagi N, Shibuya M. A novel mammalian protein kinase gene (mak) is highly expressed in testicular germ cells at and after meiosis. Mol Cell Biol 10: 2261–2268, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyata Y, Akashi M, Nishida E. Molecular cloning and characterization of a novel member of the MAP kinase superfamily. Genes Cells 4: 299–309, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Miyata Y, Nishida E. Distantly related cousins of MAP kinase: biochemical properties and possible physiological functions. Biochem Biophys Res Commun 266: 291–295, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Mutoh H, Satoh K, Kita H, Sakamoto H, Hayakawa H, Yamamoto H, Isoda N, Tamada K, Ido K, Sugano K. Cdx2 specifies the differentiation of morphological as well as functional absorptive enterocytes of the small intestine. Int J Dev Biol 49: 867–871, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Obaya AJ, Mateyak MK, Sedivy JM. Mysterious liaisons: the relationship between c-Myc and the cell cycle. Oncogene 18: 2934–2941, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Okazaki N, Takahashi N, Kojima S, Masuho Y, Koga H. Protocadherin LKC, a new candidate for a tumor suppressor of colon and liver cancers, its association with contact inhibition of cell proliferation. Carcinogenesis 23: 1139–1148, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Perryman SV, Sylvester KG. Repair and regeneration: opportunities for carcinogenesis from tissue stem cells. J Cell Mol Med 10: 292–308, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature 434: 843–850, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Rosenwald IB, Rhoads DB, Callanan LD, Isselbacher KJ, Schmidt EV. Increased expression of eukaryotic translation initiation factors eIF-4E and eIF-2 alpha in response to growth induction by c-myc. Proc Natl Acad Sci USA 90: 6175–6178, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rousseau D, Kaspar R, Rosenwald I, Gehrke L, Sonenberg N. Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc Natl Acad Sci USA 93: 1065–1070, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell 103: 253–262, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Shinkai Y, Satoh H, Takeda N, Fukuda M, Chiba E, Kato T, Kuramochi T, Araki Y. A testicular germ cell-associated serine-threonine kinase, MAK, is dispensable for sperm formation. Mol Cell Biol 22: 3276–3280, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol 16: 619–625, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Togawa K, Yan YX, Inomoto T, Slaugenhaupt S, Rustgi AK. Intestinal cell kinase (ICK) localizes to the crypt region and requires a dual phosphorylation site found in map kinases. J Cell Physiol 183: 129–139, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Tong C, Yin Z, Song Z, Dockendorff A, Huang C, Mariadason J, Flavell RA, Davis RJ, Augenlicht LH, Yang W. c-Jun NH2-terminal kinase 1 plays a critical role in intestinal homeostasis and tumor suppression. Am J Pathol 171: 297–303, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111: 241–250, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J 20: 4370–4379, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia L, Robinson D, Ma AH, Chen HC, Wu F, Qiu Y, Kung HJ. Identification of human male germ cell-associated kinase, a kinase transcriptionally activated by androgen in prostate cancer cells. J Biol Chem 277: 35422–35433, 2002 [DOI] [PubMed] [Google Scholar]