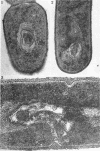
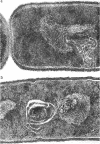
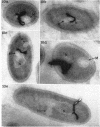
Abstract
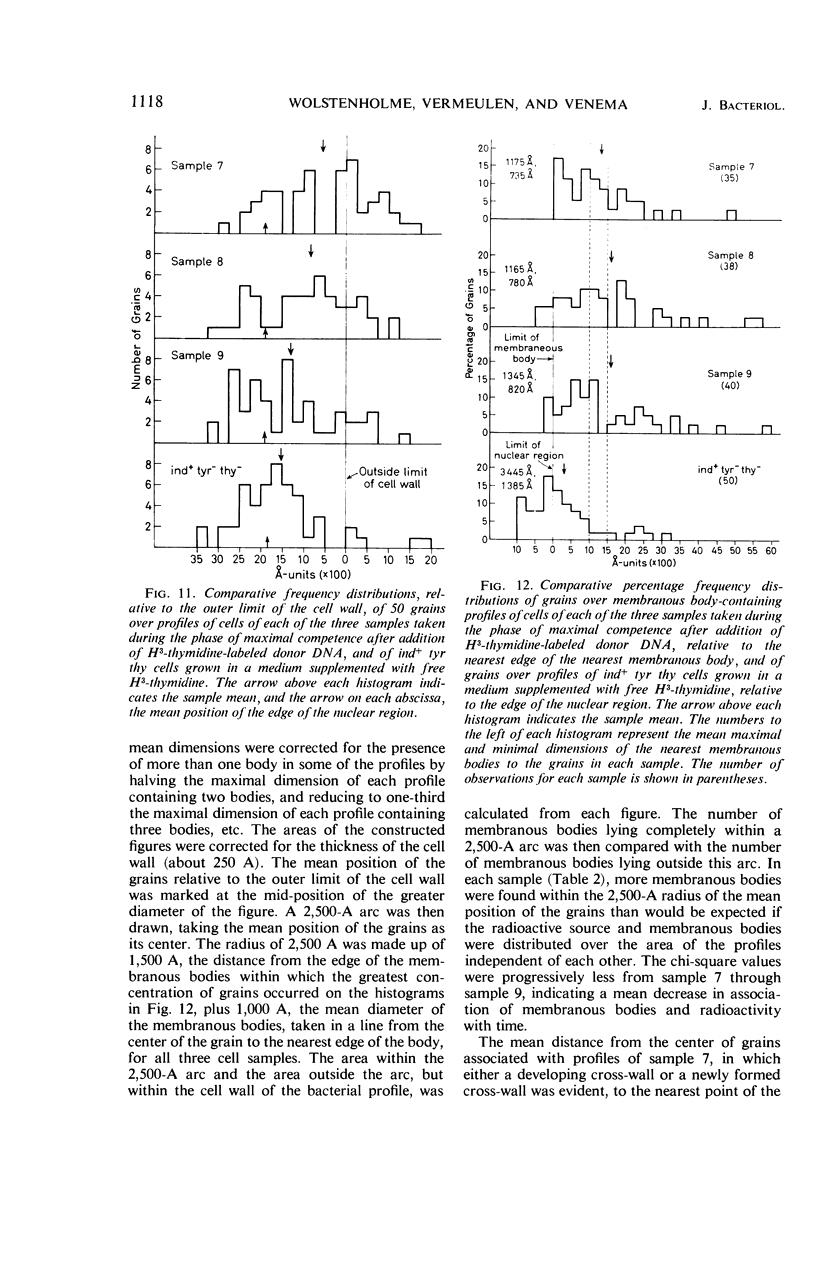
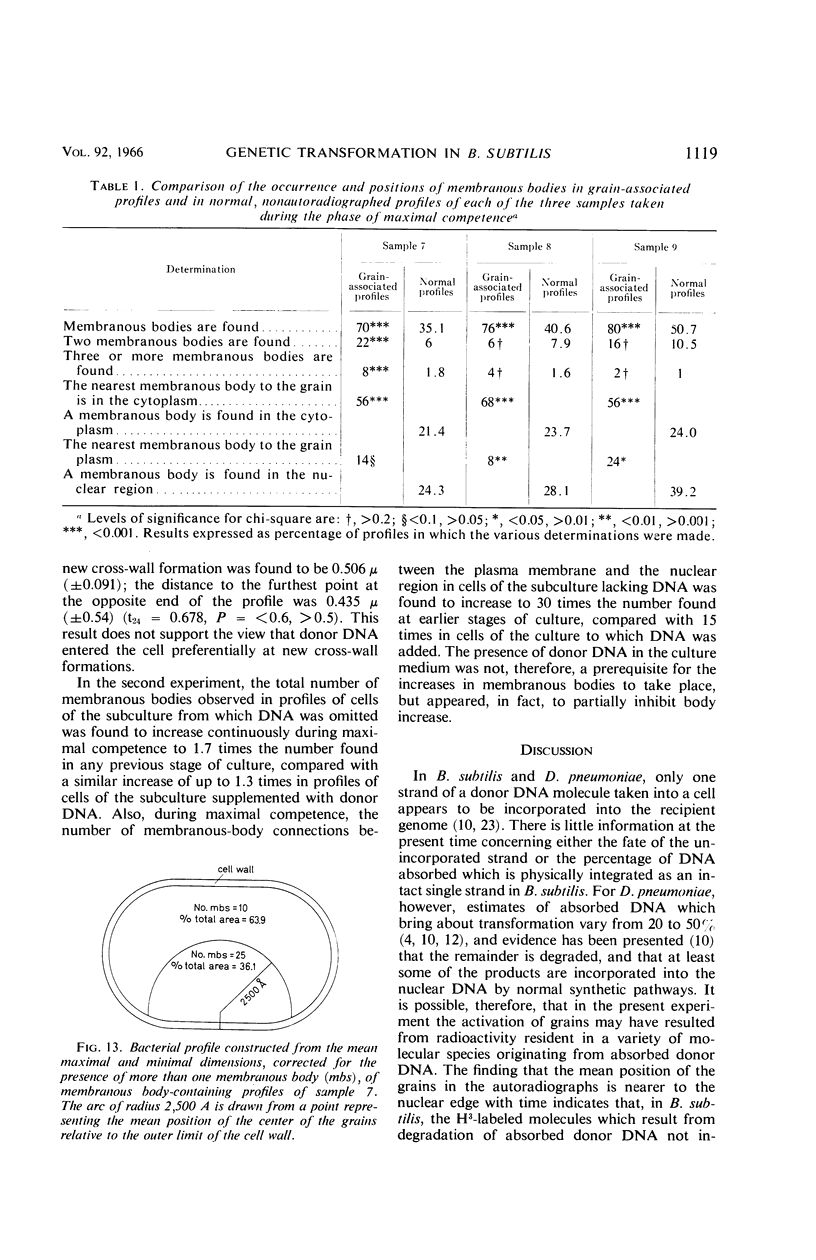
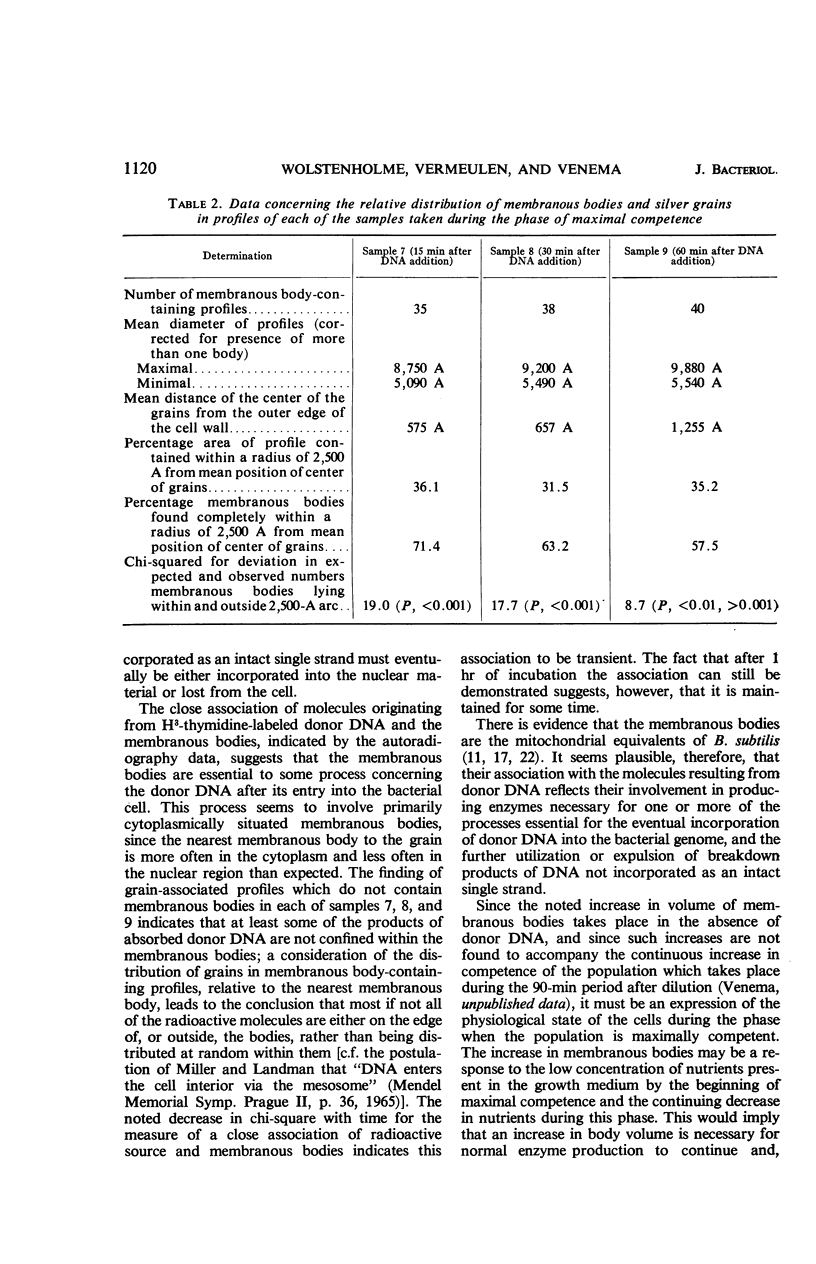
Wolstenholme, David R. (Max-Planck-Institut für Biologie, Tübingen, Germany), Cornelius A. Vermeulen, and Gerhardus Venema. Evidence for the involvement of membranous bodies in the processes leading to genetic transformation in Bacillus subtilis. J. Bacteriol. 92:1111–1121. 1966.—Data obtained from electron microscopic autoradiographs of profiles of cells of a Bacillus subtilis population exposed to H3-thymidine-labeled donor deoxyribonucleic acid (DNA) during the phase of maximal competence indicated that molecules originating from absorbed DNA are closely associated with membranous bodies, particularly with those situated in the cytoplasm, but that most if not all of the radioactive molecules are outside the bodies. It is suggested that membranous bodies produce enzymes essential to the eventual incorporation of transforming DNA into the bacterial genome, or to the breakdown and utilization or expulsion of absorbed DNA not incorporated as transformant (or to both processes). During the phase of maximal competence, the total number of membranous bodies seen in profiles increased continuously to as much as 2.3 times the numbers found during earlier stages of culture. This increase was not accounted for by a decrease in bacterial cell volume, but resulted from an actual increase in total volume of membranous bodies. The number of membranous bodies visibly connecting plasma membrane and nuclear region increased during maximal competence to as much as 30 times the numbers found in earlier stages. As both increases were found in the absence of donor DNA and only began after maximal competence was attained, it seemed most probable that they were an expression of a physiological state influenced by the continuing deficiency of nutrients in the growth medium during this phase of culture.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARO L. G. High-resolution autoradiogaphy. II. The problem of resolution. J Cell Biol. 1962 Nov;15:189–199. doi: 10.1083/jcb.15.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARO L. G., VAN TUBERGEN R. P., KOLB J. A. High-resolution autoradiography. I. Methods. J Cell Biol. 1962 Nov;15:173–188. doi: 10.1083/jcb.15.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S. Deoxyribonucleic acid incorporation by transformed bacteria. Biochim Biophys Acta. 1957 Oct;26(1):83–85. doi: 10.1016/0006-3002(57)90056-2. [DOI] [PubMed] [Google Scholar]
- GLAUERT A. M., BRIEGER E. M., ALLEN J. M. The fine structure of vegetative cells of Bacillus subtilis. Exp Cell Res. 1961 Jan;22:73–85. doi: 10.1016/0014-4827(61)90087-8. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R. D. CYCLICAL BEHAVIOR IN PNEUMOCOCCAL GROWTH AND TRANSFORMABILITY OCCASIONED BY ENVIRONMENTAL CHANGES. Proc Natl Acad Sci U S A. 1954 Feb;40(2):49–55. doi: 10.1073/pnas.40.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F., Ryter A., Cuzin F. On the association between DNA and membrane in bacteria. Proc R Soc Lond B Biol Sci. 1966 Mar 22;164(995):267–278. doi: 10.1098/rspb.1966.0029. [DOI] [PubMed] [Google Scholar]
- KIRBY K. S. A new method for the isolation of deoxyribonucleic acids; evidence on the nature of bonds between deoxyribonucleic acid and protein. Biochem J. 1957 Jul;66(3):495–504. doi: 10.1042/bj0660495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACKS S. Molecular fate of DNA in genetic transformation of Pneumococcus. J Mol Biol. 1962 Jul;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S., TOLMACH L. J. Genetic transformation. I. Cellular incorporation of DNA accompanying transformation in Pneumococcus. Biochim Biophys Acta. 1957 Oct;26(1):68–82. doi: 10.1016/0006-3002(57)90055-0. [DOI] [PubMed] [Google Scholar]
- Leene W., van Iterson W. Tetranitro--blue tetrazolium reduction in Bacillus subtilis. J Cell Biol. 1965 Oct;27(1):237–241. doi: 10.1083/jcb.27.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVIN A. W. The genetics of transformation. Adv Genet. 1961;10:61–163. doi: 10.1016/s0065-2660(08)60116-9. [DOI] [PubMed] [Google Scholar]
- RYTER A., JACOB F. ETUDE AU MICROSCOPE 'ELECTRONIQUE DE LA LIAISON ENTRE NOYAU ET M'ESOSOME CHEZ BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1964 Sep;107:384–400. [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- SPIZIZEN J. Genetic activity of deoxyribonucleic acid in the reconstitution of biosynthetic pathways. Fed Proc. 1959 Dec;18:957–965. [PubMed] [Google Scholar]
- Sedar A. W., Burde R. M. The demonstration of the succinic dehydrogenase system in Bacillus subtilis using tetranitro--blue tetrazolium combined with techniques of electron microscopy. J Cell Biol. 1965 Oct;27(1):53–66. doi: 10.1083/jcb.27.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASZ A., JAMIESON J. D., OTTOLENGHI E. THE FINE STRUCTURE OF DIPLOCOCCUS PNEUMONIAE. J Cell Biol. 1964 Aug;22:453–467. doi: 10.1083/jcb.22.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ITERSON, LEENE W. A CYTOCHEMICAL LOCALIZATION OF REDUCTIVE SITES IN A GRAM-POSITIVE BACTERIUM. TELLURITE REDUCTION IN BACILLUS SUBTILIS. J Cell Biol. 1964 Mar;20:361–375. doi: 10.1083/jcb.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ITERSON W. Some features of a remarkable organelle in Bacillus subtilis. J Biophys Biochem Cytol. 1961 Jan;9:183–192. doi: 10.1083/jcb.9.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENEMA G., PRITCHARD R. H., VENEMA-SCHROEDER T. FATE OF TRANSFORMING DEOXYRIBONUCLEIC ACID IN BACILLUS SUBTILIS. J Bacteriol. 1965 May;89:1250–1255. doi: 10.1128/jb.89.5.1250-1255.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]