Abstract
Background
Glandular trichomes produce a wide variety of commercially important secondary metabolites in many plant species. The most prominent anti-malarial drug artemisinin, a sesquiterpene lactone, is produced in glandular trichomes of Artemisia annua. However, only limited genomic information is currently available in this non-model plant species.
Results
We present a global characterization of A. annua glandular trichome transcriptome using 454 pyrosequencing. Sequencing runs using two normalized cDNA collections from glandular trichomes yielded 406,044 expressed sequence tags (average length = 210 nucleotides), which assembled into 42,678 contigs and 147,699 singletons. Performing a second sequencing run only increased the number of genes identified by ~30%, indicating that massively parallel pyrosequencing provides deep coverage of the A. annua trichome transcriptome. By BLAST search against the NCBI non-redundant protein database, putative functions were assigned to over 28,573 unigenes, including previously undescribed enzymes likely involved in sesquiterpene biosynthesis. Comparison with ESTs derived from trichome collections of other plant species revealed expressed genes in common functional categories across different plant species. RT-PCR analysis confirmed the expression of selected unigenes and novel transcripts in A. annua glandular trichomes.
Conclusion
The presence of contigs corresponding to enzymes for terpenoids and flavonoids biosynthesis suggests important metabolic activity in A. annua glandular trichomes. Our comprehensive survey of genes expressed in glandular trichome will facilitate new gene discovery and shed light on the regulatory mechanism of artemisinin metabolism and trichome function in A. annua.
Background
Secreting glandular trichomes (GTs) are a major site for biosynthesis and accumulation of a wide range of plant natural products. These plant natural products often function to protect the plants against insect predation [1,2], and contribute to the flavour and aroma of plants. Many of the natural products also have pharmacological effects, such as the analgesic drug morphine, the anticancer compound taxol, and the antimalarial drug artemisinin. Artemisinin, a sesquiterpene lactone, is currently recognized as one of the most prominent anti-malarial treatment [3]. A complete understanding of the artemisinin biosynthetic pathway and its regulatory mechanism holds the key to efficient metabolic engineering for increased artemisinin yield. In the past decades, research efforts have been dedicated to identification of enzymes and intermediate compounds leading to artemisinin production. Many genes encoding enzymes participate in the pathway have been cloned and functionally characterized [4-10]. However, little is known about the regulatory aspects of sesquiterpene metabolism. This is partly due to the fact that A. annua is a non-model plant with limited genomic information available, and sequencing of limited number of randomly selected cDNA clones often have insufficient coverage of less abundant transcripts, including important regulatory transcription factors (TFs). In addition, genes uniquely or preferentially expressed in trichomes may be under-represented in non-tissue-targeted EST sequencing projects. A comprehensive survey of genes expressed in glandular trichome will facilitate new gene discovery and contribute significantly to elucidating the terpenoid pathway regulation and trichome function in A. annua.
Whole genome or transcriptome sequencing enables functional genomic studies based on global gene expression. The newly developed high throughput pyrosequencing technology allows rapid production of sequence data with dramatically reduced time, labor, and cost [11-15]. So far, most applications of pyrosequencing have involved analysis of genomic DNA [16]. Published reports on 454 pyrosequencing of transcriptomes have been mostly restricted to model species with genomic or comprehensive Sanger EST data available [11,17-19]. Previous studies [11,19] using genome or Sanger EST sequences for mapping and annotation of 454 ESTs were not able to accomplish de novo assembly of their 454 ESTs. We here present the global transcriptome characterization of A. annua glandular trichome, the so called biofactory for the production of artemisinin and other plant secondary metabolites. We assigned putative function to 28,573 unigenes, including previously undescribed enzymes likely involved in sesquiterpene biosynthesis. We verified the expression of 32 selected unigenes and novel transcripts in glandular trichomes using semi-quantitative RT-PCR. These 454 ESTs were linked to metabolic process specific in glandular trichomes and form the basis for further investigation.
Results
Sequencing and assembly of 454 pyrosequencing ESTs
Totally 406,044 ESTs (minimal size > 50 bp) averaging 210 bp were generated from two consecutive pyrosequencing runs. Cleaning (removal of primer, polyA tail, etc.) of the raw sequences resulted in a total of 386,881 high quality reads with an average length of 205 nucleotides totalling 85 Mb. After clustering and assembly using TGICL CAP3 clustering tools [20,21], these reads were assembled into 42,678 contigs and 147,699 singletons. The average length for contigs and singletons are 334 bp and 191 bp respectively. The contigs and singletons are collectively referred to as unigenes. The length distribution of unigenes and their component reads are summarized in Table 1 and Table 2.
Table 1.
Length distribution of assembled contigs and singletons
| Nucleotides length (bp) | Contigs | Singletons |
| 50-99 | 276 | 22,730 |
| 100-199 | 2,534 | 41,936 |
| 200-299 | 19,220 | 82,169 |
| 300-399 | 11,568 | 863 |
| 400-499 | 4,940 | 1 |
| 500-599 | 1,991 | 0 |
| 600-699 | 980 | 0 |
| 700-799 | 529 | 0 |
| 800-899 | 296 | 0 |
| 900-999 | 142 | 0 |
| 1,000-1,499 | 173 | 0 |
| 1,500-1,999 | 22 | 0 |
| > 2,000 | 7 | 0 |
| Total | 42,678 | 147,699 |
| Maximum length | 2,366 bp | 411 bp |
| Average length | 334 bp | 191 bp |
Table 2.
Summary of component reads per assembly
| Number of reads | Number of contigs |
| 2 to 10 | 39,112 |
| 11 to 20 | 2,142 |
| 21-30 | 585 |
| 31-40 | 289 |
| 41-50 | 164 |
| 51-100 | 250 |
| 101-150 | 63 |
| 151-200 | 27 |
| > 200 | 46 |
Pyrosequencing provides deep coverage of the A. annua trichome transcriptome
The contigs were searched against the NCBI non-redundant (NR) protein database using the blastx algorithm. Among the 190,377 contigs and singletons, 29,577(15.5%) had at least one significant alignment to existing gene model in blastx searches (E-value cutoff, e-10) (see Additional file 1). A majority (84.5%) of the pyrosequencing assemblies did not match any known sequences in the existing database and thus likely represent novel (E-value cutoff, pts sion of 17 transcripts identified in this study. Performing a second sequencing run increased the number of genes identified by approximately 30% (Table 3), suggesting that two pyrosequencing runs detect a substantial fraction of genes expressed in glandular trichomes and provide deep coverage of the A. annua trichome transcriptome.
Table 3.
Summary of blast hits from two pyrosequencing runs
| Pyrosequencing run | NCBI database unique hits |
| 1st run | 266,976 |
| 2nd run | 289,467 |
| Total | 357, 843 |
Characterization and GO annotation of novel transcripts
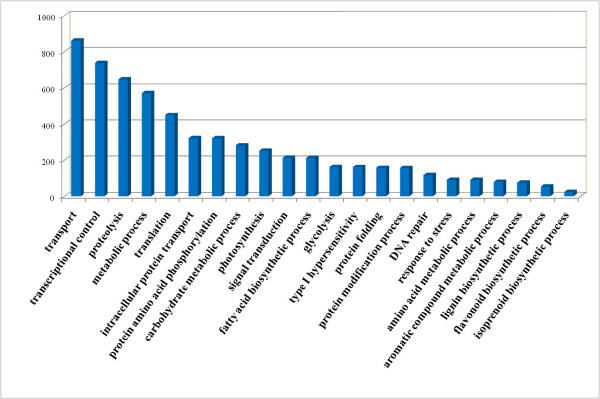
The 357,843 sequences that had matches with protein sequences in the NCBI protein database http://www.ncbi.nlm.nih.gov/sites/entrez?db=protein could be condensed into 29,577 clusters based on their top protein hits. Each 454 contig was assigned a putative gene description and a GO classification based on the 'best hit' blastx search (bitscore > 45, e-value < 1-10), using the 'inferred from sequence similarity' (ISS) level of evidence [22]. The unigenes were classified into three major functional categories: biological process, molecular function, and cellular component, according to the standard Gene Ontology terms (GO; http://www.geneontology.org). The assigned functionality of genes covers a broad range of GO categories. The top 20 most highly represented GO categories are illustrated in Figure 1. Under the category of biological process, transport, transcriptional control, and metabolic process were among the most highly represented categories, indicating the important metabolic activities in A. annua glandular trichomes. Other categories include photosynthesis, secondary metabolism (lignin, flavonoid, and isoprenoid biosynthesis process) and primary metabolism (fatty acid, glycolysis, carbohydrateprocess etc.).
Figure 1.
Top-ranked GO categories (molecular function) of assembled pyrosequencing ESTs.
Comparison of 454 sequence contigs to trichome ESTs from other plant species
TrichOME http://trichome.noble.org/trichomedb/ is a publicly available database of genes and metabolites expressed in plant trichomes. It currently contains 37, 017 conventional ESTs derived from 8 plant species, including Medicago sativa, Humulus lupulus, Mentha × piperita, Nicotiana benthamiana, Ocimum basilicum, Solanum habrochaites, Solanum lycopersicum and Solanum pennellii. A tblastx search against TrichomeDB showed that only 17,372(9%) of our 454 contigs had best blast hits (e-value < 1e-10) to 8,095 EST clusters with unique descriptions. Thus 454 sequencing has revealed many transcripts not previously detected in A. annua. ESTs homologous to photosynthesis-related proteins (chlorophyll a/b binding protein, ribulose bisphosphate carboxylase small subunit) are among the top 10 most highly expressed transcripts. The top ranked common molecular function of ESTs identified from all 9 plant species are listed in Table 4. Regulation of metabolic process, metabolic process, oxidation reduction, and transport categories has the highest number of contigs. Trichomes are known to be active in photosynthesis, as well as for their roles in storage and secretion of toxic compounds e.g. heavy metals [23,24], which requires the function of transporters. In our assembled pyrosequencing EST collections, we identified a large number of contigs homologous to ABC transporter, which is one of the most important families of membrane transport proteins that may play critical roles in the transmembrane transport of secondary metabolites in plants. The large amount of transporters can be linked to the secretion and transport function of glandular trichomes.
Table 4.
Shared common GO terms (biological process) in all trichome EST databases
| GO ID | GO term | No. of unigenes |
| GO:0006464 | Positive regulation of protein metabolic process | 147 |
| GO:0006730 | Metabolic process | 36 |
| GO:0008152 | Positive regulation of metabolic process | 28 |
| GO:0055114 | Oxidation reduction | 21 |
| GO:0006006 | Glucose metabolic process | 21 |
| GO:0006334 | Nucleosome assembly | 16 |
| GO:0006412 | Positive regulation of biosynthetic process | 14 |
| GO:0006096 | Positive regulation of glycolysis | 5 |
| GO:0006810 | Transport | 5 |
| GO:0006869 | Positive regulation of lipid transport | 2 |
Representation of genes related to secondary metabolism
Numerous sesquiterpene and monoterpene compounds have been identified in A. annua leaves, stems [5,25,26] and isolated glandular trichomes [27]. The genes corresponding to enzymes involved in the biosynthesis of major sesquiterpenes have been cloned and characterized [5,25-33]. To investigate the trichome function in secondary metabolism, the annotated unigenes were searched for enzymes participate in terpenoids biosynthesis. As shown in Additional file 2, unigenes corresponding to all the known enzymes in the terpenoids MEP and MVA pathway were identified. In higher plants, terpenoids precursor isopentenyl diphosphate (IPP) can be produced from both MVA and MEP routes, which is then converted to its isomer DMAPP (Figure 2) [34]. The cytosolic MVA terpenoids pathway, which starts from acetyl-CoA and proceeds through the intermediate mevalonate (MVA), provides the precursors for sterols and ubiquinone [35]. The plastidial MEP pathway, which involves a condensation of pyruvate and glyceraldehyde-3-phosphate, is used for the synthesis of isoprene, carotenoids, abscisic acid, and the side chains of chlorophylls and plastoquinone [36-39]. Although the subcellular compartmentation allows both pathways to operate independently, there is ample evidence that cross-talk exist between these two pathways [40,41].
Figure 2.
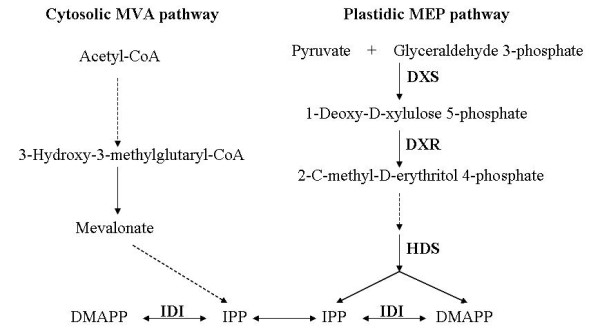
Simplified graphical representation of terpenoid biosynthetic pathway in A. annua. DXR: deoxy-D-xylulose 5-phosphate synthase; DXP: 1-deoxy-Dxylulose-5-phosphate reductoisomerase; HDS: 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate synthase; IDI: isopentenyl diphosphate/dimethylallyl diphosphate isomerase. DMAPP: Dimethylallyl Diphosphate. IPP: isopentenyl diphosphate.
Unigenes encoding the MEP and MVA pathway enzymes and all the sesquiterpene artemisinin pathway enzymes were present in our pyrosequencing collection. It is noteworthy that although the sequences were derived from normalized cDNA collections, unigenes corresponding to MEP pathway enzymes were two fold more abundant as compared with MVA pathway transcripts. This likely suggests that the MEP pathway may serve as a major route for DMAPP/IPP production in the A. annua trichomes. The MEP pathway has previously been shown to provide precursors for both mono-and sesqui-terpene biosynthesis in snapdragon flowers [42]. In a recent report on hops, the ESTs encoding MEP pathway enzymes are also found more abundant than those of MVA pathways [43].
Except for those well characterized terpenoid pathway genes, other unigenes annotated as sesquiterpene synthase and monoterpene synthase were identified. Three unigenes (Contig02039, Contig16267, Contig14765) annotated as sesquiterpine synthases were selected for RACE PCR to retrieve the full length cDNAs. Sequence analysis indicated that the conserved sesquiterpene synthase functional domain exists in all three genes (Figure 3). Further functional characterization of these enzymes will be reported elsewhere.
Figure 3.
Alignment of putative sesquiterpene synthases with other homologs from A. annua (accession no. DQ447636 and AY006482). Identical amino acids are highlighted. The functional motifs are underlined.
Furthermore, large amount of unigenes annotated as phenylpropanoids and flavanoids pathway enzymes were present in the assemebled pyrosequencing EST collection (ss Additional file 3), indicating the metabolic function of glandular trichomes in A. annua secondary metabolism.
RT-PCR validation
A set of 17 contigs were selected for semi-quantitative RT-PCR analysis to confirm their expression (Figure 4A). The selected contigs encode enzymes involved in artemisinin biosynthesis, and putativetranscription factors. PCR experiments were conducted on four pools of cDNAs derived from (1) glandular trichomes, (2) non-glandular trichomes (3) leaves, and (4) roots. The results demonstrate that all of the novel transcripts detected among the 454-ESTs are indeed expressed in glandular trichomes, including those with low expression levels. This suggests that deep pyrosequencing is effective in revealing the expression of many rare transcripts, e.g. transcription factors. Most of the tested contigs were also expressed in leaf and non glandular trichome cDNA pools, except for one contig40477, which was only expressed in glandular trichomes and roots. Interestingly, three contigs likely encode enzymes needed in sesquiterpene biosynthesis were also strongly expressed in non-glandular type of trichomes. This raises the question as to whether glandular trichome is the sole site for the biosynthesis of artemisinin and other sesquiterpenes in A. annua.
Figure 4.
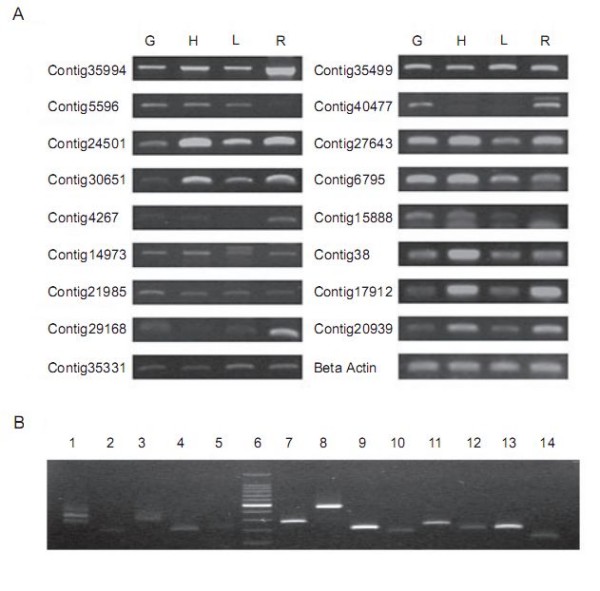
Semi-quantitative RT-PCR analysis of selected unigenes and novel transcripts. A. Expression of selected contigs in different tissue types. G: Glandular trichome, N: Non-glandular tirhcome, L: Leaf, R: Root. bHLH family proteins: Contig35994 and Contig5596; WD family proteins: Contig24501 and Contig30651; Myb family proteins: Contig14973, Contig21985, Contig29168, Contig35331, Contig35499, and Contig40477; Terpene synthases: Contig27643; amorpha-4,11-diene synthase [Artemisia annua] ABM88787: Contig 6795; sesquiterpene cyclase [Artemisia annua] AAG24640: Contig15888 (3R)- linalool synthase [Artemisia annua] AAF13356; WRKY Proteins: Contig38, Contig17912, Contig20939. B. Expression of novel transcripts and singletons in GT. Lane 1-5: singletons S122859, S078690, S091943, S154166, and S174533; Lane 6: DNA marker; Lane 7-14: novel transcripts C3719, C13021, C15708, C1441, C20920, C29103, C445, and C14916.
RT-PCR was also used to confirm the expression of novel transcripts and singletons. A set of 18 novel transcripts and singletons was randomly selected to test if they are indeed expressed in GT (Figure 4B). Of the 20 primer pairs, 13 produced RT-PCR products that were of the correct size and whose sequence matched the sequences from which the primers were designed. Based on these results, we conclude that many of the novel transcripts and singletons detected among the 454-ESTs are not due to the sequencing artifacts. This result provides further evidence for the value of tissue specific 454 sequencing for gene discovery.
Discussion
As the sole plant source for artemisinin production, the A. annua has been studied extensively for the past decades. Like most other non-model plant species, it has lacked genetic and genomic resources necessary for mechanistic study. Although a precise estimate of transcriptome coverage is unattainable without full genomic sequence, we appear to have recovered a significantly portion of the A. annua glandular trichome transcriptome. Novel transcripts detected highlights the hypothesis-expanding aspects of 454 deep pyrosequencing approach, which potentially facilitate the understanding of glandular trichome metabolic function. The assembled sequence data also provided a rich source of information for further investigation.
Two consecutive pyrosequencing runs identified a large number of genes expressed in glandular trichomes. In data analysis, approximately 85% of the pyrosequencing assemblies did not align to any ESTs available in GenBank. This high proportion could reflect the specialized cell type that was sampled or perhaps the greater complexity of the A. annua genome. Because our priority goal in this study is gene discovery, we therefore chose normalized cDNA population to reduce oversampling of abundant transcripts and to maximize coverage of less abundant transcripts present in the sample. The average contig length was fairly short (~334 bp), and only 62% of the sequence reads assembled into contigs, leaving 147,699 singletons.
Genes involved in plant secondary metabolism have frequently been identified by EST approach [44]. The lower cost and greater sequence coverage offered by pyrosequencing makes it possible to identify more candidate genes involved in plant natural product biosynthetic pathways, esp. those with low abundance and often missed by conventional EST projects. For non-model species with little or no genomic data available, such as A. annua, pyrosequencing offers rapid characterization of a large portion of the transcriptome and therefore provides a comprehensive tool for gene discovery. However, one limitation of pyrosequencing is that one must rely on RACE PCR in order to obtain full-length sequence data for a given gene of interest.
Comparison between our glandular trichome 454 ESTs with conventional ESTs generated from trichomes of other plant species revealed likely common function in non-glandular and glandular trichomes. In addition, some unigenes corresponding to enzymes in sesquiterpene biosynthesis were found to be highly expressed in both trichome types in our RT-PCR analysis. Although it has been suggested that glandular trichomes are the site for synthesis and accumulation of plant secondary metabolites, it will be interesting to further investigate the different functional roles of non-glandular trichomes in artemisinin biosynthesis.
Conclusion
In conclusion, we describe the global analysis of glandular trichome in A. annua using massively parallel pyrosequencing. Mining the pyrosequencing ESTs resulted in the identification of many contigs likely involved in terpenoid biosynthesis and trichome function. Functional characterizations of selected genes are being carried out. These pyrosequencing data form the basis for further characterization of the molecular mechanism of glandular trichome function in A. annua. The results also highlight the value of using tissue-specific high throughput pyrosequencing technology for gene discovery in non-model plants. Access to all EST contigs obtained in this study is facilitated through a file available in the supplemental data (see Additional file 4).
Methods
Plant Materials
A. annua seeds were purchased from Youyang, Sichuang province of China. Seeds were sown into commercial potting mixture for germination. The germinated plantlets were grown under natural light conditions in the greenhouse located at The Chinese University of Hong Kong. Flower buds were collected for trichome isolation before flowering.
Isolation of glandular trichomes
Trichome cells were gently abraded from the surface of flower buds using glass beads and a commercial cell disrupter (BioSpec Products). The isolated secretary cells were separated from other cells and tissue fragments in the mixture by sequentially passing through a 40 μm and a 30 μm nylon sieves. Glandular cells were finally collected in 30 μm meshes with minimum contamination of non-glandular trichomes (Figure 5).
Figure 5.
Isolation of glandular trichomes. A: Extracted glandular trichomes B: Crude extracts containing both glandular and non-glandular trichomes.
RNA extraction, cDNA synthesis and normalization
Total RNA was extracted from glandular trichomes isolated from 30 g flower buds following the standard protocol of RNeasy Plant Mini Kit (Qiagen). cDNA was synthesized using the BD SMARTM PCR cDNA Synthesis Kit (Clontech). First-strand cDNA synthesis was performed with oligo(dT) primer as described in the provided protocol using 500 ng total RNA. Double-strand cDNA was prepared from 2 μl of the first-strand reaction by PCR with provided primers in a 100 μL reaction. cDNA was purified using Qiagen QIAquick PCR purification spin columns. Normalization was performed using TRIMMER cDNA normalization kit (EVR_GEN) to decrease the prevalence of abundant transcripts before sequencing. Approximately 1 μg of normalized double stranded cDNA was used for 454 pyrosequencing.
454 pyrosequencing, data pre-process and assembly
Approximately 1 μg of the adaptor-ligated cDNA population was sheared by nebulization and DNA sequencing was performed following protocols for the Genome Sequencer GS FLX System (Roche Diagnostic). Reads generated by the FLX sequencer were trimmed of low quality, low complexity [poly(A)] and adaptor sequences using the SeqClean software http://compbio.dfci.harvard.edu/tgi/. The cleaned sequences were subject to CAP3 program [20] for clustering and assembly using default parameters.
Gene annotation using GO terms
After assembly, the resulting contigs and singlets were aligned with NCBI non-redundant protein database using blast2go software with a cut-off e-value of 1e-10. The GI accessions of best hits were retrieved, and the GO accessions were mapped to GO terms according to molecular function, biological process and cellular component ontologies http://www.geneontology.org/.
Semi-quantitative RT-PCR analysis
To verify the presence of pyrosequencing ESTs in glandular trichomes, we totally selected 35 unigenes and novel transcripts for RT-PCR analysis. Total RNA were extracted from glandular trichomes, non-glandular hairy trichomes, leaves and hairy roots respectively. The first-strand cDNA was synthesized from 10 μL (about 1 μg) total RNA using SuperScript™ II Reverse Transcriptase (Invitrogen) with Oligo(dT)12-18 Primer. PCR was performed using 0.5 to 2 μL of the cDNA in a total of 50 μL reaction volume. The PCR conditions were 2 min at 95°C, 30 s at 95°C, 30 s at 47-56°C, 1 min at 72°C for 30 cycles, followed by 5 min at 72°C. These conditions were chosen because none of the samples analyzed reached a plateau at the end of the amplification (i.e. they were at the exponential phase of the amplification). Actin was used as a loading control, and loading was estimated by staining the gel with ethidium bromide. Expression analysis of each gene was confirmed in at least 2 independent RT-reactions using forward and reverse primers.
List of abbreviations used
GTs: glandular trichomes; TFs: transcription factors; NR: non-redundant; EST: expressed sequence tag; ABC transporter: ATP-binding cassette transponer; A. annua: Artemisia annua; GO: gene ontology; ISS: inferred from sequence similarity; MEP pathway: 2-C-methyl-d-erythritol 4-phosphate pathway; MVA pathway: mevalonic acid pathway; IPP: isopentenyl diphosphate; DMAPP: Dimethylallyl pyrophosphate.
Authors' contributions
WW carried out the trichome isolation, RT-PCR, and participated in the sequence analysis and drafted the manuscript. YW and QZ carried out EST assembly, data annotation and bioinformatics analysis. YQ participated in the trichome isolation and sequence analysis. Dianjing Guo conceived of the study and and participated in its design and coordination. All authors read and approved the final manuscript.
Supplementary Material
Contigs with at least one significant alignment to existing gene model. The data represent all the assembled contigs with at least one significant alignment to the existing gene model according to BlastX search.
Unigenes encoding putative enzymes in terpenoids metabolism. The data represent all the unigenes encoding putative enzymes in terpenoids metabolism.
Unigenes annotated as phenylpropanoids and flavanoids pathway enzymes presented in assembled pyrosequencing EST collection. The data represent all the unigenes annotated as phenylpropanoids and flavanoids pathway enzymes.
Assembled pyrosequencing ESTs. The data represent all the assembled pyrosequencing ESTs.
Acknowledgments
Acknowledgements
We thank Mr. Patrick Lau from the core facility in the Faculty of Science at CUHK for performing the 454 pyrosequencing. The work was fully supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China (Project no. CUHK 4603/06M).
Contributor Information
Wei Wang, Email: wangwei@cuhk.edu.hk.
Yejun Wang, Email: s0802282@cuhk.edu.hk.
Qing Zhang, Email: zhqingfu@gmail.com.
Yan Qi, Email: s061155@cuhk.edu.hk.
Dianjing Guo, Email: djguo@cuhk.edu.hk.
References
- Ranger CM, Hower AA. Role of the glandular trichomes in resistance of perennial alfalfa to the potato leafhopper (Homoptera: Cicadellidae) J Econ Entomol. 2001;94:950–957. doi: 10.1603/0022-0493-94.4.950. [DOI] [PubMed] [Google Scholar]
- Wagner GJ, Wang E, Shepherd RW. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann Bot (Lond) 2004;93:3–11. doi: 10.1093/aob/mch011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke SO, Paul RN. Development and fine-structure of the glandular trichomes of Artemisia annua L. International Journal of Plant Sciences. 1993;154:107–118. doi: 10.1086/297096. [DOI] [Google Scholar]
- Bertea CM, Freije JR, Woude H vander, Verstappen FWA, Perk L, Marquez V, De Kraker JW, Posthumus MA, Jansen BJM, de Groot A. Identification of intermediates and enzymes involved in the early steps of artemisinin biosynthesis in Artemisia annua. Planta Medica. 2005;71:40–47. doi: 10.1055/s-2005-837749. [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Wallaart TE, Janssen MH, van Loo B, Jansen BJ, Posthumus MA, Schmidt CO, De Kraker JW, Konig WA, Franssen MC. Amorpha-4,11-diene synthase catalyses the first probable step in artemisinin biosynthesis. Phytochemistry. 1999;52:843–854. doi: 10.1016/S0031-9422(99)00206-X. [DOI] [PubMed] [Google Scholar]
- Mercke P, Bengtsson M, Bouwmeester HJ, Posthumus MA, Brodelius PE. Molecular cloning, expression, and characterization of amorpha-4,11-diene synthase, a key enzyme of artemisinin biosynthesis in Artemisia annua L. Archives of Biochemistry and Biophysics. 2000;381:173–180. doi: 10.1006/abbi.2000.1962. [DOI] [PubMed] [Google Scholar]
- Chang YJ, Song SH, Park SH, Kim SU. Amorpha-4,11-diene synthase of Artemisia annua: cDNA isolation and bacterial expression of a terpene synthase involved in artemisinin biosynthesis. Archives of Biochemistry and Biophysics. 2000;383:178–184. doi: 10.1006/abbi.2000.2061. [DOI] [PubMed] [Google Scholar]
- Teoh KH, Polichuk DR, Reed DW, Nowak G, Covello PS. Artemisia annua L. (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett. 2006;580:1411–1416. doi: 10.1016/j.febslet.2006.01.065. [DOI] [PubMed] [Google Scholar]
- Ro DK PE, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Teoh KH, Reed DW, Maes L, Goossens A, Olson DJH, Ross ARS, Covello PS. The molecular cloning of artemisinic aldehyde Delta 11(13) reductase and its role in glandular trichomedependent biosynthesis of artemisinin in Artemisia annua. Journal of Biological Chemistry. 2008;283:21501–21508. doi: 10.1074/jbc.M803090200. [DOI] [PubMed] [Google Scholar]
- Weber AP, Weber KL, Carr K, Wilkerson C, Ohlrogge JB. Sampling the Arabidopsis transcriptome with massively parallel pyrosequencing. Plant Physiol. 2007;144:32–42. doi: 10.1104/pp.107.096677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MJ, Dhingra A, Soltis PS, Shaw R, Farmerie WG, Folta KM, Soltis DE. Rapid and accurate pyrosequencing of angiosperm plastid genomes. BMC Plant Biol. 2006;6:17. doi: 10.1186/1471-2229-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse SM, Huber JA, Morrison HG, Sogin ML, Welch DM. Accuracy and quality of massively parallel DNA pyrosequencing. Genome Biol. 2007;8:R143. doi: 10.1186/gb-2007-8-7-r143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker T, Schlagenhauf E, Graner A, Close TJ, Keller B, Stein N. 454 sequencing put to the test using the complex genome of barley. BMC Genomics. 2006;7:275. doi: 10.1186/1471-2164-7-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinar HN, Schwarz C, Qi J, Shapiro B, Macphee RD, Buigues B, Tikhonov A, Huson DH, Tomsho LP, Auch A. Metagenomics to paleogenomics: large-scale sequencing of mammoth DNA. Science. 2006;311:392–394. doi: 10.1126/science.1123360. [DOI] [PubMed] [Google Scholar]
- Emrich SJ, Barbazuk WB, Li L, Schnable PS. Gene discovery and annotation using LCM-454 transcriptome sequencing. Genome Research. 2007;17:69–73. doi: 10.1101/gr.5145806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge MN, Warren RL, Hirst M, Romanuik T, Zeng T, Go A, Delaney A, Griffith M, Hickenbotham M, Magrini V. Analysis of the prostate cancer cell line LNCaP transcriptome using a sequencing-by-synthesis approach. BMC Genomics. 2006;7:246. doi: 10.1186/1471-2164-7-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung F, Haas BJ, Goldberg SMD, May GD, Xiao YL, Town CD. Sequencing Medicago truncatula expressed sequenced tags using 454 Life Sciences technology. BMC Genomics. 2006;7:272. doi: 10.1186/1471-2164-7-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Madan A. CAP3: A DNA sequence assembly program. Genome Research. 1999;9:868–877. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea G, Huang X, Liang F, Antonescu V, Sultana R, Karamycheva S, Lee Y, White J, Cheung F, Parvizi B. TIGR Gene Indices clustering tools (TGICL): a software system for fast clustering of large EST datasets. Bioinformatics. 2003;19:651–652. doi: 10.1093/bioinformatics/btg034. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper H, Lombi E, Zhao FJ, McGrath SP. Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta. 2000;212:75–84. doi: 10.1007/s004250000366. [DOI] [PubMed] [Google Scholar]
- Choi YE, Harada E, Wada M, Tsuboi H, Morita Y, Kusano T, Sano H. Detoxification of cadmium in tobacco plants: formation and active excretion of crystals containing cadmium and calcium through trichomes. Planta. 2001;213:45–50. doi: 10.1007/s004250000487. [DOI] [PubMed] [Google Scholar]
- Ma CF, Wang HH, Lu X, Li HF, Liu BY, Xu GW. Analysis of Artemisia annua L. volatile oil by comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. Journal of Chromatography A. 2007;1150:50–53. doi: 10.1016/j.chroma.2006.08.080. [DOI] [PubMed] [Google Scholar]
- Ma CF, Wang HH, Lu X, Xu GW, Liu BY. Metabolic fingerprinting investigation of Artemisia annua L. in different stages of development by gas chromatography and gas chromatographymass spectrometry. J Chromatogr A. 2008;1186:412–419. doi: 10.1016/j.chroma.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Bertea CM, Voster A, Verstappen FW, Maffei M, Beekwilder J, Bouwmeester HJ. Isoprenoid biosynthesis in Artemisia annua: cloning and heterologous expression of a germacrene A synthase from a glandular trichome cDNA library. Arch Biochem Biophys. 2006;448:3–12. doi: 10.1016/j.abb.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Matsushita Y, Kang W, Charlwood BV. Cloning and analysis of a cDNA encoding farnesyl diphosphate synthase from Artemisia annua. Gene. 1996;172:207–209. doi: 10.1016/0378-1119(96)00054-6. [DOI] [PubMed] [Google Scholar]
- Cai Y, Jia JW, Crock J, Lin ZX, Chen XY, Croteau R. A cDNA clone for beta-caryophyllene synthase from Artemisia annua. Phytochemistry. 2002;61:523–529. doi: 10.1016/S0031-9422(02)00265-0. [DOI] [PubMed] [Google Scholar]
- Picaud S, Brodelius M, Brodelius PE. Expression, purification and characterization of recombinant (E)-beta-farnesene synthase from Artemisia annua. Phytochemistry. 2005;66:961–967. doi: 10.1016/j.phytochem.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Mercke P, Crock J, Croteau R, Brodelius PE. Cloning, expression, and characterization of epi-cedrol synthase, a sesquiterpene cyclase from Artemisia annua L. Arch Biochem Biophys. 1999;369:213–222. doi: 10.1006/abbi.1999.1358. [DOI] [PubMed] [Google Scholar]
- Hua L, Matsuda SP. The molecular cloning of 8-epicedrol synthase from Artemisia annua. Arch Biochem Biophys. 1999;369:208–212. doi: 10.1006/abbi.1999.1357. [DOI] [PubMed] [Google Scholar]
- Jia JW, Crock J, Lu S, Croteau R, Chen XY. (3R)-Linalool synthase from Artemisia annua L.: cDNA isolation, characterization, and wound induction. Arch Biochem Biophys. 1999;372:143–149. doi: 10.1006/abbi.1999.1466. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Schwender J, Disch A, Rohmer M. Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via amevalonate- independent pathway. FEBS Lett. 1997;400:271–274. doi: 10.1016/S0014-5793(96)01404-4. [DOI] [PubMed] [Google Scholar]
- Disch A, Hemmerlin A, Bach TJ, Rohmer M. Mevalonate-derived isopentenyl diphosphate is the biosynthetic precursor of ubiquinone prenyl side chain in tobacco BY-2 cells. Biochemical Journal. 1998;331:615–621. doi: 10.1042/bj3310615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwender J, Zeidler J, Groner R, Muller C, Focke M, Braun S, Lichtenthaler FW, Lichtenthaler HK. Incorporation of 1-deoxy-Dxylulose into isoprene and phytol by higher plants and algae. FEBS Letters. 1997;414:129–134. doi: 10.1016/S0014-5793(97)01002-8. [DOI] [PubMed] [Google Scholar]
- Arigoni D, Sagner S, Latzel C, Eisenreich W, Bacher A, Zenk MH. Terpenoid biosynthesis from 1-deoxy-D-xylulose in higher plants by intramolecular skeletal rearrangement. Proc Natl Acad Sci USA. 1997;94:10600–10605. doi: 10.1073/pnas.94.20.10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milborrow BV, Lee HS. Endogenous biosynthetic precursors of (+)- abscisic acid. VI - Carotenoids and ABA are formed by the 'nonmevalonate' triose-pyruvate pathway in chloroplasts. Australian Journal of Plant Physiology. 1998;25:507–512. doi: 10.1071/PP98006. [DOI] [Google Scholar]
- Hirai N, Yoshida R, Todoroki Y, Ohigashi H. Biosynthesis of abscisic acid by the non-mevalonate pathway in plants, and by the mevalonate pathway in fungi. Biosci Biotechnol Biochem. 2000;64:1448–1458. doi: 10.1271/bbb.64.1448. [DOI] [PubMed] [Google Scholar]
- Piel J, Donath J, Bandemer K, Boland W. Mevalonate-independent biosynthesis of terpenoid volatiles in plants: Induced and constitutive emission of volatiles. Angewandte Chemie-International Edition. 1998;37:2478–2481. doi: 10.1002/(SICI)1521-3773(19981002)37:18<2478::AID-ANIE2478>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Laule O, Furholz A, Chang HS, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange BM. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6866–6871. doi: 10.1073/pnas.1031755100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Andersson S, Orlova I, Gatto N, Reichelt M, Rhodes D, Boland W, Gershenzon J. The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:933–938. doi: 10.1073/pnas.0407360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GD, Tian L, Aziz N, Broun P, Dai XB, He J, King A, Zhao PX, Dixon RA. Terpene Biosynthesis in Glandular Trichomes of Hop. Plant Physiology. 2008;148:1254–1266. doi: 10.1104/pp.108.125187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao XM, Katz S, Pollard M, Ohlrogge J. Carbocyclic fatty acids in plants: Biochemical and molecular genetic characterization of cyclopropane fatty acid synthesis of Sterculia foetida. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7172–7177. doi: 10.1073/pnas.092152999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contigs with at least one significant alignment to existing gene model. The data represent all the assembled contigs with at least one significant alignment to the existing gene model according to BlastX search.
Unigenes encoding putative enzymes in terpenoids metabolism. The data represent all the unigenes encoding putative enzymes in terpenoids metabolism.
Unigenes annotated as phenylpropanoids and flavanoids pathway enzymes presented in assembled pyrosequencing EST collection. The data represent all the unigenes annotated as phenylpropanoids and flavanoids pathway enzymes.
Assembled pyrosequencing ESTs. The data represent all the assembled pyrosequencing ESTs.