Abstract
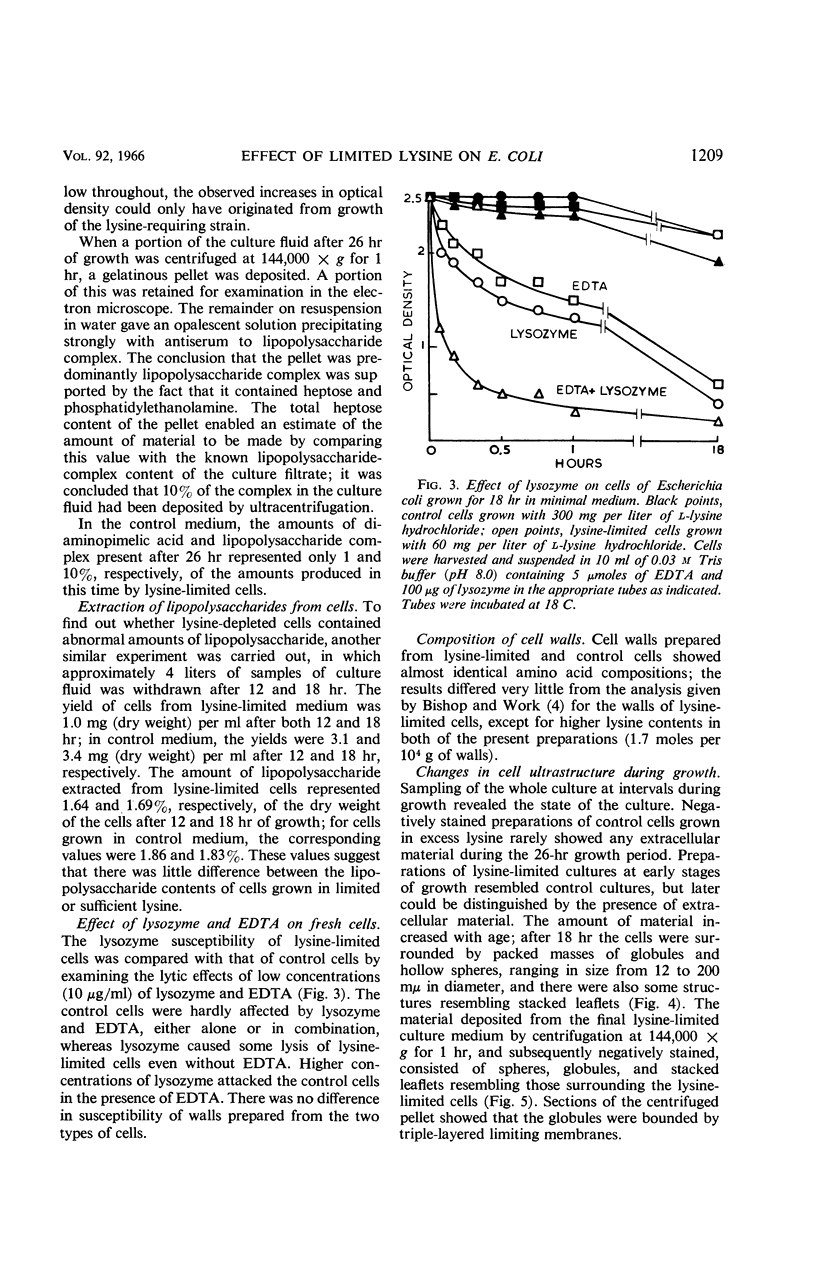
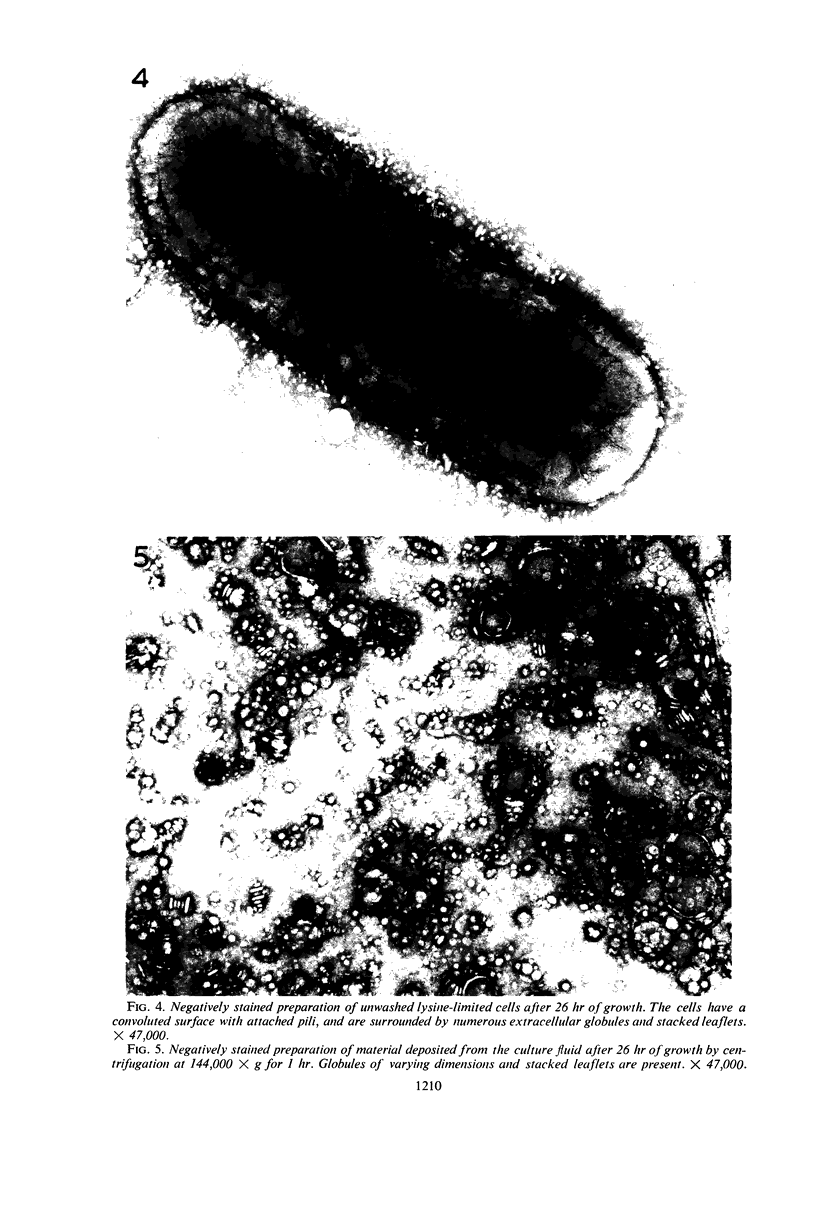
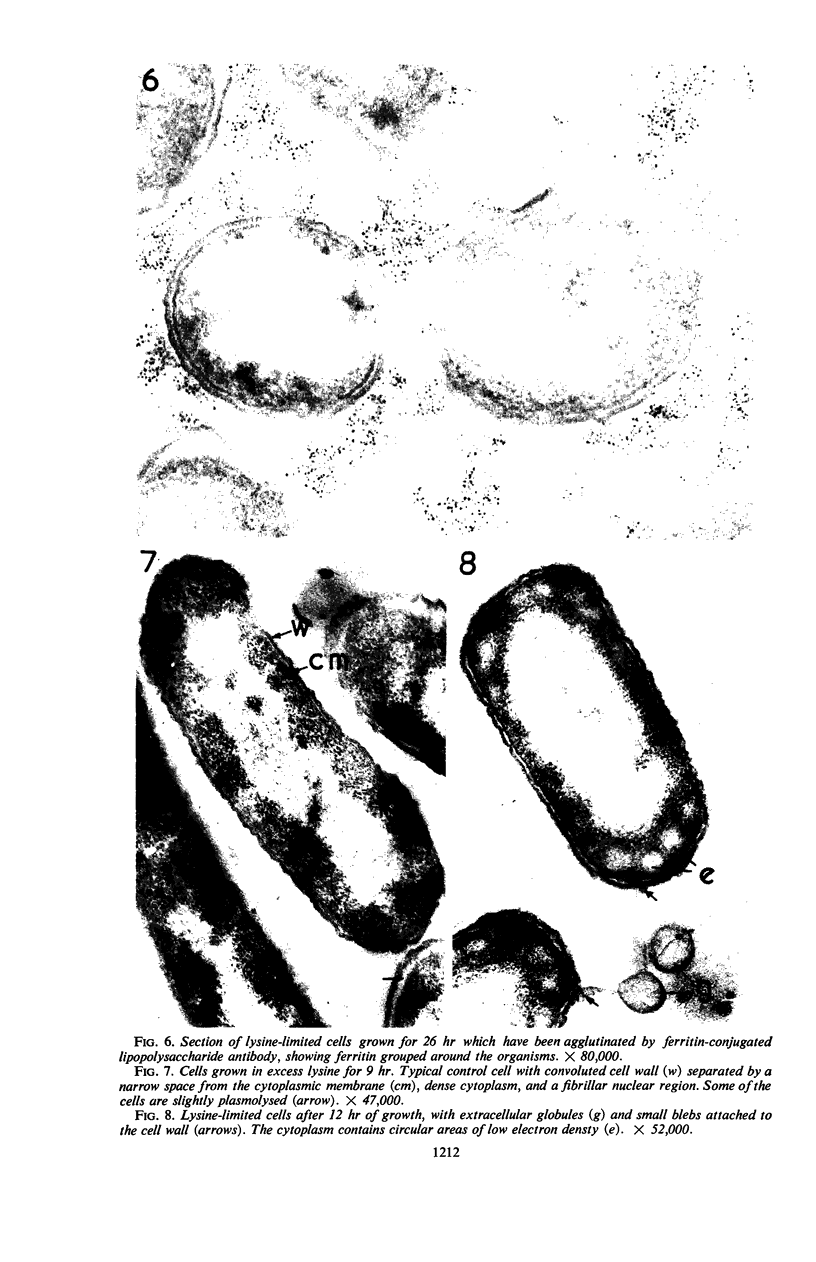
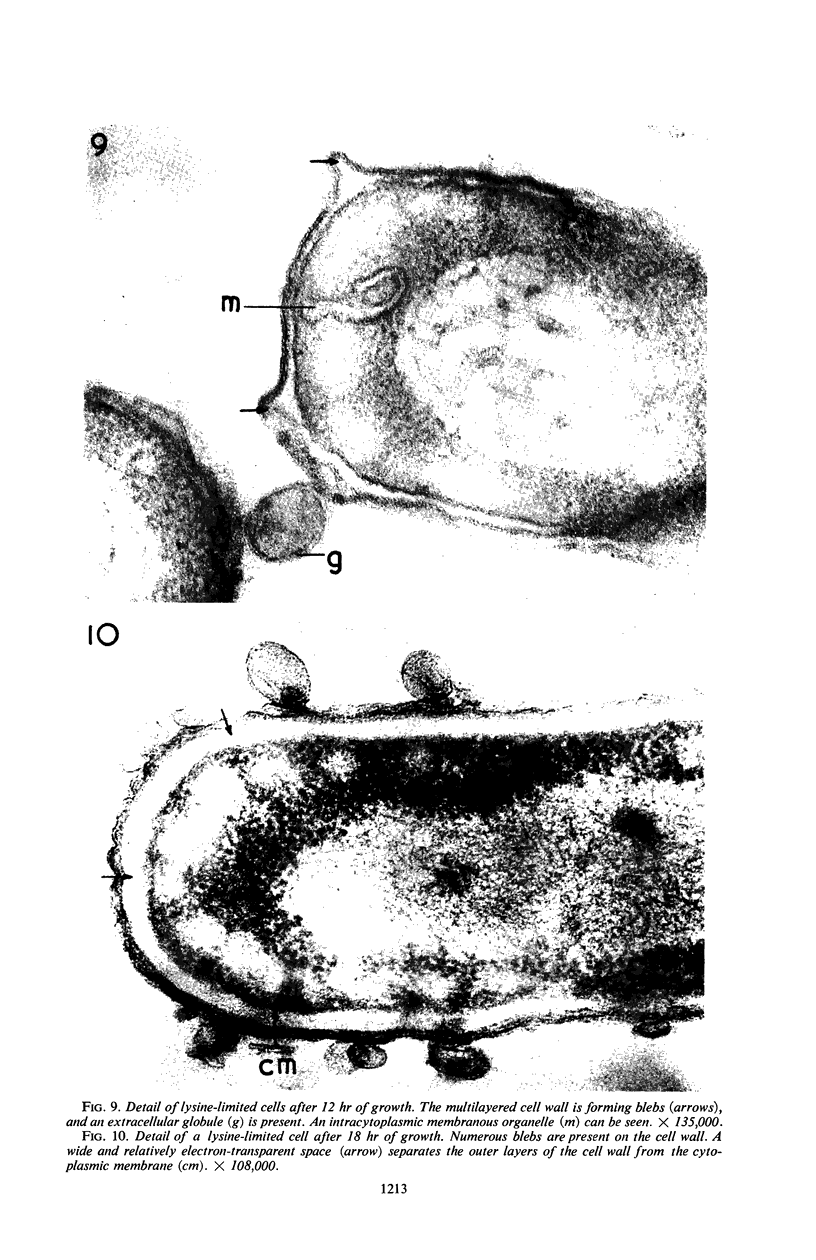
Knox, K. W. (Twyford Laboratories, London, England), Maret Vesk, and Elizabeth Work. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J. Bacteriol. 92:1206–1217. 1966.—The lysine-requiring mutant Escherichia coli 12408, when grown in 15 liters of defined medium containing a suboptimal amount of lysine, showed a biphasic type of growth. During a long stationary phase of 15 hr, there was a steady accumulation of diaminopimelic acid (DAP) and an antigenic complex of lipopolysaccharide (LPS) and lipoprotein; the accumulation continued unchanged until the end of the second growth phase. The rapid rate of DAP excretion suggested that it was the result of a derepressed state of a biosynthetic pathway. LPS excretion was such that the amount in the culture fluid was doubled during a period corresponding to the normal generation time for the organism; this suggested that the LPS-lipoprotein complex was a product of unbalanced growth. Surface defects were suggested by the action of lysozyme, which, in low concentrations (10 μg/ml), lysed the lysine-limited cells even in the absence of ethylenediaminetetraacetic acid, but had no effect at 10 μg/ml on cells grown with adequate lysine. Electron microscopy of cells excreting the LPS complex showed them to be surrounded by a mass of stacked leaflets and globules, some of which were bounded by triple membranes. Sections showed no lysis but changes in cell surfaces; outer layers of the walls had numerous blebs whose outer membranes were sometimes continuous with the outer triple membrane of the wall. LPS-lipoprotein probably originates from these blebs.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLSOP J WORK E. Cell walls of Propionibacterium species: fractionation and composition. Biochem J. 1963 Jun;87:512–519. doi: 10.1042/bj0870512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLADEN H. A., MERGENHAGEN S. E. ULTRASTRUCTURE OF VEILLONELLA AND MORPHOLOGICAL CORRELATION OF AN OUTER MEMBRANE WITH PARTICLES ASSOCIATED WITH ENDOTOXIC ACTIVITY. J Bacteriol. 1964 Nov;88:1482–1492. doi: 10.1128/jb.88.5.1482-1492.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN A. D. ASPECTS OF BACTERIAL RESPONSE TO THE IONIC ENVIRONMENT. Bacteriol Rev. 1964 Sep;28:296–329. doi: 10.1128/br.28.3.296-329.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Anderson T. F. The surface structure of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1592–1599. doi: 10.1073/pnas.54.6.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. G., Work E. An extracellular glycolipid produced by Escherichia coli grown under lysine-limiting conditions. Biochem J. 1965 Aug;96(2):567–576. doi: 10.1042/bj0960567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEPETRIS S. ULTRASTRUCTURE OF THE CELL WALL OF ESCHERICHIA COLI. J Ultrastruct Res. 1965 Apr;12:247–262. doi: 10.1016/s0022-5320(65)80098-3. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- Green D. E., Perdue J. F. Membranes as expressions of repeating units. Proc Natl Acad Sci U S A. 1966 May;55(5):1295–1302. doi: 10.1073/pnas.55.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIVE L. ACTINOMYCIN SENSITIVITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Biochem Biophys Res Commun. 1965 Jan 4;18:13–17. doi: 10.1016/0006-291x(65)90874-0. [DOI] [PubMed] [Google Scholar]
- LILLY M. D., CLARKE P. H., MEADOW P. M. THE ACCUMULATION OF NUCLEOTIDES BY ESCHERICHIA COLI STRAIN 26-26. J Gen Microbiol. 1963 Jul;32:103–116. doi: 10.1099/00221287-32-1-103. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leive L., Davis B. D. The transport of diaminopimelate and cystine in Escherichia coli. J Biol Chem. 1965 Nov;240(11):4362–4369. [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative organisms and the role of versene. Biochim Biophys Acta. 1958 Nov;30(2):225–232. doi: 10.1016/0006-3002(58)90044-1. [DOI] [PubMed] [Google Scholar]
- ROBERTSON J. D. The ultrastructure of cell membranes and their derivatives. Biochem Soc Symp. 1959;16:3–43. [PubMed] [Google Scholar]
- Shands J. W. Localization of Somatic Antigen on Gram-Negative Bacteria by Electron Microscopy. J Bacteriol. 1965 Jul;90(1):266–270. doi: 10.1128/jb.90.1.266-270.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A., Knox K. W., Work E. Chemical and biological properties of an extracellular lipopolysaccharide from Escherichia coli grown under lysine-limiting conditions. Biochem J. 1966 Apr;99(1):53–61. doi: 10.1042/bj0990053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDERWINKEL E., MURRAY R. G. [Bacterial intracytoplasmic organelles and the site of oxidation-reduction activity]. J Ultrastruct Res. 1962 Aug;7:185–199. doi: 10.1016/s0022-5320(62)80035-5. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., MARTIN H. H. The rigid layer of the cell wall of Escherichia coli strain B. J Gen Microbiol. 1960 Feb;22:158–166. doi: 10.1099/00221287-22-1-158. [DOI] [PubMed] [Google Scholar]
- WORK E., DENMAN R. F. The use of a bacterial culture fluid as a source of alpha-diaminopimelic acid. Biochim Biophys Acta. 1953 Jan;10(1):183–183. doi: 10.1016/0006-3002(53)90226-1. [DOI] [PubMed] [Google Scholar]
- WORK E. Reaction of ninhydrin in acid solution with straight-chain amino acids containing two amino groups and its application to the estimation of alpha epsilon-diaminopimelic acid. Biochem J. 1957 Nov;67(3):416–423. doi: 10.1042/bj0670416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. J., Kelly B., Suffling A., Work E. Variation of activity of bacterial diaminopimelate decarboxylase under different conditions of growth. Biochem J. 1964 Jun;91(3):600–610. doi: 10.1042/bj0910600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Work E., Knox K. W., Vesk M. The chemistry and electron microscopy of an extracellular lipopolysaccharide from Escherichia coli. Ann N Y Acad Sci. 1966 Jun 30;133(2):438–449. doi: 10.1111/j.1749-6632.1966.tb52382.x. [DOI] [PubMed] [Google Scholar]
- YUGARI Y., GILVARG C. Coordinate end-product inhibition in lysine synthesis in Escherichia coli. Biochim Biophys Acta. 1962 Aug 27;62:612–614. doi: 10.1016/0006-3002(62)90256-1. [DOI] [PubMed] [Google Scholar]






