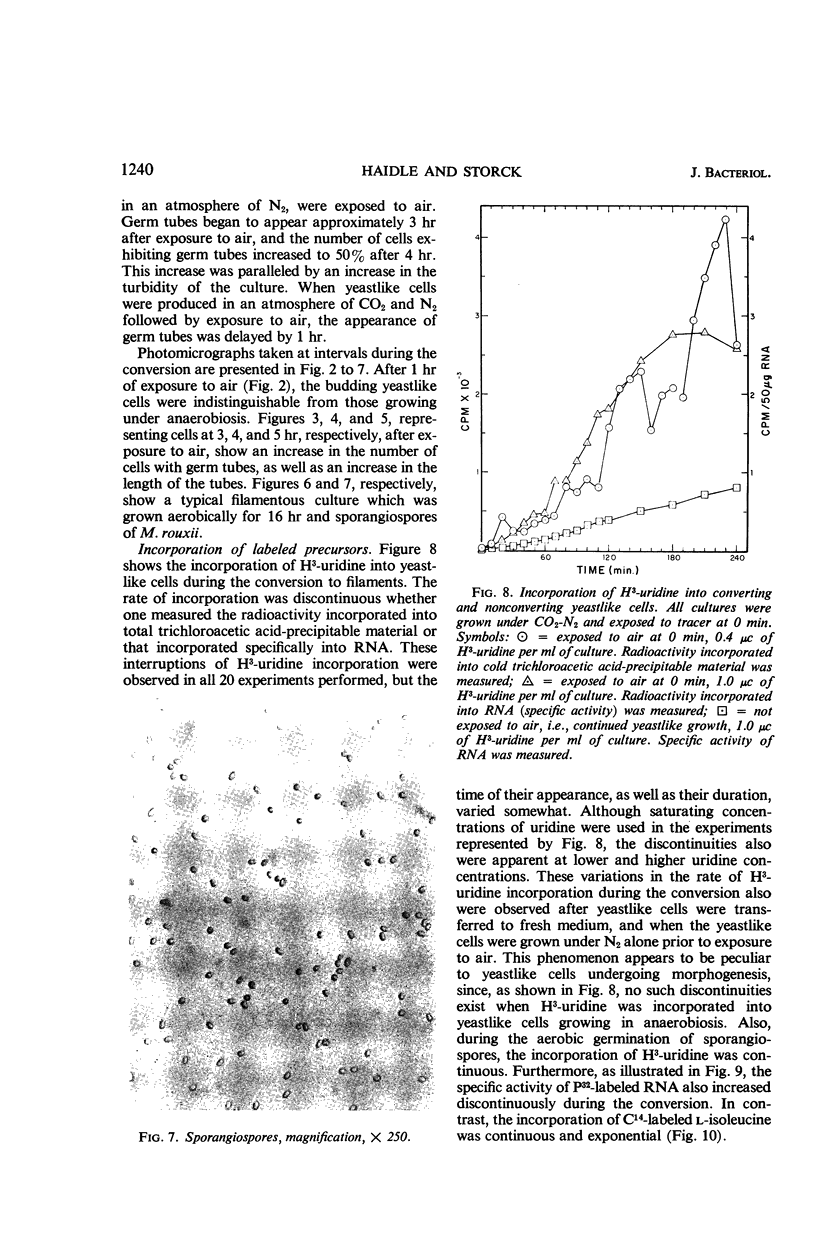
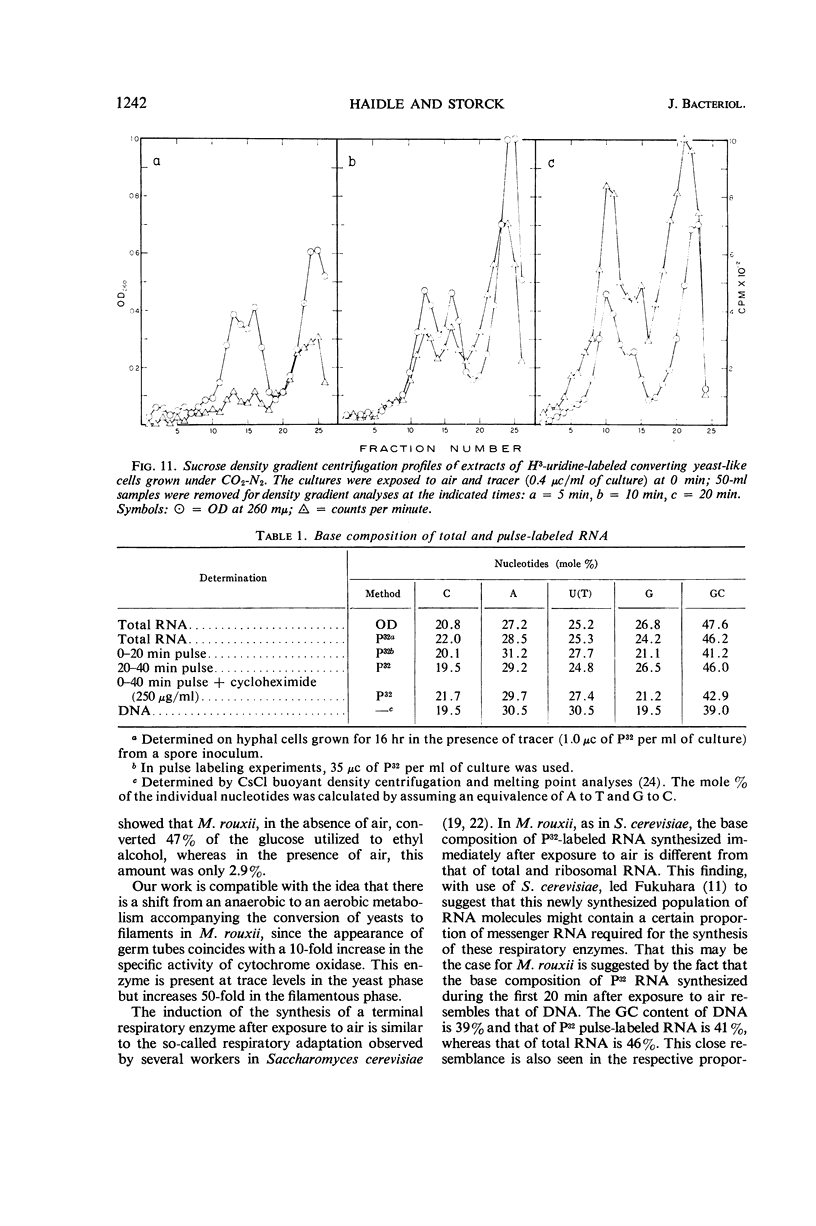
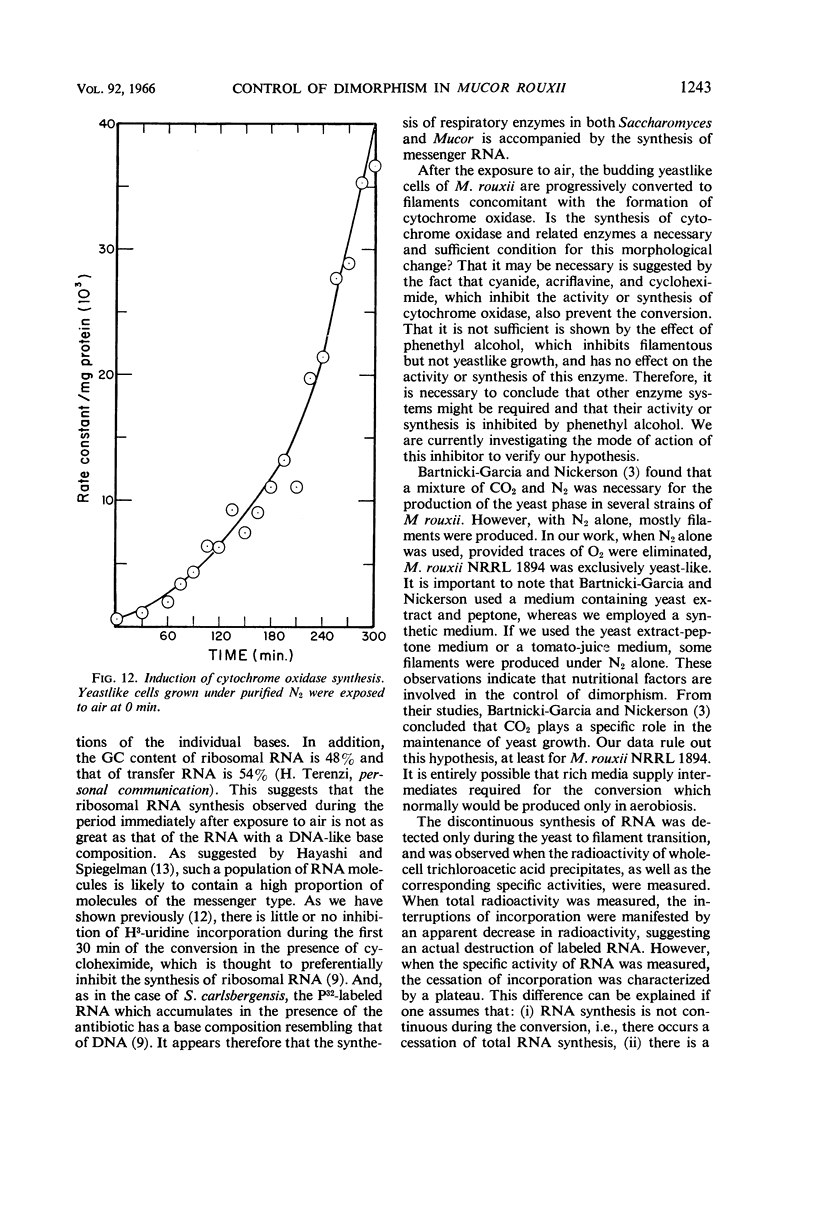
Abstract
Haidle, C. W. (The University of Texas, Austin), and R. Storck. Control of dimorphism in Mucor rouxii. J. Bacteriol. 92:1236–1244. 1966.—Yeastlike cells of Mucor rouxii NRRL 1894 were converted to filaments in a medium containing glucose, mineral salts, casein hydrolysate, nicotinic acid, and thiamine when the gas phase was changed from CO2-N2 or N2 alone to air. Germ tubes began to appear 3 to 4 hr after exposure to air. Ribonucleic acid (RNA) precursors were incorporated into RNA in a discontinuous fashion during this conversion, but the incorporation was continuous during the anaerobic growth of yeastlike cells and during the aerobic germination of sporangiospores. The incorporation of labeled amino acids during the conversion was exponential. Labeling of ribosomal RNA occurred as shortly as 5 min after replacement of CO2-N2 with air. However, P32-labeled RNA isolated 20 min after exposure to air had a guanine plus cytosine (GC) content of 41% (mole%) as compared with the 47% found for labeled and unlabeled RNA isolated at other stages of the life cycle of this organism or later during the conversion. In addition, the overall base composition of this 20-min pulse-labeled RNA resembled that of deoxyribonucleic acid (GC = 39%), suggesting that a significant proportion of this RNA is of the messenger type. Furthermore, the synthesis of cytochrome oxidase was induced upon exposure of yeastlike cells to air. Cyanide, acriflavine, and cycloheximide, which inhibited the action or synthesis of cytochrome oxidase, also inhibited the yeast to filament transition.
Full text
PDF
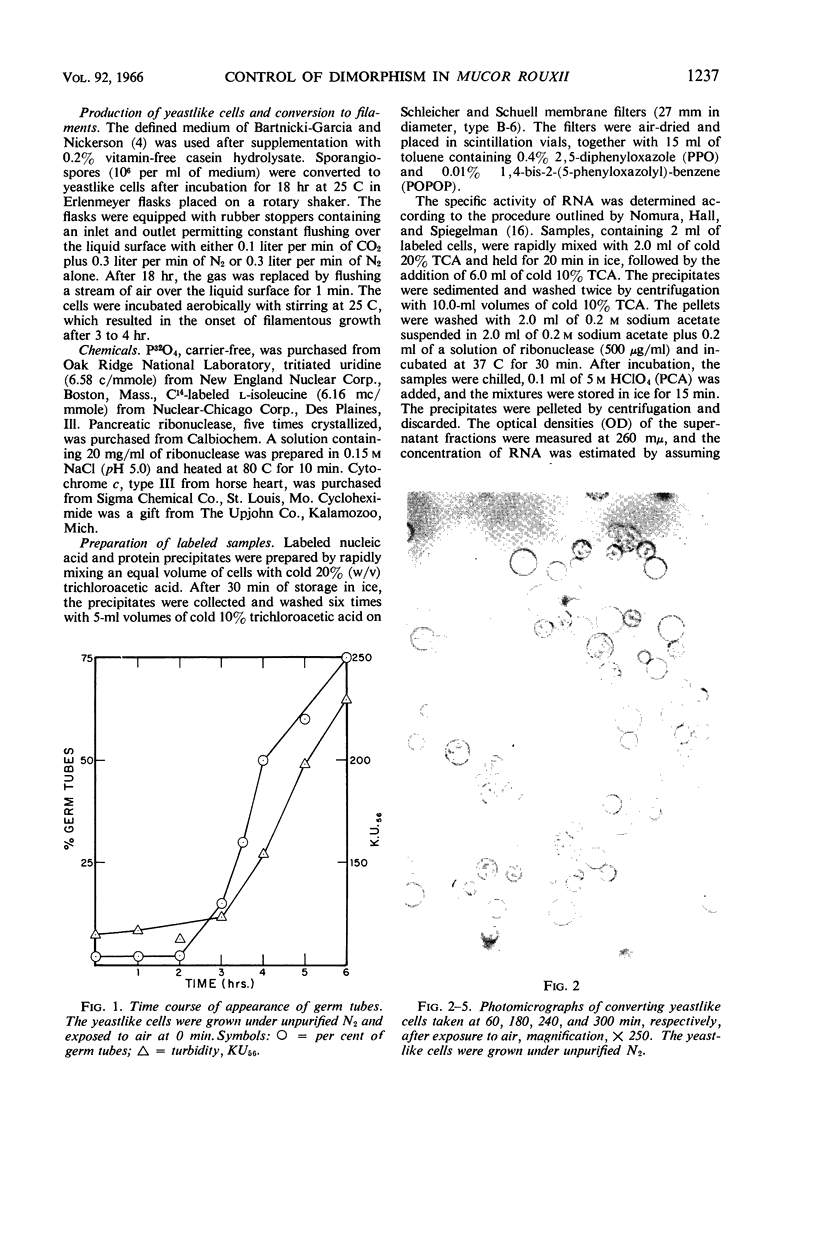
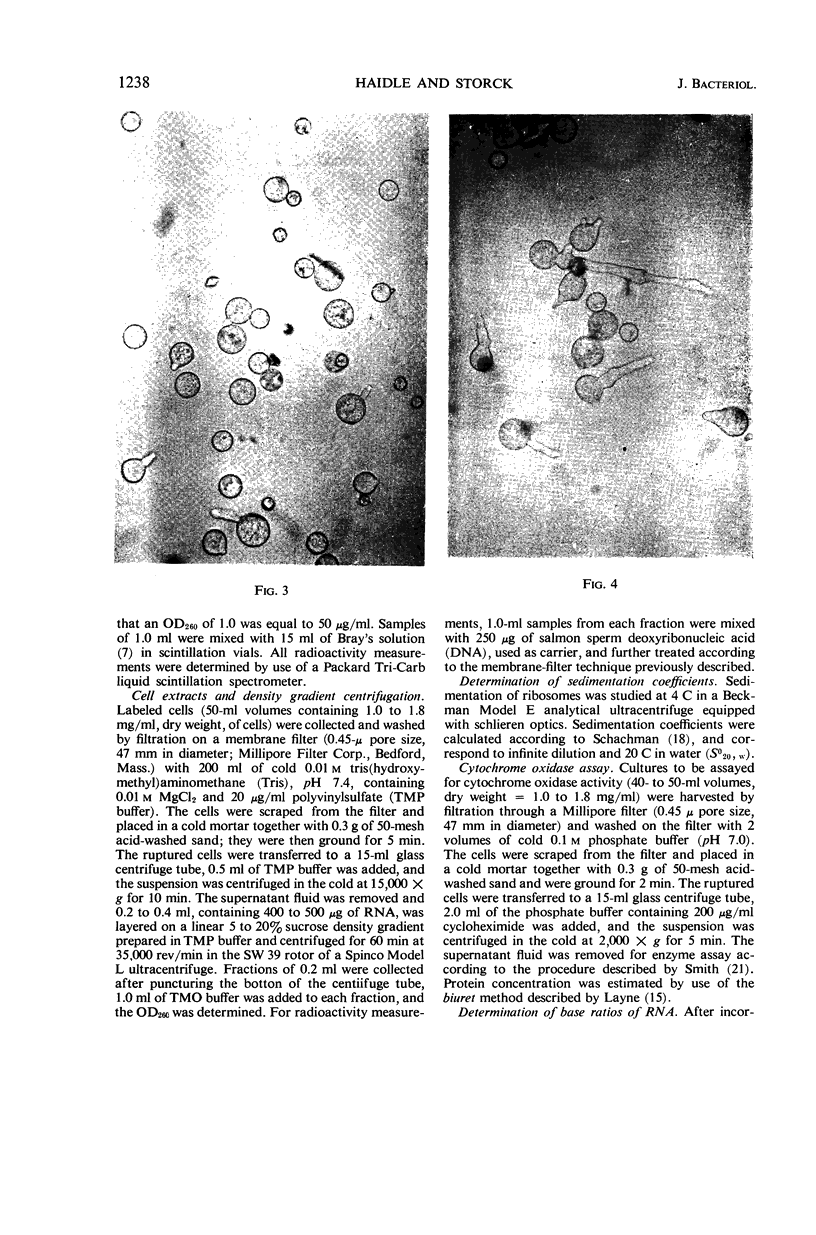
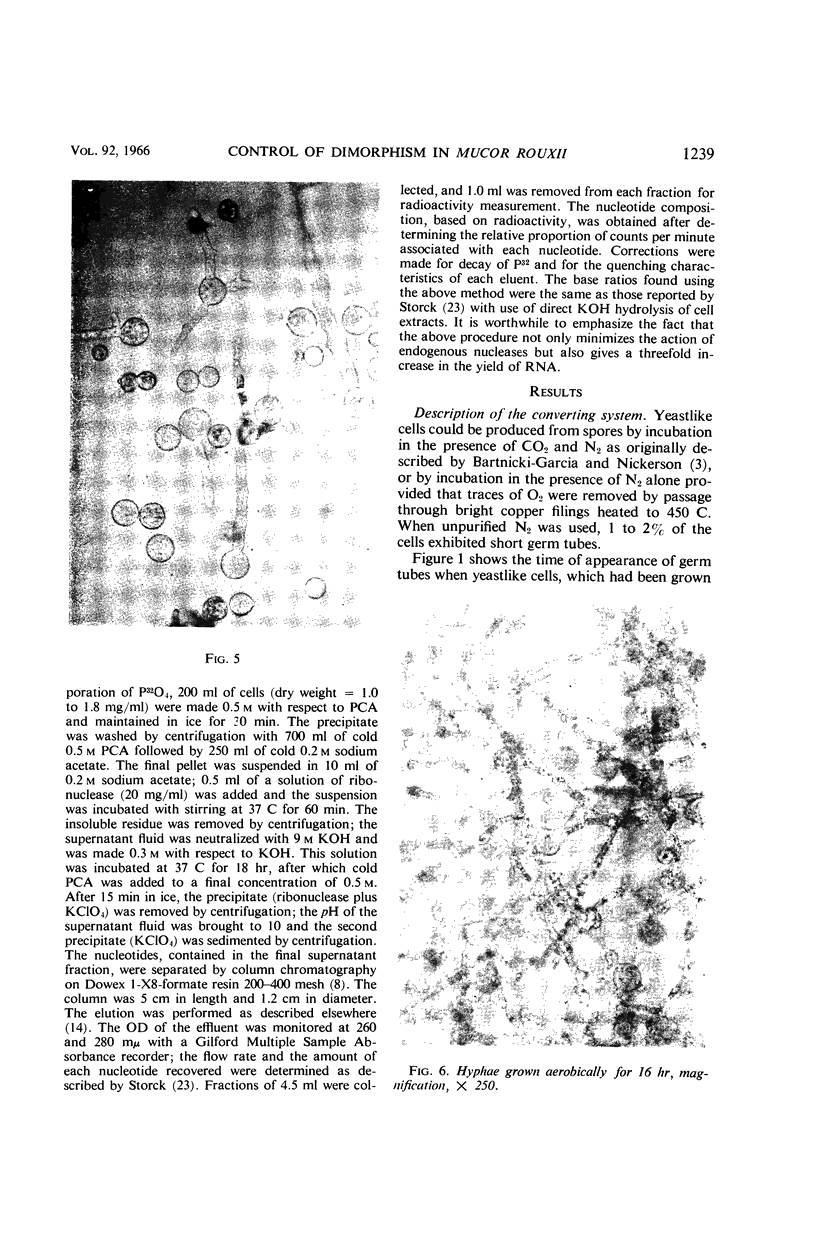

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTNICKI GARCIA S. SYMPOSIUM ON BIOCHEMICAL BASES OF MORPHOGENESIS IN FUNGI. III. MOLD-YEAST DIMORPHISM OF MUCOR. Bacteriol Rev. 1963 Sep;27:293–304. doi: 10.1128/br.27.3.293-304.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Assimilation of carbon dioxide and morphogenesis Mucor rouxii. Biochim Biophys Acta. 1962 Nov 5;64:548–551. doi: 10.1016/0006-3002(62)90314-1. [DOI] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Induction of yeast-like development in Mucor by carbon dioxide. J Bacteriol. 1962 Oct;84:829–840. doi: 10.1128/jb.84.4.829-840.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Isolation, composition, and structure of cell walls of filamentous and yeast-like forms of Mucor rouxii. Biochim Biophys Acta. 1962 Mar 26;58:102–119. doi: 10.1016/0006-3002(62)90822-3. [DOI] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Nutrition, growth, and morphogenesis of Mucor rouxii. J Bacteriol. 1962 Oct;84:841–858. doi: 10.1128/jb.84.4.841-858.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnicki-Garcia S., Nickerson W. J. THIAMINE AND NICOTINIC ACID: ANAEROBIC GROWTH FACTORS FOR MUCOR ROUXII. J Bacteriol. 1961 Jul;82(1):142–148. doi: 10.1128/jb.82.1.142-148.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIALA E. S., DAVIS F. F. PREFERENTIAL INHIBITION OF SYNTHESIS AND METHYLATION OF RIBOSOMAL RNA IN NEUROSPORA CRASSA BY ACTIDIONE. Biochem Biophys Res Commun. 1965 Jan 4;18:115–118. doi: 10.1016/0006-291x(65)90892-2. [DOI] [PubMed] [Google Scholar]
- FUKUHARA H. RNA SYNTHESIS OF YEAST IN THE PRESENCE OF CYCLOHEXIMIDE. Biochem Biophys Res Commun. 1965 Jan 18;18:297–301. doi: 10.1016/0006-291x(65)90757-6. [DOI] [PubMed] [Google Scholar]
- HAYASHI M., SPIEGELMAN S. The selective synthesis of informational RNA in bacteria. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1564–1580. doi: 10.1073/pnas.47.10.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNEY H., STORCK R. NUCLEOTIDE COMPOSITION OF RIBONUCLEIC ACID FROM NEUROSPORA CRASSA. J Bacteriol. 1963 Apr;85:822–826. doi: 10.1128/jb.85.4.822-826.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidle C. W., Storck R. Inhibition by cycloheximide of protein and RNA synthesis in Mucor rouxii. Biochem Biophys Res Commun. 1966 Jan 24;22(2):175–180. doi: 10.1016/0006-291x(66)90428-1. [DOI] [PubMed] [Google Scholar]
- SHORTMAN K., FUKUHARA H. METABOLISM OF RIBONUCLEIC ACID DURING RESPIRATORY ADAPTATION OF YEAST. Biochim Biophys Acta. 1963 Dec 20;76:501–524. [PubMed] [Google Scholar]
- SIEGEL M. R., SISLER H. D. SITE OF ACTION OF CYCLOHEXIMIDE IN CELLS OF SACCHAROMYCES PASTORIANUS. I. EFFECT OF THE ANTIBIOTIC ON CELLULAR METABOLISM. Biochim Biophys Acta. 1964 May 18;87:70–82. doi: 10.1016/0926-6550(64)90048-9. [DOI] [PubMed] [Google Scholar]
- Somlo M., Fukuhara H. On the necessity of molecular oxygen for the synthesis of respiratory enzymes in yeast. Biochem Biophys Res Commun. 1965 May 18;19(5):587–591. doi: 10.1016/0006-291x(65)90379-7. [DOI] [PubMed] [Google Scholar]
- Storck R. Nucleotide composition of nucleic acids of fungi. I. Ribonucleic acids. J Bacteriol. 1965 Nov;90(5):1260–1264. doi: 10.1128/jb.90.5.1260-1264.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storck R. Nucleotide composition of nucleic acids of fungi. II. Deoxyribonucleic acids. J Bacteriol. 1966 Jan;91(1):227–230. doi: 10.1128/jb.91.1.227-230.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet S. R. Accumulation of RNA with a DNA like base composition in Saccharomyces carlsbergensis in the presence of cycloheximide. Biochem Biophys Res Commun. 1965 May 18;19(5):582–586. doi: 10.1016/0006-291x(65)90378-5. [DOI] [PubMed] [Google Scholar]