Abstract
The aim of this study was to assess the effects of replacement with recombinant human growth hormone (rhGH) in patients with GH deficiency (GHD) after treatment of acromegaly. Intervention study. Sixteen patients (8 men, age 56 years), treated for acromegaly by surgery and radiotherapy, with an insufficient GH response to insulin-induced hypoglycaemia, were treated with 1 year of rhGH replacement. Study parameters were assessed at baseline and after 1 year of rhGH replacement. Study parameters were cardiac function, body composition, bone mineral density (BMD), fasting lipids, glucose, bone turnover markers, and Quality of Life (QoL). During rhGH replacement IGF-I concentrations increased from −0.4 ± 0.7 to 1.0 ± 1.5 SD (P = 0.001), with a mean daily dose of 0.2 ± 0.1 mg in men and 0.3 ± 0.2 mg in women. Nonetheless, rhGH replacement did not alter cardiac function, lipid and glucose concentrations, body composition or QoL. Bone turnover markers (PINP and β crosslaps) levels increased (P = 0.005 and P = 0.021, respectively), paralleled by a small, but significant decrease in BMD of the hip. The beneficial effects of rhGH replacement in patients with GHD during cure from acromegaly are limited in this study.
Keywords: Growth hormone deficiency, Growth hormone replacement, Acromegaly
Introduction
Growth hormone deficiency (GHD) in adults is characterized by an adverse cardiovascular metabolic profile, altered body composition (reflected in reduced muscle strength and mass, and visceral obesity), decreased bone mass, decreased cardiac function and decreased quality of life (reviewed in [1]). Treatment with recombinant human growth hormone (rhGH) ameliorates symptoms and signs of the GHD syndrome in the short [2] and in the long-term [3, 4]. GHD is a well-known sequel of pituitary radiotherapy, e.g. for non-functioning adenomas, adrenocorticotrope hormone- or prolactin-secreting adenomas [5]. Remarkably, GHD can also be induced by treatment of active acromegaly. We have documented a diminished GH increase to insulin-induced hypoglycemia during long-term follow-up in 36% of the patients with acromegaly after postsurgical radiotherapy [6].
Almost all randomized controlled studies on the efficacy of rhGH replacement in adult GHD have excluded patients previously treated for acromegaly. Nonetheless, two intervention studies have reported on the effects of rhGH replacement in patients with GHD after previous treatment of acromegaly. In a subanalysis of acromegalic patients with GHD extracted from the large KIMS database, 6 months of rhGH replacement had no significant beneficial effects in the acromegalic patients [7]. In addition, a recent study compared the effects of 2 years of rhGH replacement on body composition, muscle strength, bone mass and metabolic parameters between ten patients previously treated for acromegaly and ten patients treated for non-functioning pituitary disease [8]. At baseline, patients with acromegaly had decreased muscle endurance and increased LDL concentrations compared to the other patients, but after 2 years of rhGH replacement there were no differences between both groups [8].
The aim of this study was to evaluate 1 year of rhGH replacement on heart function, quality of life, glucose and lipid metabolism, body composition and bone mass and turnover in order to extend the exploration whether GH replacement is beneficial in acromegalic patients with GHD during long-term biochemical cure.
Patients and methods
Patients
We enrolled 16 acromegalic patients (8 men and 8 women), who developed GHD after combined pituitary surgery and radiotherapy in the study. Inclusion criteria were previous treatment for acromegaly by surgery and/or radiotherapy and an insufficient GH increase to insulin-induced hypoglycemia (short-acting insulin 0.05–0.1 U/kg body weight, blood samples drawn at 0, 20, 30, 45, 60 and 90 min; nadir glucose levels below 2.2 mmol/l) [9]. The increase in GH concentrations was considered insufficient, when the peak GH response was below 3 μg/l [10].
Fifteen patients had been treated with primary surgery and secondary conventional radiotherapy mean interval after radiotherapy 18 years (range 4–29 years). The other patient was diagnosed with pituitary apoplexy of a GH producing adenoma. Because GH concentrations remained elevated, he underwent surgery and subsequently developed complete anterior pituitary failure. Clinical details were published previously [11].
Additional hormone replacement therapy was kept stable for at least 3 months prior to study inclusion, and was only adjusted thereafter when necessary. The purpose, nature, and possible risks of the study were explained to all subjects and written informed consent was obtained. The study protocol was approved by the ethics committee of the Leiden University Medical Center.
Study design
Study parameters were assessed both at baseline and after 1 year of rhGH replacement. The following variables were measured: fasting concentrations of lipoproteins, glucose, and IGF-I, body composition, bone turnover markers and bone mass, echocardiography, and Quality of Life parameters.
Growth hormone vials of 1 ml were manufactured and provided by Novo Nordisk Pharma, Denmark. Growth hormone replacement dose was started at 0.2 mg/day and, subsequently, titrated in the first 12 weeks of the study to obtain an IGF-I concentration within the age- and gender-adjusted reference range, according to Growth Hormone Research Society guidelines [10].
The mean age of the patients was 56 years (range 34–75 years). The interval between radiotherapy and the start of the study was 18 years (range 4–29 years). TSH deficiency was present in five patients, ACTH deficiency in nine patients, and LH-FSH deficiency in eight patients (see Table 1).
Table 1.
Clinical characteristics of the 16 patients with growth hormone deficiency after acromegaly
| Age (year) | Gender | Substitution therapy |
|---|---|---|
| 65 | Male | None |
| 65 | Male | None |
| 59 | Male | Thyroxine, Testosterone, Hydrocortisone |
| 75 | Female | Hydrocortisone |
| 66 | Female | None |
| 62 | Female | Hydrocortisone |
| 66 | Female | Thyroxine, Estradiol, Hydrocortisone |
| 40 | Female | Thyroxine, Estradiol, Hydrocortisone |
| 43 | Male | Testosterone |
| 56 | Male | Thyroxine, Testosterone, Hydrocortisone |
| 48 | Male | Thyroxine, Testosterone, Hydrocortisone |
| 51 | Female | None |
| 34 | Female | Hydrocortisone |
| 74 | Male | Testosterone |
| 45 | Male | Testosterone |
| 50 | Female | Hydrocortisone |
Body composition
Body weight and height, waist circumference, hip circumference, systolic and diastolic blood pressure (SBP and DBP, respectively) were measured. Waist-hip (WH) ratio was calculated. Body weight was measured to the nearest 0.1 kg, and body height was measured barefoot to the nearest 0.001 m. Lean body mass and fat mass were measured with DXA (Hologic 4500; Hologic Inc., Waltham, MA, USA).
Markers for bone turnover and bone mass
The following serum markers of bone turnover were measured: N-terminal propeptides of type I collagen (PINP), as a marker for bone synthesis, and β-crosslaps as a marker for bone resorption. Bone mineral density (BMD) was measured by DXA (Hologic 4500; Hologic Inc., Waltham, MA, USA). Sites measured were the lumbar spine (L1-L4) and the femoral neck (left and right). Mean BMD of the left and right femoral neck was calculated. Mean T and Z scores were calculated for total left and right hip using the NHANES reference values. The CV of BMD measurements was 1% and the machine was cross-calibrated at regular interval.
Echocardiography
Echocardiography was performed while the patients were in the left lateral decubitus position using a commercially available system (Vingmed Vivid-7, General Electric-Vingmed, Milwaukee, WI, USA). Standard parasternal (long- and short-axis) and apical views (2-, and 4-, and long-axis) were obtained. M-mode images were obtained from the parasternal long-axis views for quantitative assessment of LV dimensions (inter-ventricular septum thickness (IVST), posterior wall thickness (PWT), LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), fractional shortening (FS) and LV ejection fraction (LVEF)) [12].
The following parameters of diastolic function were obtained: diastolic transmitral peak velocities (E and A wave) and the E/A ratio. Quantitative diastolic data were derived from tissue Doppler imaging (TDI). For TDI analysis, the digital cine loops were analyzed using commercial software (Echopac 6.1; General Electric-Vingmed). The sample volume (4 mm2) was placed in the LV basal portion of the septum (using the 4-chamber views). The following parameters (mean values calculated from three consecutive heartbeats) were derived: early diastolic velocity (E′), late diastolic velocity (A′) and the E′/A′ ratio. The severity of valvular regurgitation was assessed by two independent expert readers blinded to the clinical data on a qualitative scale of trace, mild, moderate, or severe, using previously described methods [13, 14]. Left ventricular mass (LVM) was calculated by the cube formula, and using the correction formula proposed by Devereux et al. [15]: 0.8 × {1.04 [(LVEDD + PWT + IVST)3 − LVEDD3]} + 0.6. The data were assessed by two independent observers, blinded for the clinical data of the patients.
Quality of life
Quality of life was assessed using four different validated health-related quality of life questionnaires
Hospital anxiety and depression scale (HADS): The HADS consists of 14 items pertaining to anxiety and depression. Each item is measured on a 4-point scale. Scores for the anxiety and depression subscale range from 0 to 21 and for the total score from 0 to 42. A high score points to more severe anxiety and depression [16].
Multidimensional fatigue index (MFI-20): The MFI-20 contains 20 statements to assess fatigue [17]. Five different dimensions of fatigue (four items each) are calculated from these statements; (1) general fatigue; (2) physical fatigue; (3) reduced activity; (4) reduced motivation and (5) mental fatigue. Every statement is measured on a 5-point scale; scores vary from 0 to 20. Higher scores indicate higher experienced fatigue.
Nottingham health profile (NHP): The NHP is frequently used in patients with pituitary disease to assess general well-being and QoL. The survey consists of 38 yes/no questions, which are subdivided in 6 scales assessing impairments, i.e. pain (8 items), energy level (3 items), sleep (5 items), emotional reactions (9 items), social isolation (5 items) and disability/functioning, i.e. physical mobility (8 items) [18, 19]. Subscale scores are calculated as a weighted mean of the associated items and are expressed as a value between 0 and 100. The total score is the mean of the six subscales.
Quality of life-assessment of growth hormone deficiency in adults (QoL-AGDHA): This disease specific quality of life questionnaire has been developed specifically for the detection of deficits in needs achievements in areas which have shown to be commonly affected in adults with GHD [20]. The questionnaire comprises 25 items, which are summed to form a total score. Higher numerical scores (to a maximum of 25) denote poorer quality of life.
Assays and dynamic tests
Growth hormone reserve was evaluated by the insulin tolerance test in fasting conditions (short-acting insulin 0.05–0.1 U/kg body weight, blood samples drawn at 0, 20, 30, 45, 60 and 90 min; the nadir glucose concentration should drop below 2.2 mmol/l) [9]. The increase in GH concentration was considered insufficient, when the peak GH concentration was below 3 μg/l [10].
Serum IGF-I concentration was measured with the Immulite 2500 system (Diagnostic Products Corporation, Los Angeles, USA). The intra-assay variation was 5.0 and 7.5% at mean serum levels of 8 and 75 nmol/l, respectively. IGF-I levels are expressed as standard deviation-scores (SDS), using lambda-mu-sigma (LMS) smoothed reference curves based on measurements in 906 healthy individuals [21, 22].
IGFBP-3 was measured using an immunometric technique on an IMMULITE Analyzer (Diagnostic Products Corporation, Los Angeles, USA). The lower limit of detection was 0.02 mg/l and inter-assay variation was 4.4 and 4.8% at 0.91 and 8.83 mg/l. A Hitachi P800 auto analyzer (Roche, Mannheim, Germany) was used to quantify serum concentrations of glucose, total cholesterol and TG. HDL was measured with a homogenous enzymatic assay (Hitachi 911, Roche, Mannheim, Germany). LDL cholesterol concentrations (LDL) were calculated using the Friedewald formula. C-crosslinking terminal telopeptide of type I collagen (β-crosslaps) and procollagen type I aminoterminal propeptide (PINP) by chemoluminescence immunoassay with the Modular Analytics E-170 system (Roche Diagnostics, Almere, The Netherlands).
Statistics
Statistical analysis was performed using SPSS for Windows, version 14.0 (SPSS Inc. Chicago, Illinois, USA). Results are scored as the mean ± standard deviation (SD), unless specified otherwise. The data were analyzed with the paired samples Student’s t-test. Statistical significance was set at P < 0.05.
Results
IGF-1 and IGFBP-3 concentrations
One year of rhGH replacement increased IGF-I SD scores and IGF-BP3 levels (baseline IGF-I SD score: −0.4 ± 1.7 and 1.0 ± 1.5 at 1 year, P < 0.001; baseline IGFBP-3: 4.2 ± 1.2 mg/l and 5.2 ± 1.4 mg/l after 1 year, P < 0.001).
Cardiovascular risk parameters and body composition
During rhGH replacement lipid profiles did not change. In addition, blood pressure (systolic and diastolic) and fasting glucose concentrations did not change (Table 2). Mean lean body mass increased by almost 4 kg and total fat mass decreased by approximately 3 kg, but these differences did not reach statistical significance.
Table 2.
Metabolic and antroprometric parameters before and after 1 year of rhGH replacement
| Before | After | P-value | |
|---|---|---|---|
| Total cholesterol (mmol/l) | 6.0 ± 1.0 | 5.7 ± 1.2 | 0.317 |
| TG (mmol/l) | 1.8 ± 1.0 | 2.1 ± 1.3 | 0.222 |
| HDL-cholesterol (mmol/l) | 1.4 ± 0.5 | 1.4 ± 0.4 | 0.597 |
| LDL-cholesterol (mmol/l) | 4.1 ± 0.8 | 3.9 ± 1.0 | 0.250 |
| Glucose (mmol/l) | 4.6 ± 0.6 | 4.7 ± 0.5 | 0.138 |
| SBP (mm Hg) | 138 ± 17 | 135 ± 10 | 0.222 |
| DBP (mm Hg) | 87 ± 9 | 88 ± 8 | 0.924 |
| Waist circumference (cm) | 102.8 ± 11.4 | 103.6 ± 12.5 | 0.599 |
| WH ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.921 |
| LBM (kg) | 57.1 ± 13.0 | 61.7 ± 13.4 | 0.102 |
| Fat mass (kg) | 34.8 ± 16.0 | 31.3 ± 14.7 | 0.367 |
TG triglycerides; HDL high-density lipoprotein; LDL low-density lipoprotein; SBP systolic blood pressure; DBP diastolic blood pressure; WH ratio waist-to-hip ratio; LBM lean body mass
Bone parameters
RhGH replacement increased plasma concentrations of bone turnover markers (PINP and β-crosslaps) in all patients (Table 3; Fig. 1). During rhGH replacement bone mass at the lumbar spine remained unchanged in all patients, but decreased significantly at the femoral neck by 4% (Table 3; Fig. 1).
Table 3.
Bone markers and biochemical parameters of bone turnover before and after 1 year rhGH replacement
| Before | After | P-value | |
|---|---|---|---|
| PINP (ng/ml) | 29.1 ± 19.5 | 44.3 ± 33.4 | 0.005 |
| β crosslaps (ng/ml) | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.021 |
| BMD lumbar spine (g/cm²) | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.640 |
| BMD femoral neck (g/cm²) | 0.85 ± 0.17 | 0.81 ± 0.15 | <0.001 |
| T score total hip | −0.30 ± 1.5 | −0.24 ± 1.4 | 0.434 |
| Z score total hip | 0.26 ± 1.6 | 0.37 ± 1.5 | 0.125 |
PINP N-terminal propeptides of type I collagen; BMD bone mineral density
Fig. 1.
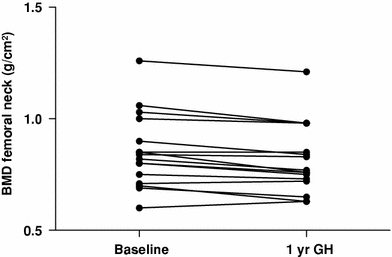
Bone mineral density decreased in all patients during 1 year rhGH replacement (n = 16, P < 0.001)
Cardiac parameters and quality of life parameters
During rhGH replacement there were no significant changes in cardiac parameters or QoL parameters (Tables 4, 5).
Table 4.
Cardiac parameters before and after 1 year rhGH replacement
| Before | After | P-value | |
|---|---|---|---|
| LVEDD (mm) | 51.6 ± 5.8 | 47.0 ± 15.5 | 0.165 |
| LVESD (mm) | 34.3 ± 4.4 | 32.6 ± 7.5 | 0.222 |
| IVST (mm) | 12.2 ± 3.4 | 12.4 ± 3.9 | 0.704 |
| PWT (mm) | 10.2 ± 1.6 | 10.0 ± 1.3 | 0.594 |
| FS (%) | 35.1 ± 6.7 | 37.0 ± 6.1 | 0.319 |
| LVEF (%) | 63.5 ± 8.4 | 66.3 ± 7.5 | 0.244 |
| E (mm/s) | 0.5 ± 0.2 | 0.5 ± 0.1 | 0.745 |
| A (mm/s) | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.789 |
| E/A ratio | 1.0 ± 0.3 | 0.9 ± 0.3 | 0.589 |
| E′ (cm/s) | 0.6 ± 0.2 | 0.5 ± 0.3 | 0.629 |
| A′ (cm/s) | 0.7 ± 0.1 | 0.6 ± 0.4 | 0.300 |
| E′/A′ ratio | 0.8 ± 0.4 | 0.9 ± 0.3 | 0.589 |
| LVM (g) | 233 ± 64 | 215 ± 105 | 0.307 |
LVEDD left ventricular end-diastolic diameter; LVESD left ventricular end-systolic diameter; FS fractional shortening; LVEF left ventricular ejection fraction; IVST inter-ventricular septum thickness; PWT posterior wall thickness; LVM left-ventricular mass
Table 5.
Quality of life parameters at baseline and after 1 year rhGH replacement in patients with GHD after previous treatment for acromegaly
| Before | After | P-value | ||
|---|---|---|---|---|
| QoL NHP | Energy | 55.2 ± 38.2 | 41.8 ± 35.4 | 0.125 |
| Pain | 19.4 ± 21.8 | 14.1 ± 18.8 | 0.278 | |
| Emotional reaction | 12.0 ± 16.4 | 13.2 ± 20.0 | 0.603 | |
| Sleep | 13.6 ± 26.9 | 14.1 ± 24.4 | 0.913 | |
| Physical mobility | 20.1 ± 20.8 | 16.0 ± 23.7 | 0.258 | |
| Social isolation | 12.0 ± 17.1 | 10.6 ± 17.1 | 0.586 | |
| QoL MFI-20 | General fatigue | 15.4 ± 4.5 | 14.2 ± 4.7 | 0.154 |
| Physical fatigue | 12.9 ± 5.2 | 13.2 ± 5.2 | 0.747 | |
| Reduction in activity | 11.4 ± 4.9 | 11.8 ± 4.9 | 0.625 | |
| Reduction in motivation | 10.8 ± 4.4 | 10.1 ± 4.5 | 0.415 | |
| Mental fatigue | 9.3 ± 4.6 | 9.8 ± 4.3 | 0.650 | |
| QoL HADS | Anxiety | 4.6 ± 2.4 | 4.9 ± 2.3 | 0.453 |
| Depression | 6.0 ± 4.4 | 6.1 ± 4.3 | 0.933 | |
| Total score | 10.6 ± 5.0 | 11.0 ± 5.3 | 0.674 | |
| QoL-AGDHA | 7.6 ± 6.1 | 7.7 ± 5.9 | 0.880 |
HADS, NHP, MFI-20, QoL-AGDHA higher scores: more impairment
Discussion
In this prospective study, we evaluated the effect of rhGH treatment on a range of relevant parameters in GHD patients, previously treated for acromegaly. During rhGH replacement IGF-I concentrations increased into the age- and gender-adjusted normal range, but neither cardiac parameters, nor any of the cardiovascular risk parameters or quality of life parameters changed during rhGH treatment. Bone turnover markers increased during rhGH replacement, which was associated with a decrease of bone mineral density at the femoral neck of 4%, whereas the bone mass of the lumbar spine remained unchanged. These data indicate that the effects of rhGH treatment of GHD patients previously treated for acromegaly are limited.
Data on the manifestations of GHD after treatment for acromegaly are limited. Two previous studies reported several clinical manifestations in this particular patient group [7, 8]. The first study compared patients with GHD after treatment for acromegaly and Cushing’s disease with patients with GHD due to other etiologies [7]. No differences in body mass index, waist-hip ratio, serum lipid concentrations, bone mineral density (at the lumbar spine and femoral neck), or IGF-I SD score were found in patients with GHD after acromegaly compared with patients with GHD due to other etiologies [7]. In the second study, muscle strength, bone mass and metabolic indices were compared between ten patients previously treated for acromegaly and ten patients treated for non-functioning pituitary disease [8]. Although there were no differences between both groups after 2 years of rhGH replacement, at baseline, patients with acromegaly had a decreased muscle endurance and increased LDL concentrations compared to the other patients, which points towards differences in their response to the treatment [8]. However, body fat decreased and lean body mass increased in that study in the patients with non-functioning pituitary disease, whereas it did not change in the same number of patients with GHD after acromegaly simultaneously studied [8], in agreement with our findings.
In adult patients with GHD, rhGH replacement increases bone mineral density [23], left ventricular mass and stroke volume [24], lean body mass [1], and quality of life [25], whereas it improves the serum lipid profile [26]. These effects are apparent within 6–12 months and are maintained during continued treatment with rhGH in the long-term [4, 24, 26–29]. However, it appears that these abnormalities associated with GHD in adults are not always reversed completely solely by rhGH replacement [29, 30] and that some patients might benefit more from combined treatment of rhGH with, for instance, lipid-lowering agents and bisfosfonates [31, 32].
In our study, parameters of both bone resorption and bone formation increased, paralleled by a net decrease in bone mineral density at the femoral neck, in agreement with the observed increase in bone turnover found during 2 years of rhGH replacement in these patients by Norrman et al. [8]. In this latter study, however, no treatment differences in the response of bone mineral density between patients previously treated for acromegaly and patients previously treated for non-functioning pituitary disease were found [8]. It is important to note, however, that there are several small differences with our study. The patients in the study of Norrman et al. were included between 1991 and 1997. Hence, the initial dose previously applied before the consensus statement of the Growth Hormone Research Society in 1998 was first based on weight in some patients, but was subsequently gradually lowered when the weight based dose regime was abandoned. In addition, almost all patients studied (90%) were female [8]. These differences could explain the discrepant effects of rhGH replacement found on bone mineral density between the present study and the study by Norrman et al. [8].
The decrease in BMD found in our study could point towards a different response to rhGH replacement in patients previously exposed to persistently increased GH concentrations. Alternatively, this observation may indicate that the possible beneficial effect of rhGH replacement on bone in these patients is insufficient to compensate the ongoing bone loss after previous GH excess in these specific patients. In active acromegaly, bone mineral density is increased [33] and this favorable effect seems to persist after successful biochemical cure [34]. However, in patients with biochemical cure of acromegaly, radiotherapy was an independent negative predictor of bone mineral density at the femoral neck [34]. Almost all patients in our cohort had been treated previously by radiotherapy. On the other hand, in patients with adult-onset GHD due to other etiologies, some, but not all, studies have found a decreased bone mass at the lumbar spine (reviewed in [1]). Replacement with rhGH in those patients seems to modestly increase bone mineral density after 1 year [27]. However, the lack of the increase in BMD, usually seen during rhGH replacement but absent in our specific patients, is in accordance with the only other study performed in patients with GHD after treatment of acromegaly [7]. Further longer-term studies are needed to clarify this issue.
Body composition did not change during rhGH replacement, whereas it has been consistently found to be altered by rhGH replacement in patients with GHD due to other diseases (an increase in lean body mass and a decrease in body fat [1]). However, the trends seen in our study point towards similar changes in body composition in patients previously treated for acromegaly. Interestingly, body fat decreased and lean body mass increased in the study of Norrman et al. [8] in the patients with non-functioning pituitary disease, whereas it did not change in the same number of patients with GHD after acromegaly simultaneously studied. Replacement with rhGH did not improve QoL parameters. Various aspects of QoL seem to improve slightly during rhGH replacement in adults with GHD due to other diseases [25]. Therefore, it is likely that other factors in our patients with GHD after treatment for acromegaly explain the lack of effect on QoL during rhGH replacement, such as persisting joint related complaints [35] and/or unfavorable late effects of previous radiotherapy [36].
Considering the beneficial effects of rhGH replacement in patients with GHD due to other causes than acromegaly, substitution in this particular subgroup is warranted. We did not find many marked beneficial effects of rhGH replacement in these patients. Higher, non-physiological, doses of rhGH could possibly result in detectable changes in the targeted parameters, as has been extensively documented in patients with GHD not pre-exposed to acromegaly. In clinical practice, however, current treatment guidelines from the Growth Hormone Research Society, advocate to titrate rhGH dose to target IGF-I concentrations within the normal age-related reference range, to ensure a therapeutic dose also in those patients with severe GHD, and avoid side effects of rhGH replacement.
In conclusion, the effects of rhGH replacement in patients with GHD after treatment for acromegaly seem to be limited. The observed effect on bone resorption and in bone mineral density might be affected by ongoing bone loss despite rhGH replacement seen in acromegaly after radiotherapy, or by the response to rhGH of bone after previous long-term exposure to GH excess. Larger long-term studies in this specific patient group are warranted to clarify the issue whether the effects of rhGH replacement in GHD might be altered by previous acromegaly. However, these patients will most probably become increasingly rare since the introduction of effective drug treatment for acromegaly.
Acknowledgments
Disclosure summary
The authors have nothing to disclose.
Grants or fellowships
A. A. van der Klaauw is supported by an AGIKO grant of The Netherlands Organisation for Health Research and Development (grant number: 92003423). N. R. Biermasz is supported by an AGIKO and clinical fellowship grant of The Netherlands Organization for Health Research and Development (grant numbers 92003150, 90700195). The other authors have nothing to disclose.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.de Boer H, Blok GJ, Van der Veen EA. Clinical aspects of growth hormone deficiency in adults. Endocr Rev. 1995;16:63–86. doi: 10.1210/edrv-16-1-63. [DOI] [PubMed] [Google Scholar]
- 2.Maison P, Demolis P, Young J, Schaison G, Giudicelli JF, Chanson P. Vascular reactivity in acromegalic patients: preliminary evidence for regional endothelial dysfunction and increased sympathetic vasoconstriction. Clin Endocrinol (Oxf) 2000;53:445–451. doi: 10.1046/j.1365-2265.2000.01127.x. [DOI] [PubMed] [Google Scholar]
- 3.Chrisoulidou A, Beshyah SA, Rutherford O, Spinks TJ, Mayet J, Kyd P, Anyaoku V, Haida A, Ariff B, Murphy M, Thomas E, Robinson S, Foale R, Johnston DG. Effects of 7 years of growth hormone replacement therapy in hypopituitary adults. J Clin Endocrinol Metab. 2000;85:3762–3769. doi: 10.1210/jc.85.10.3762. [DOI] [PubMed] [Google Scholar]
- 4.Gotherstrom G, Svensson J, Koranyi J, Alpsten M, Bosaeus I, Bengtsson B, Johannsson G. A prospective study of 5 years of GH replacement therapy in GH-deficient adults: sustained effects on body composition, bone mass, and metabolic indices. J Clin Endocrinol Metab. 2001;86:4657–4665. doi: 10.1210/jc.86.10.4657. [DOI] [PubMed] [Google Scholar]
- 5.Littley MD, Shalet SM, Beardwell CG, Ahmed SR, Applegate G, Sutton ML. Hypopituitarism following external radiotherapy for pituitary tumours in adults. Q J Med. 1989;70:145–160. [PubMed] [Google Scholar]
- 6.Biermasz NR, van Dulken H, Roelfsema F. Long-term follow-up results of postoperative radiotherapy in 36 patients with acromegaly. J Clin Endocrinol Metab. 2000;85:2476–2482. doi: 10.1210/jc.85.7.2476. [DOI] [PubMed] [Google Scholar]
- 7.Feldt-Rasmussen U, Abs R, Bengtsson BA, Bennmarker H, Bramnert M, Hernberg-Stahl E, Monson JP, Westberg B, Wilton P, Wuster C. Growth hormone deficiency and replacement in hypopituitary patients previously treated for acromegaly or Cushing’s disease. Eur J Endocrinol. 2002;146:67–74. doi: 10.1530/eje.0.1460067. [DOI] [PubMed] [Google Scholar]
- 8.Norrman LL, Johannsson G, Sunnerhagen KS, Svensson J. Baseline characteristics and the effects of two years of growth hormone (GH) replacement therapy in adults with GH deficiency previously treated for acromegaly. J Clin Endocrinol Metab. 2008;63:2531–2538. doi: 10.1210/jc.2007-2673. [DOI] [PubMed] [Google Scholar]
- 9.van der Klaauw AA, Pereira AM, van Thiel SW, Smit JW, Corssmit EP, Biermasz NR, Frolich M, Iranmanesh A, Veldhuis JD, Roelfsema F, Romijn JA. GH deficiency in patients irradiated for acromegaly: significance of GH stimulatory tests in relation to the 24 h GH secretion. Eur J Endocrinol. 2006;154:851–858. doi: 10.1530/eje.1.02163. [DOI] [PubMed] [Google Scholar]
- 10.Consensus guidelines for the diagnosis and treatment of adults with growth hormone deficiency: summary statement of the Growth Hormone Research Society Workshop on Adult Growth Hormone Deficiency (1998). J Clin Endocrinol Metab 83:379–381 [DOI] [PubMed]
- 11.Roelfsema F, van den Berg G, van Dulken H, Veldhuis JD, Pincus SM. Pituitary apoplexy in acromegaly, a long-term follow-up study in two patients. J Endocrinol Invest. 1998;21:298–303. doi: 10.1007/BF03350332. [DOI] [PubMed] [Google Scholar]
- 12.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American society of echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 13.Perry GJ, Helmcke F, Nanda NC, Byard C, Soto B. Evaluation of aortic insufficiency by Doppler color flow mapping. J Am Coll Cardiol. 1987;9:952–959. doi: 10.1016/s0735-1097(87)80254-1. [DOI] [PubMed] [Google Scholar]
- 14.Thomas JD. How leaky is that mitral valve? Simplified Doppler methods to measure regurgitant orifice area. Circulation. 1997;95:548–550. doi: 10.1161/01.cir.95.3.548. [DOI] [PubMed] [Google Scholar]
- 15.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-X. [DOI] [PubMed] [Google Scholar]
- 16.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 17.Smets EM, Garssen B, Bonke B, De Haes JC. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-O. [DOI] [PubMed] [Google Scholar]
- 18.Hunt SM, McEwen J. The development of a subjective health indicator. Sociol Health Illn. 1980;2:231–246. doi: 10.1111/1467-9566.ep11340686. [DOI] [PubMed] [Google Scholar]
- 19.Hunt SM, McKenna SP, McEwen J, Backett EM, Williams J, Papp E. A quantitative approach to perceived health status: a validation study. J Epidemiol Community Health. 1980;34:281–286. doi: 10.1136/jech.34.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKenna SP, Doward LC, Alonso J, Kohlmann T, Niero M, Prieto L, Wiren L. The QoL-AGHDA: an instrument for the assessment of quality of life in adults with growth hormone deficiency. Qual Life Res. 1999;8:373–383. doi: 10.1023/A:1008987922774. [DOI] [PubMed] [Google Scholar]
- 21.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60. [PubMed] [Google Scholar]
- 22.Rikken B, van Doorn J, Ringeling A, Van den Brande JL, Massa G, Wit JM. Plasma levels of insulin-like growth factor (IGF)-I, IGF-II and IGF-binding protein-3 in the evaluation of childhood growth hormone deficiency. Horm Res. 1998;50:166–176. doi: 10.1159/000023268. [DOI] [PubMed] [Google Scholar]
- 23.Gotherstrom G, Bengtsson BA, Bosaeus I, Johannsson G, Svensson J. Ten-year GH replacement increases bone mineral density in hypopituitary patients with adult onset GH deficiency. Eur J Endocrinol. 2007;156:55–64. doi: 10.1530/eje.1.02317. [DOI] [PubMed] [Google Scholar]
- 24.Maison P, Chanson P. Cardiac effects of growth hormone in adults with growth hormone deficiency: a meta-analysis. Circulation. 2003;108:2648–2652. doi: 10.1161/01.CIR.0000100720.01867.1D. [DOI] [PubMed] [Google Scholar]
- 25.Deijen JB, Arwert LI, Witlox J, Drent ML. Differential effect sizes of growth hormone replacement on quality of life, well-being and health status in growth hormone deficient patients: a meta-analysis. Health Qual Life Outcomes. 2005;3:63. doi: 10.1186/1477-7525-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maison P, Griffin S, Nicoue-Beglah M, Haddad N, Balkau B, Chanson P. Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH-deficient adults: a metaanalysis of blinded, randomized, placebo-controlled trials. J Clin Endocrinol Metab. 2004;89:2192–2199. doi: 10.1210/jc.2003-030840. [DOI] [PubMed] [Google Scholar]
- 27.Davidson P, Milne R, Chase D, Cooper C. Growth hormone replacement in adults and bone mineral density: a systematic review and meta-analysis. Clin Endocrinol (Oxf) 2004;60:92–98. doi: 10.1111/j.1365-2265.2004.01935.x. [DOI] [PubMed] [Google Scholar]
- 28.Gotherstrom G, Bengtsson BA, Bosaeus I, Johannsson G, Svensson J. A 10-year, prospective study of the metabolic effects of growth hormone replacement in adults. J Clin Endocrinol Metab. 2007;92:1442–1445. doi: 10.1210/jc.2006-1487. [DOI] [PubMed] [Google Scholar]
- 29.van der Klaauw AA, Romijn JA, Biermasz NR, Smit JW, van Doorn J, Dekkers OM, Roelfsema F, Pereira AM. Sustained effects of recombinant GH replacement after 7 years of treatment in adults with GH deficiency. Eur J Endocrinol. 2006;155:701–708. doi: 10.1530/eje.1.02283. [DOI] [PubMed] [Google Scholar]
- 30.van der Klaauw AA, Biermasz NR, Feskens EJ, Bos MB, Smit JW, Roelfsema F, Corssmit EP, Pijl H, Romijn JA, Pereira AM. The prevalence of the metabolic syndrome is increased in patients with GH deficiency, irrespective of long-term substitution with recombinant human GH. Eur J Endocrinol. 2007;156:455–462. doi: 10.1530/EJE-06-0699. [DOI] [PubMed] [Google Scholar]
- 31.Monson JP, Jonsson P, Koltowska-Haggstrom M, Kourides I. Growth hormone (GH) replacement decreases serum total and LDL-cholesterol in hypopituitary patients on maintenance HMG CoA reductase inhibitor (statin) therapy. Clin Endocrinol (Oxf) 2007;67:623–628. doi: 10.1111/j.1365-2265.2007.02935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biermasz NR, Hamdy NA, Janssen YJ, Roelfsema F. Additional beneficial effects of alendronate in growth hormone (GH)-deficient adults with osteoporosis receiving long-term recombinant human GH replacement therapy: a randomized controlled trial. J Clin Endocrinol Metab. 2001;86:3079–3085. doi: 10.1210/jc.86.7.3079. [DOI] [PubMed] [Google Scholar]
- 33.Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004;25:102–152. doi: 10.1210/er.2002-0022. [DOI] [PubMed] [Google Scholar]
- 34.Biermasz NR, Hamdy NA, Pereira AM, Romijn JA, Roelfsema F. Long-term maintenance of the anabolic effects of GH on the skeleton in successfully treated patients with acromegaly. Eur J Endocrinol. 2005;152:53–60. doi: 10.1530/eje.1.01820. [DOI] [PubMed] [Google Scholar]
- 35.Biermasz NR, Pereira AM, Smit JW, Romijn JA, Roelfsema F. Morbidity after long-term remission for acromegaly; persisting joint-related complaints cause reduced quality of life. J Clin Endocrinol Metab. 2005;90:2731–2739. doi: 10.1210/jc.2004-2297. [DOI] [PubMed] [Google Scholar]
- 36.van der Klaauw AA, Biermasz N, Hoftijzer HC, Pereira AM, Romijn JA. Previous radiotherapy negatively influences quality of life during four years of follow-up in patients cured from acromegaly. Clin Endocrinol (Oxf) 2008;69(1):123–128. doi: 10.1111/j.1365-2265.2007.03169.x. [DOI] [PubMed] [Google Scholar]


