Abstract
Glycerolipid biosynthesis in Leishmania initiates with the acylation of glycerol-3-phosphate by a single glycerol-3-phosphate acyltransferase, LmGAT, or of dihydroxyacetonephosphate by a dihydroxyacetonephosphate acyltransferase, LmDAT. We previously reported that acylation of the precursor dihydroxyacetonephosphate rather than glycerol-3-phosphate is the physiologically relevant pathway for Leishmania parasites. We demonstrated that LmDAT is important for normal growth, survival during the stationary phase, and for virulence. Here, we assessed the role of LmDAT in glycerolipid metabolism and metacyclogenesis. LmDAT was found to be implicated in the biosynthesis of ether glycerolipids, including the ether-lipid derived virulence factor lipophosphoglycan and glycosylphosphatidylinositol-anchored proteins. The null mutant produced longer lipophosphoglycan molecules that were not released in the medium, and augmented levels of glycosylphosphatidylinositol-anchored proteins. In addition, the integrity of detergent resistant membranes was not affected by the absence of the LmDAT gene. Further, our genetic analyses strongly suggest that LmDAT was colethal with the glycerol-3-phosphate acyltransferase encoding gene LmGAT, implying that Leishmania expresses only two acyltransferases that initiate the biosynthesis of its cellular glycerolipids. Last, despite the fact that LmDAT is important for virulence the null mutant still exhibited the typical characteristics of metacyclics.
Keywords: Leishmania, Ether glycerolipid, Lipophosphoglycan, Detergent resistant membranes, Metacyclogenesis
1. Introduction
Protozoan parasites of the genus Leishmania cause a large spectrum of important human diseases worldwide, collectively named leishmaniases. These parasites develop within the digestive tract of the sand fly vector as flagellated, mobile promastigotes, and differentiate into and multiply as non-motile amastigotes within the phagolysosomal compartment of vertebrate host macrophages.
Glycerolipids constitute 70% of total lipids in the protozoan parasite Leishmania [1-3]. They are classified into ester and ether lipids depending on the substitution at position 1 of the glycerol backbone. Ester lipids harbor an acyl group while ether lipids carry a fatty alcohol moiety. Glycerolipid and particularly ether lipid biosynthesis in Leishmania parasites has been a focus of extensive studies because some of their derivatives, such as lipophosphoglycan (LPG) and glycosylphosphatidylinositol(GPI)-anchored protease gp63 were shown to be important for parasite virulence and development (reviewed in [4-8]). LPG is an unusual complex glycolipid that bears a 1-alkyl-phosphatidylinositol lipid anchor linked to an hexasaccharide followed by 15-30 repeats of the disaccharide mannose-galactose-phosphate (phosphoglycan repeat) and ends with a small oligosaccharide (reviewed in [7, 9-11]). Likewise, GPI-anchored proteins are tethered to the membrane by an ether lipid based 1-alkyl-2-acyl-phosphatidylinositol anchor [7, 10-12]. Lipids are also essential cell constituents and therefore must be constantly synthesized to allow multiplication of the parasite. This suggests that the pathways leading to their synthesis are essential for parasite proliferation and pathogenesis, and thus, offer a reasonable target for rational design of novel antileishmanial drugs. In fact, a lipid-based drug, miltefosine, is a potent antileishmanial compound that inhibits parasite growth in vitro and in vivo, and is currently used for treatment of visceral and muco-cutaneous forms of leishmaniasis [13-16].
The acylation of dihydroxyacetonephosphate (DHAP) by a DHAP acyltransferase (DHAPAT) represents the initial and obligatory step for the biosynthesis of ether lipids in most organisms that synthesize alkylglycerolipids [17]. The product of this first acylation reaction, 1-acyl-DHAP, is then converted to 1-alkyl-DHAP by a FAD-dependent alkyl DHAP synthase [18], which is further reduced to 1-alkyl-glycerol-3-phosphate (1-alkyl-G3P) by a NADPH-dependent alkyl/acyl-DHAP reductase. The intermediate 1-alkyl-G3P serves as the obligate precursor for all ether phospholipids. Alternatively, 1-acyl-DHAP can be reduced to 1-acyl-G3P by an NADPH-dependent alkyl/acyl-DHAP reductase, which is subsequently used for the biosynthesis of ester glycerolipids. The relative contribution of the DHAP acylation step in the biosynthesis of ester phospholipids has not yet been firmly established [19, 20].
DHAPAT activity has been characterized biochemically in several organisms [21-23]. In most animal tissues, DHAPAT is found in a membrane-associated fraction [21, 23] and localized to the luminal side of peroxisomes [19, 24]. This enzyme was also found to be part of a heterotrimeric complex that includes the 1-alkyl-DHAP synthase [25, 26]. Alterations in DHAPAT function have been associated with various human diseases such as neonatal adrenoleukodystrophy, infantile Refsum disease, hyperpipecolic acidemia and rhizomelic chondrodysplasia punctata [27-29].
We have previously reported the characterization of two initial acyltransferases in L. major, LmGAT and LmDAT, specific for the lipid precursors G3P and DHAP, respectively [30, 31]. Despite the fact that LmGAT is the sole G3P acyltransferase in Leishmania, it was dispensable for viability and virulence of the parasite. Furthermore, deletion of this gene did not significantly impair the lipid composition of the parasite [31]. Our previous studies established that LmDAT localized to peroxisome-like organelles, termed glycosomes in Leishmania and related parasites [30]. The null mutant of LmDAT was viable, but grew slower than the wild type, died rapidly during the stationary phase, and more importantly, was attenuated for virulence in mice [30].
This work reports the role of LmDAT in glycerolipid metabolism and metacyclogenesis. We show that LmDAT was involved in ether lipid synthesis, including the formation of the ether lipid based virulence factors LPG and GPI-anchored proteins, but was dispensable for the integrity of detergent resistant membranes (DRM). In addition, we provide strong genetic evidences supporting the idea that LmDAT was colethal with the sole G3P acyltransferase gene LmGAT [31]. Last, LmDAT was dispensable for the expression of metacyclic phenotypes.
2. Material and methods
2.1. Strains and growth conditions
Promastigotes of Leishmania major Friedlin V1 strain (MHOM/IL/80/Friedlin) were grown in liquid and semi-solid M199-derived medium [32]. The null mutant Δlmdat/Δlmdat and complemented strain Δlmdat/Δlmdat [LmDAT BSD] were described in reference [30]. Transfection was performed according to Ngo and colleagues [33] and selection was applied as appropriate in the presence of G418, blasticidin, puromycin, hygromycin and nourseothricin (40, 20, 50, 50 and 100 μg/ml, respectively).
2.2. Plasmids
To construct pXG2.LdSAcP1 (Ec471), pXG2 (Ec401) was first created as follows. pXG1a [34] was linealized with BamHI and ligated to two phosphorylated, complementary oligonucleotides O211 (‘5-GATCCGGTACCAGATCTGGGCCC-3′) and O212 (‘5-GATCGGGCCCAGATCTGGTACCG-3′) bearing BamHI, KpnI, BglII, and ApaI restriction sites. We screened, by enzymatic digestion analysis and sequencing, for plasmids that carry a single oligonucleotide with the BamHI site at the 5′ end, and termed the resulting plasmid, pXG2. Then, LdSAcP1 was subcloned from pX63PAC.LdSAcP1 [32] as a 3-kb BamHI-BglII DNA fragment into the respective BamHI and BglII sites (sense orientation) of pXG2, to yield pXG2.LdSAcP1.
The episome pXG.LmDAT (Ec212) was constructed by subcloning the LmDAT gene as a 4.3 kb BamHI fragment from pUC.LmDAT (Ec207; [30]) in sense orientation into the BamHI site of pXG1a [33].
The plasmid pBS.LmDAT:BSD (Ec223) was created by inserting the BSD cassette excised from pL.BSD (Ec221; [30]) as a 1.6 kb SacI-EcoRI fragment and ligated into the corresponding sites of pBS.53U-LmDAT (Ec220; [30]).
2.3. Creation of homozygous double mutants of LmGAT and LmDAT genes
The null mutant Δlmgat/Δlmgat [31] was electroporated with the LmDAT:PAC cassette described in [30] and transformants were selected in the presence of puromycin. The genomic integration was verified by polymerase chain reaction (PCR) and Southern blot analysis. The resulting line Δlmgat/Δlmgat/Δlmdat/LmDAT was then transformed with a LmDAT:BSD cassette to inactivate the second LmDAT allele, and parasites resistant to both puromycin and blasticidin were selected. Alternatively, the Δlmgat/Δlmgat/Δlmdat/LmDAT strain was first transformed with the episome pXG.LmDAT (Ec212) and selected in the presence of neomycin. The resulting transformant Δlmgat/Δlmgat/Δlmdat/LmDAT [LmDAT NEO] was finally transformed with the LmDAT:BSD cassette and selected in the presence of puromycin, neomycin and blasticidin. The genotype of the resulting Δlmgat/Δlmgat/Δlmdat/Δlmdat [LmDAT NEO] clones was analyzed by PCR.
2.4. Electrophoresis
Western blot analysis was carried out in the presence of BiP (generous gift of J. Bangs; [35]), gp63-325 and WIC79.3 (generous gifts of S. Turco) monoclonal antibodies [34, 35]. Native gel electrophoresis (6%/4%) was performed similarly as sodium dodecylsulfate polyacryamide gel electrophoresis (SDS-PAGE), except that SDS was omitted. Acid phosphatase assay was performed as described in [32].
2.5. Lipid purification and analysis
Parasites were grown in triplicate cultures to end-log phase, washed three times in cold PBS. The resulting cell pellets were frozen to -75°C until use. Bulk cellular lipids were purified by the Folch method [36] and analyzed by an automated electrospray ionization-tandem mass spectrometry (ESI-MS/MS) approach. Data acquisition, analysis and acyl group identification were carried out as described previously [37] with modifications. The samples were dissolved in 1 ml chloroform. An aliquot of 50 μl of extract in chloroform was used. Precise amounts of internal standards, obtained and quantified as previously described [38], were added in the following quantities (with some small variation in amounts in different batches of internal standards): 0.66 nmol di14:0-phosphatidylcholine (PC), 0.66 nmol di24:1-PC, 0.66 nmol 13:0-lysoPC, 0.66 nmol 19:0-lysoPC, 0.36 nmol di14:0-phosphatidylethanolamine (PE), 0.36 nmol di24:1-PE, 0.36 nmol 14:0-lysoPE, 0.36 nmol 18:0-lysoPE, 0.36 nmol 14:0-lysoPG, 0.36 nmol 18:0-lysophosphatidylglycerol, 0.36 nmol di14:0-phosphatidic acid (PA), 0.36 nmol di20:0(phytanoyl)-PA, 0.24 nmol di14:0-phosphatidylserine (PS), 0.24 nmol di20:0(phytanoyl)-PS, 0.20 nmol 16:0-18:0-phosphatidylinositol (PI), and 0.16 nmol di18:0-PI. The sample and internal standard mixture was combined with solvents, such that the ratio of chloroform/methanol/300 mM ammonium acetate in water was 300/665/35, and the final volume was 1.4 ml.
Unfractionated lipid extracts were introduced by continuous infusion into the ESI source on a triple quadrupole MS/MS (API 4000, Applied Biosystems, Foster City, CA). Samples were introduced using an autosampler (LC Mini PAL, CTC Analytics AG, Zwingen, Switzerland) fitted with the required injection loop for the acquisition time and presented to the ESI needle at 30 μl/min.
Sequential Precursor (Pre) and Neutral Loss (NL, [36]) scans of the extracts produce a series of spectra with each spectrum revealing a set of lipid species containing a common head group fragment. Lipid species were detected with the following scans: PC, sphingomyelin (SM), and lysoPC, [M+H]+ ions in positive ion mode with Pre of 184.1 (Pre 184.1); PE and lysoPE, [M+H]+ ions in positive ion mode with NL of 141.0 (NL 141.0); PI, [M+NH4]+ in positive ion mode with NL 277.0; PS, [M+H]+ in positive ion mode with NL 185.0; and PA, [M+NH4]+ in positive ion mode with NL of 115.0. SM was determined from the same mass spectrum as PC (Pre of m/z 184 in positive mode) [39, 40] and by comparison with PC internal standards (di14:0-PC and di24:1-PC). In a separate experiment, 16:0-SM was determined to produce a peak 0.39 times as large as a PC of the same m/z. Thus, a molar response factor for SM (in comparison with PC) of 0.39 was applied for quantification of the SM species. The scan speed was 50 or 100 u per sec. The collision gas pressure was set at 2 (arbitrary units). The collision energies, with nitrogen in the collision cell, were +28 V for PE, +40 V for PC (and SM), +25 V for PI, PS and PA, +20 V. Declustering potentials were +100 V. Entrance potentials were +15 V for PE, +14 V for PC (and SM), PI, PA, and PS. Exit potentials were +11 V for PE, +14 V for PC (and SM), PI, PA, PS. The mass analyzers were adjusted to a resolution of 0.7 u full width at half height. For each spectrum, 9 to 150 continuum scans were averaged in multiple channel analyzer mode. The source temperature (heated nebulizer) was 100°C, the interface heater was on, +5.5 kV or -4.5 kV were applied to the electrospray capillary, the curtain gas was set at 20 (arbitrary units), and the two ion source gases were set at 45 (arbitrary units).
The background of each spectrum was subtracted, the data were smoothed, and peak areas integrated using a custom script and Applied Biosystems Analyst software. The lipids in each class were quantified in comparison to the two internal standards of that class. The first and typically every 11th set of mass spectra were acquired on the internal standard mixture only. Peaks corresponding to the target lipids in these spectra were identified and molar amounts calculated in comparison to the internal standards on the same lipid class. To correct for chemical or instrumental noise in the samples, the molar amount of each lipid metabolite detected in the “internal standards only” spectra was subtracted from the molar amount of each metabolite calculated in each set of sample spectra. The data from each “internal standards only” set of spectra was used to correct the data from the following 10 samples. Finally, the data were expressed in percentage of total lipids analyzed.
2.6. Triton X-114 fractionation and DRM isolation
Triton X-114 fractionation was carried out as followed. Equivalent of 2×107 cells were lyzed in 100 μl of PBS containing 2% Triton X-114 for 10 min on ice. Lyzed cells were then heated for 5 min at 37°C, centrifuged at 15,000 g during 5 min, which leads to the formation of two phases. Equal volume equivalent of the soluble and lower phases were loaded on a SDS-PAGE for Western blot analysis in the presence of WIC79.3 or gp63 immunoglobulins.
For the isolation of DRMs, Triton X-100 partitioning was performed as described in [41]. Soluble and insoluble fractions were then analyzed by Western blot analysis in the presence of gp63 and phosphoglycan (WIC79.3) specific immunoglobulins as described above.
2.7. Immunofluorescence assays
Indirect immunofluorescence assays with anti-phosphoglycan (WIC79.3) and polyclonal anti-gp63 antibody (generous gift of B. McGuire) were performed as described previously [32]. The images were taken with a Leica fluorescence microscope.
2.8. Ficol gradient and Northern blot
Metacyclic parasites were isolated by Ficoll gradient centrifugation [42]. Northern blot analysis was performed as described in [32]. The probe specific to the metacyclic gene SHERP [43] was obtained by PCR amplification with the primers O114 (‘5- GATCCGCGCAGACCAAGATG-3′) and O115 (‘5-CAGAGAACGGCGAAGGGACTG-3′) using genomic DNA from L. major Friedlin V1 as a template. The PCR product was purified and then labeled by random primer using a kit from Roche Applied Science.
3. Results and discussion
3.1. The dihydroxyacetonephosphate acyltransferase LmDAT is essential for the synthesis of ether glycerolipids
LmDAT initiates the first step of the ether glycerolipid biosynthetic pathway, and possibly also contributes to the production of ester glycerolipids [30, 31]. To assess the role of LmDAT in glycerolipid metabolism, total cellular lipids were purified from wild type, null mutant and complemented line, and analyzed by electrospray ionization tandem mass spectrometry (ESI-MS/MS) in positive and negative mode as described in Materials and methods.
Previous analyses of total cellular lipids of L. major by electrospray ionization mass spectrometry have established that the most common ether glycerolipid species are found in the PE, more specifically the alkenylacylPEs with a total of 36 carbons containing two or three degree of unsaturation [32]. In positive spectrum, these lipid species corresponds to masses of 730 and 728, respectively. We focused our studies on the presence of these masses in the mass spectra derived from analysis of total cellular lipids purified from wild type, null mutant and complemented strain. These ether PE species were present in the wild type while they were almost non-existent in the null mutant (Fig. 1). Restoration of LmDAT expression in the null mutant background led to the reappearance of these ether PE species in the complemented line, as expected. These results corroborate the fact that de novo synthesis by LmDAT is critical for the biosynthesis of cellular ether lipids. The low signals at masses 730 and 728 in the knock-out line may represent ether lipids taken up from the extracellular medium (fetal bovine serum) or formed from catabolism and remodeling of extracellular ether lipids. However, it is unclear whether fetal bovine serum contains ether lipids, and as well as whether Leishmania possesses the enzymatic machinery that cleaves the polar group of ether glycerolipids for subsequent replacement with an ethanolamine phosphate. Alternatively, these masses may represent non ether lipids, such as PEs bearing an even- and odd-chain fatty acids that contain in total 35 carbon atoms with two and three double bonds, respectively. Because the presence of such PE species have been reported for L. tarentolae, donovani, brazilienseis and adleri [46], it is not excluded that L. major may also contain such lipids.
Fig. 1.
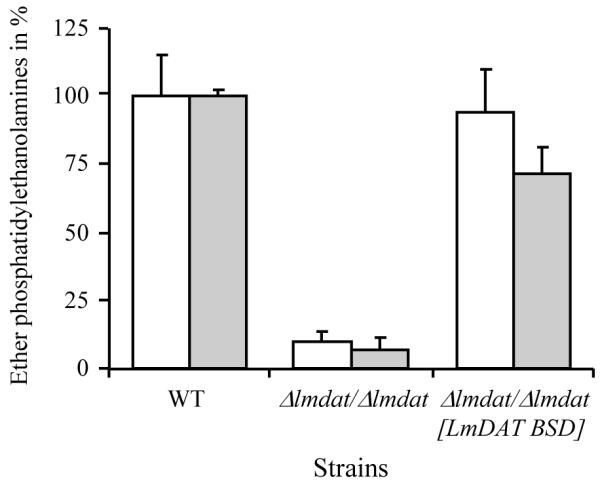
Lipid analysis of wild type, null mutant (Δlmdat/Δlmdat) and complemented strain (Δlmdat/Δlmdat [LmDAT BSD]). Ether PE were identified and quantified by ESI-MS/MS as described in Materials and methods. Most abundant one was alkylacylPE with 36 carbon atoms and two or three degree of unsaturation (36:2 and 36:3; [32]). White and grey bars represent alkylacylPEs 36:3 and 36:2, respectively, and are expressed in percentage of wild-type levels. The experiment was done twice in triplicate and a representative graph is shown with standard deviations.
LmDAT may also be involved in ester glycerolipid production, because a null mutant lacking the sole G3P acyltransferase LmGAT, that initiates the ester glycerolipid metabolic route, was viable and its lipid composition was not significantly altered ([31]; data not shown). However, comprehensive analysis of glycerolipid by mass spectrometry failed to identify significant differences between the ester glycerolipid profiles of PC, PE, and PI; (PS are below detection levels; [32]) of wild type and null mutant Δlmdat/Δlmdat (data not shown). This indicates that in the presence of the G3P acyltransferase LmGAT, LmDAT does not significantly participate in the production of ester glycerolipids. In contrast, in the absence of LmGAT, LmDAT can overtake the function of LmGAT, as cells grew normally, and the ESI MS/MS glycerolipid profile of wild type and null mutant Δlmgat/Δlmgat did not exhibit any substantial differences ([31]; data not shown).
The Δlmdat/Δlmdat strain was defected in the production of ether lipids similar to the null mutant Δads/Δads that lacks the second enzyme of the ether lipid biosynthetic pathway, the alkyl-DHAP synthase [32]. The fact that the Δads/Δads knock-out strain was not affected in its growth rate and survival during the stationary phase suggests that LmDAT is involved in an additional function that is absent in ADS [32]. One possibility is that LmDAT is implicated in the production of minor but physiologically important acyl-DHAP derived ester glycerolipid species not detected by our methodology. Alternatively, acyl-DHAP per se might be important for the parasite growth and stationary phase survival. However, beyond its role as a low concentration metabolic intermediate, no alternative functions have been described in any organism. Further, deletion of LmDAT may lead to the accumulation of DHAP and/or acyl-CoAs within the peroxisomes, which may be toxic to the cell. Last, the slow growth and poor survival during the stationary phase phenotypes are not related to the capacity of LmDAT to acylate DHAP. Notably, the N-terminal extension of approximately 700 amino acids may provide an additional function to LmDAT beside its acyltransferase activity.
3.2. The null mutant Δlmdat/Δlmdat synthesizes an altered form of LPG
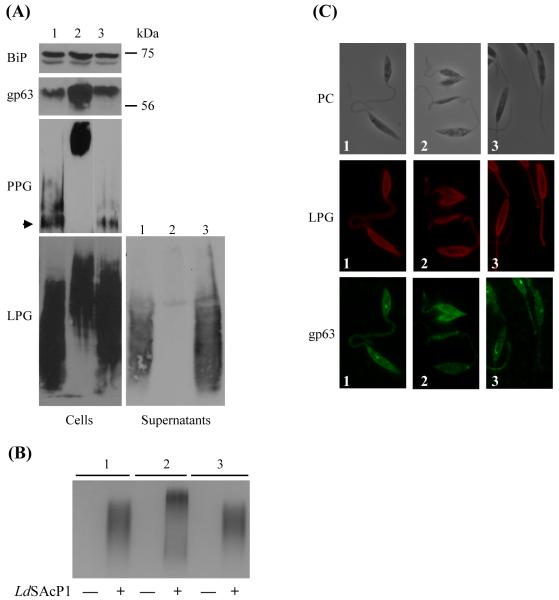
Because LPG is an ether lipid derivative [44], we tested whether its structure was affected in a null mutant of LmDAT. Western blot analysis was performed in the presence of the phosphoglycan specific monoclonal antibody WIC79.3 [35]. Wild type and complemented line expressed a LPG with similar apparent migration behavior (Fig. 2A). In contrast, the null mutant synthesized a LPG that migrated much slower than that of the wild type and complemented strain.
Fig. 2.
The Δlmdat/Δlmdat mutant synthesized altered forms of LPG and augmented levels of the GPI-anchored protein gp63. (A) Western blot analyses were performed with whole Leishmania cell extracts. Equivalent of 2×107 cells or 20 μl of culture supernatant were loaded per lane. Antibodies specific to gp63, phosphoglycan (WIC79.3; [37]) to detect LPG and PPG were used, respectively. In the case of PPG, the stacking gel is shown and the arrow indicates the separation between the stacking and separating gels. The membrane was also incubated with BiP immunoglubulins as a loading control. The ladder is shown on the right. (B) Native protein gel followed by in situ acid phosphatase assay. Each lane was loaded with 20 μl of cell supernatant on a native PAGE gel (SDS omitted) which was subsequently stained for acid phosphatase activity. “-“: non transfected cells; “+”: transfected with pXG2.LdSAcP1 (LdSAcP1); (C) Immunofluorescence assays were performed with WIC79.3 (LPG, red) and gp63 specific (green) antibody. PC, phase contrast. (A,B,C): 1, wild type; 2, null mutant Δlmdat/Δlmdat and 3, complemented line Δlmdat/Δlmdat [LmDAT BSD].
The slower migration may be due to hyperglycosylation of the phosphoglycan repeat domain, as it has been observed in metacyclic parasites and in the ether lipid mutant Δads/Δads [32, 45]. To assess whether the phosphoglycan repeat domain of LPG is longer in the null Δlmdat/Δlmdat mutant, we used the secretory acid phosphatase, that shares the same phosphoglycan repeat structure as LPG (reviewed in [46]), as a phosphoglycan reporter. Because L. major endogenous expression levels of secretory acid phosphatase are very low, we expressed the L. donovani secretory acid phosphatase LdSAcP1 in wild type, null mutant and complemented strains by transformation of the plasmid pXG2.LdSAcP1 [47]. Mid-log phase culture supernatants were loaded on a native gel followed by in situ acid phosphatase activity assay. As expected, this enzymatic activity was not observed in non transformed cells while cell lines expressing LdSAcP1 exhibited detectable enzymatic activity (Fig. 2B). The secretory acid phosphatase synthesized in wild type and complemented line had a similar migration behavior, while the secretory acid phosphatase expressed in the null mutant migrated much slower in the native gel. This assay demonstrates that the null mutant expressed glycoconjugates with a longer phosphoglycan repeat domain (Fig. 2B). To corroborate this result, endogenous expression of the phosphoglycan containing glycoprotein proteophosphoglycan (PPG; [46, 48]) revealed that it migrated much slower than that of the wild type and complemented line (Fig. 2A). In addition, levels of PPG were increased in the Δlmdat/Δlmdat null mutant compared to that of the wild type and complemented line (Fig. 2A). Hyperglycosylation of LPG, LdSAcP1 and PPG in the null mutant may be due to hyperactive glycosyltransferases implicated in the addition of the sugar precursors for phosphoglycan synthesis or because of slow transit through the secretory pathway, as ether lipids have been shown to play a role in membrane fusion [49-52]. The latter explanation is less likely because only phosphoglycosylation seems to be affected in the null mutant while N-glycosylation appears normal as the apparent size of the N-glycosylated surface protease gp63 was unaltered in the absence of LmDAT (Fig. 2A and below; [53, 54]).
Immunofluorescence assays revealed that the null mutant kept the ability to secrete LPG to the plasma membrane and flagellum as the wild type and the complemented line (Fig. 2C), suggesting that LPG made in the null mutant still bears a lipid anchor. This is further supported by Triton X-114 fractionation of LPG. This assay demonstrated that LPG partitioned into the lipid phase in all three strains (data not shown).
McConville and colleagues reported that LPG is released in the media very rapidly, with a half time of 20 min, due to the presence of only one fatty alcohol in its lipid anchor [55]. We tested whether the null mutant Δlmdat/Δlmdat is defective in releasing LPG. Media supernatants were probed for the presence of LPG by Western blot analysis using the phosphoglycan specific antibody WIC79.3. Only the wild type and complemented strain released LPG in the medium, while no LPG was detected in the null mutant supernatant (Fig. 2A). This assay demonstrates that the null mutant lost its ability to release LPG, very likely due to an altered lipid anchor. This suggests that this altered glycolipid may be more hydrophobic due to the presence of a diacylphosphatidylinositol or possibly of an inositolphophoceramide anchor. Alternatively, the altered form of LPG might be more strongly associated to other cell surface molecules due to its hyperglycosylation. Attempts to purify sufficient amounts of LPG from the null mutant failed due to the difficulty of growing enough cells for this assay. The properties of the structurally altered forms of LPG produced by the Δlmdat/Δlmdat mutant are reminiscent of that made by the ether lipid mutant Δads/Δads lacking the second enzyme of the ether lipid biosynthetic route [32].
3.3. GPI-anchored protein levels are higher in the null mutant
GPI-anchored proteins, such as the metalloprotease gp63, are 1-alkyl-2-acyl-PI derivatives in L. major [12]. Therefore, we assessed whether GPI-anchored proteins are made in the null mutant by Western blot analysis using gp63-specific antibodies. Gp63 levels were increased in the null mutant compared to wild type and complemented lines. Triton X-114 partitioning revealed that gp63 fractionated in the lipid phase, demonstrating that the null mutant still carried a lipid moiety (data not shown). Consistent with the latter result, immunofluorescence assays showed that gp63 was present in intracellular membranes as well as secreted to the plasma membrane in wild type, null mutant and complemented strains (Fig. 2C). Attempts to purify gp63 from the null mutant Δlmdat/Δlmdat to determine its lipid anchor structure were unsuccessful. Because ether lipids have been shown to be important for membrane fusion [49-52], a slower transport through the secretory pathway may account for higher levels of gp63 proteins in the null mutant. Alternatively, higher levels of gp63 protein may be due to augmented protein synthesis, slower degradation, or a combination of both phenomena. The mechanism(s) underlying this observation was not sought.
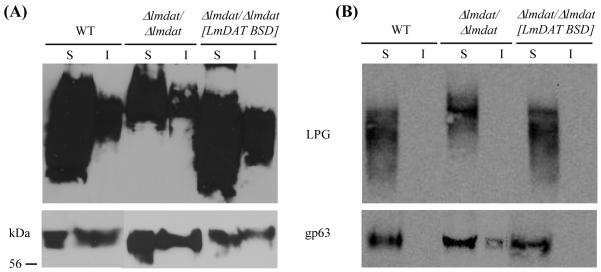
3.4. LmDAT is dispensable for DRM integrity
Ether lipids have been shown to be associated with DRM, and thus, may also promote their formation [41, 56-58]. Therefore, we investigated the role of LmDAT in DRM formation. Membrane DRM were isolated by cold Triton X-100 fractionation followed by differential centrifugation as described in Material and methods. The resulting supernatant and insoluble (pellet) fractions were subjected to Western blot analysis in the presence of anti-gp63 and anti-phosphoglycan (WIC79.3 to detect LPG) monoclonal antibodies. As previously reported, LPG enriched preferentially in the soluble fraction while gp63 can be found in equal amounts in the soluble and insoluble fractions in the wild type and complemented line when the detergent extraction was performed at 4°C (Fig. 3A, [41]). As a control, the experiment was performed at 37°C. As expected, both LPG and gp63 fractionated in the soluble fraction (Fig. 3B; [41]). The partitioning of both LPG and the GPI-anchored protein gp63 was not affected in the null mutant at both 4°C and 37°C (Fig. 3), indicating that LmDAT is dispensable for DRM’s formation, consistent with results obtained with the ether lipid null mutant Δads/Δads [32]. Notably, Leishmania mutants lacking sphingolipids formed normal DRM [41, 59]. Thus, this parasite seems to tolerate a great variability in lipid composition for the formation of lipid DRM.
Fig. 3.
DRM structures are conserved in the null mutant. Whole cells were extracted in the presence of Triton X-100 at 4°C (A) or 37°C (B), and soluble and insoluble fractions were separated by differential centrifugation as described in Material and methods. Western blot analyses were performed in the presence of LPG (WIC79.3) and gp63 specific immunoglobulins. Equivalent of 4×106 cells were loaded in each lane. I, insoluble fraction; S, soluble phase. WT, wild type; Δlmdat/Δlmdat, null mutant; Δlmdat/Δlmdat [LmDAT BSD], complemented strain. The protein ladder is shown on the left.
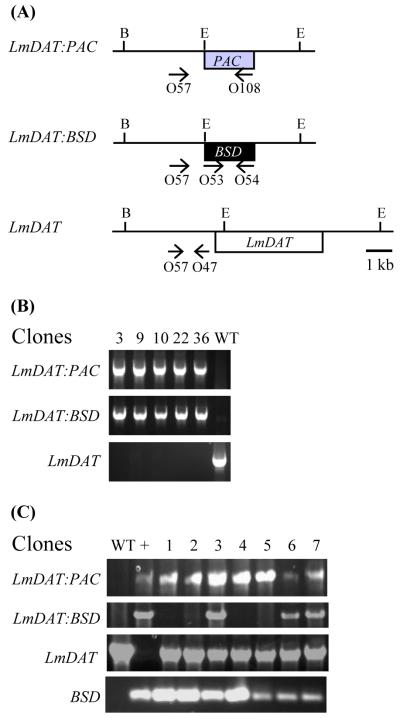
3.5. LmGAT and LmDAT of Leishmania major are colethal
Leishmania harbors a single G3P acyltransferase, LmGAT, and a single DHAPAT, LmDAT [30, 31]. To assess whether LmGAT and LmDAT are the sole initial acyltranferases in Leishmania, we attempted to create a double knock-out line based on the strategy described in Materials and methods. Double deletion mutants of LmGAT and LmDAT alleles were obtained only when LmDAT was provided on an episome (Fig. 4A and B). However, no double null mutants of LmGAT and LmDAT were recovered in the absence of LmDAT (Fig. 4A and C). From the seven candidates obtained, three aneuploids still carried the wild-type LmDAT gene in addition to the PAC and BSD disrupted LmDAT alleles. In contrast, the four remaining candidates bear the BSD cassette integrated in a non specific locus while one wild-type LmDAT allele was still present. These results suggest that LmGAT and LmDAT are colethal. Therefore, it is concluded that Leishmania does not possess alternative initial acyltransferases for the biosynthesis of its glycerolipids, or the potential alternative acyltransferases are not active enough to support growth of the parasite in the absence of both LmGAT and LmDAT genes. Thus, Leishmania represents a prototype eukaryote with one G3P acyltransferase and a single DHAPAT enzyme, and the double mutant can be used for genetic complementation studies with G3P acyltransferase or DHAPAT encoding genes.
Fig. 4.
LmGAT and LmDAT are colethal. PCR analysis of the genomic organization of transgenic Leishmania lines. (A) Schematic representation of the LmDAT locus with the approximate location of the oligonucleotides used in (B) and (C). Agarose gel electrophoresis of PCR products generated with Δlmgat/Δlmgat/Δlmdat/Δlmdat [LmDAT NEO] (B) and Δlmgat/Δlmgat/Δlmdat/Δlmdat (C) candidates as genomic DNA templates. The LmDAT:PAC locus was amplified with the oligonucleotides O57 (‘5-CTCTGTGCCTGCTGTCAC-3′; position -1960 of the start of the LmDAT gene) and O108 (‘5-GACGGGAAGCTTTCAGGCACCGGGCTTGCG-3′), the LmDAT:BSD allele with primers O57 (described above) and O54 (‘5-GCTCTAGATTAGCCCTCCCACACATAAC-3′), the LmDAT gene with oligonucleotides O57 (described above) and O47 (‘5-CGTGTAAATGAGGGATGGG-3′; position -455 of the start of the LmDAT gene), and the BSD cassette with primers O53 (‘5-GCTCTAGATGCCTTTGTCTCAAGAAGAATC-3′) and O54 (described above). +: strain Δlmgat/Δlmgat/Δlmdat/Δlmdat [LmDAT NEO].
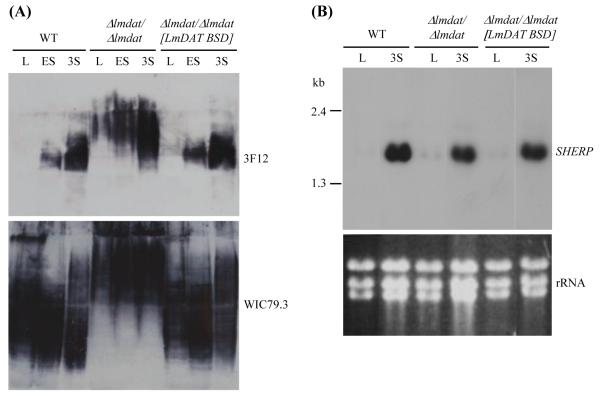
3.6. LmDAT is dispensable for the manifestation of properties specific of metacyclics
To understand the molecular basis of Δlmdat/Δlmdat attenuated virulence [30], we assessed whether LmDAT is important for differentiation into metacyclics. Three properties have been associated with metacyclic parasites: i) production of arabinosylated forms of LPG, ii) expression of the metacyclic gene SHERP, and iii) ability to fractionate in the top 10 fraction of a Ficoll gradient based on their slender morphology [42, 43, 60]. Western blot analysis in the presence of the monoclonal antibody 3F12 that recognizes arabinosylated polysaccharides [61] showed that the null mutant expressed arabinosylated forms of LPG as parasites enter the stationary phase (Fig. 5A). As a positive loading control, total LPG was revealed with the monoclonal WIC79.3 antibody [35]. The glycolipid was present in all strains and all phases of growth, demonstrating that a lack of signal with 3F12 antibody in dividing parasites is not due to its absence or low abundance.
Fig. 5.
LmDAT is dispensable for the expression of metacyclic markers. (A) Western blot analyses were carried out in the presence of phosphoglycan specific WIC79.3 (lower panel) and arabinosylated carbohydrate specific 3F12 (upper panel) monoclonal antibodies. Equivalent of 2×106 cells were loaded in each lane. L, mid-log; ES, early stationary phase; 3S, three day stationary phase. (B) Northern blot analysis of total RNA isolated from mid-log (L) and three day stationary phase (3S) cultures. The membrane was hybridized in the presence of a SHERP-specific probe as described in Materials and methods (upper panel). As a loading control, ethidium bromide stained ribosomal RNA is shown (lower panel; rRNA). The assay was performed twice and a representative experiment is shown. (A,B) WT, wild type; Δlmdat/Δlmdat, null mutant; Δlmdat/Δlmdat [LmDAT BSD], complemented strain.
The expression pattern of SHERP was then investigated by Northern blot analysis. RNAs from mid-log and three day stationary phase cultures of wild type, null mutant and complemented lines were isolated and hybridized with a SHERP-specific probe. As expected, SHERP could hardly be detected in dividing parasites of wild type, null mutant and complemented strains. In contrast, SHERP expression was robust in three day stationary cells of all three strains (Fig. 5B).
Because the null mutant expressed altered forms of LPG (see above), metacyclics were isolated by Ficoll gradient centrifugation, a method that allows parasite separation based on cell morphology rather than on LPG [42, 62, 63]. Metacyclics were quantified from three day stationary phase cultures of wild type, null mutant and complemented line. The percentage of metacyclics was similar for wild type and complemented strains while the null mutant formed approximately twice as less metacyclics compared to wild type (Table 1).
Table 1.
Percentage of metacyclics present in three day stationary phase cultures of wild type, null mutant (Δlmdat/Δlmdat) and complemented strain (Δlmdat/Δlmdat [LmDAT BSD]). Results were obtained from two independent experiments performed in duplicate and standard deviations are shown
| Strains | Metacyclics (%) |
|---|---|
| Wild type | 3.79 ± 0.41 |
| Δlmdat/Δlmdat | 2.00 ± 0.35 |
| Δlmdat/Δlmdat [LmDAT BSD] | 3.65 ± 0.21 |
Despite the fact that LmDAT was shown to be important for promastigote virulence [30], the Δlmdat/Δlmdat mutant expressed the metacyclic gene SHERP, produced arabinosylated forms of LPG in a growth stage specific fashion, and exhibited a normal slender morphology based on Ficoll gradient centrifugation. These data are similar to those obtained from the ether lipid mutant Δads/Δads [32] but in marked contrast to mutants defective in sphingolipids which were defective in metacyclogenesis and establishment of virulence [41, 59]. Thus, caution should be exercised as to link expression of metacyclic phenotypes to virulence.
In conclusion, we provided genetic evidences that Leishmania harbors only two initial acyltransferases, LmDAT and LmGAT, that are specific for DHAP and G3P, respectively. We demonstrated that the role of LmDAT in glycerolipid metabolism seems to be restricted to ether lipid biosynthesis in Leishmania despite the fact that it can also participate in ester lipid production under certain circumstances, such as in the absence of LmGAT [31]. We also showed that LmDAT is dispensable for the integrity of DRMs and expression of metacyclic properties.
Acknowledgments
We thank Adelisa Franchitti for her technical support. We are in debt to S. Turco, J. Bangs, and B. McGwire for the generous gift of WIC79.3 and gp63 specific ascites, BiP antiserum, and polyclonal gp63 antibody, respectively. This project was supported by an American Heart Association award to RZ (0630001N), and by the NIH Grant Number P20 RR016475 from the INBRE Program of the National Center for Research Resources. The lipid analyses described in this work were performed at the Kansas Lipidomics Research Center Analytical Laboratory. Instrument acquisition and method development at the Kansas Lipidomics Research Center was supported by National Science Foundation (EPS 0236913, MCB 0455318, DBI 0521587), Kansas Technology Enterprise Corporation, K-IDeA Networks of Biomedical Research Excellence (INBRE) of National Institute of Health (P20RR16475), and Kansas State University.
Abbreviations
- DHAP
dihydroxyacetonephosphate
- DHAPAT
dihydroxyacetonephosphate acyltransferase
- DRM
detergent resistant membrane
- ESI-MS/MS
electrospray ionization-tandem mass spectrometry
- G3P
glycerol-3-phosphate
- GPI
glycosylphosphatidylinositol
- LPA
lysophosphatidic acid
- LPG
lipophosphoglycan
- NL
neutral loss
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PCR
polymerase chain reaction
- PE
phosphatidylethanolamine
- PI
phosphatidylinositol
- PPG
proteophosphoglycan
- PS
phosphatidylserine
- SDS-PAGE
sodium dodecylsulfate polyacrylamide gel electrophoresis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wassef MK, Fioretti TB, Dwyer DM. Lipid analyses of isolated surface membranes of Leishmania donovani promastigotes. Lipids. 1985;20:108–115. doi: 10.1007/BF02534216. [DOI] [PubMed] [Google Scholar]
- [2].Beach DH, Holz GG, Jr., Anekwe GE. Lipids of Leishmania promastigotes. J Parasitol. 1979;65:201–216. [PubMed] [Google Scholar]
- [3].Chaudhuri G, Banerjee AB. Lipids & fatty acids of Leishmania donovani donovani promastigotes. Indian J Med Res. 1987;85:140–148. [PubMed] [Google Scholar]
- [4].Descoteaux A, Avila HA, Zhang K, Turco SJ, Beverley SM. Leishmania LPG3 encodes a GRP94 homolog required for phosphoglycan synthesis implicated in parasite virulence but not viability. Embo J. 2002;21:4458–4469. doi: 10.1093/emboj/cdf447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ilg T. Lipophosphoglycan of the protozoan parasite Leishmania: stage- and species-specific importance for colonization of the sandfly vector, transmission and virulence to mammals. Med Microbiol Immunol (Berl) 2001;190:13–17. doi: 10.1007/s004300100071. [DOI] [PubMed] [Google Scholar]
- [6].Matlashewski G. Leishmania infection and virulence. Med Microbiol Immunol (Berl) 2001;190:37–42. doi: 10.1007/s004300100076. [DOI] [PubMed] [Google Scholar]
- [7].Naderer T, Vince JE, McConville MJ. Surface determinants of Leishmania parasites and their role in infectivity in the mammalian host. Curr Mol Med. 2004;4:649–665. doi: 10.2174/1566524043360069. [DOI] [PubMed] [Google Scholar]
- [8].Sacks DL. Leishmania-sand fly interactions controlling species-specific vector competence. Cell Microbiol. 2001;3:189–196. doi: 10.1046/j.1462-5822.2001.00115.x. [DOI] [PubMed] [Google Scholar]
- [9].Descoteaux A, Turco SJ. Functional aspects of the Leishmania donovani lipophosphoglycan during macrophage infection. Microbes Infect. 2002;4:975–981. doi: 10.1016/s1286-4579(02)01624-6. [DOI] [PubMed] [Google Scholar]
- [10].Ilgoutz SC, McConville MJ. Function and assembly of the Leishmania surface coat. Int J Parasitol. 2001;31:899–908. doi: 10.1016/s0020-7519(01)00197-7. [DOI] [PubMed] [Google Scholar]
- [11].Ferguson MA. The surface glycoconjugates of trypanosomatid parasites. Philos Trans R Soc Lond B Biol Sci. 1997;352:1295–1302. doi: 10.1098/rstb.1997.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schneider P, Ferguson MA, McConville MJ, Mehlert A, Homans SW, Bordier C. Structure of the glycosyl-phosphatidylinositol membrane anchor of the Leishmania major promastigote surface protease. J Biol Chem. 1990;265:16955–16964. [PubMed] [Google Scholar]
- [13].Croft SL, Seifert K, Duchene M. Antiprotozoal activities of phospholipid analogues. Mol Biochem Parasitol. 2003;126:165–172. doi: 10.1016/s0166-6851(02)00283-9. [DOI] [PubMed] [Google Scholar]
- [14].Soto J, Arana BA, Toledo J, Rizzo N, Vega JC, Diaz A, Luz M, Gutierrez P, Arboleda M, Berman JD, Junge K, Engel J, Sindermann H. Miltefosine for new world cutaneous leishmaniasis. Clin Infect Dis. 2004;38:1266–1272. doi: 10.1086/383321. [DOI] [PubMed] [Google Scholar]
- [15].Jha TK, Sundar S, Thakur CP, Bachmann P, Karbwang J, Fischer C, Voss A, Berman J. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N Engl J Med. 1999;341:1795–1800. doi: 10.1056/NEJM199912093412403. [DOI] [PubMed] [Google Scholar]
- [16].Sundar S, Rosenkaimer F, Makharia MK, Goyal AK, Mandal AK, Voss A, Hilgard P, Murray HW. Trial of oral miltefosine for visceral leishmaniasis. Lancet. 1998;352:1821–1823. doi: 10.1016/S0140-6736(98)04367-0. [DOI] [PubMed] [Google Scholar]
- [17].Hajra AK, Bishop JE. Glycerolipid biosynthesis in peroxisomes via the acyl dihydroxyacetone phosphate pathway. Ann N Y Acad Sci. 1982;386:170–182. doi: 10.1111/j.1749-6632.1982.tb21415.x. [DOI] [PubMed] [Google Scholar]
- [18].de Vet EC, Hilkes YH, Fraaije MW, van den Bosch H. Alkyl-dihydroxyacetonephosphate synthase. Presence and role of flavin adenine dinucleotide. J Biol Chem. 2000;275:6276–6283. doi: 10.1074/jbc.275.9.6276. [DOI] [PubMed] [Google Scholar]
- [19].Hajra AK. Glycerolipid biosynthesis in peroxisomes (microbodies) Prog Lipid Res. 1995;34:343–364. doi: 10.1016/0163-7827(95)00013-5. [DOI] [PubMed] [Google Scholar]
- [20].Liu D, Nagan N, Just WW, Rodemer C, Thai TP, Zoeller RA. Role of dihydroxyacetonephosphate acyltransferase in the biosynthesis of plasmalogens and nonether glycerolipids. J Lipid Res. 2005;46:727–735. doi: 10.1194/jlr.M400364-JLR200. [DOI] [PubMed] [Google Scholar]
- [21].Jones CL, Hajra AK. Solubilization and partial purification of dihydroxyacetone-phosphate acyltransferase from guinea pig liver. Arch Biochem Biophys. 1983;226:155–165. doi: 10.1016/0003-9861(83)90280-1. [DOI] [PubMed] [Google Scholar]
- [22].Ofman R, Wanders RJ. Purification of peroxisomal acyl-CoA: dihydroxyacetonephosphate acyltransferase from human placenta. Biochim Biophys Acta. 1994;1206:27–34. doi: 10.1016/0167-4838(94)90068-x. [DOI] [PubMed] [Google Scholar]
- [23].Webber KO, Hajra AK. Purification of dihydroxyacetone phosphate acyltransferase from guinea pig liver peroxisomes. Arch Biochem Biophys. 1993;300:88–97. doi: 10.1006/abbi.1993.1013. [DOI] [PubMed] [Google Scholar]
- [24].Heise N, Opperdoes FR. The dihydroxyacetonephosphate pathway for biosynthesis of ether lipids in Leishmania mexicana promastigotes. Mol Biochem Parasitol. 1997;89:61–72. doi: 10.1016/s0166-6851(97)00101-1. [DOI] [PubMed] [Google Scholar]
- [25].Biermann J, Just WW, Wanders RJ, Van Den Bosch H. Alkyl-dihydroxyacetone phosphate synthase and dihydroxyacetone phosphate acyltransferase form a protein complex in peroxisomes. Eur J Biochem. 1999;261:492–499. doi: 10.1046/j.1432-1327.1999.00295.x. [DOI] [PubMed] [Google Scholar]
- [26].Thai TP, Heid H, Rackwitz HR, Hunziker A, Gorgas K, Just WW. Ether lipid biosynthesis: isolation and molecular characterization of human dihydroxyacetonephosphate acyltransferase. FEBS Lett. 1997;420:205–211. doi: 10.1016/s0014-5793(97)01495-6. [DOI] [PubMed] [Google Scholar]
- [27].Ofman R, Hettema EH, Hogenhout EM, Caruso U, Muijsers AO, Wanders RJ. Acyl-CoA:dihydroxyacetonephosphate acyltransferase: cloning of the human cDNA and resolution of the molecular basis in rhizomelic chondrodysplasia punctata type 2. Hum Mol Genet. 1998;7:847–853. doi: 10.1093/hmg/7.5.847. [DOI] [PubMed] [Google Scholar]
- [28].Barth PG, Wanders RJ, Schutgens RB, Staalman CR. Variant rhizomelic chondrodysplasia punctata (RCDP) with normal plasma phytanic acid: clinico-biochemical delineation of a subtype and complementation studies. Am J Med Genet. 1996;62:164–168. doi: 10.1002/(SICI)1096-8628(19960315)62:2<164::AID-AJMG9>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- [29].Moser HW. Peroxisomal diseases. Adv Hum Genet. 1993;21:1–106. 443–151. [PubMed] [Google Scholar]
- [30].Zufferey R, Ben Mamoun C. Leishmania major expresses a single dihydroxyacetone phosphate acyltransferase localized in the glycosome, important for rapid growth and survival at high cell density and essential for virulence. J Biol Chem. 2006;281:7952–7959. doi: 10.1074/jbc.M512911200. [DOI] [PubMed] [Google Scholar]
- [31].Zufferey R, Mamoun CB. The initial step of glycerolipid metabolism in Leishmania major promastigotes involves a single glycerol-3-phosphate acyltransferase enzyme important for the synthesis of triacylglycerol but not essential for virulence. Mol Microbiol. 2005;56:800–810. doi: 10.1111/j.1365-2958.2005.04579.x. [DOI] [PubMed] [Google Scholar]
- [32].Zufferey R, Allen S, Barron T, Sullivan DR, Denny PW, Almeida IC, Smith DF, Turco SJ, Ferguson MA, Beverley SM. Ether phospholipids and glycosylinositolphospholipids are not required for amastigote virulence or for inhibition of macrophage activation by Leishmania major. J Biol Chem. 2003;278:44708–44718. doi: 10.1074/jbc.M308063200. [DOI] [PubMed] [Google Scholar]
- [33].Ha DS, Schwarz JK, Turco SJ, Beverley SM. Use of the green fluorescent protein as a marker in transfected Leishmania. Mol Biochem Parasitol. 1996;77:57–64. doi: 10.1016/0166-6851(96)02580-7. [DOI] [PubMed] [Google Scholar]
- [34].Connell ND, Medina-Acosta E, McMaster WR, Bloom BR, Russell DG. Effective immunization against cutaneous leishmaniasis with recombinant bacille Calmette-Guerin expressing the Leishmania surface proteinase gp63. Proc Natl Acad Sci U S A. 1993;90:11473–11477. doi: 10.1073/pnas.90.24.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].de Ibarra AA, Howard JG, Snary D. Monoclonal antibodies to Leishmania tropica major: specificities and antigen location. Parasitology. 1982;85(Pt 3):523–531. doi: 10.1017/s0031182000056304. [DOI] [PubMed] [Google Scholar]
- [36].Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- [37].Bartz R, Li WH, Venables B, Zehmer JK, Roth MR, Welti R, Anderson RG, Liu P, Chapman KD. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J Lipid Res. 2007;48:837–847. doi: 10.1194/jlr.M600413-JLR200. [DOI] [PubMed] [Google Scholar]
- [38].Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou HE, Rajashekar CB, Williams TD, Wang X. Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. J Biol Chem. 2002;277:31994–32002. doi: 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- [39].Brugger B, Erben G, Sandhoff R, Wieland FT, Lehmann WD. Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proc Natl Acad Sci U S A. 1997;94:2339–2344. doi: 10.1073/pnas.94.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Liebisch G, Lieser B, Rathenberg J, Drobnik W, Schmitz G. High-throughput quantification of phosphatidylcholine and sphingomyelin by electrospray ionization tandem mass spectrometry coupled with isotope correction algorithm. Biochim Biophys Acta. 2004;1686:108–117. doi: 10.1016/j.bbalip.2004.09.003. [DOI] [PubMed] [Google Scholar]
- [41].Zhang K, Showalter M, Revollo J, Hsu FF, Turk J, Beverley SM. Sphingolipids are essential for differentiation but not growth in Leishmania. Embo J. 2003;22:6016–6026. doi: 10.1093/emboj/cdg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- [43].Knuepfer E, Stierhof YD, McKean PG, Smith DF. Characterization of a differentially expressed protein that shows an unusual localization to intracellular membranes in Leishmania major. Biochem J. 2001;356:335–344. doi: 10.1042/0264-6021:3560335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].McConville MJ, Bacic A, Mitchell GF, Handman E. Lipophosphoglycan of Leishmania major that vaccinates against cutaneous leishmaniasis contains an alkylglycerophosphoinositol lipid anchor. Proc Natl Acad Sci U S A. 1987;84:8941–8945. doi: 10.1073/pnas.84.24.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].McConville MJ, Turco SJ, Ferguson MA, Sacks DL. Developmental modification of lipophosphoglycan during the differentiation of Leishmania major promastigotes to an infectious stage. Embo J. 1992;11:3593–3600. doi: 10.1002/j.1460-2075.1992.tb05443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ilg T, Stierhof YD, Wiese M, McConville MJ, Overath P. Characterization of phosphoglycan-containing secretory products of Leishmania. Parasitology. 1994;108(Suppl):S63–71. doi: 10.1017/s0031182000075739. [DOI] [PubMed] [Google Scholar]
- [47].Shakarian AM, Dwyer DM. Structurally conserved soluble acid phosphatases are synthesized and released by Leishmania major promastigotes. Exp Parasitol. 2000;95:79–84. doi: 10.1006/expr.2000.4511. [DOI] [PubMed] [Google Scholar]
- [48].Ilg T, Stierhof YD, Craik D, Simpson R, Handman E, Bacic A. Purification and structural characterization of a filamentous, mucin-like proteophosphoglycan secreted by Leishmania parasites. J Biol Chem. 1996;271:21583–21596. doi: 10.1074/jbc.271.35.21583. [DOI] [PubMed] [Google Scholar]
- [49].Glaser PE, Gross RW. Rapid plasmenylethanolamine-selective fusion of membrane bilayers catalyzed by an isoform of glyceraldehyde-3-phosphate dehydrogenase: discrimination between glycolytic and fusogenic roles of individual isoforms. Biochemistry. 1995;34:12193–12203. doi: 10.1021/bi00038a013. [DOI] [PubMed] [Google Scholar]
- [50].Thai TP, Rodemer C, Jauch A, Hunziker A, Moser A, Gorgas K, Just WW. Impaired membrane traffic in defective ether lipid biosynthesis. Hum Mol Genet. 2001;10:127–136. doi: 10.1093/hmg/10.2.127. [DOI] [PubMed] [Google Scholar]
- [51].Munn NJ, Arnio E, Liu D, Zoeller RA, Liscum L. Deficiency in ethanolamine plasmalogen leads to altered cholesterol transport. J Lipid Res. 2003;44:182–192. doi: 10.1194/jlr.m200363-jlr200. [DOI] [PubMed] [Google Scholar]
- [52].Gorgas K, Teigler A, Komljenovic D, Just WW. The ether lipid-deficient mouse: tracking down plasmalogen functions. Biochim Biophys Acta. 2006;1763:1511–1526. doi: 10.1016/j.bbamcr.2006.08.038. [DOI] [PubMed] [Google Scholar]
- [53].Funk VA, Jardim A, Olafson RW. An investigation into the significance of the N-linked oligosaccharides of Leishmania gp63. Mol Biochem Parasitol. 1994;63:23–35. doi: 10.1016/0166-6851(94)90005-1. [DOI] [PubMed] [Google Scholar]
- [54].Funk VA, Thomas-Oates JE, Kielland SL, Bates PA, Olafson RW. A unique, terminally glucosylated oligosaccharide is a common feature on Leishmania cell surfaces. Mol Biochem Parasitol. 1997;84:33–48. doi: 10.1016/s0166-6851(96)02780-6. [DOI] [PubMed] [Google Scholar]
- [55].Proudfoot L, Schneider P, Ferguson MA, McConville MJ. Biosynthesis of the glycolipid anchor of lipophosphoglycan and the structurally related glycoinositolphospholipids from Leishmania major. Biochem J. 1995;308(Pt 1):45–55. doi: 10.1042/bj3080045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mattjus P, Slotte JP. Does cholesterol discriminate between sphingomyelin and phosphatidylcholine in mixed monolayers containing both phospholipids? Chem Phys Lipids. 1996;81:69–80. doi: 10.1016/0009-3084(96)02535-2. [DOI] [PubMed] [Google Scholar]
- [57].Ohvo-Rekila H, Ramstedt B, Leppimaki P, Slotte JP. Cholesterol interactions with phospholipids in membranes. Prog Lipid Res. 2002;41:66–97. doi: 10.1016/s0163-7827(01)00020-0. [DOI] [PubMed] [Google Scholar]
- [58].Pike LJ, Han X, Chung KN, Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41:2075–2088. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- [59].Denny PW, Goulding D, Ferguson MA, Smith DF. Sphingolipid-free Leishmania are defective in membrane trafficking, differentiation and infectivity. Mol Microbiol. 2004;52:313–327. doi: 10.1111/j.1365-2958.2003.03975.x. [DOI] [PubMed] [Google Scholar]
- [60].Sacks DL, Brodin TN, Turco SJ. Developmental modification of the lipophosphoglycan from Leishmania major promastigotes during metacyclogenesis. Mol Biochem Parasitol. 1990;42:225–233. doi: 10.1016/0166-6851(90)90165-i. [DOI] [PubMed] [Google Scholar]
- [61].Kelleher M, Curtis JM, Sacks DL, Handman E, Bacic A. Epitope mapping of monoclonal antibodies directed against lipophosphoglycan of Leishmania major promastigotes. Mol Biochem Parasitol. 1994;66:187–200. doi: 10.1016/0166-6851(94)90146-5. [DOI] [PubMed] [Google Scholar]
- [62].Sacks DL, Perkins PV. Identification of an infective stage of Leishmania promastigotes. Science. 1984;223:1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- [63].Zakai HA, Chance ML, Bates PA. In vitro stimulation of metacyclogenesis in Leishmania braziliensis, L. donovani, L. major and L. mexicana. Parasitology. 1998;116(Pt 4):305–309. doi: 10.1017/s0031182097002382. [DOI] [PubMed] [Google Scholar]
- [64].Sacks DL, Saraiva EM, Rowton E, Turco SJ, Pimenta PF. The role of the lipophosphoglycan of Leishmania in vector competence. Parasitology. 1994;108(Suppl):S55–62. doi: 10.1017/s0031182000075727. [DOI] [PubMed] [Google Scholar]