Abstract
Background:
The ICH Score is a commonly used clinical grading scale for outcome after acute intracerebral hemorrhage (ICH) and has been validated for 30-day mortality, but not long-term functional outcome. The goals of this study were to assess whether the ICH Score accurately stratifies patients with regard to 12-month functional outcome and to further delineate the pace of recovery of patients during the first year post-ICH.
Methods:
We performed a prospective observational cohort study of all patients with acute ICH admitted to the emergency departments of San Francisco General Hospital and UCSF Medical Center from June 1, 2001, through May 31, 2004. Components of the ICH Score (admission Glasgow Coma Scale score, initial hematoma volume, presence of intraventricular hemorrhage, infratentorial ICH origin, and age) were recorded along with other clinical characteristics. Patients were then assessed with the modified Rankin Scale (mRS) at hospital discharge, 30 days, and 3, 6, and 12 months post-ICH.
Results:
Of 243 patients, 95 (39%) died during initial acute hospitalization. The ICH Score accurately stratified patients with regard to 12-month functional outcome for various dichotomous cutpoints along the mRS (p < 0.05). Many patients continued to improve across the first year, with a small number of patients becoming disabled or dying due to late events unrelated to the initial ICH.
Conclusions:
The ICH Score is a valid clinical grading scale for long-term functional outcome after acute intracerebral hemorrhage (ICH). Many ICH patients improve after hospital discharge and this improvement may continue even after 6 months post-ICH.
GLOSSARY
- CHR
= Committee on Human Research;
- ED
= emergency department;
- GCS
= Glasgow Coma Scale;
- ICH
= intracerebral hemorrhage;
- IVH
= intraventricular hemorrhage;
- mRS
= modified Rankin Scale;
- SFGH
= San Francisco General Hospital.
The ICH Score is the most commonly used clinical grading scale for outcome after intracerebral hemorrhage (ICH).1 Comprised of factors related to age, initial level of consciousness, and neuroimaging findings, the ICH Score was initially developed to provide a simple, reliable way of stratifying outcome early after acute ICH for the purpose of improving communication for clinical care and clinical research. Outcome for development of the ICH Score was mortality at 30 days. The ICH Score has subsequently been externally validated in numerous other cohorts2–6 and has been used as a stratification tool in a clinical trial of neuroprotection in order to improve balance of baseline characteristics.7 However, the reliability of the ICH Score in stratifying patients regarding long-term functional outcome has not been systematically assessed.
Despite several recent large clinical trials of medical or surgical interventions,8,9 ICH remains without a treatment of proven benefit. Even so, there remains great interest in understanding and using outcome predictors for ICH. Numerous observational studies have been performed to develop prediction models and scales for ICH outcome,2,6,10–17 although few besides the ICH Score have been externally validated. Just as the use of clinical grading scales has improved communication and consistency in other neurocritical care disorders such as acute ischemic stroke (NIH Stroke Scale), traumatic brain injury (Glasgow Coma Scale), and subarachnoid hemorrhage (Hunt/Hess and World Federation of Neurological Surgeons Scales), the availability of a validated reliable grading scale which is predictive of both early mortality and long-term functional outcome would likely improve consistency in ICH.
The overall purpose of this study was to determine whether the ICH Score reliably stratifies patients with acute ICH with regard to 12-month functional outcome as assessed by the modified Rankin Scale (mRS). Given recent concerns that early care limitations such as early do-not-resuscitate orders or withdrawal of medical support may create self-fulfilling prophecies of poor outcome,18–20 an additional aim was to determine whether this influenced the reliability of the ICH Score on risk stratification. A final goal was to provide insight into the pace of recovery of ICH survivors, in order to assess the optimal time point during the first year for the purposes of outcome assessment. In order to accomplish these aims, we conducted a prospective study of acute ICH patients who were then followed for outcome for 12 months.
METHODS
We performed a prospective longitudinal observational study involving all patients with acute nontraumatic intracerebral hemorrhage who presented to the emergency departments (ED) of San Francisco General Hospital (SFGH) or UCSF Medical Center from June 1, 2001, through May 31, 2004. Patients who were transferred from another hospital were excluded in order to ensure availability of records related to initial evaluation and to exclude potential differential bias if transferred patients had different characteristics than those presenting directly to the ED for initial evaluation. Patients were identified during initial hospitalization and aspects related to initial evaluation and in-hospital care were recorded on case report forms. This included the elements which comprise the ICH Score: age, Glasgow Coma Scale (GCS) score at the time of hospital admission from the ED, hematoma volume on the initial CT scan (as measured manually using the ABC/2 method21 by a single examiner [J.C.H.]), presence of intraventricular hemorrhage (IVH) on the initial CT scan, and hematoma origin (either infratentorial or supratentorial). Patients were then followed for up to 12 months and clinical status (alive/dead and scores on the mRS22 [a commonly used stroke ordinal outcome scale with scores ranging from 0 (no symptoms at all) to 6 (dead)]) was assessed at hospital discharge, 30 days, and 3, 6, and 12 months post-ICH either in person or via telephone by a physician or research coordinator trained in the administration of the mRS.23 If contact could not be made then medical records were reviewed (n = 22 for 12-month evaluation; 9% of cohort). For patients who were lost to follow-up prior to the 12-month time point, a Social Security Death Index search was performed 2 years after the study close. If death was not identified, then the clinical status from the last known evaluation point was carried forward (n = 10 for lost to follow-up for 12-month evaluation).
All aspects of patient care were left to the treating physicians. Clinical care was provided in accordance with the American Heart Association Guidelines for the Management of Spontaneous Intracerebral Hemorrhage from 1999. As this was an observational study only, no information gathered or analyzed solely for purposes of this study were provided to treating physicians during the course of patient care. Cause of ICH and cause of death (if applicable) were taken from that attributed by the attending treating physician. No patient received recombinant Factor VIIa or was enrolled in an ICH treatment trial; 7 patients were also enrolled in a concurrent observational study of brain tissue oxygen monitoring. Only the initial ICH occurring during the study period was considered for each patient. Of 248 cases of ICH, 2 patients refused all participation and 3 patients had an additional ICH leading to an ED admission at SFGH or UCSF during the 12-month follow-up period after initial ICH. Thus, the cohort for study consisted of 243 patients.
The ICH Score was determined as previously described by creating a sumscore of points assigned for individual components: GCS (3–4 = 2, 5–12 = 1, 13–15 = 0), hematoma volume (≥30 mL = 1, <30 mL = 0), presence of IVH (yes = 1, no = 0), infratentorial origin (yes = 1, no = 0), and patient age ≥80 (yes = 1, no = 0). Patient ICH Scores ranged from 0 to 5; no patient in this cohort achieved an ICH Score of 6. Cuzick’s24 nonparametric test for trend was used to assess association of increasing points on the ICH Score with increased risk of mortality or decreased likelihood of favorable functional outcome as determined by the mRS, which was dichotomized at various cutpoints in order to determine whether the ICH Score reliably stratified patients across the spectrum of mRS outcomes. Receiver operating characteristic curves were constructed for mRS dichotomous cutpoints as well as for mortality and associated c-statistics were determined. Statistical analysis was performed using Stata (Version 9.0, College Station, TX), and significance was considered as p < 0.05.
Standard protocol approvals, registrations, and patient consents.
All aspects of this study were approved by the Committee on Human Research (CHR) of the UCSF prior to initiating any human subjects research. The CHR is the institutional review board for UCSF Medical Center and SFGH. Per conditions of the UCSF CHR approval, informed consent was obtained from all subjects or their authorized surrogates for follow-up after hospital discharge.
RESULTS
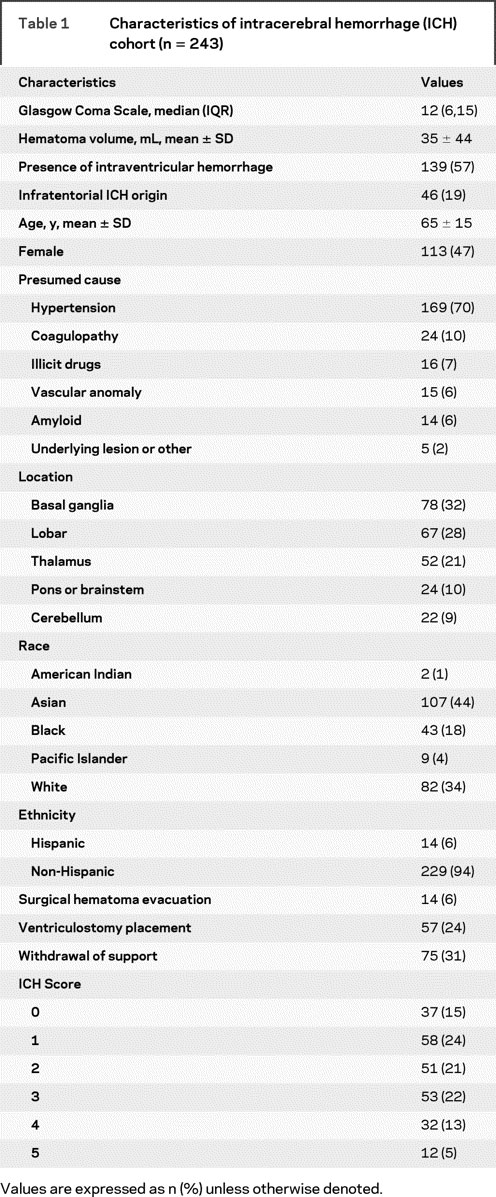
Table 1 summarizes the baseline and initial treatment characteristics for the entire cohort of 243 patients. The majority of ICH cases were presumed due to chronic hypertension, with coagulopathy being the second most common cause. Surgical hematoma evacuation was performed in only 14 cases: 5 cerebellar (23%), 8 lobar (12%), and 1 basal ganglia (1%). ICP monitoring via a ventricular catheter was performed in 24% of patients. Thirty-nine percent of patients died during the initial hospitalization; 78% of patients who died during initial hospitalization had withdrawal of support. Components of the ICH Score were widely distributed, with 20% of patients aged 80 or older, 38% of patients having an initial hematoma volume greater than or equal to 30 mL, 19% of patients with infratentorial origin, and IVH present in 57% of cases. GCS was 3–5 in 22%, 5–12 in 30%, and 13–15 in 47% of patients.
Table 1 Characteristics of intracerebral hemorrhage (ICH) cohort (n = 243)
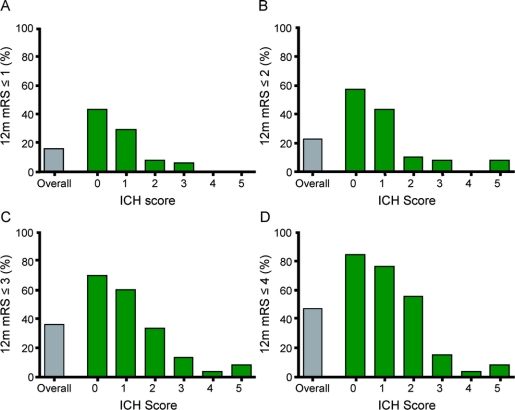
As demonstrated in figure 1, the ICH Score accurately stratified patients with regard to 12-month functional outcome on the mRS. While the overall likelihood of a favorable outcome decreased as lower numbered mRS cutpoints were used to define favorable outcome, the ICH Score accurately stratified patients at each of these cutpoints (p < 0.05, test for trend) as well as across the entire mRS (p < 0.05, test for trend). This means that, in general, an increasing ICH Score was associated with a lower likelihood of favorable outcome regardless of the specific cutpoint used to define outcome. c-Statistics for the various mRS cutpoints were 0.80 for mRS ≤1, 0.81 for mRS ≤2, 0.80 for mRS ≤3, and 0.84 for mRS ≤4. Sensitivity analyses excluding those patients who were unavailable for 12-month follow-up, had medical records reviewed for 12-month timepoint assessment, underwent surgical hematoma evacuation, or had intracranial pressure monitoring did not affect the conclusions of the study. Of note, one patient with an ICH Score of 5 achieved a 12-month mRS of 2. This 49-year-old patient presented to the ED with a GCS of 12 and rapidly deteriorated to a GCS of 3; he was then taken for urgent head CT which demonstrated a 30 mL cerebellar hematoma that was immediately surgically evacuated.
Figure 1 Favorable 12-month outcome based on various dichotomized cutpoints
Distribution of modified Rankin Scale (mRS) scores at 12 months after intracerebral hemorrhage (ICH) with favorable outcome defined using various dichotomized cutpoints. The Y-axis indicates the percentage of patients who achieved the indicated outcome. (A) considers a favorable outcome as a 12-month mRS of ≤1 (able to carry out all usual duties or activities); (B) as an mRS of ≤2 (slight disability); (C) as an mRS of ≤3 (moderate disability but ambulatory); (D) as an mRS of ≤4 (moderate to severe disability; nonambulatory). For each outcome cutpoint, rising ICH Scores are associated with lower likelihood of favorable outcome (p < 0.05, test for trend). Note that one patient (out of 12) with an ICH Score of 5 achieved a 12-month mRS of 2 after evacuation of a cerebellar hematoma.
This same significant trend for ICH Score outcome stratification was present for these same mRS cutpoints even when functional outcome was measured at 30 days, 3 months, or 6 months post-ICH (data not shown). In order to determine if withdrawal of support affected the ability of the ICH Score to accurately stratify patients with regard to functional outcome, sensitivity analysis was done excluding patients who died during initial hospitalization. For each of the 4 mRS cutpoints in figure 1, the ICH Score accurately stratified surviving patients with regard to functional outcome at all measured timepoints, indicating that the ability of the ICH Score to accurately stratify long-term outcome is not affected by withdrawal of support (p < 0.05, test for trend). The ICH Score remained a significant predictor of mortality risk at 30 days (c = 0.86), as well as 3 (c = 0.88), 6 (c = 0.87), and 12 months (c = 0.87) post-ICH (p < 0.001 for all timepoints, test for trend).
Table 2 describes the proportion of patients with various outcomes, as measured by the mRS, at various timepoints during the first year post-ICH, irrespective of ICH Score. The vast majority of patients who died did so early after ICH. Of the 16 patients who died between hospital discharge and 3 months, only 2 patients were known to die of causes not directly following from the initial ICH (1 cancer, 1 renal failure). Of the 6 patients who died after 3 months, none was known to clearly follow from the initial ICH (1 each with brain tumor, multiorgan dysfunction from sepsis, collagen vascular disease, and cardiovascular disease, 2 with cause not known).
Table 2 Modified Rankin Scale (mRS) score at various timepoints (n = 243)
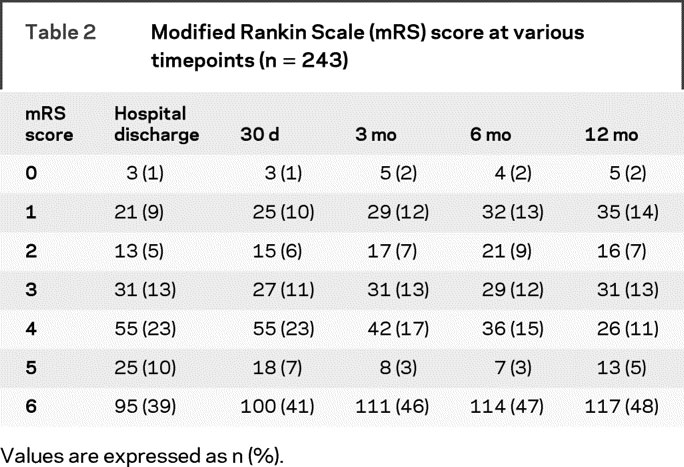
Overall, 51 of the 148 (34%) patients who survived to hospital discharge improved in mRS score between hospital discharge and 12 months. Eleven of these patients (13% of hospital survivors) improved at least 2 points on the mRS. Thirty-two patients (22% of hospital survivors) worsened on the mRS between hospital discharge and 12 months, with 15 of these patients (10% of hospital survivors) worsening by 2 or more points. Of these 15 patients, 13 died after hospital discharge as described above, 1 patient deteriorated from a hospital discharge mRS of 3 to a 12-month mRS of 5, and 1 patient developed a new ICH between 3 and 6 months after initial ICH. Forty-four percent (n = 65) of hospital survivors did not change in mRS between hospital discharge and 12 months.
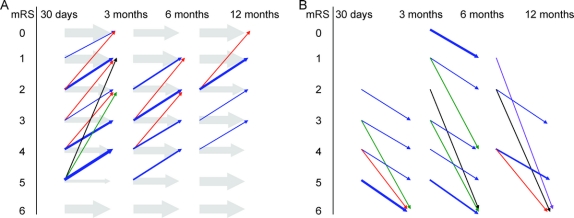
Figure 2 demonstrates the change in mRS for patients across different timepoints during the first year after ICH indicating that change in clinical status was common. For example, from table 2, 31 patients (13%) had an mRS of 3 at hospital discharge and 31 patients (13%) had an mRS of 3 at 12 months. However, only 12 of these were the same patients, information not readily apparent from summary statistics such as in table 2. In figure 2, arrow thickness is used to illustrate the proportion of patients with a specific mRS score who remain the same or change until the next assessment point, whereas arrow color is used to illustrate the absolute change in mRS scale points. As seen in panel A, most patients remain at the same mRS value from one timepoint to the next (gray shaded arrow). However, many patients demonstrate improvement and this improvement may occur throughout the first year post-ICH. This is especially notable during the first 3 months in severely impaired patients (mRS 4 or 5). Improvement in moderately affected patients (mRS ≤3) continued through 12 months, whereas improvement in more severely affected patients (mRS 4 or 5) was rare after 6 months. Panel B demonstrates that some patients do worsen throughout the year, but most of this is modest (1 point on the mRS) except for those patients who die from their initial ICH (usually within 3 months) or who develop a new unrelated medical problem resulting in death or disability.
Figure 2 Change in modified Rankin Scale (mRS) score during first year after intracerebral hemorrhage (ICH)
(A) Patients who remained the same or improved their mRS score across various timepoints assessed during the first year post-ICH. While most patients remained the same, substantial numbers of patients improved throughout the year. (B) Patients who worsened their mRS during the first year post-ICH. Most of those who worsened functionally did so by one step on the mRS. However, several patients developed new unrelated events after 3 months which led to death. Arrow thickness is used to represent the proportion of patients with a specific mRS score who change or stay the same to the next timepoint. Arrow color is used to depict change in mRS score from one timepoint to next (no change = gray, 1-point change = blue, 2 = red, 3 = green, 4 = black, 5 = purple).
DISCUSSION
This study demonstrates that the ICH Score is a valid clinical grading scale for stratifying likelihood of favorable functional outcome throughout the first year after acute ICH, out to 12 months. This is in addition to prior studies which have demonstrated the ICH Score as a validated predictor of risk of 30-day mortality. Importantly, in this study the ICH Score was valid and significant regardless of the specific cutpoint chosen to define favorable functional outcome. This is important since different clinical research studies or clinical care contexts may define favorable outcome differently, as has been the case for several different recent ICH treatment clinical trials.7–9 Additionally, this study demonstrates that a substantial proportion of patients with acute ICH improve after hospital discharge and that this improvement may occur throughout the first year post-ICH.
The purpose of this study was not to develop a new ICH outcome prediction model or to test whether various prediction models were more accurate with regard to predicting individual patient outcome. Rather, the point of this study was to assess the validity of an existing, simple, and previously validated scale, the ICH Score. We believe that the specific ability of a scale such as the ICH Score to predict individual patient outcome is less important than the overall trend of increasing scale scores in stratifying increased overall probability of unfavorable outcome. Because outcome prediction models are developed from populations of patients, they can provide overall probabilities of the likelihood of a specific outcome, but these point estimates have inherent uncertainty and confidence intervals. Concern has been raised for the possibility of self-fulfilling prophecies of poor outcome if early prognostication of a negative outcome in an individual patient leads to care limitations.18–20
This study has also helped to elucidate the course of improvement (or lack thereof) after acute ICH. This is important clinically because it helps patients, families, and providers plan for ongoing care needs. It is also important in the context of clinical treatment trials for ICH, since the optimal definition of favorable outcome and the optimal time for this assessment is paramount. This study suggests that a large proportion of survivors improve during the first year after ICH and that the pace of this improvement takes several forms. First, many patients show profound improvement, often from very severe disability, during the first 3 months. However, improvement also occurs in many patients across the disability spectrum from 3 to 6 months. Improvement after 6 months was seen, but principally in patients who were less disabled. Whether this represents a lack of continued aggressive care of chronically disabled patients is unclear. Additionally, some patients worsened somewhat (often by 1 point on the mRS) throughout the year and a few patients (5% in this study) died or became disabled after 6 months due to new events remote from the initial ICH. In considering the optimal outcome assessment timepoint for clinical trials, a balance must be struck between allowing adequate time for meaningful recovery to occur and avoiding the occurrence of unrelated events which decrease study power. Of note, studies of traumatic brain injury have tended to use 6-month outcome,25 as opposed to 3-month outcome, which is commonly used in ischemic stroke trials.26 Whether ICH clinical trials should use a longer duration of follow-up should be considered and may potentially be influenced by the specific cutpoint used to define favorable outcome.
This study has numerous limitations. First, although it was a prospective study in which patients were identified and followed, 10 patients (4%) were lost to follow-up for 12-month functional outcome. Also, because this study was performed in the institutions where the ICH Score was developed, the possibility of bias exists. While the ICH Score was not formally used as a prognostic tool in clinical decision-making, residents and faculty were aware of it. However, the staff performing outcome assessments were not aware of a patient’s specific ICH Score as this was not calculated from study records until follow-up was completed. Additionally, the reasons for worsening in mRS scores by 1 point were not clear in many cases. While the possibility of variability in mRS assessment exists due to interrater differences or the fact that telephone interviews or medical record reviews were used when in-person assessments were not able to be conducted,27,28 a small group of 5 trained research personnel conducted all follow-up assessments. Finally, because patients were only followed for 1 year, the possibility of continued improvement after this point was not evaluated. However, even with these limitations, this study clearly demonstrates that the ICH Score is a validated clinical grading scale for outcome considered as mortality or functional outcome throughout the first year after acute ICH.
AUTHOR CONTRIBUTIONS
Statistical analysis was performed by Dr. Hemphill (who holds a Master’s in Clinical Research from UCSF).
DISCLOSURE
Dr. Hemphill has received stock and stock options for serving on scientific advisory boards of Innercool Therapies and Ornim; has received funding for travel from Novo Nordisk; has received honoraria for non-industry sponsored activities; has served as a consultant for Novo Nordisk, UCB Pharma, and Medivance; serves on the speakers’ bureau of the Network for Continuing Medical Education; has received research support from Novo Nordisk, the NIH [NINDS K23 NS41240 (PI) and U10 NS058931 (PI)], and the University of California [#gcp06-10218]; and has performed case reviews and given expert testimony regarding stroke or neurocritical care. M. Farrant reports no disclosures. Dr. Neill has received royalties from publishing in Up To Date and has served on speakers’ bureaus of UCB, EKR Therapeutics, and Bristol-Myers Squibb.
Address correspondence and reprint requests to Dr. J. Claude Hemphill III, Neurocritical Care Program, Department of Neurology, Room 4M62, San Francisco General Hospital, 1001 Potrero Avenue, San Francisco, CA 94110 chemphill@sfgh.ucsf.edu
Editorial, page 1084
e-Pub ahead of print on September 2, 2009, at www.neurology.org.
Supported by grant K23 NS41240 from NIH/NINDS.
Disclosure: Author disclosures are provided at the end of the article.
Received December 24, 2008. Accepted in final form June 17, 2009.
REFERENCES
- 1.Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–897. [DOI] [PubMed] [Google Scholar]
- 2.Cheung RT, Zou LY. Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke 2003;34:1717–1722. [DOI] [PubMed] [Google Scholar]
- 3.Clarke JL, Johnston SC, Farrant M, Bernstein R, Tong D, Hemphill JC 3rd. External validation of the ICH score. Neurocrit Care 2004;1:53–60. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes H, Gregson BA, Siddique MS, Mendelow AD. Testing the ICH score. Stroke 2002;33:1455–1456. [DOI] [PubMed] [Google Scholar]
- 5.Jamora RD, Kishi-Generao EM, Jr., Bitanga ES, Gan RN, Apaga NE, San Jose MC. The ICH score: predicting mortality and functional outcome in an Asian population. Stroke 2003;34:6–7. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Sandoval JL, Chiquete E, Romero-Vargas S, Padilla-Martinez JJ, Gonzalez-Cornejo S. Grading scale for prediction of outcome in primary intracerebral hemorrhages. Stroke 2007;38:1641–1644. [DOI] [PubMed] [Google Scholar]
- 7.Lyden PD, Shuaib A, Lees KR, et al. Safety and tolerability of NXY-059 for acute intracerebral hemorrhage: the CHANT Trial. Stroke 2007;38:2262–2269. [DOI] [PubMed] [Google Scholar]
- 8.Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008;358:2127–2137. [DOI] [PubMed] [Google Scholar]
- 9.Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005;365:387–397. [DOI] [PubMed] [Google Scholar]
- 10.Ariesen MJ, Algra A, van der Worp HB, Rinkel GJ. Applicability and relevance of models that predict short term outcome after intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 2005;76:839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality [see comments]. Stroke 1993;24:987–993. [DOI] [PubMed] [Google Scholar]
- 12.Cho DY, Chen CC, Lee WY, Lee HC, Ho LH. A new Modified Intracerebral Hemorrhage score for treatment decisions in basal ganglia hemorrhage: a randomized trial. Crit Care Med 2008;36:2151–2156. [DOI] [PubMed] [Google Scholar]
- 13.Rost NS, Smith EE, Chang Y, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke 2008;39:2304–2309. [DOI] [PubMed] [Google Scholar]
- 14.Tuhrim S, Dambrosia JM, Price TR, et al. Intracerebral hemorrhage: external validation and extension of a model for prediction of 30-day survival [see comments]. Ann Neurol 1991;29:658–663. [DOI] [PubMed] [Google Scholar]
- 15.Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Validation and comparison of models predicting survival following intracerebral hemorrhage. Crit Care Med 1995;23:950–954. [DOI] [PubMed] [Google Scholar]
- 16.Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage [see comments]. Crit Care Med 1999;27:617–621. [DOI] [PubMed] [Google Scholar]
- 17.Weimar C, Benemann J, Diener HC. Development and validation of the Essen Intracerebral Haemorrhage Score. J Neurol Neurosurg Psychiatry 2006;77:601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker KJ, Baxter AB, Cohen WA, et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology 2001;56:766–772. [DOI] [PubMed] [Google Scholar]
- 19.Hemphill JC 3rd, Newman J, Zhao S, Johnston SC. Hospital usage of early do-not-resuscitate orders and outcome after intracerebral hemorrhage. Stroke 2004;35:1130–1134. [DOI] [PubMed] [Google Scholar]
- 20.Zahuranec DB, Brown DL, Lisabeth LD, et al. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology 2007;68:1651–1657. [DOI] [PubMed] [Google Scholar]
- 21.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996;27:1304–1305. [DOI] [PubMed] [Google Scholar]
- 22.Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke 1988;19:1497–1500. [DOI] [PubMed] [Google Scholar]
- 23.Merino JG, Lattimore SU, Warach S. Telephone assessment of stroke outcome is reliable. Stroke 2005;36:232–233. [DOI] [PubMed] [Google Scholar]
- 24.Cuzick J. A Wilcoxon-type test for trend. Stat Med 1985;4:87–90. [DOI] [PubMed] [Google Scholar]
- 25.Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med 2001;344:556–563. [DOI] [PubMed] [Google Scholar]
- 26.Tissue plasminogen activator for acute ischemic stroke: The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 27.Newcommon NJ, Green TL, Haley E, Cooke T, Hill MD. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke 2003;34:377–378. [DOI] [PubMed] [Google Scholar]
- 28.Quinn TJ, Ray G, Atula S, Walters MR, Dawson J, Lees KR. Deriving modified Rankin scores from medical case-records. Stroke 2008;39:3421–3423. [DOI] [PubMed] [Google Scholar]