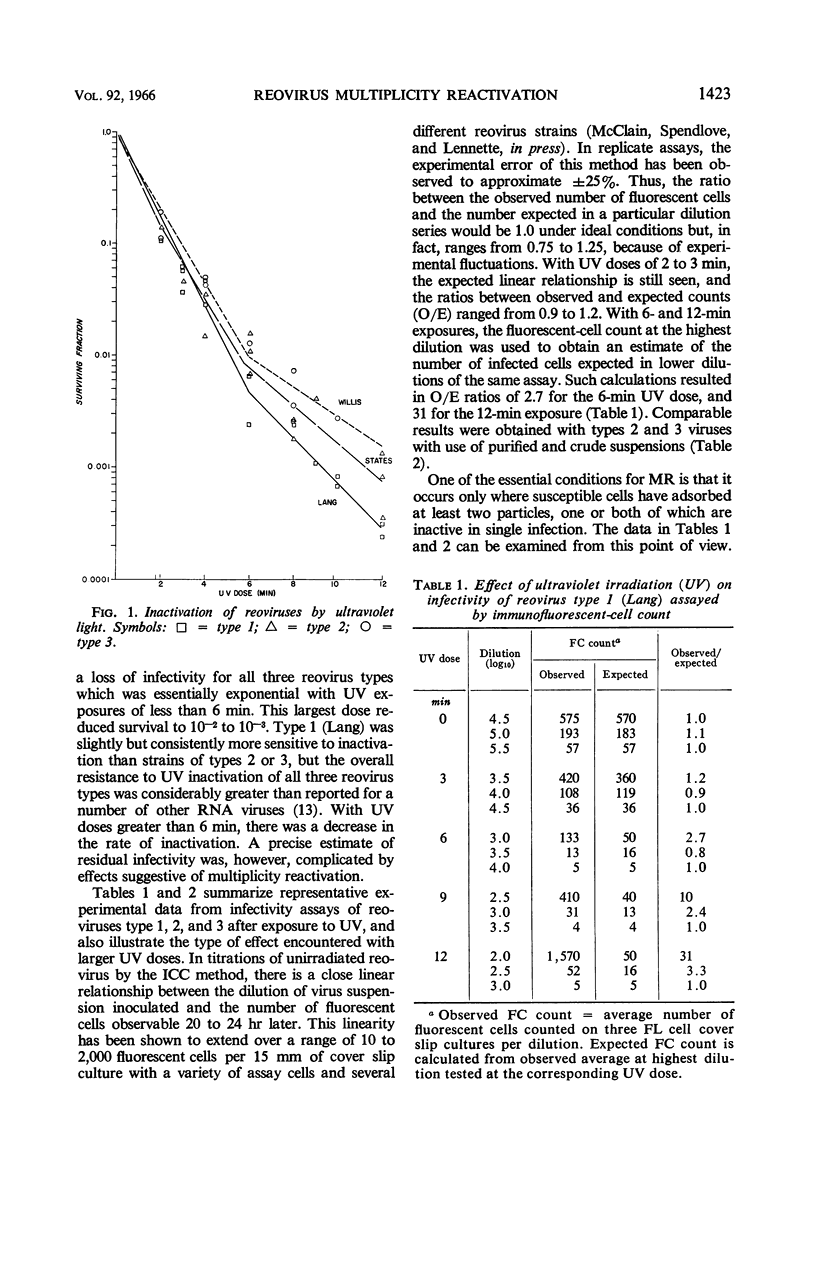
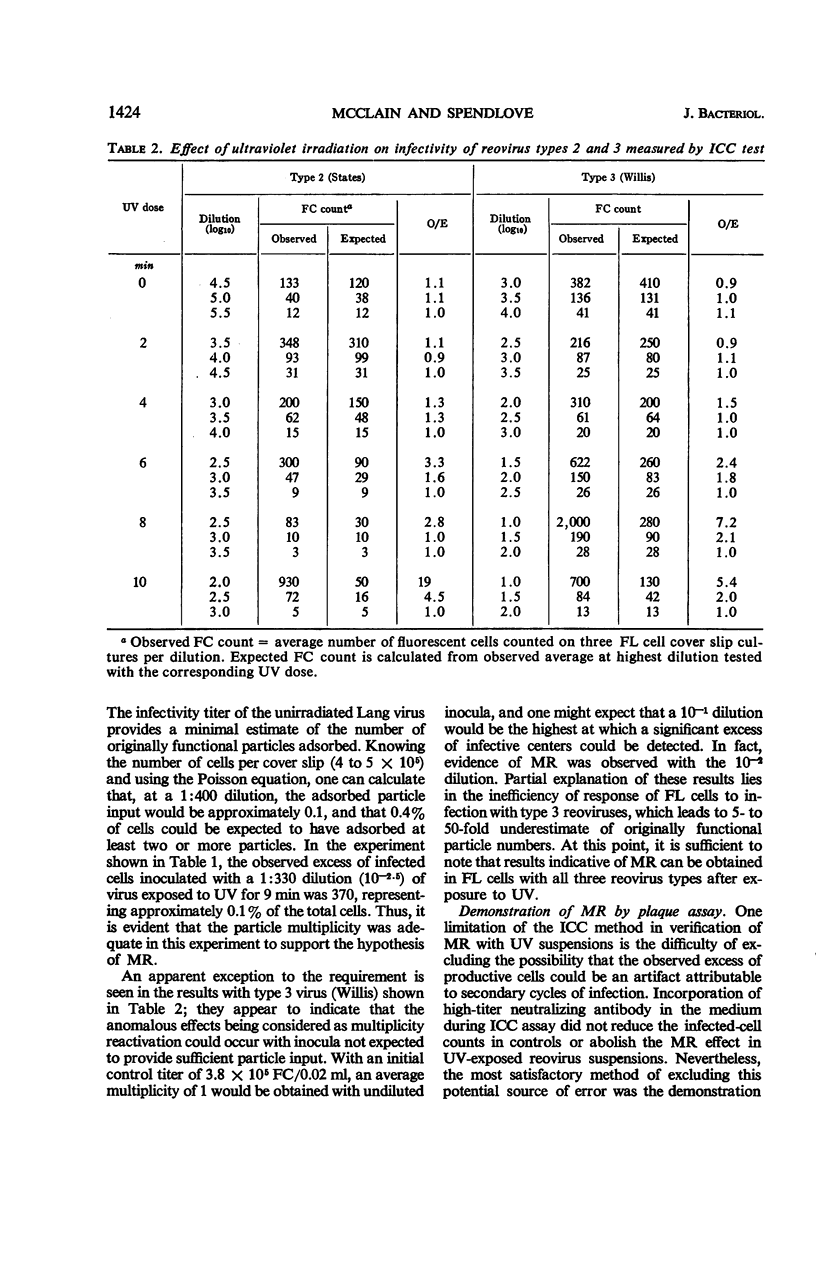
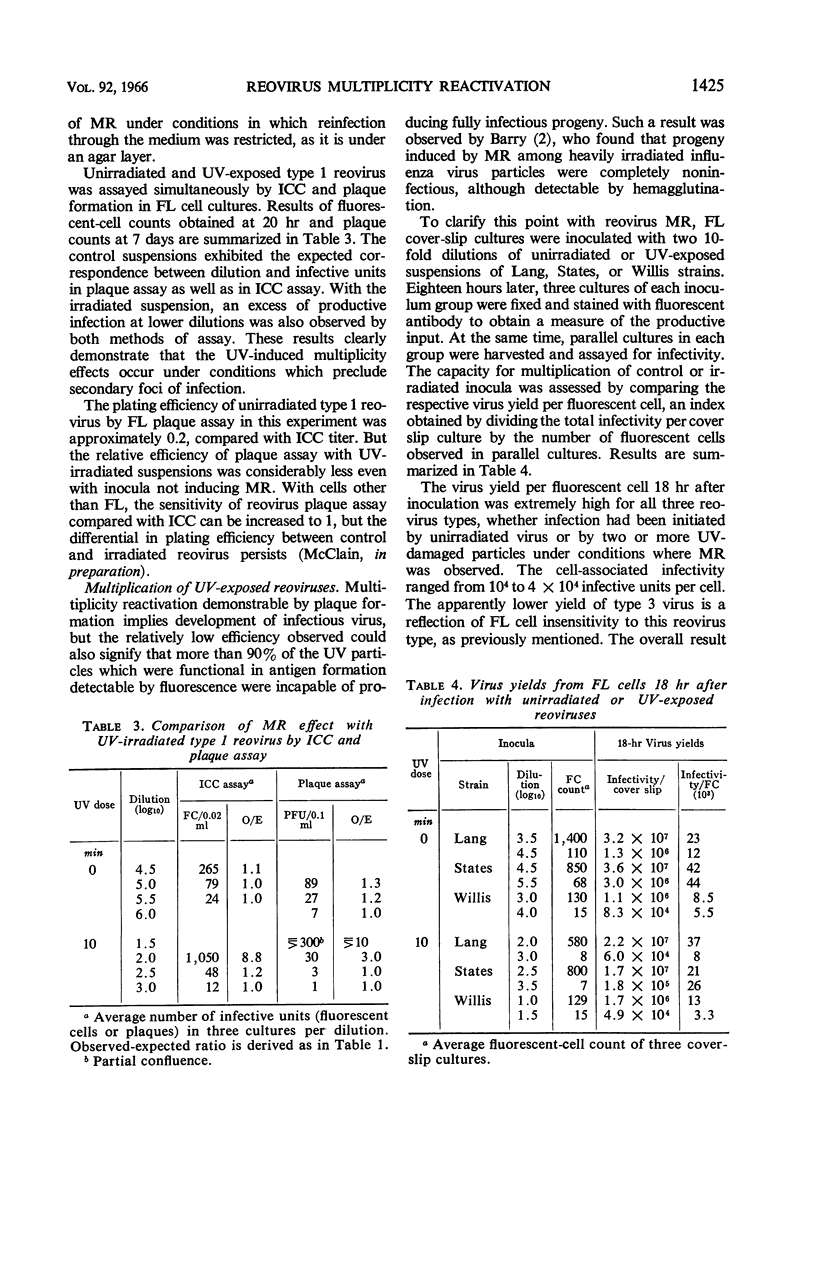
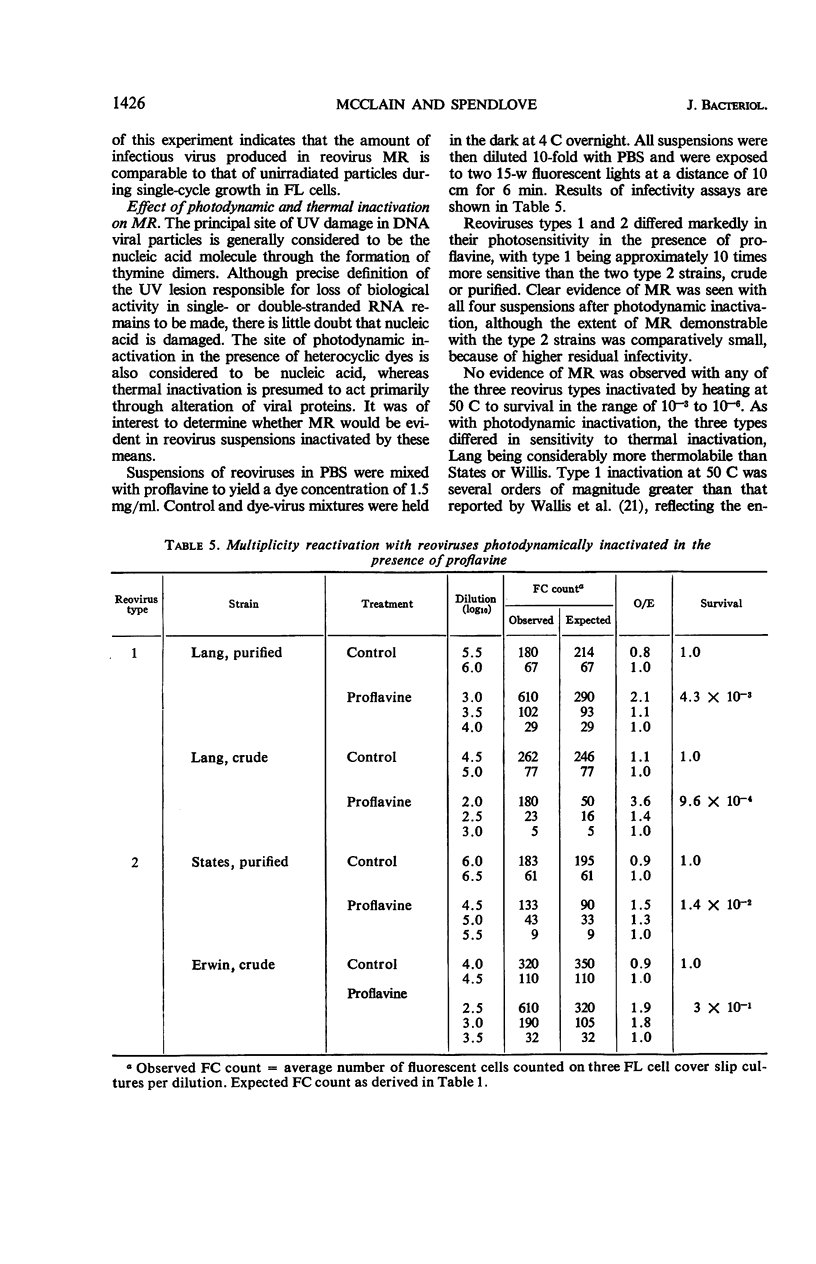
Abstract
McClain, Mary E. (California State Department of Public Health, Berkeley), and Rex S. Spendlove. Multiplicity reactivation of reovirus particles after exposure to ultraviolet light. J. Bacteriol. 92:1422–1429. 1966.—Exposure of reovirus suspensions to moderate doses of ultraviolet light results in essentially exponential inactivation of infectivity to survivals of 10−2 to 10−3. With suspensions of sufficiently high particle concentration, larger doses of ultraviolet light (6 to 12 min) are associated with multiplicity reactivation (MR) which is demonstrable both by immunofluorescent-cell count and by plaque assay in FL human amnion cells. Similar effects are produced by photodynamic inactivation in the presence of proflavine, but not by thermal inactivation at 50 C. All three reovirus types exhibit MR under appropriate conditions, and all three interact in mixed ultraviolet suspensions with high efficiency. Progeny from FL cells infected under conditions of MR were as infectious as those of unirradiated inocula, with yields per cell ranging from 104 to 4 × 104 infective units.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABEL P. Multiplicity reactivation and marker rescue with vaccinia virus. Virology. 1962 Aug;17:511–519. doi: 10.1016/0042-6822(62)90150-2. [DOI] [PubMed] [Google Scholar]
- BARRY R. D. The multiplication of influenza virus. II. Multiplicity reactivation of ultraviolet irradiated virus. Virology. 1961 Aug;14:398–405. doi: 10.1016/0042-6822(61)90330-0. [DOI] [PubMed] [Google Scholar]
- BOLLUM F. J., SETLOW R. B. Ultraviolet inactivation of DNA primer activity. I. Effects of different wavelengths and doses. Biochim Biophys Acta. 1963 Apr 30;68:599–607. doi: 10.1016/0006-3002(63)90189-6. [DOI] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAKE J. W. Interference and multiplicity reactivation in polioviruses. Virology. 1958 Aug;6(1):244–264. doi: 10.1016/0042-6822(58)90073-4. [DOI] [PubMed] [Google Scholar]
- DRAKE J. W. Multiplicity reactivation of Newcastle disease virus. J Bacteriol. 1962 Aug;84:352–356. doi: 10.1128/jb.84.2.352-356.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLISON S. A., FEINER R. R., HILL R. F. A host effect on bacteriophage survival after ultraviolet irradiation. Virology. 1960 May;11:294–296. doi: 10.1016/0042-6822(60)90069-6. [DOI] [PubMed] [Google Scholar]
- GALASSO G. J., SHARP D. G. HOMOLOGOUS INHIBITION, TOXICITY, AND MULTIPLICITY REACTIVATION WITH ULTRAVIOLET-IRRADIATED VACCINIA VIRUS. J Bacteriol. 1963 Jun;85:1309–1314. doi: 10.1128/jb.85.6.1309-1314.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirvaitis J., Simon E. H. A radiobiological study of the development of Newcastle disease virus. Virology. 1965 Aug;26(4):545–553. doi: 10.1016/0042-6822(65)90316-8. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Dulbecco R. Genetic Recombinations Leading to Production of Active Bacteriophage from Ultraviolet Inactivated Bacteriophage Particles. Genetics. 1949 Mar;34(2):93–125. doi: 10.1093/genetics/34.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E. Reactivation of Irradiated Bacteriophage by Transfer of Self-Reproducing Units. Proc Natl Acad Sci U S A. 1947 Sep;33(9):253–264. doi: 10.1073/pnas.33.9.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUPERT C. S. Photoenzymatic repair of ultraviolet damage in DNA. I. Kinetics of the reaction. J Gen Physiol. 1962 Mar;45:703–724. doi: 10.1085/jgp.45.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUPERT C. S. Photoenzymatic repair of ultraviolet damage in DNA. II. Formation of an enzyme-substrate complex. J Gen Physiol. 1962 Mar;45:725–741. doi: 10.1085/jgp.45.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauth A. M. The Physical State of Viral Nucleic Acid and the Sensitivity of Viruses to Ultraviolet Light. Biophys J. 1965 May;5(3):257–273. doi: 10.1016/s0006-3495(65)86715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETLOW R. B., SETLOW J. K. Evidence that ultraviolet-induced thymine dimers in DNA cause biological damage. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1250–1257. doi: 10.1073/pnas.48.7.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENDLOVE R. S., LENNETTE E. H., CHIN J. N., KNIGHT C. O. EFFECT OF ANTIMITOTIC AGENTS ON INTRACELLULAR REOVIRUS ANTIGEN. Cancer Res. 1964 Nov;24:1826–1833. [PubMed] [Google Scholar]
- SPENDLOVE R. S., SCHAFFER F. L. ENZYMATIC ENHANCEMENT OF INFECTIVITY OF REOVIRUS. J Bacteriol. 1965 Mar;89:597–602. doi: 10.1128/jb.89.3.597-602.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLIS C., MELNICK J. L. IRREVERSIBLE PHOTOSENSITIZATION OF VIRUSES. Virology. 1964 Aug;23:520–527. doi: 10.1016/0042-6822(64)90236-3. [DOI] [PubMed] [Google Scholar]
- WALLIS C., SMITH K. O., MELNICH J. L. REOVIRUS ACTIVATION BY HEATING AND INACTIVATION BY COOLING IN MGC12 SOLUTIONS. Virology. 1964 Apr;22:608–619. doi: 10.1016/0042-6822(64)90083-2. [DOI] [PubMed] [Google Scholar]


